Immune control by amino acid catabolism during tumorigenesis and therapy
Immune control by amino acid catabolism during tumorigenesis and therapy"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
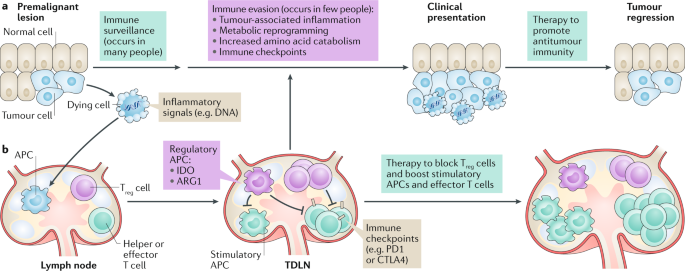
ABSTRACT Immune checkpoints arise from physiological changes during tumorigenesis that reprogramme inflammatory, immunological and metabolic processes in malignant lesions and local lymphoid
tissues, which constitute the immunological tumour microenvironment (TME). Improving clinical responses to immune checkpoint blockade will require deeper understanding of factors that
impact local immune balance in the TME. Elevated catabolism of the amino acids tryptophan (Trp) and arginine (Arg) is a common TME hallmark at clinical presentation of cancer. Cells
catabolizing Trp and Arg suppress effector T cells and stabilize regulatory T cells to suppress immunity in chronic inflammatory diseases of clinical importance, including cancers. Processes
that induce Trp and Arg catabolism in the TME remain incompletely defined. Indoleamine 2,3 dioxygenase (IDO) and arginase 1 (ARG1), which catabolize Trp and Arg, respectively, respond to
inflammatory cues including interferons and transforming growth factor-β (TGFβ) cytokines. Dying cells generate inflammatory signals including DNA, which is sensed to stimulate the
production of type I interferons via the stimulator of interferon genes (STING) adaptor. Thus, dying cells help establish local conditions that suppress antitumour immunity to promote
tumorigenesis. Here, we review evidence that Trp and Arg catabolism contributes to inflammatory processes that promote tumorigenesis, impede immune responses to therapy and might promote
neurological comorbidities associated with cancer. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more
Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IMMUNOMETABOLISM OF REGULATORY T CELLS IN CANCER Article Open access 04 June 2025 MYELOID-DERIVED ITACONATE SUPPRESSES
CYTOTOXIC CD8+ T CELLS AND PROMOTES TUMOUR GROWTH Article 14 November 2022 IMMUNOMETABOLISM IN CANCER: BASIC MECHANISMS AND NEW TARGETING STRATEGY Article Open access 16 May 2024 REFERENCES
* Bissell, M. J. & Hines, W. C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. _Nat. Med._ 17, 320–329 (2011). CAS PubMed
PubMed Central Google Scholar * Vesely, M. D. & Schreiber, R. D. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. _Ann. NY Acad. Sci._ 1284, 1–5
(2013). CAS PubMed Google Scholar * Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. _N. Engl. J. Med._ 315, 1650–1659
(1986). CAS PubMed Google Scholar * Casero, R. A. Jr, Murray Stewart, T. & Pegg, A. E. Polyamine metabolism and cancer: treatments, challenges and opportunities. _Nat. Rev. Cancer_
18, 681–695 (2018). CAS PubMed Google Scholar * Garber, K. A new cancer immunotherapy suffers a setback. _Science_ 360, 588 (2018). CAS PubMed Google Scholar * Mitchell, T. C. et al.
Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). _J. Clin Oncol._ 36, 3223–3230
(2018). PubMed Central Google Scholar * Muller, A. J., Manfredi, M., Zakharia, Y. & Prendergast, G. C. IDO inhibitors for cancer treatment: lessons from ECHO-301. _Semin.
Immunopathol_. 41, 41–48 (2019). PubMed Google Scholar * Seymour, R. L., Ganapathy, V., Mellor, A. L. & Munn, D. H. A high-affinity, tryptophan-selective amino acid transport system in
human macrophages. _J. Leukoc. Biol._ 80, 1320–1327 (2006). CAS PubMed Google Scholar * Ron, D. Translational control in the endoplasmic reticulum stress response. _J. Clin. Invest._
110, 1383–1388 (2002). CAS PubMed PubMed Central Google Scholar * Lehman, S. L., Ryeom, S. & Koumenis, C. Signaling through alternative Integrated Stress Response pathways
compensates for GCN2 loss in a mouse model of soft tissue sarcoma. _Sci. Rep._ 5, 11781 (2015). PubMed PubMed Central Google Scholar * Mossmann, D., Park, S. & Hall, M. N. mTOR
signalling and cellular metabolism are mutual determinants in cancer. _Nat. Rev. Cancer_ 18, 744–757 (2018). PubMed Google Scholar * Cormerais, Y. et al. Genetic disruption of the
multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. _Cancer Res._ 76, 4481–4492 (2016). CAS PubMed
Google Scholar * Esaki, N. et al. ASC amino acid transporter 2, defined by enzyme-mediated activation of radical sources, enhances malignancy of GD2-positive small-cell lung cancer. _Cancer
Sci._ 109, 141–153 (2018). CAS PubMed PubMed Central Google Scholar * Wyant, G. A. et al. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use
protein as a nutrient. _Cell_ 171, 642–654 (2017). CAS PubMed PubMed Central Google Scholar * Muller, A. J. et al. Chronic inflammation that facilitates tumor progression creates local
immune suppression by inducing indoleamine 2,3 dioxygenase. _Proc. Natl Acad. Sci. USA_ 105, 17073–17078 (2008). THIS GENETIC STUDY OF IDO ESTABLISHES ITS KEY CONTRIBUTIONS TO FORMATION OF A
PATHOGENIC INFLAMMATORY MILIEU THAT IS CRITICAL FOR MALIGNANT DEVELOPMENT. CAS PubMed Google Scholar * Smith, C. et al. IDO is a nodal pathogenic driver of lung cancer and metastasis
development. _Cancer Discov._ 2, 722–735 (2012). THIS STUDY OFFERS GENETIC EVIDENCE THAT IDO IS CRUCIAL FOR TUMOUR FORMATION, VASCULOGENESIS, METASTASIS AND MDSC ACTIVATION AND RECRUITMENT.
