Serum but not cerebrospinal fluid levels of allantoin are increased in de novo parkinson’s disease
Serum but not cerebrospinal fluid levels of allantoin are increased in de novo parkinson’s disease"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Oxidative stress supposedly plays a role in the pathogenesis of Parkinson’s disease (PD). Uric acid (UA), a powerful antioxidant, is lowered in PD while allantoin, the oxidation
product of UA and known biomarker of oxidative stress, was not systematically studied in PD. We aim to compare serum and cerebrospinal fluid (CSF) levels of UA, allantoin, and allantoin/UA
ratio in de novo PD patients and controls, and evaluate their associations with clinical severity and the degree of substantia nigra degeneration in PD. We measured serum and CSF levels of
UA, allantoin, and allantoin/UA ratio in 86 PD patients (33 females, mean age 57.9 (SD 12.6) years; CSF levels were assessed in 51 patients) and in 40 controls (19 females, 56.7 (14.1)
years). PD patients were examined using Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Montreal Cognitive Assessment (MoCA), Scales for Outcomes in Parkinson
Disease-Autonomic (SCOPA-AUT), the University of Pennsylvania Smell Identification Test (UPSIT), one-night video-polysomnography, and dopamine transporter single-photon emission computed
tomography (DAT-SPECT). Serum allantoin and allantoin/UA ratio were significantly increased in the PD group compared to controls (_p_ < 0.001 and _p_ = 0.002, respectively). Allantoin/UA
ratios in serum and CSF were positively associated with the SCOPA-AUT score (_p_ = 0.005 and 0.031, respectively) and RBD presence (_p_ = 0.044 and 0.028, respectively). In conclusion, serum
allantoin and allantoin/UA ratio are elevated in patients with de novo PD. Allantoin/UA ratio in serum and CSF is associated with autonomic dysfunction and RBD presence, indicating that
higher systemic oxidative stress occurs in PD patients with more diffuse neurodegenerative changes. SIMILAR CONTENT BEING VIEWED BY OTHERS URIC ACID AND ALTERATIONS OF PURINE RECYCLING
DISORDERS IN PARKINSON’S DISEASE: A CROSS-SECTIONAL STUDY Article Open access 09 September 2024 PARKINSON’S DISEASE IS CHARACTERIZED BY VITAMIN B6-DEPENDENT INFLAMMATORY KYNURENINE PATHWAY
DYSFUNCTION Article Open access 26 April 2025 NEUROFILAMENT LIGHT CHAIN AND Α-SYNUCLEIN RT-QUIC AS DIFFERENTIAL DIAGNOSTIC BIOMARKERS IN PARKINSONISMS AND RELATED SYNDROMES Article Open
access 11 October 2021 INTRODUCTION Parkinson’s disease (PD) is the most common form of neurodegenerative synucleinopathy. The main pathological features of PD are the loss of dopaminergic
neurons in substantia nigra pars compacta leading to the depletion of dopamine and the formation of alpha-synuclein positive intracytoplasmatic inclusions referred to as Lewy bodies in
surviving neurons1. Clinical manifestations of PD are heterogeneous, and several disease phenotypes were defined based on the spectrum of motor and non-motor symptoms. Unbiased clustering
analyses showed that patients with dysautonomia and rapid eye movement (REM) sleep behavior disorder (RBD), referred to as the “diffuse malignant” subtype, have a higher risk of cognitive
impairment and faster disease progression as compared to patients with only motor involvement, known as the “mild motor-predominant” subtype2,3. There is increasing evidence that implicates
oxidative stress as a key driver of the complex mechanism underlying neurodegeneration in PD4. Oxidative stress may be involved in the pathogenesis of PD through several mechanisms such as
oxidation of dopamine, calcium influx through L-type calcium channels and its associated excitotoxicity, mitochondrial dysfunction, protein misfolding and aggregation, or ferroptosis5.
Oxidative stress arises when the production of reactive oxygen species (ROS) exceeds clearance by endogenous antioxidants6. Uric acid (UA), the end product of purine metabolism in humans, is
one of the powerful antioxidants in the extracellular compartment with putative neuroprotective effects. UA is a scavenger of singlet oxygen, peroxy radicals, and hydroxyl radicals7. PD is
associated with lower serum UA levels, particularly in the later stages of the disease8. A series of epidemiological studies suggested that higher levels of UA are associated with a
decreased risk of disease development and a slower rate of clinical progression in PD9,10. In line with these studies, a recent dose-response meta-analysis reported that higher serum UA
levels reduced the risk for PD11. Consequently, the effects of UA were studied in preclinical disease models. UA within astrocytes prevented dopaminergic cell death and atrophy induced by
oxidative and mitochondrial toxins in cellular PD models12. UA showed attenuated toxic effects on dopaminergic cells in a mouse intrastriatal 6-hydroxydopamine model of PD13. Because of the
evolutionary loss of the functional uricase enzyme, humans are unable to further catabolize UA to the more soluble compound allantoin. However, UA can be nonenzymatically oxidized into
allantoin by ROS under conditions of increased oxidative stress14. Consequently, allantoin has emerged as a reliable biomarker for assessing the oxidative status both in vitro and in vivo15.
