Solution processed low power organic field-effect transistor bio-chemical sensor of high transconductance efficiency
Solution processed low power organic field-effect transistor bio-chemical sensor of high transconductance efficiency"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Developing organic field-effect transistor (OFET) biosensors for customizable detection of biomarkers for many diseases would provide a low-cost and convenient tool for both
biological studies and clinical diagnosis. In this work, design principles of the OFET transducer for biosensors were derived to relate the signal-to-noise ratio (_SNR_) to the
device-performance parameters. Steep subthreshold swing (_SS_), proper threshold voltage (_V_th), good-enough bias-stress stability, and mechanical durability are shown to be the key
prerequisites for realizing OFET bio-sensors of high transconductance efficiency (_g_m/_I_D) for large _SNR_. Combining a low trap-density channel and a high-_k_/low-_k_ gate dielectric
layer, low-temperature (<100 °C) solution-processed flexible OFETs can meet the performance requirements to maximize the _g_m/_I_D. An extended gate-structure OFET biosensor was further
implemented for label-free detection of miR-21, achieving a detection limit below 10 pM with high selectivity at a low operation voltage (<1 V). SIMILAR CONTENT BEING VIEWED BY OTHERS
SIGNAL TRANSDUCTION INTERFACES FOR FIELD-EFFECT TRANSISTOR-BASED BIOSENSORS Article Open access 19 February 2024 SUPER-NERNSTIAN ION SENSITIVE FIELD-EFFECT TRANSISTOR EXPLOITING CHARGE
SCREENING IN WSE2/MOS2 HETEROSTRUCTURE Article Open access 16 December 2021 BIOMOLECULE SENSORS BASED ON ORGANIC ELECTROCHEMICAL TRANSISTORS Article Open access 13 February 2025 INTRODUCTION
There are rapidly growing demands for ubiquitous perception of various biomarkers (ions or biomolecules) in blood, body fluids, secretions, excrement, and tissue cells of human for
personalized medical diagnosis and healthcare1,2. To realize that, the biosensing devices need to be highly customizable to meet diverse system requirements in terms of biocompatibility,
cost, and form factor (compact, thin, flexible, or comfortable), while producing output signal of high-enough signal-to-noise ratio (_SNR_) under strict power constraint. The most affordable
and convenient way would be based on electrical measurements, which avoid using additional bulky, power-hungry, and expensive optical components. Transducers made by integration of the
sensor interface with a field-effect transistor (FET) have been widely studied, since the FET can convert the sensed signal into an amplified output signal for potentially large _SNR_3. In
addition, with FET switches, multiplexed detection in a single reaction is convenient to be implemented for high-throughput and multi-analyte analysis. The organic FET (OFET), composed of
organic semiconductors (OSCs) and polymer dielectric layers, shows several competitive advantages over inorganic counterparts for such biosensing applications4,5. The use of
solution-printing processes and versatile structures might facilitate integration of various sensing interfaces or probes at the device level in great freedom6. With low processing
temperature and superior intrinsic mechanical flexibility of the full organic stacks, truly flexible sensor electronics can be fabricated using common low Young’s modulus thin plastic foils
with much less stress-management efforts7. In the past, OFETs have been studied for detecting various biomolecules, including enzyme, DNA, and protein8,9,10,11. As a popularly used approach,
probes are immobilized on the gate or its extended part to capture the target molecules. Once the molecules are being captured, the resulted potential change at the gate is recorded or
converted to output-current change through the OFET for further processing. These work well proved that the capability of OFETs to be designed for various biosensing functions through
material- and device-structure engineering. However, many of the devices required vacuum processes and inorganic dielectric layers, which would sacrifice the technical competence of the
OFET. Although solution-based processes at low temperature might offer attractive features for high customizability at low cost, they suffer difficulties in fine control of layer thickness,
especially for very-thin films, and formation of high-quality semiconductor/dielectric films and interfaces. With such constraints, the achievable device performance is severely limited. In
the past, there have been significant efforts on improving the carrier mobility in the OSC channels through material molecule design and crystallization-controlled processing methods12,13.
However, for biochemical sensors to detect very low concentration of analyte in various portable, wearable, or implantable scenarios, large _SNR_ with low operating voltage and power would
be a prerequisite14,15,16. However, there is lack of studies on optimal design of the OFET for such figure of merits considering the interplay between device structures and material stacks
under processing constraints. This work derives the OFET-design principles for biochemical sensor transducers of large transconductance efficiency (_g_m/_I_D) for large _SNR_ with low
operation voltage. Steep subthreshold swing (_SS_), proper threshold voltage (_V_th), and operational stability are shown to be the key parameters to determine the optimal performance. Based
on the design principles, low-voltage OFETs are then fabricated on plastic substrate in low-temperature solution processes to obtain the required performance, through formation of a low
trap-state density channel interface on high-_k_/low-_k_ bilayer-structure gate insulator. To verify its transduction performance, a plastic sensor tag is implemented through encapsulating
the OFET with a sensing electrode and a reference electrode for miRNA detection. miRNAs are key biomarkers for many diseases, and label-free miRNA detection in electrochemical ways would
provide low-cost and convenient tools for both biological fundamental studies and clinical diagnosis17. However, miRNAs are of low charge level due to their short intrinsic molecule length,
and thus challenging to be detected at very low concentration. This work demonstrates the feasibility of using the OFET for miRNA sensing. With the optimal device performance, the sensor
exhibits a 20% relative output-signal change over the background upon detection of miR-21 (a miRNA biomarker for breast cancer) at 10 pM under an operation voltage less than one volt.