CAS PubMed PubMed Central Google Scholar * Metz, R. et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by
D-1-methyl-tryptophan. _Oncoimmunology_ 1, 1460–1468 (2012). PubMed PubMed Central Google Scholar * Mautino, M. R. et al. A novel prodrug of indoximod with enhanced pharmacokinetic
properties. _Cancer Res._ 77 (Suppl. 13), 4076 (2017). Google Scholar * Friberg, M. et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. _Int.
J. Cancer_ 101, 151–155 (2002). CAS PubMed Google Scholar * Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine
2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. _Nat. Med._ 11, 312–319 (2005). THIS STUDY LINKS IDO TO A CANCER PATHWAY AND
SHOWS THAT IDO INHIBITORS CAN EXERT ROBUST ANTITUMOUR EFFECTS IF COMBINED WITH DNA-DAMAGING CHEMOTHERAPY. CAS PubMed Google Scholar * Hou, D. Y. et al. Inhibition of indoleamine
2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. _Cancer Res._ 67, 792–801 (2007). CAS PubMed Google Scholar * Lemos, H. et
al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. _Cancer Res._ 76, 2076–2081 (2016). THIS STUDY SHOWS THAT STING PROMOTES GROWTH OF POORLY
IMMUNOGENIC TUMOURS BY STIMULATING DCS IN TDLNS TO EXPRESS IDO. CAS PubMed PubMed Central Google Scholar * Weiner, G. J. CpG oligodeoxynucleotide-based therapy of lymphoid malignancies.
_Adv. Drug Deliv. Rev._ 61, 263–267 (2009). CAS PubMed Google Scholar * Unterholzner, L. The interferon response to intracellular DNA: why so many receptors? _Immunobiology_ 218,
1312–1321 (2013). CAS PubMed Google Scholar * Prendergast, G. C., Metz, R., Muller, A. J., Merlo, L. M. & Mandik-Nayak, L. IDO2 in immunomodulation and autoimmune disease. _Front.
Immunol._ 5, 585 (2014). PubMed PubMed Central Google Scholar * Badawy, A. A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus
on aging, exercise, diet and nutritional supplements. _Neuropharmacology_ 112, 248–263 (2017). CAS PubMed Google Scholar * Morris, G., Carvalho, A. F., Anderson, G., Galecki, P. &
Maes, M. The many neuroprogressive actions of tryptophan catabolites (TRYCATs) that may be associated with the pathophysiology of neuro-immune disorders. _Curr. Pharm. Des._ 22, 963–977
(2016). CAS PubMed Google Scholar * Thomas, S. R., Mohr, D. & Stocker, R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes.
_J. Biol. Chem._ 269, 14457–14464 (1994). CAS PubMed Google Scholar * Hesterberg, R. S., Cleveland, J. L. & Epling-Burnette, P. K. Role of polyamines in immune cell functions. _Med.
Sci._ 6, E22 (2018). Google Scholar * Boutard, V. et al. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage
cytotoxicity. _J. Immunol._ 155, 2077–2084 (1995). CAS PubMed Google Scholar * Pallotta, M. T. et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by
dendritic cells. _Nat. Immunol._ 12, 870–878 (2011). CAS PubMed Google Scholar * Theate, I. et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in
normal and tumoral human tissues. _Cancer Immunol. Res._ 3, 161–172 (2015). CAS PubMed Google Scholar * Munn, D. H. & Mellor, A. L. IDO in the tumor microenvironment: inflammation,
counter-regulation, and tolerance. _Trends Immunol._ 37, 193–207 (2016). CAS PubMed PubMed Central Google Scholar * El-Zaatari, M. et al. Indoleamine 2,3-dioxygenase 1, increased in
human gastric pre-neoplasia, promotes inflammation and metaplasia in mice and is associated with type II hypersensitivity/autoimmunity. _Gastroenterology_ 154, 140–153 (2018). CAS PubMed
Google Scholar * Platten, M., Wick, W. & Van den Eynde, B. J. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. _Cancer Res._ 72, 5435–5440 (2012). CAS PubMed
Google Scholar * Prendergast, G. C., Malachowski, W. P., DuHadaway, J. B. & Muller, A. J. Discovery of IDO1 inhibitors: from bench to bedside. _Cancer Res._ 77, 6795–6811 (2017). CAS
PubMed PubMed Central Google Scholar * Uyttenhove, C. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. _Nat. Med._
9, 1269–1274 (2003). THIS IS AN EARLY REPORT HIGHLIGHTING THAT ELEVATED IDO EXPRESSION IS A COMMON TME FEATURE AND THAT IDO INHIBITION CAN ENHANCE T CELL ACCUMULATION IN THE TME. CAS
PubMed Google Scholar * Witkiewicz, A. K. et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. _J. Am. Coll. Surg._ 208, 781–787;
discussion 787–789 (2009). PubMed PubMed Central Google Scholar * Brandacher, G. et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on
tumor-infiltrating T cells. _Clin. Cancer Res._ 12, 1144–1151 (2006). THIS IS ONE OF THE EARLIEST STUDIES TO ESTABLISH THAT HIGH IDO ACTIVITY IN HUMAN TUMOURS TENDS TO ASSOCIATE WITH A POOR
PROGNOSIS. CAS PubMed Google Scholar * Yu, J. et al. Upregulated expression of indoleamine 2,3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T
cells in situ and lymph node metastasis. _Clin. Dev. Immunol._ 2011, 1–10 (2011). Google Scholar * Qian, F. et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in
reversing indoleamine-2,3-dioxygenase-mediated arrest of T cell proliferation in human epithelial ovarian cancer. _Cancer Res._ 69, 5498–5504 (2009). CAS PubMed Google Scholar *
Feder-Mengus, C. et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. _Eur. J. Cancer_ 44, 2266–2275 (2008). CAS PubMed Google Scholar * Brody, J. R. et al.
Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. _Cell Cycle_ 8, 1930–1934 |(2009). CAS
PubMed Google Scholar * Corm, S. et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma.
_Leuk. Res._ 33, 490–494 (2009). CAS PubMed Google Scholar * Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. _Nature_ 478, 197–203
(2011). THIS STUDY LINKS TDO ACTIVITY WITH AHR SIGNALLING AND SHOWS THAT THIS PATHWAY PROMOTES TUMOUR DEVELOPMENT. CAS PubMed Google Scholar * D’Amato, N. C. et al. A TDO2-AhR signaling
axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. _Cancer Res._ 75, 4651–4664 (2015). PubMed PubMed Central Google Scholar * Wei, L. et al. High
indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. _Front. Immunol._ 9, 724 (2018). PubMed PubMed Central Google Scholar * Sinclair,
L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. _Nat. Immunol._ 14, 500–508 (2013). THIS STUDY
REVEALS AN OBLIGATORY REQUIREMENT FOR ACTIVATED T CELLS TO UPREGULATE AMINO ACID TRANSPORTER ACTIVITY TO STIMULATE MTOR AND DIFFERENTIATE INTO EFFECTOR T CELLS. CAS PubMed PubMed Central
Google Scholar * Lee, G. K. et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. _Immunology_ 107, 1–9 (2002). Google Scholar * Munn, D. H. et
al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. _Immunity_ 22, 1–10 (2005). THIS STUDY IDENTIFIES A CRITICAL
REQUIREMENT FOR GCN2 SIGNALLING FOR T CELLS TO PROLIFERATE AND DIFFERENTIATE. Google Scholar * Rodriguez, P. C., Quiceno, D. G. & Ochoa, A. C. L-Arginine availability regulates
T-lymphocyte cell-cycle progression. _Blood_ 109, 1568–1573 (2007). THIS STUDY LINKS ARG CATABOLISM TO BLOCKING T CELL ENTRY INTO CELL CYCLE VIA A GCN2-DEPENDENT MECHANISM. CAS PubMed
PubMed Central Google Scholar * Sharma, M. D. et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. _Sci. Adv._ 1, e1500845 (2015). PubMed
PubMed Central Google Scholar * Sharma, M. D. et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice.
_Immunity_ 33, 942–954 (2010). CAS PubMed PubMed Central Google Scholar * Sharma, M. D. et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature
Tregs via IDO. _J. Clin. Invest._ 117, 2570–2582 (2007). CAS PubMed PubMed Central Google Scholar * Sharma, M. D. et al. An inherently bi-functional subset of Foxp3 + Treg/T-helper
cells is controlled by the transcription factor Eos. _Immunity_ 38, 998–1012 (2013). CAS PubMed PubMed Central Google Scholar * Munn, D. H. et al. Expression of indoleamine
2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. _J. Clin. Invest._ 114, 280–290 (2004). THIS STUDY IDENTIFIES IDO-EXPRESSING DCS IN TDLNS AS POTENT REGULATORS
OF T CELL IMMUNITY. CAS PubMed PubMed Central Google Scholar * Munn, D. H. et al. Potential regulatory function of human dendritic cells expressing IDO. _Science_ 297, 1867–1870 (2002).
CAS PubMed Google Scholar * Chen, W., Liang, X., Peterson, A. J., Munn, D. H. & Blazar, B. R. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic
cell-induced adaptive T regulatory cell generation. _J. Immunol._ 181, 5396–5404 (2008). CAS PubMed PubMed Central Google Scholar * Lee, J. R. et al. Pattern of recruitment of
immunoregulatory antigen-presenting cells in malignant melanoma. _Lab. Invest._ 83, 1457–1466 (2003). CAS PubMed Google Scholar * Montero, A. J., Diaz-Montero, C. M., Kyriakopoulos, C.
E., Bronte, V. & Mandruzzato, S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. _J. Immunother._ 35, 107–115 (2012). PubMed Google Scholar * Bronte, V.
