Microstructure and corrosion evolution of additively manufactured aluminium alloy aa7075 as a function of ageing
Microstructure and corrosion evolution of additively manufactured aluminium alloy aa7075 as a function of ageing"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Additively manufactured high strength aluminium (Al) alloy AA7075 was prepared using selective laser melting (SLM). High strength Al-alloys prepared by SLM have not been widely
studied to date. The evolution of microstructure and hardness, with the attendant corrosion, were investigated. Additively manufactured AA7075 was investigated both in the “as-produced”
condition and as a function of artificial ageing. The microstructure of specimens prepared was studied using electron microscopy. Production of AA7075 by SLM generated a unique
microstructure, which was altered by solutionising and further altered by artificial ageing—resulting in microstructures distinctive to that of wrought AA7075-T6. The electrochemical
response of additively manufactured AA7075 was dependent on processing history, and unique to wrought AA7075-T6, whereby dissolution rates were generally lower for additively manufactured
AA7075. Furthermore, immersion exposure testing followed by microscopy, indicated different corrosion morphology for additively manufactured AA7075, whereby resultant pit size was notably
smaller, in contrast to wrought AA7075-T6. SIMILAR CONTENT BEING VIEWED BY OTHERS CORROSION MECHANISM OF AN EQUIMOLAR ALCOCRFENI HIGH-ENTROPY ALLOY ADDITIVELY MANUFACTURED BY ELECTRON BEAM
MELTING Article Open access 11 August 2020 ENHANCED AGING KINETICS IN AL-MG-SI ALLOYS BY UP-QUENCHING Article Open access 07 June 2021 NON-OXIDE PRECIPITATES IN ADDITIVELY MANUFACTURED
AUSTENITIC STAINLESS STEEL Article Open access 17 May 2021 INTRODUCTION Metal additive manufacturing (AM) is a promising technology for complex and net-shape prototyping and production, with
minimal waste generation.1,2,3,4,5 With the prospect of net shape production of high strength aluminium (Al) alloys,6,7,8,9,10,11,12 the interest in AM technology to produce components from
high strength 7xxx series (Al–Zn–Mg(–Cu)) Al-alloys is increasing.6,13,14,15,16,17,18 To date, the microstructural evolution and corrosion behaviour of AM prepared 7xxx series Al-alloys
have not been studied and hence not understood in contrast to wrought 7xxx series Al-alloys presently used in aerospace applications. The microstructure of wrought aluminium alloy AA7075,
which is conventionally produced from homogenised cast ingots by either rolling, extrusion or forging, has been extensively studied.19,20,21,22,23,24,25,26,27,28 Wrought AA7075-T6, as a
plate or sheet product, principally contains nanometre scale η′-phase (which nominally MgZn2) precipitates as the major strengthening phase. However, several second phase particles
(including dispersoid phases or non-strengthening constituent particles), such as Al7Cu2Fe, Al3Fe, Mg2Si, Al2Cu, Al2CuMg, MgZn2, Al6Mn, Al3Ti and Al3Zr, with varying size, shape and
distribution have been previously determined to populate wrought AA7075.19,20,21,22,23,24,25,26,27,28 Second phase particles influence corrosion behaviour of wrought
AA7075.26,29,30,31,32,33,34,35 The localised corrosion, and its morphology, of wrought 7xxx Al-alloys is principally associated with second phase particles, as the particles exhibit
electrochemical characteristics different from those of the Al-matrix.26,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 Electrochemical characteristics of several second phase particles
that populate in the Al-matrix of wrought AA7075 have been previously determined in solutions of various chloride concentrations and pH, either by the production of bulk intermetallic
phases, by the electrochemical microcell technique, or by electron microscopy30,38,44,45,46,47,48,49,50,51,52—providing a deterministic rationale for the of each second phases in the
localised corrosion of AA7075. It was determined that Al7Cu2Fe, Al3Fe, Al6Mn, Al3Ti, Al3Zr and Al2Cu are ‘cathodic’ to the Al-matrix; whilst, Mg2Si, Al2CuMg and MgZn2 are ‘anodic’ to the
Al-matrix. At sites-containing cathodic particles, localised corrosion develops in the form of circumferential trenches by dissolving the Al-matrix at the particles’ periphery.26,30,31,44,45
Whilst at anodic sites, ‘self-dissolution’ of the second phase particle occurs, most typically in the form of incongruent particle dissolution/dealloying of Mg from such anodic second phase
particles (Mg2Si, Al2CuMg and MgZn2) resulting in remnants of either Si-, Al2Cu- and Zn-, respectively.38,46,47,48,49,50,51,52 As has been documented previously, following its dealloying,
the remnant of Al2CuMg has been determined to behave as a local cathode, dissolving the Al-matrix,50,51,52 the role of Mg2Si and MgZn2 (the latter of which may be Cu enriched42) remnants is
not clear. Pitting and localised corrosion associated with second phase particles evolves with time, and serves as the precursor to intergranular corrosion in wrought AA7075-T6, which is an
alloy that can also suffer stress corrosion cracking in the presence of tensile stress.31,33,34,35,36,37,38,39,40,41 Further, pits in AA7075-T6 also well documented to act as initiation
sites for the nucleation of fatigue cracks as a result of cyclic loading.53,54,55 To date, studies on the microstructure and mechanical properties of SLMed 7xxx Al-alloys are
limited,6,13,14,15,16,17,18 and in the context of corrosion, no prior studies exist to date. A recent study discovered the predominant presence of a nanometre scale icosahedral
quasicrystalline particle, termed ν-phase.13 This (ν) phase was comprised of Zn–Mg–Cu(–Al), a structurally and chemically unique in the Al-matrix of as-SLMed AA7075.13 As a consequence of
