Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer
Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The GIM2 phase III trial demonstrated the benefit of dose-dense chemotherapy in node-positive early breast cancer (eBC). To better define the dose-dense effect in the hormone
receptor-positive subgroup, we evaluated its benefit through a composite measure of recurrence risk. We conducted an ancillary analysis of the GIM2 trial evaluating the absolute treatment
effect through a composite measure of recurrence risk (CPRS) in patients with hormone receptor-positive HER2-negative eBC. CPRS was estimated through Cox proportional hazards models applied
to the different clinicopathological features. The treatment effect was compared to the values of CPRS by using the Sub-population Treatment Effect Pattern Plot (STEPP) process. The
Disease-Free Survival (DFS)-oriented STEPP analysis showed distinct patterns of relative treatment effect with respect to CPRS. Overall, 5-year DFS differed across CPRS quartiles ranging
from 95.2 to 66.4%. Each CPRS quartile was characterized by a different patients’ composition, especially for age, lymph node involvement, tumor size, estrogen and progesterone receptor
expression, and Ki-67. A number needed to treat of 154 and 6 was associated with the lowest and the highest CPRS quartile, respectively. Dose-dense adjuvant chemotherapy showed a consistent
benefit in node-positive eBC patients with hormone receptor-positive HER2-negative disease, but its effect varied according to CPRS. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPACT OF
DOSE-DENSE NEOADJUVANT CHEMOTHERAPY ON PATHOLOGIC RESPONSE AND SURVIVAL FOR HER2-POSITIVE BREAST CANCER PATIENTS WHO RECEIVE TRASTUZUMAB Article Open access 11 June 2021 GAIN2 TRIAL OVERALL
SURVIVAL WITH INTENSE VERSUS TAILORED DOSE DENSE CHEMOTHERAPY IN EARLY BREAST CANCER Article Open access 30 July 2024 CLINICAL OUTCOMES IN ESTROGEN RECEPTOR-POSITIVE EARLY-STAGE BREAST
CANCER PATIENTS WITH RECURRENCE SCORE 26-30: OBSERVATIONAL REAL-WORLD COHORT STUDY Article Open access 02 June 2023 INTRODUCTION Although evidence support dose-dense chemotherapy in early
breast cancer (eBC), a clinical practice still varies around the world, and few randomized clinical trials have compared the same drugs and doses in the dose-dense and control
arms1,2,3,4,5,6. Furthermore, indirect comparison among trials could also be potentially confounded by noncompletely comparable risk profiles across the patients’ populations enrolled in
different studies. In the clinical decision-making process, careful analysis of clinical and biological characteristics is crucial to identify subgroups of patients with a favorable
risk/benefit ratio associated with the use of a dose-dense schedule. Despite the increasing evidence supporting the use of dose-dense adjuvant chemotherapy for patients with high-risk eBC,
the association between hormone receptor status and absolute benefit from a dose-dense schedule remains unclear7,8. Previous generation studies often discouraged the use of dose-dense
regimens in patients with hormone receptor-positive tumors, restricting the recommendation to the hormone receptor-negative subgroup7,9. The CALGB combined analysis of trials 8541, 9344, and
9741, highlighted that dose-dense chemotherapy was particularly effective in patients with hormone receptor-negative eBC, whereas the benefit in patients with hormone receptor-positive
disease was smaller and not significantly different from the standard schedule10. The GIM2 randomized phase III trial enrolled patients with node-positive eBC regardless of hormone receptor
and HER2 status. After a median follow-up of 7 years, the study demonstrated an improvement both in terms of disease-free survival (DFS) and overall survival (OS) for the dose-dense arm
(hazard ratio (HR) 0.77, 95% CI 0.65–0.92, _P_ = 0.004 and HR 0.65, 95% CI 0.51–0.84; _P_ = 0.001, respectively)11. Interestingly, the trial reported a homogeneous benefit among the two