& Zanovello, P. Regulation of immune responses by L-arginine metabolism. _Nat. Rev. Immunol._ 5, 641–654 (2005). CAS PubMed Google Scholar * Holmgaard, R. B. et al. Tumor-expressed
IDO recruits and activates MDSCs in a Treg-dependent manner. _Cell Rep._ 13, 412–424 (2015). THIS REPORT SHOWS THAT IDO INHIBITORS CAN PHENOCOPY IDO GENETIC BLOCKADE IN BLUNTING MDSC
RECRUITMENT AND ACTIVATION IN THE TME. CAS PubMed PubMed Central Google Scholar * Gielen, P. R. et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients
with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. _Neuro Oncol._ 18, 1253–1264 (2016). CAS PubMed PubMed Central Google Scholar * Zhang, H. et al.
Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. _Blood_ 122, 1105–1113 (2013). CAS PubMed PubMed Central Google Scholar * Mellor, A. L. et al.
Cutting edge: CpG oligonucleotides induce splenic CD19+dendritic cells to acquire potent IDO-dependent T cell regulatory functions via IFN type 1 signaling. _J. Immunol._ 175, 5601–5605
(2005). CAS PubMed Google Scholar * Ravishankar, B. et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. _Proc. Natl Acad. Sci. USA_ 109, 3909–3914 (2012). CAS
PubMed Google Scholar * Ravishankar, B. et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity.
_Proc. Natl Acad. Sci. USA_ 112, 10774–10779 (2015). CAS PubMed Google Scholar * Ravishankar, B. et al. Marginal zone CD169+macrophages coordinate apoptotic cell-driven cellular
recruitment and tolerance. _Proc. Natl Acad. Sci. USA_ 111, 4215–4220 (2014). CAS PubMed Google Scholar * Huang, L. et al. Cutting edge: DNA sensing via the STING adaptor in myeloid
dendritic cells induces potent tolerogenic responses. _J. Immunol._ 191, 3509–3513 (2013). CAS PubMed PubMed Central Google Scholar * Huang, L. et al. Engineering DNA nanoparticles as
immunomodulatory reagents that activate regulatory T cells. _J. Immunol._ 188, 4913–4920 (2012). CAS PubMed PubMed Central Google Scholar * Munn, D. H., Sharma, M. D. & Mellor, A. L.
Ligation of B7-1/B7-2 by human CD4(+) T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. _J. Immunol._ 172, 4100–4110 (2004). CAS PubMed Google Scholar * Baban, B.
et al. Physiologic control of IDO competence in splenic dendritic cells. _J. Immunol._ 187, 2329–2335 (2011). CAS PubMed PubMed Central Google Scholar * Xia, T., Konno, H., Ahn, J.
& Barber, G. N. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. _Cell Rep._ 14, 282–297 (2016). THIS STUDY
IDENTIFIES CORRELATIONS BETWEEN REDUCED STING SIGNALLING IN HUMAN COLORECTAL CARCINOMA, REDUCED RESPONSES TO DNA DAMAGE AND TUMORIGENESIS. CAS PubMed Google Scholar * Ahn, J., Xia, T.,
Rabasa Capote, A., Betancourt, D. & Barber, G. N. Extrinsic phagocyte-dependent STING signaling dictates the immunogenicity of dying cells. _Cancer Cell_ 33, 862–873 (2018). CAS PubMed
Google Scholar * Shinde, R. et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. _Nat.
Immunol._ 19, 571–582 (2018). CAS PubMed PubMed Central Google Scholar * Romani, L. & Puccetti, P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. _Trends
Microbiol._ 14, 183–189 (2006). CAS PubMed Google Scholar * DiNatale, B. C. et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces
interleukin-6 in the presence of inflammatory signaling. _Toxicol. Sci._ 115, 89–97 (2010). CAS PubMed PubMed Central Google Scholar * Duarte, J. H., Di Meglio, P., Hirota, K., Ahlfors,
H. & Stockinger, B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. _PLOS ONE_ 8, e79819 (2013). PubMed PubMed Central Google
Scholar * Mezrich, J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. _J. Immunol._ 185, 3190–3198 (2010). CAS PubMed PubMed
Central Google Scholar * Nguyen, N. T. et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. _Proc. Natl Acad. Sci.
USA_ 107, 19961–19966 (2010). CAS PubMed Google Scholar * Vogel, C. F., Goth, S. R., Dong, B., Pessah, I. N. & Matsumura, F. Aryl hydrocarbon receptor signaling mediates expression of
indoleamine 2,3-dioxygenase. _Biochem. Biophys. Res. Commun._ 375, 331–335 (2008). CAS PubMed PubMed Central Google Scholar * Litzenburger, U. M. et al. Constitutive IDO expression in
human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. _Oncotarget_ 5, 1038–1051 (2014). PubMed PubMed Central Google Scholar * Feng, S., Cao, Z.
& Wang, X. Role of aryl hydrocarbon receptor in cancer. _Biochim. Biophys. Acta_ 1836, 197–210 (2013). CAS PubMed Google Scholar * Lewis, H. C., Chinnadurai, R., Bosinger, S. E. &
Galipeau, J. The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. _Oncotarget_ 8, 91914–91927 (2017). PubMed PubMed Central
Google Scholar * Ehrlich, A. K. & Kerkvliet, N. I. Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated diseases? _Curr. Opin. Toxicol._
2, 72–78 (2017). PubMed PubMed Central Google Scholar * Hayashi, T. et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell
apoptosis. _Proc. Natl Acad. Sci. USA_ 104, 18619–18624 (2007). CAS PubMed Google Scholar * Yan, Y. et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses
encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. _J. Immunol._ 185, 5953–5961 (2010). CAS PubMed PubMed Central Google Scholar * Cronin, S. J. F. et al.