this discovery, a more detailed study upon as-SLMed AA7075, including as a function of artificial ageing, is essential in the context of understanding microstructural evolution—and also the
corrosion response in contrast to wrought AA7075-T6. Herein, the microstructure, hardness, electrochemical behaviour and localised corrosion response of SLMed AA7075 in three different
conditions, viz. as-SLMed, solutionised + quenched, and solutionised + quenched and aged at 120 °C for 24 h, were studied and compared with those of wrought AA7075-T6. RESULTS
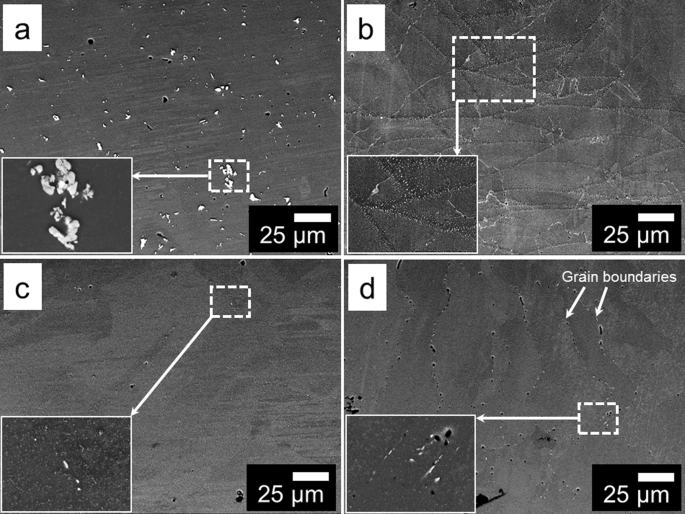
MICROSTRUCTURAL ANALYSIS SCANNING ELECTRON MICROSCOPY (SEM) ANALYSIS The microstructures of AA7075-T6 and SLMed AA7075 in three different conditions, viz. as-SLMed, solutionised + quenched,
and solutionised + quenched and aged at 120 °C for 24 h, were realised using SEM in backscattered electron (BSE) mode (Fig. 1). Specimens from wrought AA7075-T6 exhibited uniformly
distributed coarse (up to ~15 µm in size) second phase particles in the Al-matrix (Fig. 1a), with such coarse second phase particles often forming networks that may extend up to ~100 µm in
size (Fig. 1a, inset). The coarse particles were identified as Al–Cu–Fe(–Si) phase constituents, as determined from EDXS point analysis, which are nominally formed during the casting
process19 and do not adequately redissolve during subsequent thermomechanical processing. In contrast, as-SLMed AA7075 revealed a characteristic melt pool structure (synonymous with SLM
prepared alloys) with high density nanometre to sub-micrometre scale Mg–Zn–Cu(–Al) and Mg2Si (β-phase) second phase particles. The Mg–Zn–Cu(–Al) and Mg2Si (β-phase) particles were
homogenously distributed in the Al-matrix (Fig. 1b), emphasising the impact of high solidification rates (>106 °C/s3,7,16,18) that are achieved during the SLM process. The second phase
particles in as-SLMed AA7075 were mostly spherical; however, some elongated and thread-like shaped particles were also observed along melt pool boundaries (Fig. 1b). In addition, the second
phase particles were considerably finer within the core of melt pools and coarser at melt pool boundaries. Such difference in particle size, shape and distribution at melt pool boundaries
when compared to those within the core of melt pools may be rationalised by the cooling rate gradient within each melt pool. Melt pool boundaries experience lower cooling rates than the core
of melt pools,5,7,16,17,56 and thus may result in greater solute rearrangement at the boundaries (although noting that the attendant cooling rates are likely to significantly inhibit
diffusion). It is noted that the SLM process itself, led to loss of Zn and Mg, whilst Cu, Cr and Fe were not lost, as determined from ICP-OES compositional analysis of the AA7075 feedstock
powder and as-SLMed AA7075 (Table 1). In such high-power laser-based AM processing, elements with low fusion are susceptible to vaporising as a result of their higher equilibrium vapour
pressure than that of Al.57 Loss of alloying elements as vapour was determined to increase scan track instability, porosity and solidification cracking in AM specimens.57 It is also noted
that the SLM process annihilated coarse Fe-containing constituent particles in as-SLMed AA7075. The annihilation of coarse Fe-containing constituent particles in as-SLMed AA7075 is
beneficial in the context of localised corrosion susceptibility, since localised corrosion predominantly associated with such particles in wrought
AA7075-T6.26,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 In addition, the loss of Zn and Mg may also influence the artificial aging of SLMed AA7075, as Zn and Mg are essential for
forming MgZn2 phase precipitates in the Al-matrix. The effect of solutionising on the microstructure of SLMed AA7075 can be observed from Fig. 1c. The majority of the sub-micrometre-sized
particles, as well as the melt pool boundaries observed in the as-SLMed condition are no longer perceptible in the solutionised condition (at identical scales). At the SEM image scale, it is
corroborated the dissolution of the majority of particles in the Al-matrix of SLMed AA7075 resulted in super-saturated solid solution (Fig. 1c). The undissolved second phase particles, as
shown in the inset in Fig. 1c, were determined to be Al–Cu–Fe(–Si) particles, which do not dissolve upon solutionising.19 However, the size and number density of such coarse Fe-containing
constituents in solutionised SLMed AA7075 was significantly lower than in wrought AA7075-T6. Further ageing at 120 °C for 24 h resulted in the nucleation and growth of precipitates along
grain boundaries and within grains (Fig. 1d). As the diffusion of solute is higher along grain boundaries than within grains,58 precipitates were larger along grain boundaries, revealing
remnant columnar grains in the microstructure (Fig. 1d). From EDXS-SEM point analysis, it was identified that the second phase particles in aged SLMed AA7075 are Al–Cu–Fe(–Si) constituent
particles and in precipitates containing Zn, Mg and Cu (η-phase). Very fine pits (dark in contrast) corresponding to second phase particles in Fig. 1a–d were formed as a result of final
polishing in colloidal silica suspension. The SEM analysis overall revealed significant microstructural variation between wrought AA7075-T6 and SLMed AA7075. In addition, the microstructure
of as-SLMed AA7075 also varied significantly with solutionising and artificial ageing. Such microstructural variations are important to understand as they are significant in influencing the
hardness, electrochemical behaviour and localised corrosion and of AA7075 produced by SLM. TRANSMISSION ELECTRON MICROSCOPY (TEM) ANALYSIS Detailed TEM characterisation of SLMed AA7075 in
three different conditions, viz. as-SLMed, solutionised + quenched, and solutionised + quenched and aged at 120 °C for 24 h, was performed and the resulting images are shown in Figs 2–4.