Whereas serum UA level is influenced by many factors such as diet, renal function, drugs, and variants in the genes coding urate transporters16, allantoin and/or allantoin/UA ratio may be
more accurate biomarkers compared to UA alone. In contrast to UA, allantoin has been only marginally studied in PD. One study revealed no differences in 24-hour urine excretion of UA,
allantoin, and allantoin/UA ratio between PD and healthy subjects17. In our previous study, we observed increased serum allantoin and allantoin/UA ratio in patients with isolated RBD (iRBD),
which is in most cases the prodromal stage of neurodegenerative synucleinopathies, including PD18. This study aimed to compare serum and cerebrospinal fluid (CSF) levels of UA, allantoin,
and allantoin/UA ratio in PD and healthy controls, and to evaluate associations of these biochemical parameters with the severity of motor and non-motor symptoms, including the presence of
RBD and the degree of substantia nigra degeneration in PD. RESULTS Of 111 patients consecutively included in the BIO-PD study between 11/2015 and 11/2021, 103 had serum samples available in
the biobank; 17 samples were excluded due to the use of medication affecting UA levels. Thus, serum samples from 86 patients were analyzed while CSF was available in 51 of them. Serum and
CSF samples from 50 controls included between 11/2015 and 06/2021 fulfilling the inclusion criteria were available in our biobank, while 10 samples were excluded due to a history of using
medication affecting UA levels, yielding 40 control samples for comparison. Mean freezer storage-times were comparable for PD and control samples (3.3 (SD 1.7) vs 3.1 (SD 1.3) years; _p_ =
0.50). Demographic data of the PD and control groups, as well as results of biochemical analyses are listed in Table 1; The PD subgroup with available CSF had demographic and clinical
parameters comparable to the total group (Supplementary Table 1). RBD was diagnosed in 21 (24%, 16 males, 5 females) PD patients. There was a significant effect of sex (_p_ < 0.001;
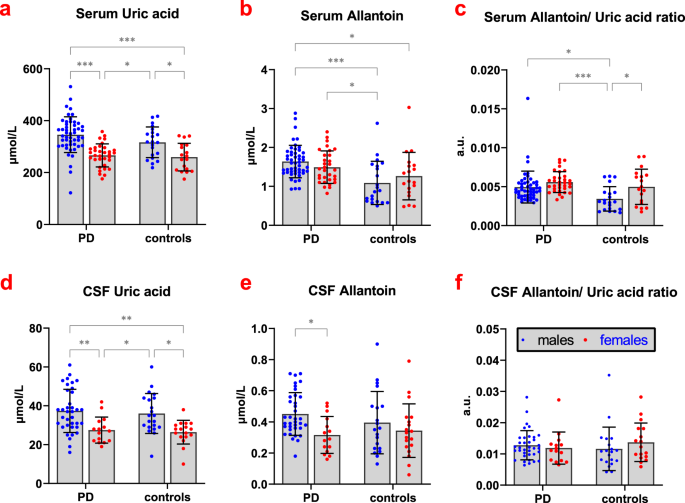
higher in men) and BMI (_p_ < 0.001; positive association) on serum UA concentrations while no between-group difference was found. Serum allantoin concentrations and serum allantoin/UA
ratio were significantly increased in the PD group compared to controls (_p_ < 0.001 and _p_ = 0.002, respectively); there was a significant effect of storage-time on serum allantoin
concentration (_p_ = 0.037; positive association) and a significant effect of sex on serum allantoin/UA ratio (_p_ = 0.007; higher in women, Fig. 1). CSF UA and allantoin concentrations were
significantly correlated with their serum concentrations (_r_ = 0.65, _p_ < 0.001 and _r_ = 0.38, _p_ < 0.001, respectively; Supplementary Table 2). There was a significant effect of
sex and age on CSF concentrations of UA (_p_ < 0.001 with higher concentrations in men and _p_ = 0.04, respectively) and allantoin (_p_ = 0.03 with higher concentrations in men and _p_
< 0.001, respectively) and significant effect of age on CSF allantoin/UA ratio (_p_ = 0.01); associations with age were positive for all parameters. No between-group differences in CSF
parameters were found. Adjusted for age and sex, allantoin/ UA ratios in serum and CSF were positively associated with SCOPA-AUT score (_p_ = 0.005 and 0.031, respectively) and RBD presence
(_p_ = 0.044 and 0.028, respectively), whereby the associations with SCOPA-AUT were mostly driven by the gastrointestinal and urinary subscores (Supplementary Table 3). CSF allantoin levels
and allantoin/ UA ratio were positively associated with disease duration (_p_ < 0.001 and 0.010, respectively). CSF UA levels were positively associated with olfactory function (_p_ =
0.013; Table 2). PD patients with RBD had higher allantoin/ UA ratios in the serum (0.006 ± 0.003 vs. 0.005 ± 0.001, _p_ = 0.039) and CSF (0.015 ± 0.006 vs. 0.011 ± 0.004, _p_ = 0.038)
compared to patients without RBD. Sensitivity analysis comparing PD and controls excluded due to medications affecting UA levels showed the same pattern of findings as the main analysis. The
excluded PD group had higher serum allantoin concentrations (_p_ < 0.001) and serum allantoin/UA ratio (_p_ < 0.001) compared to the excluded control group; no between-group
differences were observed for the CSF parameters (Supplementary Table 4). DISCUSSION Our study investigated the serum and CSF levels of UA, allantoin, and allantoin/UA ratio in patients with
PD and their associations with clinical parameters. We found significantly increased levels of serum allantoin and allantoin/UA ratio in PD patients compared to healthy controls, indicating
increased oxidative stress. Allantoin/UA ratios in serum and CSF were positively associated with autonomic dysfunction assessed using the SCOPA-AUT questionnaire and with RBD presence. CSF
UA levels were positively associated with olfactory function. Degree of motor and autonomic impairment as well as the prevalence of RBD of our cohort are comparable to previous studies in
newly diagnosed PD patients19,20 while cognitive performance is similar to the healthy Czech population in this age group21, indicating that the patient group is a representative early PD
