Dynamic responses of the haematopoietic stem cell niche to diverse stresses
Dynamic responses of the haematopoietic stem cell niche to diverse stresses"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
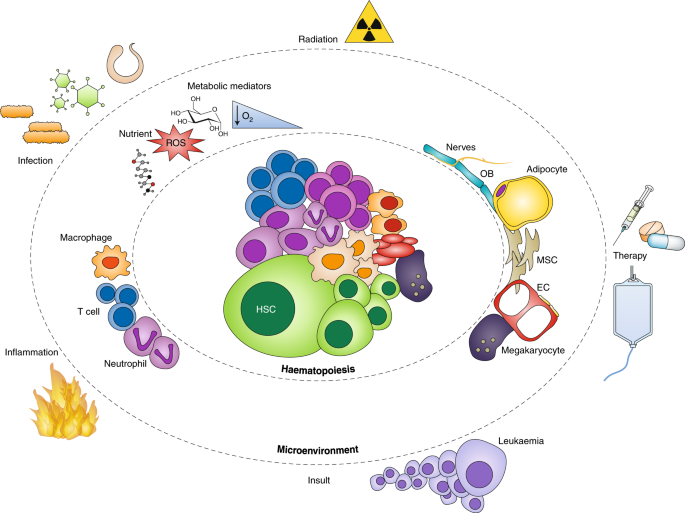
ABSTRACT Adult haematopoietic stem cells (HSCs) mainly reside in the bone marrow, where stromal and haematopoietic cells regulate their function. The steady state HSC niche has been
extensively studied. In this Review, we focus on how bone marrow microenvironment components respond to different insults including inflammation, malignant haematopoiesis and chemotherapy.
We highlight common and unique patterns among multiple cell types and their environment and discuss current limitations in our understanding of this complex and dynamic tissue. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online
access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
HEMATOPOIETIC STEM AND PROGENITOR CELL SIGNALING IN THE NICHE Article 19 October 2020 BREAST CANCER REMOTELY IMPOSES A MYELOID BIAS ON HAEMATOPOIETIC STEM CELLS BY REPROGRAMMING THE BONE
MARROW NICHE Article 30 November 2023 MICRO-ENVIRONMENTAL SENSING BY BONE MARROW STROMA IDENTIFIES IL-6 AND TGFΒ1 AS REGULATORS OF HEMATOPOIETIC AGEING Article Open access 14 August 2020
CHANGE HISTORY * _ 22 JANUARY 2020 A Correction to this paper has been published: https://doi.org/10.1038/s41556-020-0469-0 _ REFERENCES * Ding, L. & Morrison, S. J. Haematopoietic stem
cells and early lymphoid progenitors occupy distinct bone marrow niches. _Nature_ 495, 231–235 (2013). Article CAS PubMed PubMed Central Google Scholar * Greenbaum, A. et al. CXCL12 in
early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. _Nature_ 495, 227–230 (2013). Article CAS PubMed PubMed Central Google Scholar * Bilic-Curcic, I. et
al. Visualizing levels of osteoblast differentiation by a two-color promoter-GFP strategy: type I collagen-GFPcyan and osteocalcin-GFPtpz. _Genesis_ 43, 87–98 (2005). Article CAS PubMed
Google Scholar * Karsenty, G., Kronenberg, H. M. & Settembre, C. Genetic control of bone formation. _Annu. Rev. Cell Dev. Biol._ 25, 629–648 (2009). Article CAS PubMed Google Scholar
* Yamazaki, S. et al. Nonmyelinating schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. _Cell_ 147, 1146–1158 (2011). Article CAS PubMed Google Scholar
* Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. _Nature_ 460, 259–263 (2009). Article CAS PubMed PubMed Central Google
Scholar * Ishitobi, H. et al. Flk1-GFP BAC Tg mice: an animal model for the study of blood vessel development. _Exp. Anim._ 59, 615–622 (2010). Article CAS PubMed Google Scholar *
Claxton, S. et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. _Genesis_ 46, 74–80 (2008). Article CAS PubMed Google Scholar * Alva, J. A. et al.
VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. _Dev. Dyn._ 235, 759–767 (2006). Article CAS PubMed Google Scholar *
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow.
_Cell Stem Cell_ 15, 154–168 (2014). Article CAS PubMed PubMed Central Google Scholar * Joseph, C. et al. Deciphering hematopoietic stem cells in their niches: a critical appraisal of
genetic models, lineage tracing, and imaging strategies. _Cell Stem Cell_ 13, 520–533 (2013). Article CAS PubMed Google Scholar * Tjin, G. et al. Imaging methods used to study mouse and
human HSC niches: current and emerging technologies. _Bone_ 119, 19–35 (2019). Article CAS PubMed Google Scholar * Mizoguchi, T. et al. Osterix marks distinct waves of primitive and
definitive stromal progenitors during bone marrow development. _Dev. Cell_ 29, 340–349 (2014). Article CAS PubMed PubMed Central Google Scholar * Ding, L., Saunders, T. L., Enikolopov,
G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. _Nature_ 481, 457–462 (2012). Article CAS PubMed PubMed Central Google Scholar * Liu, Y.