The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. _Nature_ 563, 564–568 (2018). CAS PubMed Google Scholar * Adams, S. et al. Involvement of the kynurenine
pathway in human glioma pathophysiology. _PLOS ONE_ 9, e112945 (2014). PubMed PubMed Central Google Scholar * Sahm, F. et al. The endogenous tryptophan metabolite and NAD+precursor
quinolinic acid confers resistance of gliomas to oxidative stress. _Cancer Res._ 73, 3225–3234 (2013). CAS PubMed Google Scholar * Triplett, T. A. et al. Reversal of indoleamine
2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. _Nat. Biotechnol._ 36, 758–764 (2018). CAS PubMed PubMed Central Google
Scholar * Sculier, J. P. et al. Medical anticancer treatment of lung cancer associated with comorbidities: a review. _Lung Cancer_ 87, 241–248 (2015). CAS PubMed Google Scholar *
Capuron, L. & Dantzer, R. Cytokines and depression: the need for a new paradigm. _Brain Behav. Immun._ 17, S119–S124 (2003). CAS PubMed Google Scholar * Sui, H. et al.
5-Hydroxytryptamine receptor (5-HT1DR) promotes colorectal cancer metastasis by regulating Axin1/beta-catenin/MMP-7 signaling pathway. _Oncotarget_ 6, 25975–25987 (2015). PubMed PubMed
Central Google Scholar * Gwynne, W. D. et al. Serotonergic system antagonists target breast tumor initiating cells and synergize with chemotherapy to shrink human breast tumor xenografts.
_Oncotarget_ 8, 32101–32116 (2017). PubMed PubMed Central Google Scholar * Kim, H. et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. _J.
Clin. Invest._ 122, 2940–2954 (2012). CAS PubMed PubMed Central Google Scholar * Huang, L. et al. Virus infections incite pain hypersensitivity by inducing indoleamine 2,3 dioxygenase.
_PLOS Pathog._ 12, e1005615 (2016). PubMed PubMed Central Google Scholar * LaVoy, E. C., Fagundes, C. P. & Dantzer, R. Exercise, inflammation, and fatigue in cancer survivors. _Exerc.
Immunol. Rev._ 22, 82–93 (2016). PubMed PubMed Central Google Scholar * Beatty, G. L. et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1
epacadostat (INCB024360) in patients with advanced solid malignancies. _Clin. Cancer Res._ 23, 3269–3276 (2017). CAS PubMed PubMed Central Google Scholar * Cheong, J. E., Ekkati, A.
& Sun, L. A patent review of IDO1 inhibitors for cancer. _Expert Opin. Ther. Pat._ 28, 317–330 (2018). CAS PubMed Google Scholar * Fuertes, M. B. et al. Host type I IFN signals are
required for antitumor CD8+T cell responses through CD8{alpha}+dendritic cells. _J. Exp. Med._ 208, 2005–2016 (2011). THIS STUDY SHOWS THAT TYPE I INTERFERON SIGNALS MEDIATED BY DCS IN THE
TME PROMOTE EFFECTOR T CELL RESPONSES. CAS PubMed PubMed Central Google Scholar * Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of
immunogenic tumors. _Immunity_ 41, 830–842 (2014). CAS PubMed PubMed Central Google Scholar * Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I
interferon-dependent antitumor immunity in immunogenic tumors. _Immunity_ 41, 843–852 (2014). CAS PubMed PubMed Central Google Scholar * Corrales, L. et al. Direct activation of STING in
the tumor microenvironment leads to potent and systemic tumor regression and immunity. _Cell Rep._ 11, 1018–1030 (2015). REFERENCES 102–104 SHOW THAT STING–TYPE I INTERFERON SIGNALLING
INCITES IMMUNITY DIRECTED AT IMMUNOGENIC TUMOURS AND THAT SYNTHETIC STING AGONISTS AMPLIFY THIS ANTITUMOUR RESPONSE. CAS PubMed PubMed Central Google Scholar * Li, T. et al. Antitumor
activity of cGAMP via stimulation of cGAS-cGAMP-STING-IRF3 mediated innate immune response. _Sci. Rep._ 6, 19049 (2016). CAS PubMed PubMed Central Google Scholar * Lemos, H. et al.
Activation of the STING adaptor attenuates experimental autoimmune encephalitis. _J. Immunol._ 192, 5571–5578 (2014). CAS PubMed PubMed Central Google Scholar * Aya, F. et al.
Life-threatening colitis and complete response with ipilimumab in a patient with metastatic BRAF-mutant melanoma and rheumatoid arthritis. _ESMO Open_ 1, e000032 (2016). PubMed PubMed
Central Google Scholar * De Martin, E. et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. _J. Hepatol._ 68, 1181–1190 (2018).