hormone receptor subgroups (_P_ for interaction of 0.43), with an HR 0.69 for hormone receptor-negative (95% CI 0.48–0.99) and 0.80 for hormone receptor-positive (95% CI 0.65–0.98)11. To
further refine the evidence of treatment effect in the hormone receptor-positive subgroup and to better inform selection in node-positive patients between dose-dense versus standard-interval
adjuvant chemotherapy, we evaluated treatment benefit according to a quantitative composite measure of recurrence risk based on clinicopathological features. RESULTS COHORT CHARACTERISTICS
AND PROGNOSTIC FACTORS Characteristics of the 1131 patients in the HER2-negative hormone receptor-positive analysis population according to cohort, as defined by chemotherapy schedule
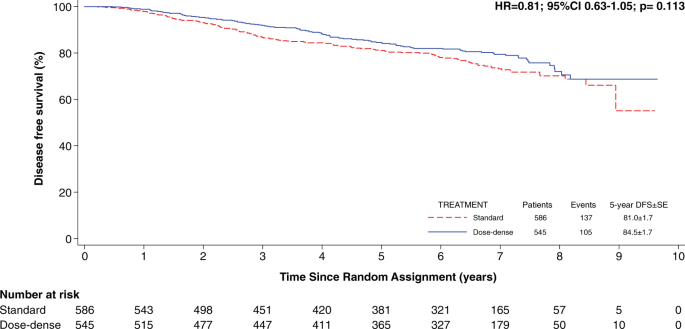
(dose-dense or standard interval), are summarized in Table 1. The median follow-up was 6.4 years (interquartile range 3.8–7.3 years). The overall 5-year DFS was 84.5% among patients treated
with dose-dense chemotherapy versus 81% among patients treated with standard-interval chemotherapy (Fig. 1). The impact of prognostic factors in terms of DFS, independently from treatment
assignment, are described in Fig. 2. The multivariate analysis highlighted that number of positive nodes (≥4 vs 1–3; HR 2.09, 95% CI 1.61–2.71, _P_ < 0.001), tumor size (>2 vs ≤2 cm;
HR 1.78, 95% CI 1.36–2.34, _P_ < 0.001) and histological grade (respectively, G2 vs G1 and G3 vs G1; HR 2.78, 95% CI 1.13–6.80, _P_ = 0.026; HR 2.98, 95% CI 1.20–7.40, _P_ = 0.018) were
the most important prognostic factors in terms of DFS and thus contributed the most to the composite measure of recurrence risk (Table 2). CPRS QUARTILES DEFINED SUBGROUPS WITH DIFFERENTIAL
RISK AND CONSEQUENT BENEFIT FROM DOSE-DENSE CHEMOTHERAPY Table 3 shows the features of the 17 subpopulations identified using the STEPP methodology. As adjacent subpopulations share
patients, an overlap in the CPRS range is present. The DFS-oriented STEPP analysis showed different patterns of relative treatment effect with respect to CPRS (Fig. 3a and Table 3). In
subpopulations with lower risk scores, the efficacy of dose-dense therapy appears to be absent while for CPRS median values around 2.20 we observed significantly reduced hazard ratios to
testify the maximum efficacy of therapy. The hazard ratio increased in patients with a worse anamnestic profile. Overall, DFS differed widely across CPRS quartiles ranging from 95.2% to
66.4% (Fig. 3b). Each CPRS quartile was characterized by a different patients’ composition (Table 4). Considering the observed and expected frequency of each cell estimated from the two-way
Table 4, the first CPRS quartile was characterized by age between 45 and 49 years (90 observed vs 51 expected), less than four lymph nodes involved (275 observed vs 177 expected) a tumor
size ≤2 (264 observed vs 155 expected) and a PGR > 20 (respectively, 25 observed vs 60 expected and 242 observed vs 170 expected for the PGR 20–49 and ≥50 subgroups). The second CPRS
quartile was mainly characterized by age between 40 and 49 years (105 observed vs 91 expected), less than four lymph nodes involved (255 observed vs 177 expected), a tumor size ≤2 (182
observed vs 155 expected), and G2 (169 observed vs 154 expected) (Fig. 3c). Patients included in the third CPRS quartile showed ≥4 lymph nodes involved (132 observed vs 106 expected), a
tumor size >2 (141 observed vs 128 expected), a PGR < 20 (69 observed vs 53 expected), and G3 (125 observed vs 107 expected) (Fig. 3c). The fourth CPRS was characterized by an age <
40 years (respectively 12 observed vs 9 expected and 48 observed vs 21 expected for the <35 years and 35–39 years subgroups), ≥4 lymph nodes involved (255 observed vs 105 expected) a
tumor size >2 (250 observed vs 127 expected), ER < 50 (70 observed vs 45 expected), PGR < 20 (78 observed vs 52 expected), G3 (155 observed vs 106 expected), and a Ki-67 ≥ 26 (129