sample. The determination of allantoin is difficult due to its low concentrations in body fluids, high polarity, and lack of chromophore for UV detection. In previous studies, allantoin was
measured in diverse clinical samples, mostly in plasma, with highly variable mean concentrations in healthy controls ranging from 0.9 to 22.0 μmol/l22. We employed the UHPLC-MS/MS method
using an isotopically labeled internal standard that proved to be precise23. Elevated levels of allantoin, supposedly related to increased systemic oxidative stress, were previously reported
in patients with various disorders, including bacterial meningitis, Behçet’s disease, recurrent aphthous stomatitis or chronic renal failure24,25. Plasmatic allantoin and allantoin/UA ratio
were unrelated to levels of lipid peroxidation product malondialdehyde (MDA)26 and may be associated with the protective antioxidant activities under systemic oxidative stress. In line with
our results, previous research on oxidative stress markers in PD showed significantly higher levels of blood 8-hydroxyguanosine, nitrite, and MDA and lower levels of catalase, glutathione,
and total-cholesterol, indicating the exhaustion of the antioxidative defense system in PD27. In contrast to results from two meta-analyses that revealed lower UA levels in PD patients8,28,
we did not find a significant difference in serum and CSF UA levels between PD patients and controls. This may be due to the inclusion of newly diagnosed, untreated PD patients - a clinical
population that did not show consistent alterations in UA levels in previous studies29. Indeed, a meta-analysis confirmed that lower UA levels may be detectable only in later disease
stages8. Due to its neuroprotective effects, UA was proposed as a potential therapeutic target in PD12,13. However, a clinical trial with urate-elevating compound inosine in early PD has not
shown any difference in the rate of clinical disease progression over placebo30. Moreover, a genome-wide association study of genetic variants underlying urate levels found no causal
associations between UA and PD risk, age at onset, or disease progression severity31. Hence, UA may not be directly involved in the pathogenesis of PD but rather its decreased levels could
represent an epiphenomenon32. In PD patients only one study examining allantoin was conducted until now and no difference between PD and healthy subjects in 24-hour urine excretion of UA,
allantoin, and allantoin/UA ratio was found17. In contrast to the latter finding, our previous study revealed increased serum allantoin and allantoin/UA ratio in patients with iRBD, implying
increased systemic oxidative stress in prodromal synucleinopathy18. In the current study, we have also found increased serum allantoin and allantoin/UA ratio in PD, but the effect was
smaller, yielding 45% increase in serum allantoin in PD compared to its 86% increase in iRBD males. Although allantoin levels were slightly higher in PD patients with RBD compared to those
without RBD, we speculate that a larger increase of allantoin/UA ratio in iRBD compared to PD implies that high serum allantoin in synucleinopathies is most pronounced in the prodromal phase
and gradually declines during later disease stages when low UA levels become apparent. We have observed moderate associations of UA, allantoin or allantoin/UA ratios in serum and CSF with
non-motor symptoms, i.e. autonomic and olfactory function and RBD presence. These results are only exploratory and need to be treated with caution since statistical correction for multiple
comparisons was not applied. Autonomic dysfunction as well as RBD are associated with a diffuse spread of synuclein pathology in the nervous system, i.e. the diffuse malignant disease
subtype33. Hence, the increased allantoin/UA ratio may be the systemic reaction to the spread of α-synuclein in the peripheral nervous system34. The association with autonomic symptoms were
mostly found in the gastrointestinal and urinary domains, which may be related to their early occurrence in synucleinopathies. Interestingly, the association between high allantoin levels
and autonomic dysfunction mirrors previous findings related to low UA and non-motor symptoms such as fatigue, attention deficit, anxiety, and cardiovascular disturbances in PD32,35, which
we, however, could not replicate. We can speculate that higher oxidative stress levels herald a more severe course in the early stage of disease and it may be worth to further study
allantoin/UA ratio as a prognostic biomarker in PD. In our current work, higher UA and allantoin levels in serum and CSF were observed in PD patients taking medication affecting UA levels
(mostly thiazide diuretics). In contrast to UA and allantoin levels, allantoin/UA ratio seems to be relatively independent on the use of medication affecting UA in PD and controls. This fact
together with its stronger associations with the severity of non-motor symptoms indicates that allantoin/UA ratio may be a more universal and useful marker in synucleinopathies compared to
UA or allantoin alone. We did not find significant between-group differences in allantoin or allantoin/UA ratio in CSF. The fact that between group difference in allantoin and allantoin/UA
levels in serum was not paralleled in CSF could reflect a higher extent of oxidative stress on a systemic level possibly reflecting alpha-synuclein spread in the peripheral nervous system in