et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. _PLoS One_ 8, e71318 (2013). Article CAS PubMed PubMed Central Google
Scholar * Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. _Nature_ 502, 637–643 (2013). Article CAS PubMed PubMed Central Google Scholar * Visnjic,
D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. _Blood_ 103, 3258–3264 (2004). Article CAS PubMed Google Scholar * Strecker, S., Fu, Y., Liu,
Y. & Maye, P. Generation and characterization of _Osterix-_ Cherry reporter mice. _Genesis_ 51, 246–258 (2013). Article CAS PubMed Google Scholar * Crane, G. M., Jeffery, E. &
Morrison, S. J. Adult haematopoietic stem cell niches. _Nat. Rev. Immunol._ 17, 573–590 (2017). Article CAS PubMed Google Scholar * Asada, N. et al. Differential cytokine contributions
of perivascular haematopoietic stem cell niches. _Nat. Cell Biol._ 19, 214–223 (2017). Article CAS PubMed PubMed Central Google Scholar * Yu, V. W. C. & Scadden, D. T. Heterogeneity
of the bone marrow niche. _Curr. Opin. Hematol._ 23, 331–338 (2016). Article CAS PubMed PubMed Central Google Scholar * Wei, Q. & Frenette, P. S. Niches for hematopoietic stem
cells and their progeny. _Immunity_ 48, 632–648 (2018). Article CAS PubMed PubMed Central Google Scholar * Takizawa, H., Boettcher, S. & Manz, M. G. Demand-adapted regulation of
early hematopoiesis in infection and inflammation. _Blood_ 119, 2991–3002 (2012). Article CAS PubMed Google Scholar * Nagai, Y. et al. Toll-like receptors on hematopoietic progenitor
cells stimulate innate immune system replenishment. _Immunity_ 24, 801–812 (2006). Article CAS PubMed PubMed Central Google Scholar * Massberg, S. et al. Immunosurveillance by
hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. _Cell_ 131, 994–1008 (2007). Article CAS PubMed PubMed Central Google Scholar * Baldridge, M.
T., King, K. Y., Boles, N. C., Weksberg, D. C. & Goodell, M. A. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. _Nature_ 465, 793–797
(2010). Article CAS PubMed PubMed Central Google Scholar * Vainieri, M. L. et al. Systematic tracking of altered haematopoiesis during sporozoite-mediated malaria development reveals
multiple response points. _Open Biol._ 6, 160038 (2016). Article PubMed PubMed Central CAS Google Scholar * Matatall, K. A. et al. Chronic infection depletes hematopoietic stem cells
through stress-induced terminal differentiation. _Cell Rep._ 17, 2584–2595 (2016). Article CAS PubMed PubMed Central Google Scholar * Rashidi, N. M. et al. In vivo time-lapse imaging
shows diverse niche engagement by quiescent and naturally activated hematopoietic stem cells. _Blood_ 124, 79–83 (2014). Article CAS PubMed PubMed Central Google Scholar * Boettcher, S.
et al. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. _Blood_ 124, 1393–1403 (2014). Article CAS PubMed PubMed Central Google Scholar *
Khakpour, S., Wilhelmsen, K. & Hellman, J. Vascular endothelial cell Toll-like receptor pathways in sepsis. _Innate Immun._ 21, 827–846 (2015). Article CAS PubMed Google Scholar *
Prendergast, A. M. et al. IFNα-mediated remodeling of endothelial cells in the bone marrow niche. _Haematologica_ 102, 445–453 (2017). Article CAS PubMed PubMed Central Google Scholar *
Andonegui, G. et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. _J. Clin. Invest._ 119, 1921–1930
(2009). CAS PubMed PubMed Central Google Scholar * Casanova-Acebes, M. et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. _Cell_ 153, 1025–1035 (2013).
Article CAS PubMed PubMed Central Google Scholar * Shi, C. et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like
receptor ligands. _Immunity_ 34, 590–601 (2011). Article CAS PubMed PubMed Central Google Scholar * Chou, D. B. et al. Stromal-derived IL-6 alters the balance of myeloerythroid
progenitors during _Toxoplasma gondii_ infection. _J. Leukoc. Biol._ 92, 123–131 (2012). Article CAS PubMed PubMed Central Google Scholar * Schurch, C. M., Riether, C. & Ochsenbein,
A. F. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. _Cell Stem Cell_ 14, 460–472 (2014). Article CAS
PubMed Google Scholar * Day, R. B., Bhattacharya, D., Nagasawa, T. & Link, D. C. Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B
lymphopoiesis in mice. _Blood_ 125, 3114–3117 (2015). Article CAS PubMed PubMed Central Google Scholar * Schajnovitz, A. et al. CXCL12 secretion by bone marrow stromal cells is
dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. _Nat. Immunol._ 12, 391–398 (2011). Article CAS PubMed Google Scholar * Petit, I. et al. G-CSF
induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. _Nat. Immunol._ 3, 687–694 (2002). Article CAS PubMed Google Scholar * Calvi, L. et al.