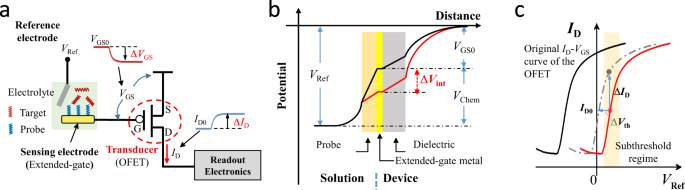
RESULTS AND DISCUSSION DESIGN PRINCIPLES Figure 1a illustrates the structure of an OFET-based biochemical sensor, consisting of an OFET transducer with an extended-gate sensing electrode
(SE), and a reference electrode (RE). The probe-immobilized SE is to capture analyte targets in solution through specific target/probe combination. For measurement, a constant voltage bias
(_V_Ref) is applied to the RE, inducing an initial potential at the OFET gate (_V_GS0) through the solution and in turn a channel current in the OFET (_I_D0). As depicted in Fig. 1b, _V_GS0
can be expressed as: $$V_{{\mathrm{GS}}0} = V_{\mathrm{Ref}} - V_{\mathrm{Chem}}$$ (1) where _V_Chem is the voltage between the RE and the SE, and can be assumed to be a sum of a constant
_V_Chem0 and an interfacial potential drop (_V_int0)18. The interfacial potential drop _V_int0, arising from the electrode/electrolyte interfacial dipole, is modulated with the captured
charged biomolecules and can be measured by using the open circuit potential method19. When the SE detects a certain concentration of analyte targets in the solution, the captured negatively
(or positively) charged targets would induce a change of the interfacial potential drop (Δ_V_int), and thus Δ_V_GS. Correspondingly, an output-current change (Δ_I_D) can be measured by the
subsequent readout circuit for digitalization, as shown in Fig. 1a. The detection limit of the whole system is determined by the signal-to-noise ratio (_SNR_) of the OFET transducer, which
is given as16,20 $$SNR = 10\log \left( {\frac{{P_{{{{\mathrm{signal}}}}}}}{{P_{{{{\mathrm{noise}}}}}}}} \right)$$ (2) where _P_signal is the signal power and _P_noise is the noise power.
According to the aforementioned analysis, _P_signal is contributed by Δ_I_D upon detection of a certain concentration of analyte. For low-frequency biochemical sensing, _P_noise is mainly
dominated by 1/_f_ noise and can be described as7 $$i_{1/{{{\mathrm{f}}}}}^2 = K\frac{{I^{\upbeta}}}{{f^{\upalpha}}}$$ (3) where _I_ is the operation current, _K_ is a process-dependent
coefficient, _f_ is the frequency, and _α_ and _β_ are noise parameters (with _α_ = 1 and _β_ = 2 in theory in subthreshold regime). Correspondingly, the _SNR_ for an OFET transducer
operating in subthreshold regime at low frequency is deduced to be: $$SNR = 10\log \left( {\frac{{P_{{{{\mathrm{signal}}}}}}}{{P_{{{{\mathrm{noise}}}}}}}} \right) = 10\log \left(
{\frac{{\Delta I^2}}{{i_{{{{\mathrm{tot}}}}}^2}}} \right) \approx 10\log \left( {\frac{{\Delta I^2}}{{i_{1/{{{\mathrm{f}}}}}^2}}} \right) = 10\log \left[ {\frac{f}{K}\left( {\frac{{\Delta
I}}{I}} \right)^2} \right]$$ (4) According to (4), the _SNR_ depends on the relative current-output signal change over the background level (∆_I_/_I_). As a result, to improve the detection
limit, it would be important to maximize Δ_I_D/_I_D0 of the OFET transducer for a certain concentration of analytes. Δ_I_D/_I_D0 can be described as $$\frac{{\Delta
I_{{{\mathrm{D}}}}}}{{I_{{{{\mathrm{D}}}}0}}} = \frac{{g_{{{\mathrm{m}}}}\Delta V_{{{{\mathrm{GS}}}}}}}{{I_{{{{\mathrm{D}}}}0}}} = - \frac{{g_{{{\mathrm{m}}}}}}{{I_{{{{\mathrm{D}}}}0}}}
\cdot \Delta V_{{{{\mathrm{int}}}}} = - \left( {\frac{{\ln 10}}{{SS}}} \right) \cdot \Delta V_{{{{\mathrm{int}}}}}$$ (5) where _g_m is transconductance and _SS_ is the subthreshold swing.