early-stage PD. The antioxidative defense in the CNS may also involve different substances than UA. Despite not being increased, the CSF allantoin/UA ratio was significantly associated with
SCOPA-AUT score and RBD status in PD patients. It is, however, not clear whether CSF levels of UA and allantoin are truly related to dysautonomia and RBD, or rather these associations
reflect just high cross-correlation between serum and CSF levels of UA and allantoin. This finding is in line with previous reports showing that CSF UA is predominantly determined by serum
UA, and is modified by the blood-brain barrier integrity36. Yet, under pathological conditions, additional formation of UA from the catabolism of nucleotides, nucleosides, and purine
precursors within CNS may occur, as was suggested in patients with bacterial meningitis37. Theoretically, allantoin in the CSF could increase at later PD stages with higher oxidative stress
in the CNS compartment, which may be supported by the positive association between CSF allantoin and disease duration in our current study. To our knowledge, this is the first study
investigating allantoin and allantoin/UA ratio in serum and CSF in PD patients. The limitation of this study is the relatively small size of the control group. Additionally, only a clinical
control group could be used for comparison due to difficulties in obtaining CSF from healthy volunteers. This clinical control group partially consisted of patients with altered CNS
functioning, such as sleep disorders, anxiety, or chronic pain, conditions where little is known about oxidative stress involvement. However, the inclusion of heterogeneous control samples
from patients with various disorders minimizes the likelihood of significant bias. In conclusion, we have shown that serum allantoin and allantoin/UA ratio are elevated in patients with de
novo untreated PD. Moreover, we found a positive association of allantoin/UA ratio in serum and CSF with autonomic dysfunction and RBD, indicating that allantoin and allantoin/UA ratio may
be biomarkers of a more severe PD subtype with accentuated non-motor symptoms. METHODS PARTICIPANTS This study is part of a longitudinal project “biomarkers in PD (BIO-PD)” aimed at
collecting a large representative sample of de novo PD patients38. All PD patients were diagnosed based on the Movement Disorder Society clinical diagnostic criteria for PD39 and
comprehensively examined before the initiation of dopaminergic treatment. Paired serum/CSF samples from age- and sex-comparable spinal anesthesia subjects and symptomatic controls with
noninflammatory, non-neurodegenerative conditions (defined as CSF cell count ≤15 elements/unit of volume and CSF protein concentration ≤1 g/l) were selected from our CSF biobank operated
according to Consensus Guidelines for CSF and Blood Biobanking for CNS Biomarker Studies40. To minimize potential bias associated with a homogeneous control group, the selected controls
included patients with various neurological disorders including functional movement disorder (_n_ = 14), narcolepsy (_n_ = 3), idiopathic hypersomnia (_n_ = 3), headache (_n_ = 1), nerve
root compression (_n_ = 1), anxiety (_n_ = 1), spinal stenosis (_n_ = 1), and patients undergoing surgery in spinal anesthesia for nonmalignant urologic disorders including prostatic
hyperplasia (_n_ = 9), hydrocele (_n_ = 6), bladder tumor (_n_ = 6), urethral stricture (_n_ = 3), and phimosis (_n_ = 2). To be eligible for this study, all participants had to be free from
medical conditions (i.e. gout, chronic kidney disease, restless legs syndrome, and multiple sclerosis) and medications (i.e, thiazide diuretics or xanthine oxidase inhibitors) that
potentially affect UA levels. In controls, a history of neurodegenerative disorders manifesting with a movement disorder or dementia was also an exclusion criterion. Another requirement was
the availability of >200 μl serum and (in the case of controls) CSF aliquots stored in the biobank. This study was approved by the ethics committee of the General University Hospital in
Prague. Participants with PD were informed about the study and provided written informed consent; participants from the control group provided samples for biomarker research as per universal
biobank informed consent. CLINICAL EXAMINATIONS The protocol in PD patients included a structured interview, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)
part III41, Montreal Cognitive Assessment (MoCA)21, Scales for Outcomes in Parkinson Disease-Autonomic (SCOPA-AUT) questionnaire42, olfactory testing using the University of Pennsylvania
Smell Identification Test (UPSIT)43, one-night video-polysomnography, and dopamine transporter single-photon emission computed tomography (DAT-SPECT). DAT-SPECT was performed using the
[123I]-2-b-carbomethoxy-3b-(4-iodophenyl)-N-(3-fluoropropyl) nortropane ([123I]FP-CIT, DaTscan®, GE Healthcare, Little Chalfont, Buckinghamshire, UK) tracer according to European Association
of Nuclear Medicine (EANM) procedure guidelines44. The acquisition parameters were as follows: rotational radius 13–15 cm, image matrix 128 × 128, angular sampling with 120 projections at
3° interval and 40 s per view, zoom 1.3, energy window 159 ± 10% keV. Reconstruction of the raw SPECT projection data was performed using the ordered subset expectation maximization (OSEM)