PubMed Google Scholar * Menzies, A. M. et al. Anti-PD1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. _Ann. Oncol._ 28,
368–376 (2017). CAS PubMed Google Scholar * Johnson, D. B. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. _JAMA Oncol._ 2, 234–240
(2016). PubMed Google Scholar * Banerjee, T. et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. _Oncogene_
27, 2851–2857 (2008). CAS PubMed Google Scholar * Ursu, R. et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric,
randomised study. _Eur. J. Cancer_ 73, 30–37 (2017). CAS PubMed Google Scholar * Moreno Ayala, M. A. et al. Dual activation of Toll-like receptors 7 and 9 impairs the efficacy of
antitumor vaccines in murine models of metastatic breast cancer. _J. Cancer Res. Clin. Oncol._ 143, 1713–1732 (2017). CAS PubMed Google Scholar * Tarhini, A. A., Gogas, H. & Kirkwood,
J. M. IFN-alpha in the treatment of melanoma. _J. Immunol._ 189, 3789–3793 (2012). CAS PubMed PubMed Central Google Scholar * Mojic, M., Takeda, K. & Hayakawa, Y. The dark side of
IFN-gamma: its role in promoting cancer immunoevasion. _Int. J. Mol. Sci._ 19, 89 (2017). PubMed Central Google Scholar * McMasters, K. M. et al. Final results of the Sunbelt melanoma
trial: a multi-institutional prospective randomized phase III study evaluating the role of adjuvant high-dose interferon alfa-2b and completion lymph node dissection for patients staged by
sentinel lymph node biopsy. _J. Clin. Oncol._ 34, 1079–1086 (2016). CAS PubMed PubMed Central Google Scholar * Mautino, M. R. et al. NLG919, a novel indoleamine-2,3-dioxygenase
(IDO)-pathway inhibitor drug candidate for cancer therapy. _Cancer Res._ 73 (Suppl. 8), 491 (2013). Google Scholar * Nayak-Kapoor, A. et al. Phase Ia study of the indoleamine
2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. _J. Immunother.Cancer._ 6, 61 (2018). PubMed PubMed Central Google Scholar * Siu,
L. L. et al. BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab
(nivo) in advanced cancers in a phase 1/2a trial. _Cancer Res._ 77 (Suppl. 13), CT116 (2017). Google Scholar * Reardon, D. et al. ATIM-29. A phase 1 study of PF-06840003, an oral indole
2,3-dioxygenase 1 (IDO1) inhibitor in patients with malignant gliomas. _Neuro Oncol._ 19, vi32 (2017). PubMed Central Google Scholar * Sahebjam, S. et al. KHK2455, a long-acting selective
IDO-1 inhibitor, in combination with mogamulizumab, an anti-CCR4 monoclonal antibody, in patients with advanced solid tumors: preliminary safety report and pharmacodynamic activity from a
first-in-human study [abstract P148]. Presented at the 2017 Society for Immunotherapy of Cancer (SITC) Annual Meeting (2017). * Mautino, M. et al. A novel prodrug of indoximod with enhanced
pharmacokinetic properties. _Cancer Res._ 77, 4076 (2017). Google Scholar * Herbst, R. S. et al. Predictive correlates of response to the anti-PDL1 antibody MPDL3280A in cancer patients.
_Nature_ 515, 563–567 (2014). CAS PubMed PubMed Central Google Scholar * Spranger, S. et al. Up-regulation of PDL1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by
CD8(+) T cells. _Sci. Transl Med._ 5, 200ra116 (2013). PubMed PubMed Central Google Scholar * Holmgaard, R. B., Zamarin, D., Munn, D. H., Wolchok, J. D. & Allison, J. P. Indoleamine
2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. _J. Exp. Med._ 210, 1389–1402 (2013). THIS STUDY SUGGESTS THAT IDO BLOCKADE CAN EMPOWER
IMMUNE CHECKPOINT THERAPY. CAS PubMed PubMed Central Google Scholar * Spranger, S. et al. Mechanism of tumor rejection with doublets of CTLA-4, PD1/PDL1, or IDO blockade involves
restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. _J. Immunother. Cancer_ 2, 3 (2014). PubMed PubMed Central Google Scholar *
Wainwright, D. A. et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PDL1 in mice with brain tumors. _Clin. Cancer Res._ 20, 5290–5301 (2014). CAS
PubMed PubMed Central Google Scholar * Zakharia, Y. et al. Interim analysis of the phase 2 clinical trial of the IDO pathway inhibitor indoximod in combination with pembrolizumab for
patients with advanced melanoma. _Cancer Res._ 77 (Suppl. 13), CT117 (2017). Google Scholar * Zakharia, Y. et al. Updates on phase1b/2 trial of the indoleamine 2,3-dioxygenase pathway
inhibitor indoximod plus checkpoint inhibitors for the treatment of unresectable stage 3 or 4 melanoma. _J. Clin. Oncol._ 34 (Suppl.), 3075 (2016). Google Scholar * Zakharia, Y., Munn, D.,
Link, C., Vahanian, N. & Kennedy, E. ACTR-53. Interim analysis of Phase 1b/2 combination of the IDO pathway inhibitor indoximod with temozolomide for adult patients with
temozolomide-refractory primary malignant brain tumors. _Neuro Oncol._ 18, vi13–vi14 (2016). Google Scholar * Smith, D. C. et al. Epacadostat plus pembrolizumab in patients with advanced
urothelial carcinoma: preliminary phase I/II results of ECHO-202/KEYNOTE-037. _J. Clin. Oncol._ 35 (Suppl.), 4503 (2017). Google Scholar * Lara, P. et al. Epacadostat plus pembrolizumab in
patients with advanced RCC: preliminary phase I/II results from ECHO-202/KEYNOTE-037. _J. Clin. Oncol._ 35 (Suppl.), 4515 (2017). Google Scholar * Gangadhar, T. C. et al. Efficacy and
safety of epacadostat plus pembrolizumab treatment of NSCLC: preliminary phase I/II results of ECHO-202/KEYNOTE-037. _J. Clin. Oncol._ 35, 9014 (2017). Google Scholar * Hamid, O. et al.