Therefore, to maximize Δ_I_D/_I_D0, enlarging the transconductance efficiency (_g_m/_I_D0) is as important as achieving effective immobilization of specific targets on the gate electrode
(increasing Δ_V_int). In other words, _g_m/_I_D0 is an important figure-of-merit to benchmark the sensing capability of extended-gate OFET sensors. Since the maximum of _g_m/_I_D0 occurs in
the subthreshold regime, and is proportional to the inverse of _SS_7, steep _SS_ would thus be required to design OFET transducers of high _g_m/_I_D for large _SNR_. As illustrated in Fig.
1c, when the OFET is operated in the sensor system, the obtained transfer curve by sweeping _V_Ref would have a shift compared with the original _I_D–_V_GS curve of the OFET. Such a shift is
due to the presence of an interfacial potential between the surface of the gate electrode and the aqueous solution21. To enable operation of the OFET in the subthreshold regime with a small
_V_Ref, a proper near-zero threshold voltage (_V_th) is also required. Operational stability is another concern to be considered, since a certain waiting time (i.e., tens of minutes) is
often required for interaction between the probes and the target biomolecules22. As illustrated in Fig. 1c, when the OFET is biased in the subthreshold regime, even a slight _V_th shift
would induce significant output-current change (Δ_I_D), causing false-positive or false-negative results. Similar to the electrical bias stress, since the flexible device is inevitably
subject to some bending state, attention should also be paid to the mechanical durability during bending stress. According to the above analysis, it is concluded that steep _SS_, proper
threshold voltage, and operational stability under electrical bias and mechanical stress are key prerequisites for designing low-power OFET biochemical sensor transducers of large _SNR_.
DEVICE FABRICATION AND CHARACTERIZATIONS To meet the requirements as discussed above, an OFET design combining a low trap-state density channel and a high-_k_/low-_k_ gate dielectric layer
is implemented, to achieve steep _SS_ and near-zero _V_th, while using a thick-enough dielectric layer for large-area solution processability. As shown in Fig. 2a, the devices were
fabricated in a bottom-gate bottom-contact structure on a polyethylene naphthalate (PEN) plastic substrate. The gate and source/drain electrodes were formed by thermal evaporation of silver
(Ag) through shadow masks. The gate dielectric was composed of a low-_k_ poly(vinyl cinnamate) (PVCN) layer and a high-_k_ poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene)
(P(VDF–TrFE–CFE)) layer with total thickness of about 420 nm, as shown by the cross-sectional scanning electron microscopy (SEM) image (Fig. 2b). Both layers were deposited by spin-coating
processes followed by low-temperature annealing. The surfaces of the source/drain electrodes were treated by perfluorobenzenethiol (PFBT) before deposition of the organic semiconductor (OSC)
layer. The OSC layer was deposited using a blade-coating process from a blended solution of 6,13-bis(triisopropylsilylethynyl)-pentacene (TIPS-pentacene) and polystyrene (PS)23.
Well-crystalline structure channels can be formed as shown in Fig. 2c to obtain a low-trap OSC/dielectric interface for steep _SS_ at small-gate dielectric capacitance (20.1 nF·cm−2). A
CYTOP passivation layer was finally deposited by drop-casting to protect the channel from the air ambient. Details of the fabrication processes are described in “METHODS”. The maximum
processing temperature is kept below 100 °C. The measured representative transfer (_I_D–_V_GS) and output (_I_D–_V_DS) characteristics of the sealed OFET are shown in Fig. 2d, presenting
well-behaved FET behaviors with a steep _SS_ of about 80 mV·dec−1, a nearly zero _V_th (0.3 V), and an ON/OFF ratio larger than 105. From the _SS_ value, the effective subgap trap density is
estimated to be 4.3 × 1010 eV−1·cm−2, similar to previous work using a single low-_k_ gate dielectric layer24 and also superior to those used in extended-gate OFET biosensors (Supplementary
Table 1). The measured _g_m/_I_D versus _V_GS is plotted in Fig. 2e, with its maximum value of 28.8 V−1 exceeding those of previously reported extended-gate OFET biosensors (Supplementary
Table 1). The maximum _g_m/_I_D occurring at _V_GS = 0.35 V enables the OFET to be used in the sensor system as depicted in Fig. 1a for biomolecule detection of high sensitivity and low
detection limit at low _V_Ref (<1 V). The operational stabilities under continuous switching and constant bias stress were characterized and shown in Fig. 2f. The device exhibits nearly
identical transfer characteristics after sweeping between OFF and ON for 120 times. With a bias stress at a _V_GS near threshold voltage for 3000 s, there is negligible _V_th shift (less
than 0.01 V). The relative current change (Δ_I_D(t)/_I_D(0)) is less than 15% after constant bias stress for more than 4 hours. Such a device design owns low-trap OSC/dielectric interface,