algorithm with 8 iterations and 10 subsets including Chang attenuation correction (μ = 0.11 cm−1) and 3D Butterworth post-filtering with FWHM = 8 mm45. Automated semi-quantitative analysis
was performed using the BasGan V2 software and specific binding ratios (SBR) in both putamina were calculated according to the formula [(putamen binding–background binding)/background
binding]; the lower value from both hemispheres was used for further analyses46. RBD was diagnosed based on clinical history and video-polysomnography according to the International
Classification of Sleep Disorders, third edition (ICSD-3)47. LABORATORY ANALYSES In PD patients, venous blood and optionally CSF samples were drawn at the baseline visit in the morning after
an overnight fast. In controls, sampling was performed in the morning after an overnight fast either at the neurology or urology department. Lumbar punctures were performed using 22 G
atraumatic needles in PD patients and neurologic controls and by 25 G needles in patients undergoing spinal anesthesia. Sera were separated within 60 minutes after collection and then
aliquoted. CSF was immediately centrifuged at 3000 rpm under 4 °C, and the supernatant was aliquoted. All samples were frozen at −80 °C until further biochemical analysis. The concentration
of UA in serum and CSF was determined using the Beckman Coulter AU system with Beckman Coulter Uric Acid kits (Beckman Coulter, Brea, CA, USA) in a standard biochemical laboratory at the
Institute of Rheumatology in Prague. Allantoin concentration in serum and CSF was determined by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) with an
isotopically labeled internal standard. Before the analysis, protein precipitation was used. Samples were processed as follows: 60 µL of 100% acetonitrile (containing 1.33 μM allantoin-13C2,
15N4 as an internal standard) was added to 20 µL of the sample. The mixture was vortexed and centrifuged at 16500×g for 6 min. 50 µL of supernatant was transferred into an LC vial. For the
UHPLC-MS/MS analysis, a UHPLC system Agilent 1290 with a Triple Quad 6460 mass spectrometer (Agilent Technologies, Waldbronn, Germany) was used. The hydrophilic interaction liquid
chromatography (HILIC) conditions consisted of the Acquity BEH Amide column; isocratic elution by a mobile phase composed of acetonitrile and 0.1% formic acid 90/10 (v/v). The flow rate of
the mobile phase was maintained at 0.3 mL/min, and the injection volume was 2 µL. The temperature of the column was kept at 30 °C and samples were thermostated at 10 °C. The MS/MS
spectrometer was operated in a positive mode. The applied conditions of the electrospray ion source were as follows: gas temperature: 300 °C, gas flow: 7 L/min, sheath gas temperature: 300
°C, sheath gas flow: 8 L/min, nebulizer pressure: 310 kPa and capillary voltage: 6000 V. The MS/MS measurement was performed in multiple reaction-monitoring mode (MRM). Two MRM transitions
were monitored for allantoin: Quantifier transition was 159 > 116 (collision energy 2 V and fragmentor voltage 50 V) and a qualifier transition was 159 > 99 (collision energy 8 V and
fragmentor voltage 50 V). The transition 165 > 120 (collision energy 2 V and fragmentor voltage 50 V) was monitored for allantoin-13C2, 15N4.This method, which has been validated in terms
of linearity, lower limit of quantification (LLOQ), upper limit of quantification (ULOQ), accuracy, precision, selectivity, recovery, carry-over effect, matrix effects, robustness, and
stability of both quality control (QC) samples and clinical patient samples, was demonstrated to be suitable for its intended purpose. The UHPLC-MS/MS method with one-step sample preparation
provided high sensitivity and high sample throughput23. STATISTICAL ANALYSES Comparisons of UA, allantoin, and allantoin/UA ratio in the serum and CSF between PD and controls were performed
using a univariate general linear model with group and sex as fixed factors, and with age, body mass index (BMI), and freezer storage-time as covariates. Difference in sample storage-times
may influence the concentration of biomolecules and was thus suggested to be included as a covariate in longitudinal cohort studies48. Post-hoc multiple comparisons were performed using
Tukey’s HSD. Chi-square statistics were used to analyze binary variables. Pearson partial correlation coefficients controlling for age and sex were calculated to examine relationships
between biochemical and clinical variables. Correlation analyses were considered exploratory and corrections for multiple comparisons were thus not applied. IBM SPSS statistics version 25
(IBM, Armonk, NY, USA) was used for statistical analysis; graphs were plotted using Graphpad Prism version 9.0 (Graphpad software, San Diego, CA, USA). REPORTING SUMMARY Further information
on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY Individual participant data that underlie the findings of this study are
available upon request to the corresponding author by qualified researchers (i.e., affiliated to a respected university or research institution/hospital). CODE AVAILABILITY Not relevant.
REFERENCES * Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. _Nature_ 388, 839–840 (1997). Article CAS PubMed Google Scholar * Fereshtehnejad, S. M., Zeighami, Y., Dagher, A.