Second phase particles with sizes <200 nm were observed in SLMed AA7075 (Fig. 2a–c). Another significant microstructural feature, cellular structures were observed in SLMed7075, as
pointed out by solid arrows in Fig. 2a′–c′. The size of such cellular structure was found to increase following solutionising. Cellular structures were realised by the segregation of high
atomic number elements, most probably Zn and Cu, along the structure boundaries, as evident from the HAADF images. Following ageing at 120 °C for 24 h, nanoscale η′-phase (MgZn2)
precipitates evolved in the Al-matrix, mostly along cellular structure boundaries, as shown by the dotted arrow in Fig. 2c′, indicating that the solute segregated along the cellular
structure boundaries aid in nucleating η′-phase precipitates via solute diffusion and redistribution. In fact, η′-phase precipitates were also found to contain Cu, as previously reported in
wrought Cu-containing 7xxx Al-alloys.38,42,59 Second phase particles in as-SLMed AA7075 were often found to co-exist together (rather vividly as shown in Fig. 3a, b), indicating that the
first nucleated phases were likely to provide sites for the heterogeneous nucleation of other phases. The mechanism of such nucleation processes is complex, considering high cooling rates
and subsequently negligible solute diffusion rates during the SLM process.1,2,3,4,5,6,7,57 However, most of the particles in as-SLMed AA7075 were characterised to be of icosahedral
quasicrystalline phase (Fig. 3c), termed as ν-phase (Mg–Zn–Cu(–Al)).13 As a result of coexistence of different phase particles at a single position, the determination of accurate composition
of such particles is challenging. However, an estimation of phases based on the compositional analysis via the EDXS-STEM technique can be realised (Table 2). It is noted that dissolution of
quasicrystalline ν-phase following solutionising was discovered, implying that ν-phase is a metastable phase, most likely only existing in as-SLMed AA7075. Such important characteristics of
quasicrystalline ν-phase were not previously determined, nor the relevance in the context of designing SLMed AA7xxx alloys. A nanoscale platelet-shaped phase (<1 nm in thickness) that is
considered to be the precursor to η′-phase was formed on the {111}α planes of the Al-matrix in as-SLMed AA7075, as determined from the FFT analysis and atomic-resolution image (Fig. 4a).
The precursor η′-phase could have formed either during rapid solidification achieved via the SLM process or potentially during localised reheating of an already solidified layer during the
SLM process. However, the phase was less in number and not uniformly distributed in the Al-matrix. Precursor η′-phase was not observed following solutionising (Fig. 4b), indicating the
dissolution of the phase. Nanoscale η′-phase precipitates evolved in the Al-matrix following ageing at 120 °C (Fig. 4c), caused by solute diffusion and redistribution. It is noted that
η′-phase precipitates, which evolved during the ageing process, were not uniformly distributed in the Al-matrix. In contrast, such precipitates evolve uniformly in the Al-matrix of wrought
AA7075-T6.19,20,23,25,27,28,59 The orientation relationship of the η′-phase precipitate with the Al-matrix of 24 h aged SLMed AA7075 was identified, and was similar to that for wrought
AA7xxx alloys:19,20 (0001)η′//{111}α, 〈1120〉η′//〈112〉α. HARDNESS STUDIES The variation in Vickers hardness of SLMed AA7075 as a function of ageing is presented in Fig. 5. The average
hardness of as-SLMed AA7075 decreased from ~107 to 64 VHN following solutionising. Ageing at 120 °C caused the hardness to gradually increase with time, and following 24 h the hardness
reached to that of the value recorded for as-SLMed specimens. The hardness further increased with time and reached a maximum value of ~126 VHN following 110 h of ageing. In contrast, the
average Vickers hardness of wrought AA7075-T6 was determined to be 197 ± 5 VHN. The variation in hardness between SLMed AA7075 and wrought AA7075-T6, is a result of the corresponding
microstructural variation and porosity of <2%, but also it is noted that the bulk composition of SLMed AA7075 is slightly alloy lean by comparison, and that SLMed AA7075 has not undergone
any cold (or hot) working. The second phase particles, nanoscale precipitates and cellular structures in the Al-matrix of SLMed AA7075 as shown in Figs 1–4 and Table 2, contributed to
hardness. The decrease in hardness following solutionising is mainly attributed to the predominant dissolution of quasicrystalline ν-phase and nanometre-scale precursors of η′-phase, along
with the growth of cellular structures. The evolution of η′-phase precipitates in the Al-matrix following ageing at 120 °C resulted in an increase in hardness. However, the number density
and distribution of η′-phase precipitates in artificially aged SLMed AA7075 were significantly lower and non-uniform, respectively, than those reported in wrought AA7075-T6
alloys.19,20,23,25,27,28,59 ELECTROCHEMICAL ANALYSIS POTENTIODYNAMIC POLARISATION Separate anodic and cathodic potentiodynamic scans of SLMed AA7075 and wrought AA7075-T6 were conducted, and
the results are presented in Fig. 6. The anodic and cathodic polarisation responses of SLMed AA7075 indicate a shift in the corrosion potential (_E_corr) from − 0.9 to − 1.3 _V_SCE
following solutionising (where the maximum level of Mg and Zn is in solid solution) and then to −1.2 _V_SCE following 24 h ageing. In addition, in the case of as-SLMed AA7075, the anodic
current significantly increased at potentials above _E_corr. Following solutionising and also after ageing, a distinct shift between _E_corr and _E_pit (a potential above which stable
pitting can occur) is notable. The significant shift of _E_corr following solutionising and ageing coincides with the dissolution of quasicrystalline ν-phase particles in the Al matrix. More
importantly, _E_pit of SLMed AA7075 following solutionising and ageing remains similar to the _E_corr of wrought AA7075-T6, suggesting a similar electrochemical response—which corroborates
with a scenario of the closest of the microstructures between the SLMed AA7075 and the wrought AA7075-T6. The evolution of the cathodic polarisation section did not reveal a significant
difference in cathodic kinetics between as-SLMed and solutionised AA7075. In addition, the cathodic kinetics are slightly decreased (from ~10−2 to ~5 × 10–3 mA cm−2) between _E_corr and −1.4
_V_SCE between wrought AA7075-T6 and SLMed AA7075. This may be attributed to the refinement and annihilation of coarse ‘cathodic’-constituent particles in the SLMed AA7075. Similar results
were reported previously, where a minor change in the cathodic kinetics was observed for an additively manufactured AA2024.9 However, in the case of additively manufactured AA2024 although
the overall electrochemical kinetics were shown to be similar for the wrought and SLMed conditions (noting that AA2024 is Cu-rich alloy dominated by cathodic particles), the refined
microstructure obtained via SLM positively influenced the localised corrosion susceptibility of the AA2024 with a decrease of the local cathode:anode ratio, limiting the onset of local pH
increase (from oxygen reduction reaction) and Al matrix dissolution. Overall, the variation in electrochemical response of SLMed AA7075 with thermal treatment, and also compared to that of