and is operated with low charge density and weak perpendicular electrical field at low voltage attributed to a thick-gate dielectric layer25. Compared with the implemented low-voltage device
using ultrathin dielectric of high capacitance, these factors reduce the probability of charge trapping under bias stress, and thus enable the device to achieve high operational
stabilities. Large _g_m/_I_D, proper _V_th, and high operational stability make the device a promising transducer for biomolecule detection. EVALUATION OF MECHANICAL STABILITY The mechanical
stability of the flexible OFET device encapsulated by CYTOP was evaluated by measuring its electrical characteristics with changing bending angles. Figure 3a shows the measurement system
for assessing the stability of the OFET device under bending states. The investigated bending angle (_θ_) and radius (_R_) for the OFET under bending test are illustrated in Supplementary
Fig. 1. Figure 3b shows the transfer characteristics of the OFET device before and after undergoing bending at various angles, presenting negligible degradation in the subthreshold
characteristics even at a large bending angle of about 73˚. The corresponding output characteristics (Supplementary Fig. 2) also show no apparent change after bending by about 51˚, while
only a slight degradation of on-current was observed after aggressively bending by about 73˚. In this regard, the contact resistance (_R_C) during the bending test was further estimated by
using a transfer-line method (TLM). It is realized by taking the total normalized on-resistance (_R_ON·W) from the output characteristics of OFET devices with various channel lengths in the
linear regime and then extrapolating the linear fit to a channel length of zero to obtain _R_C. As shown in Supplementary Fig. 3, the bent OFETs with channel length ranging from 50 to 110 μm
present well-behaved output performance with a clear saturation of the drain current beyond the pinch-off point. Accordingly, the corresponding width-normalized _R_ON·W can be calculated
and plotted as a function of channel length for OFET under different bending conditions (Supplementary Fig. 4). As shown in Fig. 3c, the _R_C extracted by TLM at the most significant bending
angle of 73˚ is approximately 0.3 ~ 0.38 MΩ·cm. Although such _R_C is slightly dependent on the gate bias voltage, it remains nearly unchanged during the measured bending states (Fig. 3d).
Electrical measurements of the flexible device were further performed under bending stress. For this purpose, a stressing cycle was applied to the device by repeatedly bending it to radius =
10 mm and immediately releasing it to its initial state at a rate of 12 times per minute. Figure 3e shows that the flexible OFET device maintained well-functional transfer characteristics
during the bending stress. Even after 3500 complete cycles, the change in subthreshold swing was not exceeding 5 mV·dec−1, while threshold voltage shift was less than 0.03 V (Fig. 3f),
revealing negligible change in subthreshold performance during the performed bending stress. These results illustrate that the developed low-voltage OFET can sustain the inevitable bending
during usage and maintain its electrical performance for proper signal transducing. PROBE IMMOBILIZATION AND HYBRIDIZATION To verify the device concept of the designed OFET transducer for
biosensing, this work takes miR-21 detection as an example, which is one of the potential candidate biomarkers for primary breast cancer26. The scheme for immobilizing probes on the
extended-gate SE is depicted in Fig. 4a. A thiolated single-stranded DNA (ssDNA) probe was designed to match the miR-21 for specific detection. Based on the well-established thiol-gold
chemistry, the ssDNA probes and the 6-mercapto-1-hexanol (MCH) were able to self-assemble onto the gold electrode surface efficiently via a strong SH-gold binding. The ssDNA probes work as
the receptor for capturing miRNA targets, while the MCH, as a blocking layer, reduces miRNA–gold interaction and thus improves hybridization discrimination27,28. As a result, the miR-21
targets were selectively captured on the extended-gate SE through DNA/miRNA hybridization according to the law of Watson-Crick base-pairing29. To verify the hybridization-reaction procedure,
miR-21 was labeled with fluorescent dyes (FAM) for fluorescence measurement. As shown in Fig. 4b, the ssDNA-immobilized gold incubated to the 1X phosphate-buffered saline (PBS 1X) solution
shows no fluorescence. After incubation of the electrode surface to the FAM-labeled miR-21 solution, there is weak fluorescence at a miR-21 concentration of 10 pM, while obvious fluorescence
occurring at a concentration of 1 nM. With increase of the concentration to 100 μM, the signal becomes strong. The results prove the hybridization reaction between the miR-21 targets and
the ssDNA probes. IMPLEMENTATION OF MIRNA SENSOR The individual OFET was cut from the fabricated large area sample, and then encapsulated onto a carrier PEN substrate with the extended-gate