Osteoblastic cells regulate the haematopoietic stem cell niche. _Nature_ 425, 841–846 (2003). Article CAS PubMed Google Scholar * Zhang, J. et al. Identification of the haematopoietic
stem cell niche and control of the niche size. _Nature_ 425, 836–841 (2003). Article CAS PubMed Google Scholar * Terashima, A. et al. Sepsis-induced osteoblast ablation causes
immunodeficiency. _Immunity_ 44, 1434–1443 (2016). Article CAS PubMed Google Scholar * Imai, T. et al. Cytotoxic activities of CD8+ T cells collaborate with macrophages to protect
against blood-stage murine malaria. _eLife_ 4, e04232 (2015). Article PubMed Central Google Scholar * Joice, R. et al. _Plasmodium falciparum_ transmission stages accumulate in the human
bone marrow. _Sci. Transl. Med._ 6, 244re5 (2014). Article PubMed PubMed Central CAS Google Scholar * Lee, M. S. J. et al. _Plasmodium_ products persist in the bone marrow and promote
chronic bone loss. _Sci. Immunol._ 2, eaam8093 (2017). Article PubMed Google Scholar * McCabe, A. & MacNamara, K. C. Macrophages: key regulators of steady-state and demand-adapted
hematopoiesis. _Exp. Hematol._ 44, 213–222 (2016). Article CAS PubMed PubMed Central Google Scholar * Fujisaki, J. et al. In vivo imaging of Treg cells providing immune privilege to the
haematopoietic stem-cell niche. _Nature_ 474, 216–219 (2011). Article CAS PubMed PubMed Central Google Scholar * Hirata, Y. et al. CD150high bone marrow Tregs maintain hematopoietic
stem cell quiescence and immune privilege via adenosine. _Cell Stem Cell_ 22, 445–453.e5 (2018). Article CAS PubMed PubMed Central Google Scholar * Glatman Zaretsky, A. et al. T
regulatory cells support plasma cell populations in the bone marrow. _Cell Reports_ 18, 1906–1916 (2017). Article CAS PubMed Google Scholar * Chow, A. et al. Bone marrow CD169+
macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. _J. Exp. Med._ 208, 261–271 (2011). Article CAS PubMed PubMed Central
Google Scholar * Lieschke, G. J. et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired
neutrophil mobilization. _Blood_ 84, 1737–1746 (1994). Article CAS PubMed Google Scholar * Schuettpelz, L. G. et al. G-CSF regulates hematopoietic stem cell activity, in part, through
activation of Toll-like receptor signaling. _Leukemia_ 28, 1851–1860 (2014). Article CAS PubMed PubMed Central Google Scholar * Westerterp, M. et al. Regulation of hematopoietic stem
and progenitor cell mobilization by cholesterol efflux pathways. _Cell Stem Cell_ 11, 195–206 (2012). Article CAS PubMed PubMed Central Google Scholar * Winkler, I. G. et al. Bone
marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. _Blood_ 116, 4815–4828 (2010). Article CAS PubMed Google Scholar * Gregory, C. D.
& Devitt, A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? _Immunology_ 113, 1–14 (2004). Article CAS PubMed PubMed Central Google
Scholar * Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. _J. Leukoc. Biol._ 75, 163–189 (2004). Article CAS
PubMed Google Scholar * McCabe, A. et al. Macrophage-lineage cells negatively regulate the hematopoietic stem cell pool in response to interferon gamma at steady state and during
infection. _Stem Cells_ 33, 2294–2305 (2015). Article CAS PubMed PubMed Central Google Scholar * Zoller, E. E. et al. Hemophagocytosis causes a consumptive anemia of inflammation. _J.
Exp. Med._ 208, 1203–1214 (2011). Article CAS PubMed PubMed Central Google Scholar * Colgan, S. P., Campbell, E. L. & Kominsky, D. J. Hypoxia and mucosal inflammation. _Annu. Rev.
Pathol._ 11, 77–100 (2016). Article CAS PubMed PubMed Central Google Scholar * Taylor, C. T. & Colgan, S. P. Regulation of immunity and inflammation by hypoxia in immunological
niches. _Nat. Rev. Immunol._ 17, 774–785 (2017). Article CAS PubMed PubMed Central Google Scholar * Kwak, H. J. et al. Myeloid cell-derived reactive oxygen species externally regulate
the proliferation of myeloid progenitors in emergency granulopoiesis. _Immunity_ 42, 159–171 (2015). Article CAS PubMed PubMed Central Google Scholar * Zhu, H. et al. Reactive oxygen
species-producing myeloid cells act as a bone marrow niche for sterile inflammation-induced reactive granulopoiesis. _J. Immunol._ 198, 2854–2864 (2017). Article CAS PubMed Google Scholar
* Kristinsson, S. Y., Landgren, O., Samuelsson, J., Bjorkholm, M. & Goldin, L. R. Autoimmunity and the risk of myeloproliferative neoplasms. _Haematologica_ 95, 1216–1220 (2010).