wrought AA7075-T6, is a result of significant microstructural variation and corresponding compositional variation in the Al-matrix. ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY (EIS) The EIS
results of wrought AA7075-T6 and SLMed AA7075 are presented as Nyquist and Bode plots in Fig. 7a–c. Equivalent electrical circuits to fit experimental EIS data of SLMed AA7075 and wrought
AA7075-T6 are presented in Fig. 7d, e, respectively. The associated parameters obtained via fitting of EIS data were determined and presented in Table 3. In addition, the resistance (_R_4)
and inductance (_L_1) corresponding to the inductive loop of wrought AA7075-T6 were also determined (Table 3). In the tabulated data, the polarisation resistance (_R_P) corresponds to the
extrapolated intersection of the impedance data with −_Z″_ = 0 at very low frequencies (in Fig. 7a). The EIS spectrum of wrought AA7075-T6 characteristically displayed two capacitive loops
at high or moderate frequencies and an inductive loop at low frequencies (Fig. 7a). The inductive behaviour for wrought AA7075-T6 can also be observed in the Bode magnitude plot (Fig. 7c) at
low frequencies (_f_ < 0.2 Hz). Wrought 7xxx Al-alloys in NaCl were previously determined to exhibit inductive loop at low-frequency regime in the Nyquist plot.60,61 The presence of such
inductive loop indicates a degradation of passive or oxide film developed upon Al alloys,60,61 and corrosion during the duration of testing. In addition, wrought AA7075-T6 freely corrodes
at open circuit conditions in NaCl and therefore, the passive film over the alloy is not sustained. In contrast to wrought AA7075-T6, SLMed AA7075 did not exhibit a characteristic inductive
loop, indicating that the film developed upon SLMed AA7075 is expected to provide corrosion protection comparatively greater than for wrought AA7075-T6 in NaCl. In addition, it was
determined that _R_p was greater for SLMed AA7075 compared to wrought AA7075-T6. The _R_p of as-SLMed AA7075 was also found to vary with solutionising and ageing. LOCALISED CORROSION
RESPONSE To study the localised corrosion response, specimens of wrought AA7075-T6 and SLMed AA7075 were immersed in 0.1 M NaCl for 10 h and subsequently examined, both prior to and
following immersion using SEM (Figs 8–11). Upon wrought AA7075-T6, localised corrosion was most commonly associated with Al–Cu–Fe(–Si) phase constituent particles (Fig. 8b–d). Pitting can be
identified as dark the regions in Fig. 8b. Upon closer observations (Fig. 8c, d), pits were developed in the form of circumferential trenches by dissolving the Al-matrix at the periphery of
Al–Cu–Fe(–Si) phase constituent particles. The results obtained herein support the documented corrosion morphology of high strength Al-alloys with respect to the role of Fe-containing
particles serving as local cathodes (and supporting oxygen reduction at enhanced rates relative to the matrix) and leading to trenching in NaCl solutions.29,30,31,36,44,45 In addition,
enhanced oxygen reduction at local sites will generate a hydroxyl ion atmosphere at cathodic particles, thereby increasing the local pH and accelerating the dissolution of the Al-matrix at
the periphery of such particles.31 A few, and small, pits that were not associated with Al–Cu–Fe–(Si) phase constituent particles were also observed in the Al-matrix (Fig. 8b). Such pits may
have however formed at the periphery of Al–Cu–Fe(–Si) particles that would have detached following a period of Al-matrix dissolution at the particles’ periphery or could be dealloying of
active phases, such as Mg2Si and Cu-containing MgZn2, which were very fine and could not be detected under SEM. Specimens from as-SLMed AA7075 also exhibited pitting that was associated with
Mg–Zn–Cu(–Al) (ν-phase) particles in the Al-matrix (Fig. 9b–d). Pits were developed in the form of trenches by dissolving the Al-matrix at the periphery of ν-phase (Fig. 9b–d), revealing
the apparently cathodic nature of quasicrystalline ν-phase particles, whose electrochemical behaviour was previously not known. In addition, the constituent Mg2Si particles suffered
dealloying by Mg dissolution, which is consistent with previous findings.46,47,48 Pitting was more pronounced along the melt pool boundaries than that within the core of melt pools (Fig. 9b,
c). Such variation in pitting behaviour along melt pool boundaries and within the core of melt pools is a result of the microstructural variation caused by SLM production; particles along
the boundaries were larger and in a lower number density compared to those within the core of melt pools. Solutionising as-SLMed AA7075 specimens caused the subsequent pitting morphology to
evolve uniformly in the Al-matrix (Fig. 10b). In addition, the pit number density decreased in the solutionised condition when compared to the as-SLMed condition. The decrease in pit number
density and distribution following solutionising is a direct result of the dissolution of ν-phase and disappearance of melt pools. Pits in the solutionised condition were associated with
dissolution of the Al-matrix at the periphery of Al–Cu–Fe–(Si) particles (Fig. 10b–d). The overall dissolution of the Al-matrix also increased following solutionising, as evident from cracks
developed upon the oxide layer several micrometres in thickness (Fig. 10b), validating the potentiodynamic polarisation testing results in a general sense. The increased dissolution of the
Al-matrix following solutionising may be attributed to the composition of the Al-matrix, which is a supersaturated solid solution of Al principally containing the Zn and Mg in solid
solution, which was evidenced to also decrease the _E_corr rather vividly in the solutionised condition. In response to immersion for 10 h in 0.1 M NaCl, SLMed AA7075 specimens aged at 120
°C for 24 h revealed the formation of pits with greater size along grain boundaries than within grains (Fig. 11b). This is associated with larger second phase particles and precipitates
along grain boundaries, than within grains. The pits observed associated with second phase particles were also in the form of trenches, dissolving the Al-matrix at the periphery of cathodic
Al–Cu–Fe(–Si) phase constituent particles (Fig. 11c, d). Conversely, the η′-phase (MgZn2) precipitates containing Cu, which formed along grain boundaries in response to artificial aging, are
considered to have undergone dealloying by selective Mg-dissolution, however, this was unable to be resolved using SEM. The statistics related to pitting as determined from numerous SEM
images (including Figs 8–11) of post corrosion surfaces from wrought AA7075-T6 and SLMed AA7075 are presented in Table 4. Post immersion SEM (Figs 8–11) and the results in Table 4, it may be
rationalised that pits developed upon wrought AA7075-T6 and SLMed AA7075 were associated with second phase particles. In addition, the size, shape and distribution of second phase particles
influenced pit size, shape and distribution—indicating that localised corrosion is deterministic. In comparison with wrought AA7075-T6, pits developed upon SLMed AA7075 were greater in
number, however notably smaller in size as a result of the increased population and decreased size of second phase particles present in the Al-matrix of specimens generated by SLM. In
addition, the contribution of ‘cathodic corrosion’ as the mechanism for generating trenching at the periphery of second phase particles in SLMed AA7075 is also expected to be minimal, as