observed vs 83 expected) (Fig. 3c). The number needed to treat (NNT) associated with the lowest CPRS quartile was 154, whereas the NNT associated with the highest CPRS was 6 (Fig. 3c). The
second and third CPRS quartiles had, respectively, a NTT of 35 and 5. The CPRS scores’ impact across treatment types was reported in terms of 5-year and 9-year DFS (Fig. 4a, b). DISCUSSION
The present analysis conducted within the GIM2 trial assessed the differential benefit of adjuvant dose-dense chemotherapy in hormone receptor-positive HER2-negative eBC patients through a
CPRS approach. By combining the prognostic impact of age, nodal involvement, tumor size, level of ER and PGR, histological grade, and Ki-67, 17 different CPRS-based subpopulations were
defined, and their HR heterogeneity was represented by STEPP. The population was then stratified according to CPRS quartiles, suggesting four different risk subgroups with differential
benefit from dose-dense adjuvant chemotherapy and characterized by different clinical features. Of note, analyzing the extreme percentiles, it is observed that some variables (e.g., number
of lymph nodes <4, tumor size <2 cm, PGR > 50%, histological grade <3, Ki-67 < 20%) characterize subpopulations with low CPRS values which correspond to a lower therapeutic
benefit. Conversely, the efficacy of the dose-dense chemotherapy appears to be greater for subpopulations with high CPRS values where variables such as a number of lymph nodes ≥4, a tumor
size >2 cm, and Ki-67 levels ≥20% are mostly concentrated. The subsequent DFS-oriented STEPP analysis showed, moreover, an increasingly higher benefit for the dose-dense regimen across
medium–high-risk patients, with advantage mitigation for the highest risk subgroup. On the other hand, the absolute benefit was consistently higher in the third and the fourth CPRS quartile
(respectively, NNT 5 and 6) (Fig. 3c), compared to the first CPRS quartile (NNT 154), a trend that was also confirmed in terms of 5-year and 9-year DFS. These apparently counterintuitive
results could be due to a lower relative benefit linked to the specific tumor biology of the fourth CPRS quartile which is undifferentiated and therefore potentially less chemosensitive. On
the other hand, the higher clinical risk of this subgroup amplifies small relative benefits in higher absolute benefits. The use of dose-dense chemotherapy in patients with hormone
receptor-positive has been largely debated. It is generally believed that hormone receptor-positive tumors are intrinsically less responsive to chemotherapy than hormone receptor-negative
tumors. In the CALGB combined analysis, the absolute benefit due to the dose-dense schedule was greater for patients with hormone receptor-negative disease compared with that observed for
patients with hormone receptor-positive disease10. The authors have provided some possible explanations for these observations. One hypothesis considered the fact that, although the
chemotherapy regimen was randomly assigned across treatment arms, endocrine treatment was not. Different agents and treatment compliances could randomly affect overall efficacy and
consequently alter the final conclusions. Furthermore, the risk reduction that may be achieved with chemotherapy is difficult to detect in the first years after treatment when the risk of
relapse for luminal subtypes is lower than in hormone receptor-negative subgroups. Regardless, the population of hormone receptor-positive could be heterogeneous with respect to intrinsic
sensitivity to chemotherapy. Several studies have proposed gene expression analysis as a means to define the benefit of chemotherapy in patients with hormone receptor-positive HER2-negative
breast cancer12. Interestingly, an interim analysis (median follow-up: 5.1 years) of phase 3 RxPONDER trial, presented at the San Antonio Breast Cancer Symposium 2020, showed that adding
chemotherapy to endocrine therapy did not improve outcomes for postmenopausal women with early-stage, node-positive, luminal breast cancer in comparison to endocrine therapy alone.