& Postuma, R. B. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. _Brain_ 140, 1959–1976 (2017). Article PubMed Google Scholar * Riboldi,
G. M., Russo, M. J., Pan, L., Watkins, K. & Kang, U. J. Dysautonomia and REM sleep behavior disorder contributions to progression of Parkinson’s disease phenotypes. _NPJ Parkinsons Dis._
8, 110 (2022). Article PubMed PubMed Central Google Scholar * Trist, B. G., Hare, D. J. & Double, K. L. Oxidative stress in the aging substantia nigra and the etiology of
Parkinson’s disease. _Aging Cell_ 18, e13031 (2019). Article CAS PubMed PubMed Central Google Scholar * Yu, Z. et al. The significance of uric acid in the diagnosis and treatment of
Parkinson disease: An updated systemic review. _Med. (Baltim.)_ 96, e8502 (2017). Article CAS Google Scholar * Sies, H. Oxidative stress: a concept in redox biology and medicine. _Redox
Biol._ 4, 180–183 (2015). Article CAS PubMed PubMed Central Google Scholar * Ames, B. N., Cathcart, R., Schwiers, E. & Hochstein, P. Uric acid provides an antioxidant defense in
humans against oxidant- and radical-caused aging and cancer: a hypothesis. _Proc. Natl Acad. Sci._ 78, 6858–6862 (1981). Article CAS PubMed PubMed Central Google Scholar * Wen, M. et
al. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. _PLoS One_ 12, e0173731 (2017). Article PubMed PubMed Central Google Scholar * Ascherio, A. et al. Urate
as a predictor of the rate of clinical decline in Parkinson disease. _Arch. Neurol._ 66, 1460–1468 (2009). Article PubMed PubMed Central Google Scholar * Schwarzschild, M. A. et al.
Serum Urate as a Predictor of Clinical and Radiographic Progression in Parkinson Disease. _Arch. Neurol._ 65, 716–723 (2008). Article PubMed PubMed Central Google Scholar * Chang, H.,
Wang, B., Shi, Y. & Zhu, R. Dose-response meta-analysis on urate, gout, and the risk for Parkinson’s disease. _npj Parkinson’s Dis._ 8, 160 (2022). Article CAS Google Scholar *
Cipriani, S. et al. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. _PLoS One_ 7, e37331 (2012). Article CAS PubMed PubMed
Central Google Scholar * Chen, X. et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. _Proc. Natl Acad. Sci._ 110, 300–305 (2013). Article CAS
PubMed Google Scholar * Kaur, H. & Halliwell, B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. _Chem.-Biol.
Interact._ 73, 235–247 (1990). Article CAS PubMed Google Scholar * Grootveld, M. & Halliwell, B. Measurement of allantoin and uric acid in human body fluids. A potential index of
free-radical reactions in vivo? _Biochem. J._ 243, 803–808 (1987). Article CAS PubMed PubMed Central Google Scholar * Dalbeth, N., Gosling, A. L., Gaffo, A. & Abhishek, A. Gout.
_Lancet_ 397, 1843–1855 (2021). Article CAS PubMed Google Scholar * Sampat, R. et al. Potential mechanisms for low uric acid in Parkinson disease. _J. Neural Transm._ 123, 365–370
(2016). Article CAS PubMed Google Scholar * Hasíková, L. et al. Patients with REM sleep behavior disorder have higher serum levels of allantoin. _Parkinsonism Relat. Disord._ 90, 38–43
(2021). Article PubMed Google Scholar * Simuni, T. et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: the PPMI cohort. _J. Neurol.
Neurosurg. Psychiatry_ 89, 78–88 (2018). Article PubMed Google Scholar * Mollenhauer, B. et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa
cohort. _Neurology_ 81, 1226–1234 (2013). Article PubMed Google Scholar * Kopecek, M. et al. Montreal cognitive assessment (MoCA): Normative data for old and very old Czech adults. _Appl
Neuropsychol. Adult_ 24, 23–29 (2017). Article PubMed Google Scholar * Kanďár, R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress
in humans. _Biomed. Chromatogr._ 30, 13–28 (2016). Article PubMed Google Scholar * Kozlik, P., Hasikova, L., Stiburkova, B., Zavada, J. & Kalikova, K. Rapid and reliable HILIC-MS/MS
method for monitoring allantoin as a biomarker of oxidative stress. _Anal. Biochem_ 589, 113509 (2020). Article CAS PubMed Google Scholar * Kastenbauer, S., Koedel, U., Becker, B. F.