nanometre to sub-micrometre scale cathodic second phase particles do not substantially increase the local pH when compared to the same particles in micrometre size. DISCUSSION The localised
corrosion caused by pitting, even from the early stages of corrosion in wrought AA7075-T6, can initiate fatigue cracks in the presence of cyclic stress—leading to the failure of components
prior to the service life.53,54,55 Therefore, it is relevant to discuss the role of pitting developed upon SLMed AA7075 in contrast to the localised corrosion of wrought AA7075-T6. It was
previously reported that, pits with depths of 29–75 µm initiated fatigue cracks in wrought AA7075-T6 at a nominal maximum tensile stress of ~210 MPa.36,54 In addition, the total fatigue life
decreased with the severity of corrosion damage,54,55,62 although the number of cycles to initiate a fatigue crack did not depend on the depth of pits.63,64 Herein, it was identified that
pits developed upon wrought AA7075-T6 were larger, and expected to be much deeper, by several orders of magnitude when compared to those upon SLMed AA7075. Therefore, it can be understood
that the contribution of such fine pits on fatigue corrosion of SLMed AA7075 may be less significant, and therefore an important avenue for future work. Moreover, the impedance results
herein revealed the persistence of a comparatively higher corrosion resistance for SLMed AA7075. However, the results herein indicate that future systematic studies regarding the damage
tolerance of SLMed AA7075 are required, which should also include the testing of stress corrosion cracking susceptibility. The results herein however, represent a first presentation of the
corrosion of SLMed AA7075, including in response to solutionising and aging, and correlate this response with detailed microstructural analysis. Selective laser melted (SLMed) AA7075 in
three different conditions, viz. as-SLMed, solutionised + quenched, and solutionised + quenched and aged at 120 °C for 24 h, was explicitly studied in the context of microstructure,
hardness, electrochemical response and localised corrosion morphology, and compared with wrought AA7075-T6. The following conclusion were drawn from the present study: * With similar bulk
composition, the SLM technique generated a different and unique microstructure, with varying second phase particle type, size, shape, and distribution as compared to that of wrought
AA7075-T6. The as-SLMed AA7075 contained a quasicrystalline phase (ν-phase) as the principal nanostructural feature. * The microstructure of as-SLMed AA7075 varied with solutionising, and
following ageing at 120 °C for 24 h. Quasicrystalline ν-phase and melt pools were dissolved following solutionising. Ageing resulted in η′-phase (MgZn2) precipitate formation within grains
and along grain boundaries, revealing columnar grains in the alloy microstructure. * The hardness of as-SLMed AA7075 decreased with solutionising, as a result of quasicrystalline ν-phase
dissolution. Artificial ageing following solutionising increased hardness; and following ~24 h ageing, the hardness was similar to that of the as-SLMed condition, as a result of η′-phase
precipitate formation. * The electrochemical response as determined by the polarisation test conditions herein revealed passive-like behaviour for SLMed AA7075 in 0.1 M NaCl, in contrast to
wrought AA7075-T6, which is noted for free dissolving at open circuit conditions. Impedance spectroscopy validated the assertion that SLMed AA7075 presents greater corrosion resistance than
wrought AA7075-T6. * Localised corrosion following immersion in 0.1 M NaCl for 10 h was principally associated with the dissolution of Al-matrix at the periphery of second phase particles in
SLMed AA7075 and wrought AA7075-T6. However, pits generated upon SLMed AA7075 were found to be notably smaller when compared to those upon wrought AA7075-T6—owing to finer microstructural
features and the absence of large constituent particles. The pit distribution, size and number density of SLMed AA7075 varied with thermal treatment as a result of the corresponding
microstructural variation. Pitting was more pronounced along melt pool boundaries in the as-SLMed AA7075 condition. Following solutionising, pits were generated uniformly in the Al-matrix.
Ageing resulted in pits with size greater along grain boundaries than within grains. METHODS MATERIALS AND AGEING Additively manufactured AA7075 specimens were produced from AA7075 powder
using selective laser melting (SLM). The AA7075 powder, with size distribution of 20–63 µm, was supplied by LPW technology. A Concept Laser Mlab cusing-R was used for SLM, described in
detail in ref. 13 with the production conditions for specimens tested herein consisting of: laser power, 95 W; laser scan speed, 200 mm/s; and layer thickness, 25 µm. In addition to SLM
prepared specimens, wrought AA7075-T6 rod was also studied for comparison. The compositions of the AA7075 powder as supplied, as-SLMed AA7075 and wrought AA7075-T6 were determined by
inductively coupled plasma-optical emission spectroscopy (ICP-OES) at an accredited laboratory (Spectrometer services, VIC, Australia) and shown in Table 1. As-SLMed AA7075 studied herein
presented a consolidation of >98%. In order to study microstructural evolution, hardness and corrosion, as-SLMed AA7075 specimens were solutionised at 480 °C for 1.5 h in a salt bath and
quenched in water; followed by artificial aging at 120 °C (for different times up to 110 h) in an oil bath, followed by water quenching. As-SLMed specimens were naturally aged for 1 week
prior to microstructural, mechanical and electrochemical testing. MICROSTRUCTURAL CHARACTERISATION SEM was performed using a JEOL® 7001F SEM operated at 15 kV. Energy-dispersive X-ray
spectroscopy (EDXS) was performed using an Oxford Instruments® X-Max 80 silicon detector, and analysed via Aztec® software. Specimens for SEM were metallographically prepared to a 0.01 µm
surface finish under ethanol and colloidal silica suspension. TRANSMISSION ELECTRON MICROSCOPY Scanning transmission electron microscopy (STEM) was performed using an FEI® Tecnai G2 F20
S-TWIN FEG operated at 200 kV and an aberration corrected FEI® Titan3 80–300 operated at 300 kV. Imaging was performed using a high-angle annular dark-field (HAADF) detector, which provides
atomic number contrast.65 To determine the composition of different phases, EDXS was performed using a Bruker® X-flash X-ray detector (Tecnai G2 F20), and the EDXS data was analysed using
Bruker® Esprit X-ray software. Specimen preparation for TEM involved punching 3 mm discs from thin slices of SLMed AA7075 specimens followed by mechanical grinding under ethanol to a
thickness of ~60 µm, using 1200 grit SiC paper, and then argon ion milling at −50 °C using a Gatan® precision ion polishing system (PIPS). Specimens were plasma cleaned prior to imaging for
~2 min with an argon and oxygen gas mixture using a Gatan® solarus 950 advanced plasma system. HARDNESS TESTING Vickers hardness testing was conducted by applying 1 kg of load for 10 s using
a Duramin A-300 hardness tester. Specimens for hardness testing were prepared to an 800 grit SiC paper finish, and five measurements were collected for each specimen. ELECTROCHEMICAL
CHARACTERISATION Potentiodynamic polarisation testing was performed using a Bio-logic VMP-3, using a three-electrode flat cell (inclusive of a saturated calomel reference electrode (SCE) and