Article PubMed PubMed Central Google Scholar * Kristinsson, S. Y. et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or
myelodysplastic syndromes. _J. Clin. Oncol._ 29, 2897–2903 (2011). Article PubMed PubMed Central Google Scholar * Fröhling, S., Scholl, C., Gilliland, D. G. & Levine, R. L. Genetics
of myeloid malignancies: pathogenetic and clinical implications. _J. Clin. Oncol._ 23, 6285–6295 (2005). Article PubMed CAS Google Scholar * Lane, S. W., Scadden, D. T. & Gilliland,
D. G. The leukemic stem cell niche: Current concepts and therapeutic opportunities. _Blood_ 114, 1150–1157 (2009). Article CAS PubMed PubMed Central Google Scholar * Zhang, B. et al.
Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. _Cancer Cell_ 21, 577–592 (2012). Article CAS PubMed PubMed Central Google
Scholar * Kode, A. et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. _Nature_ 506, 240–244 (2014). Article CAS PubMed PubMed Central Google Scholar *
Frisch, B. J. et al. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. _Blood_ 119, 540–550 (2012). Article CAS PubMed PubMed Central Google
Scholar * Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. _Cell Stem Cell_ 13, 285–299 (2013). Article CAS
PubMed PubMed Central Google Scholar * Schmidt, T. & Carmeliet, P. Angiogenesis: a target in solid tumors, also in leukemia? _Hematology (Am. Soc. Hematol. Educ. Program)_ 2011, 1–8
(2011). Article Google Scholar * Kampen, K. R., Ter Elst, A. & de Bont, E. S. Vascular endothelial growth factor signaling in acute myeloid leukemia. _Cell. Mol. Life Sci._ 70,
1307–1317 (2013). Article CAS PubMed Google Scholar * Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. _Nature_ 532, 380–384 (2016).
Article CAS PubMed PubMed Central Google Scholar * Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. _Nature_ 532, 323–328 (2016). Article CAS
PubMed PubMed Central Google Scholar * Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. _Nature_ 507,
323–328 (2014). Article CAS PubMed PubMed Central Google Scholar * Langen, U. H. et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. _Nat. Cell
Biol._ 19, 189–201 (2017). Article CAS PubMed PubMed Central Google Scholar * Passaro, D. et al. Increased vascular permeability in the bone marrow microenvironment contributes to
disease progression and drug response in acute myeloid leukemia. _Cancer Cell_ 32, 324–341 (2017). Article CAS PubMed PubMed Central Google Scholar * Duarte, D. et al. Inhibition of
endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. _Cell Stem Cell_ 22, 64–77.e6 (2018). Article CAS PubMed PubMed Central Google Scholar * Pitt, L. A. et
al. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. _Cancer Cell_ 27, 755–768 (2015). Article CAS PubMed PubMed Central Google Scholar * Passaro,
D. et al. CXCR4 is required for leukemia-initiating cell activity in T cell acute lymphoblastic leukemia. _Cancer Cell_ 27, 769–779 (2015). Article CAS PubMed Google Scholar * Duarte,
D. et al. Defining the in vivo characteristics of acute myeloid leukemia cells behavior by intravital imaging. _Immunol. Cell Biol._ 97, 229–235 (2019). Article PubMed Google Scholar *
Goulard, M., Dosquet, C. & Bonnet, D. Role of the microenvironment in myeloid malignancies. _Cell. Mol. Life Sci._ 75, 1377–1391 (2018). Article CAS PubMed Google Scholar * Dominici,
M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. _Cytotherapy_ 8, 315–317 (2006). Article
CAS PubMed Google Scholar * Geyh, S. et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. _Leukemia_ 27, 1841–1851
(2013). Article CAS PubMed Google Scholar * von der Heide, E. K., Neumann, M. & Baldus, C. D. Targeting the leukemic bone marrow microenvironment. _Oncotarget_ 8, 96474–96475 (2017).
Article PubMed PubMed Central Google Scholar * Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. _Cell_ 177, 1915–1932.e16 (2019). Article
CAS PubMed PubMed Central Google Scholar * Manshouri, T. et al. Bone marrow stroma-secreted cytokines protect JAK2V617F-mutated cells from the effects of a JAK2 inhibitor. _Cancer Res._
71, 3831–3840 (2011). Article CAS PubMed PubMed Central Google Scholar * Guarnerio, J. et al. A non-cell-autonomous role for Pml in the maintenance of leukemia from the niche. _Nat.
Commun._ 9, 66 (2018). Article PubMed PubMed Central CAS Google Scholar * Dong, L. et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment.
_Nature_ 539, 304–308 (2016). Article PubMed PubMed Central CAS Google Scholar * Vianello, F. et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid
leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. _Haematologica_ 95, 1081–1089 (2010). Article CAS PubMed PubMed Central Google Scholar * Tavor, S. et al. CXCR4
regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. _Cancer Res._ 64, 2817–2824 (2004). Article CAS PubMed Google Scholar *
Hawkins, E. D. et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. _Nature_ 538, 518–522 (2016). Article PubMed PubMed Central CAS Google
Scholar * Battula, V. L. et al. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. _JCI Insight_ 2, e90036 (2017). Article PubMed Central Google
Scholar * Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. _Cell Stem Cell_ 13, 285–299 (2013). Article
CAS PubMed PubMed Central Google Scholar * Raaijmakers, M. H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. _Nature_ 464, 852–857 (2010). Article
CAS PubMed PubMed Central Google Scholar * Tabe, Y. et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of
acute monocytic leukemia cells. _Cancer Res._ 77, 1453–1464 (2017). Article CAS PubMed PubMed Central Google Scholar * Shafat, M. S., Gnaneswaran, B., Bowles, K. M. & Rushworth, S.