Epacadostat plus pembrolizumab in patients with SCCHN: preliminary phase I/II results from ECHO-202/KEYNOTE-037. _J. Clin. Oncol._ 35 (Suppl.), 6010 (2017). Google Scholar * US National
Library of Medicine. _ClinicalTrials.gov_ http://www.clinicaltrials.gov/ct2/show/NCT02471846 (2018). * Long, G. V. et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in
patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study. _J. Clin. Oncol._ 36 (Suppl.), 108 (2018). Google Scholar * Galluzzi, L., Buque,
A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. _Cancer Cell_ 28, 690–714 (2015). CAS PubMed Google Scholar
* Li, M. et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. _J. Immunother. Cancer_ 2, 21
(2014). CAS PubMed PubMed Central Google Scholar * Johnson, T. S. & Munn, D. H. Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance. _Immunol.
Invest._ 41, 765–797 (2012). CAS PubMed Google Scholar * Soliman, H. H. et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients
with metastatic solid tumors. _Oncotarget_ 5, 8136–8146 (2014). PubMed PubMed Central Google Scholar * Bahary, N. et al. Phase 2 trial of the indoleamine 2,3-dioxygenase pathway (IDO)
inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: interim analysis. _J. Clin. Oncol._ 34 (Suppl.), 3020 (2016). Google Scholar * Bahary,
N. et al. Results of the phase Ib portion of a phase I/II trial of the indoleamine 2, 3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of
metastatic pancreatic cancer. _J. Clin. Oncol._ 34 (Suppl.), 452 (2016). Google Scholar * Emadi, A. et al. Indoximod in combination with idarubicin and cytarabine for upfront treatment of
patients with newly diagnosed acute myeloid leukemia (AML): phase 1 report [abstract E912]. Presented at the 22nd European Hematologic Association (EHA) Congress (2017). * Johnson, T. S. et
al. PDCT-06. Radio-immunotherapy using the IDO-inhibitor indoximod in combination with re-irradiation for children with progressive brain tumors in the phase 1 setting: an updated report of
safety and tolerability (NCT02502708). _Neuro Oncol._ 19, vi185 (2017). PubMed Central Google Scholar * Johnson, T. S. et al. Safety and tolerability of combining the IDO-inhibitor
indoximod with re-irradiation for pediatric patients with progressive brain tumors treated on the NLG-2105 phase 1 trial (NCT02502708) [abstract 4027]. Presented at the 2017 American Society
of Pediatric Hematology Oncology (ASPHO) Annual Meeting (2017). * Lugade, A. A. et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor
immunity. _J. Immunol._ 180, 3132–3139 (2008). CAS PubMed Google Scholar * Ladomersky, E. et al. IDO1 inhibition synergizes with radiation and PD1 blockade to durably increase survival
against advanced glioblastoma. _Clin. Cancer Res._ 24, 2559–2573 (2018). CAS PubMed Google Scholar * Hiniker, S. M., Chen, D. S. & Knox, S. J. Abscopal effect in a patient with
melanoma. _N. Engl. J. Med._ 366, 2035; author reply 2035–2036 (2012). CAS PubMed Google Scholar * Hiniker, S. M. et al. A systemic complete response of metastatic melanoma to local
radiation and immunotherapy. _Transl Oncol._ 5, 404–407 (2012). PubMed PubMed Central Google Scholar * Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with
melanoma. _N. Engl. J. Med._ 366, 925–931 (2012). CAS PubMed PubMed Central Google Scholar * Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant
immune mechanisms in cancer. _Nature_ 520, 373–377 (2015). CAS PubMed Google Scholar * Wang, W. et al. IDO immune status after chemoradiation may predict survival in lung cancer
patients. _Cancer Res._ 78, 809–816 (2018). THIS STUDY REVEALS STRONG CORRELATIONS BETWEEN HIGH SYSTEMIC IDO ACTIVITY IN PATIENTS WITH LUNG CANCER (NSCLC) AND POOR SURVIVAL PROSPECTS AFTER
RADIOCHEMOTHERAPY. CAS PubMed Google Scholar * Gyulveszi, G. et al. RG70099: a novel, highly potent dual IDO1/TDO inhibitor to reverse metabolic suppression of immune cells in the tumor
micro-environment. _Cancer Res._ 76, LB–085 (2016). Google Scholar * Gullapalli, S. et al. EPL-1410, a novel fused heterocycle based orally active dual inhibitor of IDO1/TDO2, as a
potential immune-oncology therapeutic. _Cancer Res._ 78, 1701 (2018). Google Scholar * Wang, Y. et al. Preclinical pharmacologic and pharmacodynamic studies of a novel and potent IDO1
inhibitor D-0751. _Cancer Res._ 78 (Suppl. 13), 2736 (2018). Google Scholar * Liu, S. et al. Preclinical evaluation of TQBWX220, a small-molecule inhibitor of IDO1. _Cancer Res._ 78 (Suppl.