platinum mesh counter electrode). Specimens for polarisation testing were prepared to a 2000 grit SiC paper finish under ethanol, and ultrasonically cleaned in ethanol. Testing was carried
out in 0.1 M NaCl. Prior to polarisation testing, specimens were exposed at open circuit potential (OCP) for 30 min. Separate anodic and cathodic scans were conducted at 1 mV s−1 scan rate
by commencing at ± 20 mV vs. OCP and ceasing at absolute current of 10 mA cm−2. Each scan was reproduced at least in triplicate for reproducibility. EIS was performed in naturally aerated
0.1 M NaCl using a frequency range of 10 kHz–10 mHz (with testing at 10 points per decade) and an applied potential of ±10 mV. Each test was replicated at least in triplicates for
reproducibility. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Lewandowski, J. J. &
Seifi, M. Metal additive manufacturing: a review of mechanical properties. _Annu. Rev. Mater. Res._ 46, 151–186 (2016). Article CAS Google Scholar * Frazier, W. E. Metal additive
manufacturing: a review. _J. Mater. Eng. Perform._ 23, 1917–1928 (2014). Article CAS Google Scholar * Buchbinder, D., Schleifenbaum, H., Heidrich, S., Meiners, W. & Bultmann, J. High
power selective lase melting (HP SLM) of aluminum parts. _Phys. Proc._ 12, 271–278 (2011). Article CAS Google Scholar * Herzog, D., Seyda, V., Wycisk, E. & Emmelmann, C. Additive
manufacturing of metals. _Acta Mater._ 117, 371–392 (2016). Article CAS Google Scholar * DebRoy, T. et al. Additive manufacturing of metallic components – process, structure and
properties. _Prog. Mater. Sci._ 92, 112–224 (2018). Article CAS Google Scholar * Martin, J. H. et al. 3D printing of high-strength aluminium alloys. _Nature_ 549, 365–369 (2017). Article
CAS Google Scholar * Rometsch, P., Jia, Q., Yang, K. V. & Wu, X. Aluminum alloys for selective laser melting—towards improved performance. In _Additive Manufacturing for the
Aerospace Industry_, 1st edn (eds Froes, F. & Boyer, R.) 301–325 (Elsevier, Amsterdam, 2019). * Aboulkhair, N. T., Everitt, N. M., Maskery, I., Ashcroft, I. & Tuck, C. Selective
laser melting of aluminium alloys. _MRS Bull._ 42, 311–319 (2017). Article CAS Google Scholar * Gharbi, O. et al. On the corrosion of additively manufactured aluminium alloy AA2024
prepared by selective laser melting. _Corros. Sci._ 143, 93–106 (2018). Article CAS Google Scholar * Zhang, H., Zhu, H., Qi, T., Hu, Z. & Zeng, X. Selective laser melting of high
strength Al–Cu–Mg alloys: processing, microstructure and mechanical properties. _Mater. Sci. Eng. A_ 656, 47–54 (2016). Article CAS Google Scholar * Zhang, H. et al. Effect of zirconium
addition on crack, microstructure and mechanical behaviour of selective laser melted Al–Cu–Mg alloy. _Scr. Mater._ 134, 6–10 (2017). Article CAS Google Scholar * Caiazzo, F., Alfieri, V.,
Argenio, P. & Sergi, V. Additive manufacturing by means of laser-aided directed metal deposition of 2024 aluminium powder: investigation and optimization. _Adv. Mech. Eng._ 9, 1–12
(2017). Google Scholar * Kairy, S. K. et al. On the characterization of a hitherto unreported icosahedral quasicrystal phase in additively manufactured aluminium alloy AA7075. _Metall.
Mater. Trans. A_ 50, 529–533 (2019). Article CAS Google Scholar * Reschetnik, W. et al. Fatigue crack growth behaviour and mechanical properties of additively processed EN AW-7075
aluminium alloy. _Procedia Sruct. Integr._ 2, 3040–3048 (2016). Article Google Scholar * Kaufmann, N. et al. Influence of process parameters on the quality of aluminium alloy EN AW 7075
using selective laser melting (SLM). _Phys. Procedia_ 83, 918–926 (2016). Article CAS Google Scholar * Sistiaga, M. L. M. et al. Changing the alloy composition of Al7075 for better
processability by selective laser melting. _J. Mater. Process. Technol._ 238, 437–445 (2016). Article CAS Google Scholar * Qi, T. et al. Selective laser melting of Al7050 powder: Melting
mode transition and comparison of the characteristics between the keyhole and conduction mode. _Mater. Des._ 135, 257–266 (2017). Article CAS Google Scholar * Wang, P., Li, H. C.,
Prashanth, K. G., Eckert, J. & Scudino, S. Selective laser melting of Al–Zn–Mg–Cu: heat treatment, microstructure and mechanical properties. _J. Alloy. Compd._ 707, 287–290 (2017).
Article CAS Google Scholar * Polmear, I. J., St John, D., Nie, J. & Qian, M. _Light Alloys from Traditional Alloys to Nanocrystals_. 5th edn (Elsevier, Oxford, 2017). Google Scholar
* Nie, J. F. Physical metallurgy in light alloys. In _Physical Metallurgy_ (eds Laughlin, D. E. & Hono, K.) (Elsevier, Amsterdam, The Netherlands, 2014). * Mondolfo, L. F. _The
Aluminium–magnesium–zinc Alloys: A Review of the Literature_ (Research and Development Center, Reverse Copper and Brass Inc., 1967). * Mondolfo, L. F. _Aluminium Alloys: Structure and
Properties_. (Butterworths, London, 1976). Google Scholar * Li, X. M. & Starink, M. J. Effect of compositional variations on characteristics of coarse intermetallic particles in
overaged 7000 aluminium alloys. _Mater. Sci. Technol._ 17, 1324–1328 (2001). Article CAS Google Scholar * Gao, M., Feng, C. R. & Wei, R. P. An analytical electron microscopy study of
constituent particles in commercial 7075-T6 and 2024-T3 alloys. _Metall. Mater. Trans. A_ 29, 1145–1151 (1998). Article Google Scholar * Li, X. M. & Starink, M. J. Identification and
analysis of intermetallic phases in overaged Zr-containing and Cr-containing Al–Zn–Mg–Cu alloys. _J. Alloy. Compd._ 509, 471–476 (2011). Article CAS Google Scholar * Meng, Q. &
Frankel, G. S. Effect of Cu content on corrosion behaviour of 7xxx series aluminium alloys. _J. Electrochem. Soc._ 151, B271–B283 (2004). Article CAS Google Scholar * Deschamps, A., De
Geuser, F., Horita, Z., Lee, S. & Renou, G. Precipitation kinetics in a severely plastically deformed 7075 aluminium alloy. _Acta Mater._ 66, 105–117 (2014). Article CAS Google Scholar
* Zhang, Y. et al. Dynamic precipitation, segregation and strengthening of an Al–Zn–Mg–Cu alloy (AA7075) processed by high-pressure torsion. _Acta Mater._ 162, 19–32 (2019). Article CAS
Google Scholar * Davis, J. R. _Corrosion of Aluminium and Aluminium Alloys_, 1st edn (ASM International, Materials Park, 1999). * Wei, R. P., Liao, C. M. & Gao, M. A transmission
electron microscopy study of constituent-particle-induced corrosion in 7075-T6 and 2024-T3 aluminum alloys. _Metall. Mater. Trans. A_ 29, 1153–1160 (1998). Article Google Scholar *
Birbilis, N., Cavanaugh, M. K. & Buchheit, R. G. Electrochemical behaviour and localized corrosion associated with Al7Cu2Fe particles in aluminium alloy 7075-T651. _Corros. Sci._ 48,
4202–4215 (2006). Article CAS Google Scholar * Gupta, R. K., Hinton, B. R. W. & Birbilis, N. The effect of chromate on the pitting susceptibility of AA7075-T651 studied using
potentiostatic transients. _Corros. Sci._ 82, 197–207 (2014). Article CAS Google Scholar * Knight, S. P., Birbilis, N., Muddle, B. C., Trueman, A. R. & Lynch, S. P. Correlations
between intergranular stress corrosion cracking, grain boundary microchemistry, and grain-boundary electrochemistry for Al–Zn–Mg–Cu alloys. _Corros. Sci._ 52, 4073–4080 (2010). Article CAS
Google Scholar * Knight, S. P. et al. & Lynch, S. P. Some effects of alloy composition on stress corrosion cracking in Al–Zn–Mg–Cu alloys. _Corros. Sci._ 98, 50–62 (2015). Article
CAS Google Scholar * Doig, P., Flewitt, P. E. J. & Edington, J. W. The stress corrosion cracking susceptibility of 7075Al–Zn–Mg–Cu alloys tempered from T6 to an overaged T7X*.