A. The bone marrow microenvironment - home of the leukemic blasts. _Blood Rev._ 31, 277–286 (2017). Article PubMed Google Scholar * Cahu, X. et al. Bone marrow sites differently imprint
dormancy and chemoresistance to T-cell acute lymphoblastic leukemia. _Blood Adv._ 1, 1760–1772 (2017). Article CAS PubMed PubMed Central Google Scholar * Behan, J. W. et al. Adipocytes
impair leukemia treatment in mice. _Cancer Res._ 69, 7867–7874 (2009). Article CAS PubMed PubMed Central Google Scholar * Asano, J. et al. The serine/threonine kinase Pim-2 is a novel
anti-apoptotic mediator in myeloma cells. _Leukemia_ 25, 1182–1188 (2011). Article CAS PubMed Google Scholar * Decker, S. et al. PIM kinases are essential for chronic lymphocytic
leukemia cell survival (PIM2/3) and CXCR4-mediated microenvironmental interactions (PIM1). _Mol. Cancer Ther._ 13, 1231–1245 (2014). Article CAS PubMed Google Scholar * Ruan, J. et al.
Heparanase inhibits osteoblastogenesis and shifts bone marrow progenitor cell fate in myeloma bone disease. _Bone_ 57, 10–17 (2013). Article CAS PubMed PubMed Central Google Scholar *
Boyd, A. L. et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. _Nat. Cell Biol._ 19, 1336–1347 (2017). Article CAS
PubMed Google Scholar * Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. _Nature_ 466, 829–834 (2010). Article PubMed PubMed Central
CAS Google Scholar * Arranz, L. et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. _Nature_ 512, 78–81 (2014). Article CAS PubMed Google
Scholar * Giannopoulos, K. et al. Characterization of regulatory T cells in patients with B-cell chronic lymphocytic leukemia. _Oncol. Rep._ 20, 677–682 (2008). PubMed Google Scholar *
Muthu Raja, K. R. et al. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. _PLoS One_ 7, e47077 (2012). Article CAS
PubMed PubMed Central Google Scholar * Mansour, I., Zayed, R. A., Said, F. & Latif, L. A. Indoleamine 2,3-dioxygenase and regulatory T cells in acute myeloid leukemia. _Hematology_
21, 447–453 (2016). Article CAS PubMed Google Scholar * Kawano, Y. et al. Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. _J. Clin. Invest._ 128,
2487–2499 (2018). Article PubMed PubMed Central Google Scholar * Molldrem, J. J. et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous
leukemia. _Nat. Med._ 6, 1018–1023 (2000). Article CAS PubMed Google Scholar * Butt, N. M. et al. Circulating bcr-abl-specific CD8+ T cells in chronic myeloid leukemia patients and
healthy subjects. _Haematologica_ 90, 1315–1323 (2005). CAS PubMed Google Scholar * Schurch, C., Riether, C., Amrein, M. A. & Ochsenbein, A. F. Cytotoxic T cells induce proliferation
of chronic myeloid leukemia stem cells by secreting interferon-gamma. _J. Exp. Med._ 210, 605–621 (2013). Article CAS PubMed PubMed Central Google Scholar * Yang, L. et al. IFN-γ
negatively modulates self-renewal of repopulating human hemopoietic stem cells. _J. Immunol._ 174, 752–757 (2005). Article CAS PubMed Google Scholar * Gabrilovich, D. I. & Nagaraj,
S. Myeloid-derived suppressor cells as regulators of the immune system. _Nat. Rev. Immunol._ 9, 162–174 (2009). Article CAS PubMed PubMed Central Google Scholar * Chen, X. et al.
Induction of myelodysplasia by myeloid-derived suppressor cells. _J. Clin. Invest._ 123, 4595–4611 (2013). Article CAS PubMed PubMed Central Google Scholar * Van Valckenborgh, E. et al.
Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. _Leukemia_ 26, 2424–2428 (2012). Article PubMed
Google Scholar * Giallongo, C. et al. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in
chronic myeloid leukemia patients. _PLoS One_ 9, e101848 (2014). Article PubMed PubMed Central Google Scholar * Jitschin, R. et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived
suppressor cells that inhibit T-cell responses and promote TRegs. _Blood_ 124, 750–760 (2014). Article CAS PubMed Google Scholar * Giallongo, C. et al. Mesenchymal stem cells (MSC)
regulate activation of granulocyte-like myeloid derived suppressor cells (G-MDSC) in chronic myeloid leukemia patients. _PLoS One_ 11, e0158392 (2016). Article PubMed PubMed Central CAS
Google Scholar * Giallongo, C. et al. Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells
(MSC). _Oncotarget_ 7, 85764–85775 (2016). Article PubMed PubMed Central Google Scholar * Pyzer, A. R. et al. MUC1-mediated induction of myeloid-derived suppressor cells in patients with
acute myeloid leukemia. _Blood_ 129, 1791–1801 (2017). Article CAS PubMed PubMed Central Google Scholar * Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived
suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. _Cancer Res._ 68, 5439–5449 (2008). Article CAS PubMed PubMed Central Google Scholar * Hay,
N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? _Nat. Rev. Cancer_ 16, 635–649 (2016). Article CAS PubMed PubMed Central Google Scholar *
Valsecchi, R. et al. HIF-1alpha regulates the interaction of chronic lymphocytic leukemia cells with the tumor microenvironment. _Blood_ 127, 1987–1997 (2016). Article CAS PubMed PubMed
Central Google Scholar * Benito, J. et al. Hypoxia-activated prodrug TH-302 targets hypoxic bone marrow niches in preclinical leukemia models. _Clin. Cancer Res._ 22, 1687–1698 (2016).
Article CAS PubMed Google Scholar * Das, D. S. et al. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells.
_Leukemia_ 30, 2187–2197 (2016). Article PubMed PubMed Central CAS Google Scholar * Moschoi, R. et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid
leukemic cells during chemotherapy. _Blood_ 128, 253–264 (2016). Article CAS PubMed Google Scholar * Liu, J. et al. Stromal cell-mediated mitochondrial redox adaptation regulates drug
resistance in childhood acute lymphoblastic leukemia. _Oncotarget_ 6, 43048–43064 (2015). Article PubMed PubMed Central Google Scholar * Zhang, W. et al. Stromal control of cystine
metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. _Nat. Cell Biol._ 14, 276–286 (2012). Article CAS PubMed PubMed Central Google Scholar * Lagadinou, E. D. et
al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. _Cell Stem Cell_ 12, 329–341 (2013). Article CAS PubMed PubMed
Central Google Scholar * Cole, A. et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. _Cancer Cell_ 27, 864–876 (2015). Article
CAS PubMed PubMed Central Google Scholar * Rodriguez, A. M., Nakhle, J., Griessinger, E. & Vignais, M. L. Intercellular mitochondria trafficking highlighting the dual role of
mesenchymal stem cells as both sensors and rescuers of tissue injury. _Cell Cycle_ 17, 712–721 (2018). Article CAS PubMed PubMed Central Google Scholar * Marlein, C. R. et al. NADPH
oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. _Blood_ 130, 1649–1660 (2017). Article CAS PubMed Google Scholar *
Ricciardi, M. R. et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. _Blood_ 126, 1925–1929 (2015). Article CAS PubMed
Google Scholar * Ye, H. et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. _Cell Stem Cell_ 19, 23–37 (2016). Article CAS PubMed PubMed
Central Google Scholar * Ehsanipour, E. A. et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. _Cancer Res._ 73, 2998–3006 (2013). Article CAS
PubMed PubMed Central Google Scholar * Kalaitzidis, D. et al. Amino acid-insensitive mTORC1 regulation enables nutritional stress resilience in hematopoietic stem cells. _J. Clin.
Invest._ 127, 1405–1413 (2017). Article PubMed PubMed Central Google Scholar * Lazare, S. et al. Lifelong dietary intervention does not affect hematopoietic stem cell function. _Exp.
Hematol._ 53, 26–30 (2017). Article PubMed Google Scholar * Lu, Z. et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. _Nat.
Med._ 23, 79–90 (2017). Article CAS PubMed Google Scholar * Ghosh, J. & Kapur, R. Role of mTORC1-S6K1 signaling pathway in regulation of hematopoietic stem cell and acute myeloid
leukemia. _Exp. Hematol._ 50, 13–21 (2017). Article CAS PubMed PubMed Central Google Scholar * Agathocleous, M. et al. Ascorbate regulates haematopoietic stem cell function and
leukaemogenesis. _Nature_ 549, 476–481 (2017). Article PubMed PubMed Central Google Scholar * Cimmino, L. et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia
progression. _Cell_ 170, 1079–1095.e20 (2017). Article CAS PubMed PubMed Central Google Scholar * Wood, M. E. et al. Second malignant neoplasms: assessment and strategies for risk
reduction. _J. Clin. Oncol._ 30, 3734–3745 (2012). Article PubMed Google Scholar * Green, D. E. & Rubin, C. T. Consequences of irradiation on bone and marrow phenotypes, and its
relation to disruption of hematopoietic precursors. _Bone_ 63, 87–94 (2014). Article CAS PubMed Google Scholar * Kondo, H. et al. Total-body irradiation of postpubertal mice with 137 Cs
acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. _Radiat. Res._ 171, 283–289 (2009). Article CAS PubMed Google Scholar * Willey, J. S. et al. Early
increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. _Radiat. Res._ 170, 388–392 (2008). Article CAS PubMed PubMed Central Google Scholar * Mauch, P. et
al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. _Int. J. Radiat. Oncol. Biol. Phys._ 31, 1319–1339 (1995). Article CAS PubMed
Google Scholar * Gong, B., Oest, M. E., Mann, K. A., Damron, T. A. & Morris, M. D. Raman spectroscopy demonstrates prolonged alteration of bone chemical composition following extremity
localized irradiation. _Bone_ 57, 252–258 (2013). Article CAS PubMed PubMed Central Google Scholar * Rieger, K. et al. Mesenchymal stem cells remain of host origin even a long time
after allogeneic peripheral blood stem cell or bone marrow transplantation. _Exp. Hematol._ 33, 605–611 (2005). Article CAS PubMed Google Scholar * Dickhut, A. et al. Mesenchymal stem
cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. _Ann. Hematol._ 84, 722–727 (2005). Article PubMed Google Scholar
* Abbuehl, J.-P., Tatarova, Z., Held, W. & Huelsken, J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell
transplantation. _Cell Stem Cell_ 21, 241–255.e6 (2017). Article CAS PubMed Google Scholar * Cao, X. et al. Irradiation induces bone injury by damaging bone marrow microenvironment for
stem cells. _Proc. Natl. Acad. Sci. USA_ 108, 1609–1614 (2011). Article CAS PubMed PubMed Central Google Scholar * Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of
stem cells and haematopoiesis by secreting SCF. _Nat. Cell Biol._ 19, 891–903 (2017). Article CAS PubMed PubMed Central Google Scholar * Hooper, A. T. et al. Engraftment and
reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. _Cell Stem Cell_ 4, 263–274 (2009). Article CAS PubMed PubMed Central Google
Scholar * Poulos, M. G. et al. Endothelial jagged-1 is necessary for homeostatic and regenerative hematopoiesis. _Cell Rep._ 4, 1022–1034 (2013). Article CAS PubMed PubMed Central
Google Scholar * Bowers, E. et al. Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow. _Nat. Med._ 24, 95–102 (2018). Article CAS PubMed Google
Scholar * Kaur, S. et al. Self-repopulating recipient bone marrow resident macrophages promote long-term hematopoietic stem cell engraftment. _Blood_ 132, 735–749 (2018). Article CAS
PubMed Google Scholar * Gencheva, M. et al. Bone marrow osteoblast vulnerability to chemotherapy. _Eur. J. Haematol._ 90, 469–478 (2013). Article CAS PubMed PubMed Central Google
Scholar * Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. _Nature_ 569, 222–228 (2019). Article CAS PubMed PubMed Central Google Scholar * Itkin, T.
et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. _Blood_ 120, 1843–1855 (2012). Article
CAS PubMed Google Scholar * Zhao, M. et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. _Nat. Med._ 20, 1321–1326
(2014). Article CAS PubMed Google Scholar * Hérault, A. et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. _Nature_ 544, 53–58 (2017). Article
PubMed PubMed Central CAS Google Scholar * Lucas, D. et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. _Nat. Med._ 19, 695–703 (2013). Article CAS
PubMed PubMed Central Google Scholar * Palen, K., Thakar, M., Johnson, B. D. & Gershan, J. A. Bone marrow-derived CD8+ T cells from pediatric leukemia patients express PD1 and
expand ex vivo following induction chemotherapy. _J. Pediatr. Hematol. Oncol._ 41, 648–652 (2019). Article CAS PubMed PubMed Central Google Scholar * Wemeau, M. et al. Calreticulin
exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. _Cell Death Dis._ 1, e104 (2010). Article CAS PubMed PubMed Central
Google Scholar * Fucikova, J. et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. _Cancer Res._ 71, 4821–4833 (2011). Article CAS PubMed Google
Scholar * Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. _Science_ 334, 1573–1577 (2011). Article CAS PubMed Google
Scholar * Ersvaer, E., Liseth, K., Skavland, J., Gjertsen, B. T. & Bruserud, O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and
TREG cells. _BMC Immunol._ 11, 38 (2010). Article PubMed PubMed Central CAS Google Scholar * Salem, M. L. et al. Chemotherapy alters the increased numbers of myeloid-derived suppressor
and regulatory T cells in children with acute lymphoblastic leukemia. _Immunopharmacol. Immunotoxicol._ 40, 158–167 (2018). Article CAS PubMed Google Scholar * Barrett, D. M., Teachey,
D. T. & Grupp, S. A. Toxicity management for patients receiving novel T-cell engaging therapies. _Curr. Opin. Pediatr._ 26, 43–49 (2014). Article CAS PubMed PubMed Central Google
Scholar * Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. _N. Engl. J. Med._ 368, 1509–1518 (2013). Article CAS PubMed PubMed Central Google
Scholar * Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. _N. Engl. J. Med._ 371, 1507–1517 (2014). Article PubMed PubMed Central CAS Google
Scholar * Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. _Nat. Med._ 24, 731–738 (2018). Article CAS PubMed
PubMed Central Google Scholar * Coutu, D. L., Kokkaliaris, K. D., Kunz, L. & Schroeder, T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. _Nat.