13), 192 (2018). Google Scholar Download references ACKNOWLEDGEMENTS Research in the A.L.M. and L.H. laboratory is supported by US National Institutes of Health (NIH) (AI103347), Cancer
Research UK and the Faculty of Medical Sciences at Newcastle University. Research in the G.C.P. laboratory is supported by NIH (CA191191), the W.W. Smith Trust, the Lankenau Medical Center
Foundation and Main Line Health. G.C.P. is the Havens Chair in Biomedical Research at the Lankenau Institute for Medical Research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of
Cellular Medicine, Faculty of Medical Sciences, Framlington Place, Newcastle University, Newcastle-upon-Tyne, UK Henrique Lemos, Lei Huang & Andrew L. Mellor * Lankenau Institute for
Medical Research, Wynnewood, PA, USA George C. Prendergast Authors * Henrique Lemos View author publications You can also search for this author inPubMed Google Scholar * Lei Huang View
author publications You can also search for this author inPubMed Google Scholar * George C. Prendergast View author publications You can also search for this author inPubMed Google Scholar *
Andrew L. Mellor View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors researched data for the article, substantially contributed to
the discussion of content and wrote, reviewed and edited the manuscript. CORRESPONDING AUTHOR Correspondence to Andrew L. Mellor. ETHICS DECLARATIONS COMPETING INTERESTS A.L.M. and G.C.P.
receive remuneration as scientific consultants for NewLink Genetics Inc. and are also shareholders in this company. G.C.P. also discloses interests in Incyte as a shareholder and in Kyn
Therapeutics as a scientific adviser. A.L.M. also discloses interests as a scientific adviser to Kyn Therapeutics. The other authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS CLINICALTRIALS.GOV DATABASE:
http://clinicaltrials.gov/ KAPLAN–MEIER PLOTTER: http://kmplot.com/ THE HUMAN PROTEIN ATLAS: http://www.proteinatlas.org/ GLOSSARY * Immune checkpoints Mechanisms that suppress local
immunity in inflamed tissues such as the tumour microenvironment. * Immunological tumour microenvironment (TME). Primary tumour lesions and local draining lymph nodes where antitumour
immunity is controlled. * Integrated stress response (ISR). A cellular response to stress that impacts protein translation via effects on the eukaryotic initiation factor eIF2. *
Damage-associated molecular patterns (DAMPs). Molecules released by dead and dying cells, which are sensed by innate immune cells. * M2 macrophages A subset of macrophages typically
associated with wound healing and tissue repair. * _N_-Methyl-d-aspartate receptor signalling (NMDAR signalling). A signalling pathway that has dichotomous effects on neurons such as
promoting death or survival of neurons, resistance to trauma and synaptic plasticity and transmission. * Mechanical nociception Perception of pain in response to a mechanical stimulus.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lemos, H., Huang, L., Prendergast, G.C. _et al._ Immune control by amino acid catabolism during
tumorigenesis and therapy. _Nat Rev Cancer_ 19, 162–175 (2019). https://doi.org/10.1038/s41568-019-0106-z Download citation * Published: 29 January 2019 * Issue Date: March 2019 * DOI:
https://doi.org/10.1038/s41568-019-0106-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
The UK prepares to advance gender equality at the 58th Session of the UN Commission on the Status of Women - GOV.UKIn preparation for the forthcoming CSW, the Government Equalities Office (GEO) hosted a well-attended event on 28 Octobe...
Arun Gawli tops in Gandhi examBecome a MemberDark ModeBecome a MemberOperation SindoorRaghav's TakeState of EducationUncovering HateQisse KahaniyaanCl...
DELAYED START AT YONEX ALL ENGLAND OPEN 2021 DUE TO COVID-19 RETESTSThe Badminton World Federation (BWF) and Badminton England can confirm a significant number of COVID-19 tests conducted ...
Lakhs join 'tweet morcha' against fuel price hikeMumbai, June 2 (IANS) People across the country tweeted to Prime Minister Narendra Modi and Maharashtra Chief Minister D...
Effects of Morphine on Uptake of Glucose and Synthesis of Glycogen in Muscle of Normal and Chronically Morphinized RatsTHERE is as yet no satisfactory explanation for the phenomenon of drug addiction. Morphine-induced biological dependence...
Latests News
Immune control by amino acid catabolism during tumorigenesis and therapyABSTRACT Immune checkpoints arise from physiological changes during tumorigenesis that reprogramme inflammatory, immunol...
RNase H hydrolysis of the 5′ terminus of the avian sarcoma virus genome during reverse transcriptionNUCLEOTIDE sequence analyses of the ends of the avian retro-virus genome 1–6 have confirmed predictions of the terminall...
AARP Has Events in Your Community and VirtualEvents AARP LocalDetailsLocationsDiscover the Local Side of AARPAARP Local has been savedAARP Local has been removed ...
Huge honour to play army officer: aayush sharmaMumbai, June 5 (IANS) Actor Aayush Sharma says it is an honour to play an Army officer in his next titled film "Kwa...
Grateful for everyone’s support: pm modi after un adopts resolution, piloted by indiaNEW DELHI: Expressing happiness after the adoption of a resolution to establish a new ‘Memorial Wall’ for fallen Peaceke...