& Pfister, H. W. Oxidative stress in bacterial meningitis in humans. _Neurology_ 58, 186–191 (2002). Article CAS PubMed Google Scholar * Yardim‐Akaydin, S., Sepici, A., Özkan, Y.,
Şimşek, B. & Sepici, V. Evaluation of allantoin levels as a new marker of oxidative stress in Behçet’s disease. _Scand. J. Rheumatol._ 35, 61–64 (2006). Article PubMed Google Scholar
* Kand'ár, R., Žáková, P. & Mužáková, V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid
chromatography. _Clin. Chim. Acta_ 365, 249–256 (2006). Article PubMed Google Scholar * Wei, Z., Li, X., Li, X., Liu, Q. & Cheng, Y. Oxidative Stress in Parkinson’s Disease: A
Systematic Review and Meta-Analysis. _Front Mol. Neurosci._ 11, 236 (2018). Article PubMed PubMed Central Google Scholar * Shen, L. & Ji, H. F. Low uric acid levels in patients with
Parkinson’s disease: evidence from meta-analysis. _BMJ Open_ 3, e003620 (2013). Article PubMed PubMed Central Google Scholar * Koros, C. et al. Serum uric acid level as a putative
biomarker in Parkinson’s disease patients carrying GBA1 mutations: 2-Year data from the PPMI study. _Parkinsonism Relat. Disord._ 84, 1–4 (2021). Article CAS PubMed Google Scholar *
Schwarzschild, M. A. et al. Effect of Urate-Elevating Inosine on Early Parkinson Disease Progression: The SURE-PD3 Randomized Clinical Trial. _Jama_ 326, 926–939 (2021). Article CAS PubMed
Google Scholar * Coneys, R., Storm, C. S., Kia, D. A., Almramhi, M. & Wood, N. W. Mendelian Randomisation Finds No Causal Association between Urate and Parkinson’s Disease
Progression. _Mov. Disord._ 36, 2182–2187 (2021). Article PubMed Google Scholar * Grażyńska, A. et al. The Influence of Serum Uric Acid Level on Non-Motor Symptoms Occurrence and Severity
in Patients with Idiopathic Parkinson’s Disease and Atypical Parkinsonisms-A Systematic Review. _Medicina (Kaunas)_ 57, (2021). * De Pablo-Fernandez, E. et al. Association of Autonomic
Dysfunction With Disease Progression and Survival in Parkinson Disease. _JAMA Neurol._ 74, 970–976 (2017). Article PubMed PubMed Central Google Scholar * Chen, Z., Li, G. & Liu, J.
Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. _Neurobiol. Dis._ 134, 104700 (2020). Article PubMed Google Scholar * Moccia, M.
et al. Is serum uric acid related to non-motor symptoms in de-novo Parkinson’s disease patients? _Parkinsonism Relat. Disord._ 20, 772–775 (2014). Article PubMed Google Scholar * Bowman,
G. L., Shannon, J., Frei, B., Kaye, J. A. & Quinn, J. F. Uric acid as a CNS antioxidant. _J. Alzheimers Dis._ 19, 1331–1336 (2010). Article CAS PubMed PubMed Central Google Scholar
* Becker, B. F., Kastenbauer, S., Ködel, U., Kiesl, D. & Pfister, H. W. Urate oxidation in CSF and blood of patients with inflammatory disorders of the nervous system. _Nucleosides
Nucleotides Nucleic Acids_ 23, 1201–1204 (2004). Article CAS PubMed Google Scholar * Dušek, P. et al. Clinical characteristics of newly diagnosed Parkinson’s disease patients included in
the longitudinal BIO-PD study. _Česká a slovenská neurologie a neurochirurgie_ 83, 633–639 (2020). Article Google Scholar * Postuma, R. B. et al. MDS clinical diagnostic criteria for
Parkinson’s disease. _Mov. Disord._ 30, 1591–1601 (2015). Article PubMed Google Scholar * Teunissen, C. E. et al. A consensus protocol for the standardization of cerebrospinal fluid
collection and biobanking. _Neurology_ 73, 1914–1922 (2009). Article CAS PubMed PubMed Central Google Scholar * Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the
Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. _Mov. Disord._ 23, 2129–2170 (2008). Article PubMed Google Scholar * Visser, M.,
Marinus, J., Stiggelbout, A. M. & Van Hilten, J. J. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. _Mov. Disord._ 19, 1306–1312 (2004). Article PubMed
Google Scholar * Doty, R. L., Shaman, P. & Dann, M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function.