SE and the Ag/AgCl RE to complete a sensor tag for use as shown in Fig. 5a. The encapsulation processes are described in details in “METHODS”. After the extended-gate SE and the RE being
placed in the PBS 1X solution, the _I_D–_V_Ref curve was measured by sweeping _V_Ref at a _V_DS = − 0.5 V, presenting an obvious shift to the negative direction compared with the _I_D–_V_GS
curve of the pristine OFET, as seen in Fig. 5b. After immobilization of ssDNA probes onto the extended-gate SE, there is a small shift backward due to the negatively charged probes. As a
result, with a proper _V_th, the OFET transducer was able to be biased in the subthreshold regime for large _g_m/_I_D at a low _V_Ref (−0.1 V). Figure 5c shows the measured _I_D–_V_Ref
curves using the same sensor when the concentration of miR-21 targets varies from 0 to 1 μM. _V_th values were extracted from the _I_D–_V_Ref curves at a drain current of 1 nA. The measured
_V_th shift (Δ_V_th) at various concentrations of miR-21, calculated by using the _V_th value at 0 M of miR-21 as blank (Δ_V_th = _V_thmiRNA − _V_thBlank), is shown in Fig. 5d. The solution
containing a 3-base mismatch miRNA of a much higher concentration (100 μM) was also tested for comparison. The results show that Δ_V_th for the lowest measured miR-21 concentration (10 pM)
is 16.9 ± 5 mV, much higher than the value (0.2 ± 5 mV) for 3-base mismatch miRNAs of high concentration (100 μM), indicating high selectivity. Moreover, the limit of detection (LOD) was
used to interpret the detectability of the sensor. It is defined as the concentration that leads to a sensor response equal to three times the standard deviation of the negative control
sample (Δ_V_thmean ± 3σ), where Δ_V_thmean is the average response, and σ is the relative standard deviation30. According to this definition, the LOD was estimated to be 4.5 pM by taking the
response of 3-base mismatch miRNA as the negative control sample. Note that the response at 10 pM is beyond the LOD (Fig. 5d), verifying the OFET biosensor’s high sensing capability at such
a low concentration. The measured relative current change (∆_I_D/_I_D0) at _V_Ref = −0.1 V for various miR-21 concentrations is shown in Fig. 5e. For a low concentration of 10 pM,
∆_I_D/_I_D0 of 20% is able to be obtained for large-enough _SNR_ to be processed by the subsequent readout electronics for digitalization. Therefore, the sensor is able to achieve a
detection limit below 10 pM to the target miRNAs with good selectivity at a low operation voltage (<1 V). The overall performance shows competence over that of the previous work based on
a Si-FET in Table 1, in terms of the detection limit, the operation voltage, and the static power31. In summary, the derived design principles of the OFET transducer for biosensors build
relationships between the _SNR_ and the key OFET-performance parameters, including _g_m/_I_D, _SS_, _V_th and bias-stress stability. Combining a low trap-state density channel and
high-_k_/low-_k_ structure gate dielectric, OFETs fabricated on PEN substrate with low temperature solution-based processes can meet the optimal design requirements, exhibiting steep _SS_,
near-zero _V_th, and good-enough bias-stress stability and mechanical durability. Extended-gate structure OFET biosensors were further constructed for label-free detection of miR-21, a
potential biomarker for primary breast cancer. The results demonstrate that the sensor can achieve a detection limit below 10 pM to the target miRNAs with good selectivity at a low operation
voltage (<1 V). The overall performance is competitive over that of the previous work based on the Si-FET, in terms of the detection limit, the operation voltage and the static power.
This work would pave the way to developing low-cost and convenient biosensors based on OFETs to have large _SNR_ for customizable detection of disease biomarkers in both biological studies
and clinical diagnosis. METHODS MATERIALS AND REAGENTS In all, 125-μm-thick polyethylene naphthalate (PEN) plastic films (Teonex Q65HA) were purchased from DuPont Teijin Films. Poly (vinyl
cinnamate) (PVCN, _M_w = 40 kDa), polystyrene (PS, _M_w = 524 kDa), perfluorobenzenethiol (PFBT), and 6-mercapto-1-hexanol (MCH) were purchased from Sigma-Aldrich. Poly(vinylidene
fluoride–trifluoroethylene–chlorofluoroethylene) (56/36.5/7.5 mol%) terpolymer (P(VDF–TrFE–CFE)) was synthesized by the suspension-polymerization process32.
6,13-bis(triisopropylsilylethynyl)-pentacene (TIPS-pentacene) (FN4023) was provided by Merck Chemicals Ltd. CYTOP (CTL-809M) was obtained from Asahi Glass. Silver (Ag) pastes were obtained
from Hisense Electronics, Kunshan, China. Silicone sealant was purchased from Shanghai Qianru Building Materials. Phosphate-buffered saline (PBS 1X, pH = 7.2) solution was obtained from
Thermo Fisher Scientific. The sequences of probe single-stranded DNA (5′-SH-CCCCCCTCAACATCAGTCTGATAAGCTA-3′), fully complementary target miR-21 (5′-UAGCUUAUCAGACUGAUGUUGA-3′),
noncomplementary 3-base mismatch miRNA (5′-UAGCCUAUCAAACUGAUGAUGA-3′), and fluorescence-dyed miR-21 (5′-FAM-UAGCUUAUCAGACUGAUGUUGA-3′) used in this study were synthesized by Sangon Biotech