Specifically, these findings relate to postmenopausal patients with involvement of 1–3 lymph nodes and defined as low risk on the basis of recurrence score ≤25 on the 21-tumor gene
expression assay (Oncotype Dx). On the other hand, women with the same characteristics but premenopausal were found to have benefited from chemotherapy, probably as a result of the endocrine
effect of chemotherapy-induced ovarian suppression13. Although gene expression analysis was not performed in patients of the GIM2 trial, it seems reasonable to assume that the estimation of
the benefit from adding chemotherapy to endocrine therapy in low risk (i.e., recurrence score ≤25) patients also applies to dose-dense chemotherapy. Of note, a pooled analysis of two
randomized clinical trials that evaluated the efficacy of adjuvant dose-dense chemotherapy in premenopausal breast cancer patients did not show an increased risk of treatment-induced
amenorrhea with dose-dense chemotherapy14. Therefore, in premenopausal patients, it is difficult to hypothesize a greater chemo-induced endocrine effect of dose-dense chemotherapy compared
to standard-interval chemotherapy. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) patient-level meta-analysis further dissected the role of dose-dense adjuvant regimens in
eBC15. The pooled data of 37,298 women enrolled in 26 different randomized trials were analyzed, with recurrence and breast cancer mortality as primary outcomes. In the total population,
dose-intense strategies were associated with a reduction of both recurrences (RR 0.86; 95% CI 0.82–0.89; _P_ < 0.0001) and breast cancer mortality (RR 0.86; 95% CI 0.77–0.96). Recurrence
reduction was similar in hormone receptor-negative and hormone receptor-positive eBC patients (RR 0.82; 95% CI 0.71–0.95 and RR 0.83, 95% CI 0.75–0.93, respectively), while a slightly
different delta was highlighted in terms of 10-year recurrence (3.7% vs 3.1% in hormone receptor-negative and hormone receptor-positive eBC patients, respectively), consistently with the
assumption that, at the same level of relative risk reduction, higher-risk groups experience a greater absolute benefit. In fact, while acknowledging the value of the EBCTG meta-analysis, we
conducted an exploratory analysis of the GIM2 study in order to understand granularly how the different variables contribute to the risk of relapse and the consequent estimate of the
therapeutic benefit based on that risk. With a median follow-up of 7 years, GIM2 is the dose-dense focused trial with the longest follow-up and is therefore the best candidate to better
explore tailored treatment strategies in hormone receptor-positive eBC. By combining well-established prognostic factors in a CPRS, the present analysis was capable to represent risk as a
continuous variable and to identify and describe specific risk subgroups that could have a differential benefit from dose-dense chemotherapy. In particular, the CPRS quartile 4 is
characterized by specific biological characteristics that confer a potentially chemosensitive phenotype and are typically associated with a luminal B-like profile, such as low ER and PGR
levels and high KI67. Notably, the association with younger age could have intriguing biological implications16. It has been previously reported that breast cancer of the young, particularly
the hormone receptor-positive subtype, is a peculiar biological entity that can differ from the older counterpart in several molecular aspects and in particular through the enrichment of