Biotechnol._ 35, 1202–1210 (2017). Article CAS PubMed Google Scholar * Gligorijevic, B. et al. Intravital imaging and photoswitching in tumor invasion and intravasation
microenvironments. _Micros. Today_ 18, 34–37 (2010). Article PubMed PubMed Central Google Scholar * Carlson, A. L. et al. Tracking single cells in live animals using a photoconvertible
near-infrared cell membrane label. _PLoS One_ 8, e69257 (2013). Article CAS PubMed PubMed Central Google Scholar * Turcotte, R., Wu, J. W. & Lin, C. P. Intravital multiphoton
photoconversion with a cell membrane dye. _J. Biophotonics_ 10, 206–210 (2017). Article CAS PubMed Google Scholar * Alieva, M., Ritsma, L., Giedt, R. J., Weissleder, R. & van
Rheenen, J. Imaging windows for long-term intravital imaging: general overview and technical insights. _Intravital_ 3, e29917 (2014). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This Review was supported in parts by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001045), the UK Medical
Research Council (FC001045) and the Wellcome Trust (FC001045) to D.B. and by grants from the European Research Council (ERC STG 337066), the British Biology and Biotechnology Research
council (BB/i004033/1) and Bloodwise (15031 and 15040), Cancer Research UK (C36195/A26770) and the Wellcome Trust (212304/Z/18/Z) to C.L.C. A.B. and D.P. are recipients of the Junior EHA
fellowship. M.L.R.H. was funded by the Wellcome Trust. AUTHOR INFORMATION Author notes * These authors contributed equally: Antoniana Batsivari, Myriam Luydmila Rachelle Haltalli, Diana
Passaro, Constandina Pospori. AUTHORS AND AFFILIATIONS * Haematopoietic Stem Cell Laboratory, The Francis Crick Institute , London, UK Antoniana Batsivari, Diana Passaro, Constandina
Pospori, Cristina Lo Celso & Dominique Bonnet * Department of Life Sciences, Imperial College London, South Kensington campus, London, UK Myriam Luydmila Rachelle Haltalli, Constandina
Pospori & Cristina Lo Celso * Lo Celso Laboratory, The Francis Crick Institute, London, UK Myriam Luydmila Rachelle Haltalli, Constandina Pospori & Cristina Lo Celso Authors *
Antoniana Batsivari View author publications You can also search for this author inPubMed Google Scholar * Myriam Luydmila Rachelle Haltalli View author publications You can also search for
this author inPubMed Google Scholar * Diana Passaro View author publications You can also search for this author inPubMed Google Scholar * Constandina Pospori View author publications You
can also search for this author inPubMed Google Scholar * Cristina Lo Celso View author publications You can also search for this author inPubMed Google Scholar * Dominique Bonnet View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Cristina Lo Celso or Dominique Bonnet. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Batsivari, A., Haltalli, M.L.R., Passaro, D. _et al._ Dynamic responses of
the haematopoietic stem cell niche to diverse stresses. _Nat Cell Biol_ 22, 7–17 (2020). https://doi.org/10.1038/s41556-019-0444-9 Download citation * Received: 28 August 2018 * Accepted: 27
November 2019 * Published: 06 January 2020 * Issue Date: 01 January 2020 * DOI: https://doi.org/10.1038/s41556-019-0444-9 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
Elon Musk’s SpaceX raises an extra US$100 million and is now worth an estimated US$21.5 billionAdvertisementSpaceXTechTech leaders and foundersElon Musk’s SpaceX raises an extra US$100 million and is now worth an es...
Blood–brain barrier-traversing biologic secures regulatory approval, in japanRegulators in Japan have approved JCR Pharmaceuticals’ pabinafusp alfa for mucopolysaccharidosis type II (MPS II), or Hu...
Transformers for rapid detection of airway stenosis and stridorDownload PDF Article Open access Published: 02 May 2025 Transformers for rapid detection of airway stenosis and stridor ...
Ingham's World: 'Seabird colonies are an assault on all the senses, a smack in the face' | UK | News | Express.co.ukIngham's World: 'Seabird colonies are an assault on all the senses, a smack in the face'Seabird colonies are an assault ...
Europe weather maps turn red as 42c heat rips through spain, italy and greeceA volcanic heatwave is sweeping across Europe, with temperatures in some areas soaring as high as 42C. Maps by WXCHARTS ...
Latests News
Dynamic responses of the haematopoietic stem cell niche to diverse stressesABSTRACT Adult haematopoietic stem cells (HSCs) mainly reside in the bone marrow, where stromal and haematopoietic cells...
Ukrainian dad speaks out on pain after heartbreaking goodbye to sonA Ukrainian father has opened up about his heartwrenching goodbye to his family which was pictured and became one of the...
Why sweden is not in nato but russia could change everythingNATO has existed for more than 70 years since being formed shortly after the close of World War Two. The military allian...
Correction: urban–regional patterns of food purchasing behaviour: a cross-sectional analysis of the 2015–2016 australian household expenditure surveyCorrection to: _European Journal of Clinical Nutrition_ https://doi.org/10.1038/s41430-020-00746-9 The original version ...
Paul Conroy - WPRPaul ConroyLatest Posts...