(Shanghai) Co. Ltd., China. DEVICE FABRICATION OFET devices in a bottom-gate bottom-contact structure were fabricated on a plastic PEN foil laminated on a glass carrier. PVCN was dissolved
in chlorobenzene with a concentration of 10 mg/ml and spin-coated at 3000 rpm onto the substrate as a planarization layer, followed by UV cross-linking (UV Curer KW-4AC, CHEMAT) for 20 min
and then heating at 100 °C for 1 h. Next, 40 nm-thick silver (Ag) gate electrodes were deposited by thermal evaporation using a stainless-steel mask. Then, a high-_k_ P(VDF–TrFE–CFE)
(dissolved in methyl ethyl ketone, 40 mg/ml) and a low-_k_ PVCN (dissolved in chlorobenzene, 10 mg/ml) were subsequently spin-coated to form a thick bilayer-gate dielectric. Ag source/drain
(S/D) electrodes were obtained using the same processes as that for formation of the gate electrodes, defining a channel width of 2000 µm and a channel length of 70 µm, respectively. Devices
with the same channel width (1500 µm) and a series of channel lengths (50, 70, 90, and 110 µm) were also used to evaluate the mechanical stability. Before the deposition of the
semiconductor layer, the sample was immersed into a PFBT solution (5 mM in isopropanol) for 15 min to form self-assembled monolayers on the S/D electrodes. After this treatment, it was
carefully rinsed with isopropanol and blown by dry N2 gas, followed by annealing at 100 ˚C on a hot plate for 1 min. The semiconductor/polymer-blended solution was prepared by mixing
TIPS-pentacene and PS solutions (dissolved in chlorobenzene, 10 mg·mL−1) in 3:1 ratio by volume. The semiconducting film was formed using a soft-contact coating approach with a rotatable
steel sheet as the meniscus guide at coating speed of 20 mm/s, followed by annealing at 100 °C for 30 min23. Finally, CYTOP solution (10 µL) was drop-cast to passivate the channel and
annealed at 80 °C for 30 min. SENSOR-TAG ENCAPSULATION After completing the preparation of OFET device, its supporting PEN foil was carefully peeled off from the glass carrier. Subsequently,
individual CYTOP-passivated OFET was cut from it to mount onto another holding PEN substrate with prefabricated screen-printed silver interconnects and pads. A bonding process was developed
by flipping over the OFET device and attaching to the substrate using silver paste, followed by annealing at 80 °C for 10 min. Finally, a silicone sealant was dispensed with a dispenser
robot to seal the device. SENSING-ELECTRODE PREPARATION Extended-gate sensing electrode consisting of chromium (10 nm) and gold (100 nm) was deposited using magnetron sputtering, which was
further encapsulated with silicone sealant to define a sensing area of 9 mm2. Prior to immobilization of probes, the electrode surface was cleaned by O2 plasma for 10 min. Then, 10 μL of
5′-SH–modified capture ssDNA solution (10–4 M, dissolved in PBS 1X) was added onto the gold electrode and incubated at 4 °C in humid condition overnight, followed by rinsing thoroughly with
PBS 1X solution. After immobilization of probes, the electrode was posttreated with 10 μL of MCH aqueous solution (1 mM) for 1 h to remove nonspecifically bound oligonucleotides and block
extra-active gold surface, followed by rising with PBS 1X solution. The obtained sensing electrodes were used immediately to subject to 10 μL of fully complementary targets (miR-21 or
FAM-dyed miR-21) and noncomplementary targets (3-base mismatch miRNA) diluted to desired concentrations in PBS 1X solutions for hybridization, respectively, followed by rinsing with PBS 1X
solution carefully. CHARACTERIZATION AND MEASUREMENT The polarized optical micrograph for TIPS-pentacene crystalline was taken with a microscope (XPF-300C, Caikon). The fluorescence images
were obtained from inverted fluorescence microscopy (IX71, Olympus Life Science). The cross-sectional scanning electron microscopy (SEM) image was obtained on a Zeiss Ultra Plus Field
Emission Scanning Electron Microscope at an electric voltage of 5 kV. The cyclic bending test of the flexible OFET was performed on a stretching machine at bending radius of 10 mm with a
speed of 5 s/cycle. The sensor tag was connected to the extended-gate sensing electrodes and reference electrode (Ag/AgCl) via copper wires. The electrical characterizations of the OFETs and
biosensors were performed using a semiconductor parameter analyzer (Keithley 4200 system). All measurements were carried out at room temperature in ambient air. DATA AVAILABILITY The data
that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Sakata, T. Biologically coupled gate field-effect transistors meet
in vitro diagnostics. _ACS Omega_ 4, 11852–11862 (2019). Article CAS Google Scholar * Lee, M. Y., Lee, H. R., Park, C. H., Han, S. G. & Oh, J. H. Organic transistor-based chemical
sensors for wearable bioelectronics. _Acc. Chem. Res._ 51, 2829–2838 (2018). Article CAS Google Scholar * Syu, Y.-C., Hsu, W.-E. & Lin, C.-T. Review-field-effect transistor