biological processes related to stemness and growth factor signaling and the downregulation of apoptosis-related genes17. These aspects could deeply affect the tumors’ response to
chemotherapy which could justify the need for a more dose-intense treatment schedule14. Dose-dense adjuvant chemotherapy showed a consistent benefit in node-positive eBC patients with
hormone receptor-positive HER2-negative disease, but its effect varied according to CPRS. Therefore, dose-dense adjuvant chemotherapy should be taken into consideration also in the hormone
receptor-positive subgroup. This study offers a potential hint towards a tailored approach based on broader multiparametric criteria, capable of capturing the wide and granular risk spectrum
of hormone receptor-positive eBC. METHODS COHORT DESIGN The GIM2 is a multicenter, randomized phase 3 trial, with a 2 × 2 factorial design aiming to address both the role of the addition of
fluorouracil to epirubicin, cyclophosphamide, and paclitaxel and the role of the dose-dense schedule in the adjuvant treatment of patients with node-positive eBC11. The study population
included 2198 patients from 81 Italian centers. The primary endpoint was DFS, defined as the time from random assignment to local or distant recurrence, contralateral or ipsilateral breast
tumor (excluding ductal carcinoma in situ), second primary malignancy, death from any cause, and loss to follow-up or end of the study, whichever came first. As a sub-analysis of the GIM2
randomized multicenter clinical trial, the study was approved by ethics committees of all participating institutions. Written informed consent was obtained from all patients before study
entry. Participating centers and principal investigators are listed in Supplementary Data 1. COMPOSITE RISK SCORE ANALYSIS The present analysis examined the absolute treatment effect through
a composite measure of recurrence risk to better individualize the clinical algorithm in the choice between dose-dense and standard-interval chemotherapy in patients with hormone
receptor-positive HER2-negative eBC (_N_ = 1131). Patients with hormone receptor-positive HER2-positive eBC were excluded from the current analysis to avoid the potential confounding effect
of trastuzumab use in only a minority of these patients and the differential observed benefit of dose-dense chemotherapy in this subgroup18. Applying to hormone receptor-positive
HER2-negative patients the Cox proportional hazards model, the risk of recurrence (hazard ratio) related to each prognostic factor of the patients was estimated, such as age (<35, 35–39,
40–44, 45–49, ≥50 years), number of positive nodes (1–3, ≥4), tumor size (≤2, >2 cm), estrogen receptor (ER, < 50%, ≥50%), progesterone receptor (PGR, < 20%, 20–49%, ≥50%),
histological grade (1, 2, 3), KI67 ( < 14%, 14–19%, 20–25%, ≥26%). The groupings of prognostic factors followed the usual clinical cut-points as defined by the St Gallen Consensus
statements with the exception of estrogen receptor due to the low number of patients with values less than 10% (_n_ = 31) and KI67 where a high proportion of patients (_n_ = 294) showed
values equal to or greater than 2619. Multiple Imputation procedure was applied to handle missing values20,21. The CPRS was then computed, for each patient, through the equation CPRS =
_Β_1_X_1 + … + _Β_N_X_N (1). In Eq. (1) for each prognostic factor, _Β_I is the logs (hazard ratio) and _X_I is an indicator variable taking value 1 if the factor is present and 0 otherwise.