_Physiol. Behav._ 32, 489–502 (1984). Article CAS PubMed Google Scholar * Darcourt, J. et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine
transporter ligands, version 2. _Eur. J. Nucl. Med Mol. Imaging_ 37, 443–450 (2010). Article CAS PubMed Google Scholar * Dušek, P. et al. Relations of non-motor symptoms and dopamine
transporter binding in REM sleep behavior disorder. _Sci. Rep._ 9, 15463 (2019). Article PubMed PubMed Central Google Scholar * Calvini, P. et al. The basal ganglia matching tools
package for striatal uptake semi-quantification: description and validation. _Eur. J. Nucl. Med Mol. Imaging_ 34, 1240–1253 (2007). Article PubMed Google Scholar * American Academy of
Sleep, M. _International classification of sleep disorders_, (American Academy of Sleep Medicine, Darien, IL, 2014). * Enroth, S., Hallmans, G., Grankvist, K. & Gyllensten, U. Effects of
Long-Term Storage Time and Original Sampling Month on Biobank Plasma Protein Concentrations. _EBioMedicine_ 12, 309–314 (2016). Article PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This study was supported by the Czech Ministry of Health (Grant NU21-04-0053, and MH CZ–DRO-VFN64165), and National Institute for Neurological Research (Programme
EXCELES, ID Project No. LX22NPO5107 (MEYS): Financed by European Union – Next Generation EU) and by the project (Ministry of Health, Czech Republic) for consensual development of research
organization 023728. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Rheumatology, Prague, Czech Republic; Department of Rheumatology, First Faculty of Medicine, Charles
University, Prague, Czech Republic Lenka Hasíková & Jakub Závada * Department of Neurology and Centre of Clinical Neuroscience, First Faculty of Medicine, Charles University and General
University Hospital, Prague, Czech Republic Tereza Serranová, Karel Šonka, Evžen Růžička & Petr Dušek * Department of Analytical Chemistry, Faculty of Science, Charles University,
Prague, Czech Republic Petr Kozlík * Department of Physical and Macromolecular Chemistry, Faculty of Science, Charles University, Prague, Czech Republic Květa Kalíková * Institute of Medical
Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic Lenka Kotačková * Institute of Nuclear
Medicine, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic Jiří Trnka & David Zogala * Department of Radiology, First Faculty of
Medicine, Charles University and General University Hospital, Prague, Czech Republic Petr Dušek Authors * Lenka Hasíková View author publications You can also search for this author inPubMed
Google Scholar * Jakub Závada View author publications You can also search for this author inPubMed Google Scholar * Tereza Serranová View author publications You can also search for this
author inPubMed Google Scholar * Petr Kozlík View author publications You can also search for this author inPubMed Google Scholar * Květa Kalíková View author publications You can also
search for this author inPubMed Google Scholar * Lenka Kotačková View author publications You can also search for this author inPubMed Google Scholar * Jiří Trnka View author publications
You can also search for this author inPubMed Google Scholar * David Zogala View author publications You can also search for this author inPubMed Google Scholar * Karel Šonka View author
publications You can also search for this author inPubMed Google Scholar * Evžen Růžička View author publications You can also search for this author inPubMed Google Scholar * Petr Dušek
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors were involved in drafting the manuscript or revising it critically for content.
L.H. wrote the manuscript, contributed to the design of the study, organized the allantoin measurement and other biochemical measurements. J.Z. designed the project. T.S. contributed to the
design of the study. L.K. contributed to the biological sample collection, P.K. and K.K. carried out allantoin measurements. J.T. and D.Z. carried out DAT-SPECT analysis, K.Š. and E.R.
contributed to the biological sample and clinical data collection, P.D. supervised the project, contributed to the design of the project, conducted statistical data analysis, contributed to
the biological sample and clinical data collection and wrote the manuscript. The final manuscript has been seen and approved by all authors. CORRESPONDING AUTHOR Correspondence to Petr
Dušek. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1, TABLE 2, TABLE 3, TABLE 4 REPORTING SUMMARY RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hasíková, L., Závada, J.,
Serranová, T. _et al._ Serum but not cerebrospinal fluid levels of allantoin are increased in de novo Parkinson’s disease. _npj Parkinsons Dis._ 9, 60 (2023).
https://doi.org/10.1038/s41531-023-00505-0 Download citation * Received: 27 November 2022 * Accepted: 27 March 2023 * Published: 12 April 2023 * DOI:
https://doi.org/10.1038/s41531-023-00505-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Mumbai: mbpt developing lng terminal on west coastThe Mumbai Port Trust is developing floating LNG (liquefied natural gas) terminal on the west coast. At a distance of 6 ...
Grape-nuts to offer refunds to those who overpaid| Consumers who spent $10 or more for a box of Grape-Nuts during a recent supply shortage may be eligible for reimbursem...
Old image of farmer’s death revived amid recent protests_(If you feel suicidal or know someone in distress, please reach out to them with kindness and call these_ _numbers_ _of...
Channelnews : hisense tv’s: dumb.. But cheapHisense has unleashed 10 affordable new TV’s, starting from $299 for a 24″ to $1299 for a 55″ LED TV. The new thin LED ...
Day 2: sopore remains shut to mourn naseer’s killingSopore town observed a complete shutdown for the second consecutive day on Saturday to mourn the killing of local Tehree...
Latests News
Serum but not cerebrospinal fluid levels of allantoin are increased in de novo parkinson’s diseaseABSTRACT Oxidative stress supposedly plays a role in the pathogenesis of Parkinson’s disease (PD). Uric acid (UA), a pow...
In times of sickness, we turn to food for comfort, prevention and curePerhaps I wouldn’t have noticed the persistent threat of infection that runs through the final instalment of Hilary Mant...
Merkel says she doesn’t blame herself for not trying hard enough for ukraineMerkel says she doesn’t blame herself for not trying hard enough for Ukraine | WTVB | 1590 AM · 95.5 FM | The Voice of B...
Aarp smart guide to muscle health | members only20. EMBRACE AEROBICS Aerobic exercise and strength training are perfect bedfellows. In 2022, for example, the _British J...
'Toilet king of Punjab': AAP leaders criticised for inaugurating restroom repairs with commemorative plaqueNewsletters ePaper Sign in HomeIndiaKarnatakaOpinionWorldBusinessSportsVideoEntertainmentDH SpecialsOperation SindoorNew...