_Corrosion_ 33, 217–221 (1977). Article CAS Google Scholar * Xu, D. K., Birbilis, N., Lakshansky, D., Rometsch, P. A. & muddle, B. C. Effect of solution treatment on the corrosion
behaviour of aluminium alloy AA7150: optimisation for corrosion resistance. _Corros. Sci._ 53, 217–225 (2011). Article CAS Google Scholar * Lynch, S. P., Knight, S. P., Birbilis, N. &
Muddle, B. C. Stress-corrosion cracking of Al–Zn–Mg–Cu alloys effects of composition and heat-treatment. In _Proc. 2008 International Hydrogen Conference on Effects of Hydrogen on
Materials_ (eds Somerday, B., Sofronis, P. & Jones, R.) 243–250 (ASM International, 2009). * Ramgopal, T., Schmutz, P. & Frankel, G. S. Electrochemical behaviour of thin film analogs
of Mg(Zn,Cu,Al)2. _J. Electrochem. Soc._ 148, B348–B356 (2001). Article CAS Google Scholar * Ramgopal, T., Gouma, P. I. & Frankel, G. S. Role of grain-boundary precipitates and
solute-depleted zone on the intergranular corrosion of aluminium alloy 7150. _Corrosion_ 58, 687–697 (2002). Article CAS Google Scholar * Rao, B. V. N. Influence of ageing treatment on
the grain boundary precipitation and chemistry in 7075 Al alloy. _Metall. Mater. A_ 12, 1356–1359 (1981). Article CAS Google Scholar * Park, K. & Ardell, A. J. Microchemical analysis
of precipitate free zones in 7075-Al in the T6, T7 and RRA tempers. _Acta Metall. Mater._ 39, 591–598 (1991). Article CAS Google Scholar * Kairy, S. K., Turk, S., Birbilis, N. &
Shekhter, A. The role of microstructure and michrochemistry on intergranular corrosion of aluminium alloy AA7085-T7452. _Corros. Sci._ 143, 414–427 (2018). Article CAS Google Scholar *
Gupta, R. K., Deschamps, A., Cavanaugh, M. K., Lynch, S. P. & Birbilis, N. Relating the early evolution of microstructure with the electrochemical response and mechanical performance of
a Cu-rich and Cu-lean 7xxx aluminium alloy. _J. Electrochem. Soc._ 159, C492–C502 (2012). Article CAS Google Scholar * Birbilis, N. & Buchheit, R. G. Electrochemical characteristics
of intermetallic phases in aluminium alloys: an experimental survey and discussion. _J. Electrochem. Soc._ 152, B140–B151 (2005). Article CAS Google Scholar * Birbilis, N. & Buchheit,
R. G. Investigation and discussion of characteristics for intermetallic phases common to aluminium alloys as a function of solution pH. _J. Electrochem. Soc._ 155, C117–C126 (2008). Article
CAS Google Scholar * Gupta, R. K., Sukiman, N. L., Fleming, K. M., Gibson, M. A. & Birbilis, N. Electrochemical behaviour and localized corrosion associated with Mg2Si particles in
Al and Mg alloys. _ECS Electrochem. Lett._ 1, C1–C3 (2012). Article CAS Google Scholar * Kairy, S. K., Rometsch, P. A., Davies, C. H. J. & Birbilis, N. On the electrochemical and
quasi in-situ corrosion response of the Q-phase (AlxCuyMgzSiw) intermetallic particle in 6xxx series Al-alloys. _Corrosion_ 73, 87–99 (2017). Article CAS Google Scholar * Gharbi, O. &
Birbilis, N. Clarifying the dissolution mechanisms and electrochemistry of Mg2Si as a function of solution pH. _J. Electrochem. Soc._ 165, C497–C501 (2018). Article CAS Google Scholar *
Han, J. & Ogle, K. Dealloying of MgZn2 intermetallic in slightly alkaline chloride electrolyte and its significance in corrosion resistance. _J. Electrochem. Soc._ 164, C952–C961 (2017).
Article CAS Google Scholar * Buchheit, R. G., Martinez, M. A. & Montes, L. P. Evidence for Cu ion formation by dissolution and dealloying the Al2CuMg intermetallic compound in
rotating ring-disk collection experiments. _J. Electrochem. Soc._ 147, 199–124 (2000). Article Google Scholar * Buchheit, R. G., Montes, L. P., Martinez, M. A., Michael, J. & Hlava, P.