biosensing: devices and clinical applications. _ECS J. Solid State Sci. Technol._ 7, Q3196–Q3207 (2018). Article CAS Google Scholar * Zang, Y., Huang, D., Di, C. A. & Zhu, D. Device
engineered organic transistors for flexible sensing applications. _Adv. Mater._ 28, 4549–4555 (2016). Article CAS Google Scholar * Magliulo, M., Manoli, K., Macchia, E., Palazzo, G. &
Torsi, L. Tailoring functional interlayers in organic field-effect transistor biosensors. _Adv. Mater._ 27, 7528–7551 (2015). Article CAS Google Scholar * Khan, H. U. et al. In situ,
label-free DNA detection using organic transistor sensors. _Adv. Mater._ 22, 4452–4456 (2010). Article CAS Google Scholar * Jiang, C., Cheng, X. & Nathan, A. Flexible ultralow-power
sensor interfaces for E-skin. _Proc. IEEE_ 107, 2084–2105 (2019). * Lai, S., Barbaro, M. & Bonfiglio, A. Tailoring the sensing performances of an OFET-based biosensor. _Sens. Actuators B
Chem._ 233, 314–319 (2016). Article CAS Google Scholar * Song, J. et al. Influence of bioreceptor layer structure on myelin basic protein detection using organic field effect
transistor-based biosensors. _Adv. Funct. Mater._ 28, 1802605 (2018). Article Google Scholar * Minamiki, T. et al. Accurate and reproducible detection of proteins in water using an
extended-gate type organic transistor biosensor. _Appl. Phys. Lett._ 104, 243703 (2014). Article Google Scholar * Minami, T. et al. Selective nitrate detection by an enzymatic sensor based
on an extended-gate type organic field-effect transistor. _Biosens. Bioelectron._ 81, 87–91 (2016). Article CAS Google Scholar * Wang, C., Dong, H., Jiang, L. & Hu, W. Organic
semiconductor crystals. _Chem. Soc. Rev._ 47, 422–500 (2018). Article CAS Google Scholar * Gu, X., Shaw, L., Gu, K., Toney, M. F. & Bao, Z. The meniscus-guided deposition of
semiconducting polymers. _Nat. Commun._ 9, 534 (2018). Article Google Scholar * Moser, N., Rodriguez-Manzano, J., Lande, T. S. & Georgiou, P. A scalable ISFET sensing and memory array
with sensor auto-calibration for on-chip real-time DNA detection. _IEEE Trans. Biomed. Circuits Syst._ 12, 390–401 (2018). * Douthwaite, M., Koutsos, E., Yates, D. C., Mitcheson, P. D. &
Georgiou, P. A thermally powered ISFET array for on-body pH measurement. _IEEE Trans. Biomed. Circuits Syst._ 11, 1324–1334 (2017). Article Google Scholar * Rajan, N. K., Brower, K.,
Duan, X. & Reed, M. A. Limit of detection of field effect transistor biosensors: effects of surface modification and size dependence. _Appl. Phys. Lett._ 104, 084106 (2014). Article
Google Scholar * Kilic, T., Erdem, A., Ozsoz, M. & Carrara, S. microRNA biosensors: Opportunities and challenges among conventional and commercially available techniques. _Biosens.
Bioelectron._ 99, 525–546 (2018). * Kaisti, M. et al. Real-time wash-free detection of unlabeled PNA-DNA hybridization using discrete fet sensor. _Sci. Rep._ 7, 15734 (2017). * Thompson, M.
et al. Label-free detection of nucleic acid and protein microarrays by scanning Kelvin nanoprobe. _Biosens. Bioelectron._ 20, 1471–1481 (2005). Article CAS Google Scholar * Liao, W. et
al. pH sensing and low-frequency noise characteristics of low temperature (400 °C) p-channel SOI schottky ISFETs. _IEEE Electron Device Lett._ 38, 1146–1149 (2017). * Ishige, Y., Shimoda, M.
& Kamahori, M. Immobilization of DNA probes onto gold surface and its application to fully electric detection of DNA hybridization using field-effect transistor sensor. _Jpn. J. Appl.
Phys._ 45, 3776–3783 (2006). Article CAS Google Scholar * Xu, S. et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor.
_Nat. Commun._ 8, 14902 (2017). Article CAS Google Scholar * Huang, Y. et al. Scalable processing of low voltage organic field effect transistors with a facile soft-contact coating
approach. _IEEE Electron Device Lett._ 40, 1945–1948 (2019). Article CAS Google Scholar * Tang, W., Feng, L., Yu, P., Zhao, J. & Guo, X. Highly efficient all-solution-processed
low-voltage organic transistor with a micrometer-thick low-_k_ polymer gate dielectric layer. _Adv. Electron. Mater._ 2, 1500454 (2016). Article Google Scholar * Lee, W. H., Choi, H. H.,
Kim, D. H. & Cho, K. 25th anniversary article: microstructure dependent bias stability of organic transistors. _Adv. Mater._ 26, 1660–1680 (2014). Article CAS Google Scholar * Mittal,
S., Kaur, H., Gautam, N. & Mantha, A. K. Biosensors for breast cancer diagnosis: a review of bioreceptors, biotransducers and signal amplification strategies. _Biosens. Bioelectron._
88, 217–231 (2017). Article CAS Google Scholar * Qian, S. et al. Boronic acid functionalized Au nanoparticles for selective microRNA signal amplification in fiber-optic surface plasmon
resonance sensing system. _ACS Sens_ 3, 929–935 (2018). Article CAS Google Scholar * Yang, C.-T., Pourhassan-Moghaddam, M., Wu, L., Bai, P. & Thierry, B. Ultrasensitive detection of
cancer prognostic miRNA biomarkers based on surface plasmon enhanced light scattering. _ACS Sens._ 2, 635–640 (2017). Article CAS Google Scholar * Dorvel, B. R. et al. Silicon nanowires
with high-_k_ hafnium oxide dielectrics for sensitive detection of small nucleic acid oligomers. _ACS Nano_ 6, 6150–6164 (2012). * Lu, N. et al. CMOS-compatible silicon nanowire field-effect
transistors for ultrasensitive and label-free microRNAs sensing. _Small_ 10, 2022–2028 (2014). Article CAS Google Scholar * Ganguli, A., Watanabe, Y., Hwang, M. T., Huang, J.-C. &
Bashir, R. Robust label-free microRNA detection using one million ISFET array. _Biomed. Microdevices_ 20, 45 (2018). Article Google Scholar * Li, J., Sun, Z. & Yan, F. Solution
processable low-voltage organic thin film transistors with high-_k_ relaxor ferroelectric polymer as gate insulator. _Adv. Mater._ 24, 88–93 (2012). Download references ACKNOWLEDGEMENTS This
work was financially supported by the National Natural Science Foundation of China under Grant (61334008, 61804094, and 61974091), National Science Fund for Excellent Young Scholars under
Grant 61922057, and the Research Grants Council (RGC) of Hong Kong, China (Project No. C5015-15G). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Electronic Information and
Electrical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China Wei Tang, Yukun Huang, Yawen Song, Xin Xi, Yuezeng Su & Xiaojun Guo * Department of Applied Physics, The
Hong Kong Polytechnic University, Hong Kong, China Ying Fu, Yuanzhe Li & Feng Yan * State Key Laboratory of Integrated Optoelectronics, Institute of Semiconductors, Chinese Academy of
Sciences, Beijing, 100083, China Yude Yu Authors * Wei Tang View author publications You can also search for this author inPubMed Google Scholar * Ying Fu View author publications You can
also search for this author inPubMed Google Scholar * Yukun Huang View author publications You can also search for this author inPubMed Google Scholar * Yuanzhe Li View author publications
You can also search for this author inPubMed Google Scholar * Yawen Song View author publications You can also search for this author inPubMed Google Scholar * Xin Xi View author
publications You can also search for this author inPubMed Google Scholar * Yude Yu View author publications You can also search for this author inPubMed Google Scholar * Yuezeng Su View
author publications You can also search for this author inPubMed Google Scholar * Feng Yan View author publications You can also search for this author inPubMed Google Scholar * Xiaojun Guo
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.G., Y.Y., and F.Y. conceived the idea. W.T. and X.G. designed the experiments, analyzed
the data, and wrote the paper. Y.H. fabricated organic field-effect transistor and participated in tag preparation. Y.F. and Y.L. carried out the biosensor preparation and characterizations.
X.X. performed the SEM imaging. Y.S. and Y.S. helped to analyze the data and revise the paper. X.G. and F.Y. supervised the project. All authors discussed the results and contributed to the
preparation of the paper. W.T. and Y.F. contributed equally to this work. CORRESPONDING AUTHORS Correspondence to Feng Yan or Xiaojun Guo. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tang, W., Fu, Y., Huang, Y. _et al._ Solution processed low power organic field-effect transistor bio-chemical sensor of high
transconductance efficiency. _npj Flex Electron_ 6, 18 (2022). https://doi.org/10.1038/s41528-022-00149-9 Download citation * Received: 18 April 2021 * Accepted: 10 February 2022 *
Published: 18 March 2022 * DOI: https://doi.org/10.1038/s41528-022-00149-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
bdnews24.com404 Sorry, page not found Unfortunately the page you are looking for does not existGo back to Home Page...
10 Quick Questions for Melora HardinPhoto: Johan Jansson; Stylist: Randy Smith; Hair and Makeup: Steeve Daviault Facebook Twitter LinkedIn Melora Hardin, 55...
Remembering Louis Canelakes - The Texas ObserverAs 2014 uncorks, it’s fitting and important to celebrate the enduring legacy of an extraordinary man who influenced gene...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Multicentric validation of diagnostic tests based on bc-116 and bc-106 urine peptide biomarkers for bladder cancer in two prospective cohorts of patieABSTRACT BACKGROUND Non-invasive urine-based biomarkers can potentially improve current diagnostic and monitoring protoc...
Latests News
Solution processed low power organic field-effect transistor bio-chemical sensor of high transconductance efficiencyABSTRACT Developing organic field-effect transistor (OFET) biosensors for customizable detection of biomarkers for many ...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Research alert-barclays cuts petrobakken energy target priceOct 12 (Reuters) - Petrobakken Energy Ltd : * Barclays cuts Petrobakken Energy target price to C$14 from C$15; rating ov...
French health authority issues pneumococcal vaccine recommendation for over 65sFOUR SEASONAL VACCINES ARE NOW RECOMMENDED FOR THIS AGE GROUP France’s health advisory authority is recommending that pn...
Why RBI should plan stricter rules for unsecured digital lendingIt is time for the Reserve Bank of India (RBI) to focus on unsecured personal loans and caution banks and lenders about ...