F. The electrochemical characteristics of bulk-synthesized Al2CuMg articles. _J. Electrochem. Soc._ 146, 4424–4428 (1999). Article CAS Google Scholar * Buchheit, R. G., Grant, R. P.,
Hlava, P. F., Mckenzie, B. & Zender, G. L. Local dissolution phenomena associated with S phase (Al2CuMg) particles in aluminium alloy 2024-T3. _J. Electrochem. Soc._ 144, 2621–2628
(1997). Article CAS Google Scholar * Sankaran, K. K., Perez, R. & Jata, K. V. Effects of pitting corrosion on the fatigue behaviour of aluminium alloy 7075-T6: modelling and
experimental studies. _Mater. Sci. Eng. A_ 297, 223–229 (2001). Article Google Scholar * Jones, K. & Hoeppner, D. W. Pit-to-crack transition in pre-corroded 7075-T6 aluminum alloy
under cyclic loading. _Corros. Sci._ 47, 2185–2198 (2005). Article CAS Google Scholar * Kim, S., Burns, J. T. & Gangloff, R. P. Fatigue crack formation and growth from localized
corrosion in Al–Zn–Mg–Cu. _Eng. Fract. Mech._ 76, 651–667 (2009). Article Google Scholar * Thijs, L., Kempen, K., Kruth, J. P. & Humbeeck, J. V. Fine-structured aluminium products with
controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. _Acta Mater._ 61, 1809–1819 (2013). Article CAS Google Scholar * Olakanmi, E. O., Cochrane, R. F. &
Dalgarno, K. W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: processing, microstructure, and properties. _Prog. Mater. Sci._ 74, 401–477 (2015).
Article CAS Google Scholar * Porter, D. A., Easterling, K. E. & Sherif, M. Y. _Phase Transformations in Metals and Alloys_. 3rd edn (CRC Press, Florida, 2009). Google Scholar *
Marlaud, T., Deschamps, A., Bley, F., Lefebvre, W. & Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al–Zn–Mg–Cu alloys. _Acta Mater._ 58,
248–260 (2010). Article CAS Google Scholar * Birbilis, N., Cavanaugh, M. K. & Buchheit, R. G. Electrochemical response of AA7075-T651 following immersion in NaCl solution. _ECS
Trans._ 1, 115–126 (2006). Article CAS Google Scholar * Li, J. F., Jia, Z. Q., Li, C. X., Birbilis, N. & Cai, C. Exfoliation corrosion of 7150 Al alloy with various tempers and its
electrochemical impedance spectroscopy in EXCO solution. _Mater. Corros._ 60, 407–414 (2009). Article CAS Google Scholar * Burns, J. T., Kim, S. & Gangloff, R. P. Effect of corrosion
severity on fatigue evolution in Al–Zn–Mg–Cu. _Corros. Sci._ 52, 498–508 (2010). Article CAS Google Scholar * Co, N. E. & Burns, J. T. Galvanic corrosion-induced fatigue crack
initiation and propagation behaviour in AA7050-T7451. _Corrosion_ 72, 1215–1219 (2016). Article CAS Google Scholar * Co, N. E. & Burns, J. T. Effects of macro-scale corrosion damage
feature on fatigue crack initiation and fatigue behaviour. _Int. J. Fatigue_ 103, 234–247 (2017). Article CAS Google Scholar * Williams, D. B. & Carter, C. B. _Transmission Electron
Microscopy_. 2nd edn (Springer, New York, 2009). Book Google Scholar * _International Alloy Designations and Chemical Composition Limits for Wrought Aluminium and Wrought Aluminium Alloys_
(The Aluminum Association, Arlington, 2015). https://www.aluminum.org/sites/default/files/Teal%20Sheets.pdf. Download references ACKNOWLEDGEMENTS The authors gratefully acknowledge support
from Woodside Energy. All SEM and TEM images were collected at the Monash Centre for Electron Microscopy. AUTHOR INFORMATION Author notes * These authors contributed equally: Oumaïma Gharbi,
Shravan Kumar Kairy AUTHORS AND AFFILIATIONS * Laboratoire interfaces et systèmes électrochimiques, CNRS, Sorbonne Université, UMR8235, Paris, France Oumaïma Gharbi * Department of
Materials Science and Engineering, Monash University, Clayton, VIC, 3168, Australia Oumaïma Gharbi, Shravan Kumar Kairy, Paula Roberta De Lima, Derui Jiang & Juan Nicklaus * College of
Engineering and Computer Science, The Australian National University, Acton, ACT, 2601, Australia Nick Birbilis Authors * Oumaïma Gharbi View author publications You can also search for this
author inPubMed Google Scholar * Shravan Kumar Kairy View author publications You can also search for this author inPubMed Google Scholar * Paula Roberta De Lima View author publications
You can also search for this author inPubMed Google Scholar * Derui Jiang View author publications You can also search for this author inPubMed Google Scholar * Juan Nicklaus View author
publications You can also search for this author inPubMed Google Scholar * Nick Birbilis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
N.B. conceived the study with the assistance of O.G. and S.K.K. Data acquisition was conducted by S.K.K., P.R.D.L., J.N., D.J. and O.G. Data interpretation and discussions involved O.G.,
S.K.K. and O.G. the draft was written and edited by S.K.K., O.G. and N.B. approved the final version. CORRESPONDING AUTHOR Correspondence to Oumaïma Gharbi. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Gharbi, O., Kumar Kairy, S., De Lima, P.R. _et al._ Microstructure and corrosion evolution of additively manufactured aluminium alloy AA7075 as a function of
ageing. _npj Mater Degrad_ 3, 40 (2019). https://doi.org/10.1038/s41529-019-0101-6 Download citation * Received: 31 May 2019 * Accepted: 15 October 2019 * Published: 27 November 2019 * DOI:
https://doi.org/10.1038/s41529-019-0101-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Javascript support required...
I/O 2025 Highlights: Google Android XR Glasses, Glow AI cinema creator, new Gemini AI tools and moreNewsletters ePaper Sign in HomeIndiaKarnatakaOpinionWorldBusinessSportsVideoEntertainmentDH SpecialsOperation SindoorNew...
Road haulage association limited: pf notice and statement to members (pdf format)Corporate report ROAD HAULAGE ASSOCIATION LIMITED: PF NOTICE AND STATEMENT TO MEMBERS (PDF FORMAT) PF Notice and Members...
How ivanka’s mother ‘threatened to put her up for adoption' revealedDubbed the First Daughter due to her unusually high position in her father Donald Trump’s administration, Ivanka has bee...
Review: a reimagined 'twilight: los angeles, 1992' revival arrives at a time of protracted crisisThe most powerful moments in the revised version of “Twilight: Los Angeles, 1992,” Anna Deavere Smith’s landmark drama a...
Latests News
Microstructure and corrosion evolution of additively manufactured aluminium alloy aa7075 as a function of ageingABSTRACT Additively manufactured high strength aluminium (Al) alloy AA7075 was prepared using selective laser melting (S...
Reviews cross-journal editorial team | nature climate changeThe Reviews Cross-Journal Editorial Team comprises fifteen highly skilled and motivated editors, who combined have >1...
All inmates who escaped from rural sc detention center back in custodyThe last two of four inmates who escaped from Chesterfield County Detention Center on Oct. 15 have been arrested. All fo...
Javascript support required...
‘i don’t want to be irrelevant. ’ nc democrats hope to gain rural ground in novemberAs election day draws closer, Democrats are hoping to fend off a Republican supermajority in the North Carolina General ...