Thus, the CPRS was estimated as the sum of the logs (hazard ratio) of the prognostic factors of each patient. We explored the treatment effect compared to the values of CPRS by using the
Sub-population Treatment Effect Pattern Plot (STEPP) process21. Proportional hazards assumption was satisfied before and after multiple imputation22. The number needed to treat (NNT) was
computed as the reciprocal of the difference between the absolute risks of failure in the dose-dense and standard therapy arms, respectively. Patients were ordered from the lowest to the
highest value of CPRS. Two quantities were chosen: N1, the size of the cohorts, and N2 the maximum number of patients shared by two cohorts where N1 > N2. The process began with the
cohort of first N1 patients in the ordered list. At the next step, the N2 patients with the highest values of the CPRS in the previous cohort were moved to a new cohort in addition to the
N1–N2 patients that followed in the ordered list. The process ended when all patients were included in at least one cohort. In this analysis, it was chosen N1 = 300 and N2 = 250. For each
cohort, the HR relative to treatment was estimated. The heterogeneity of HRs along the cohorts defined by STEPP analysis was tested by means of permutation test23. Statistical analysis was
performed using R (The R Foundation for Statistical Computing. version 3.3.1 (2016-06-21)) and STATA (StataCorp. (2015) Stata Statistical Software: Release 14.2. College Station, TX:
StataCorp LP) software packages. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The
data generated and analyzed during this study are described in the following data record: https://doi.org/10.6084/m9.figshare.1454732124. The data are contained in two Excel spreadsheets:
“Patient survival data.xlsx” and “Patient baseline, tumour and treatment data.xlsx”. These spreadsheets are housed on institutional storage and are not publicly available for the following
reason: data contain information that could compromise research participant privacy. However, the data can be made available upon reasonable request to the corresponding author. The dataset
analyzed during this study is described with more details in the following manuscript: https://doi.org/10.1016/S0140-6736(14)62048-111. REFERENCES * Hall, C. S. et al. Prognostic value of
circulating tumor cells identified before surgical resection in nonmetastatic breast cancer patients. _J. Am. Coll. Surg._ 223, 20–29 (2016). Article Google Scholar * Dieci, M. V. et al.
Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. _Ann. Oncol._ 24, 101–108 (2013). Article CAS Google
Scholar * Pérez-Barrios, C. et al. Comparison of methods for circulating cell-free DNA isolation using blood from cancer patients: impact on biomarker testing. _Transl. Lung Cancer Res._ 5,
665–672 (2016). Article Google Scholar * Chung, J. H. et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with estrogen receptor-positive metastatic
breast cancer. _Ann. Oncol._ 28, 2866–2873 (2017). Article CAS Google Scholar * Khagi, Y. et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint
inhibitor–based immunotherapy. _Clin. Cancer Res._ 23, 5729–5736 (2017). Article CAS Google Scholar * Blondeaux, E. et al. Dose-dense adjuvant chemotherapy in early breast cancer
patients: 15-year results of the Phase 3 Mammella InterGruppo (MIG)-1 study. _Br. J. Cancer_ https://doi.org/10.1038/s41416-020-0816-8 (2020). * Bonilla, L. et al. Dose-Dense chemotherapy in
nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. _J. Natl. Cancer Inst._ 102, 1845–1854 (2010). Article CAS Google Scholar * Petrelli,
F. et al. Adjuvant dose-dense chemotherapy in breast cancer: a systematic review and meta-analysis of randomized trials. _Breast Cancer Res. Treat._ 151, 251–259 (2015). Article CAS Google
Scholar * Burnell, M. et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide
followed by paclitaxel in node-positive or high-risk node-negative breast cancer. _J. Clin. Oncol._ 28, 77–82 (2010). Article CAS Google Scholar * Berry, D. A. et al. Estrogen-receptor
status and outcomes of modern chemotherapy for patients with node-positive breast cancer. _J. Am. Med. Assoc._ 295, 1658–1667 (2006). Article CAS Google Scholar * Del Mastro, L. et al.
Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2×2 factorial, randomised phase 3 trial. _Lancet_ 385, 1863–1872
(2015). Article Google Scholar * Burstein, H. J. Systemic therapy for estrogen receptor-positive, HER2-negative. _Breast Cancer N. Engl. J. Med_ 383, 2557–2570 (2020). Article CAS Google
Scholar * Kalinsky, K. et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) inpatients (pts) with 1-3 positive
nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer. _Cancer Res_. 81, GS3-00 (2021). * Lambertini, M. et al. Dose-dense adjuvant chemotherapy in premenopausal
breast cancer patients: A pooled analysis of the MIG1 and GIM2 phase III studies. _Eur. J. Cancer_ 71, 34–42 (2017). Article CAS Google Scholar * Gray, R. et al. Increasing the dose
intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. _Lancet_
393, 1440–1452 (2019). Article Google Scholar * Partridge, A. H. et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. _J. Clin. Oncol._ 34,
3308–3314 (2016). Article Google Scholar * Azim, H. A. et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. _Clin. Cancer Res._
18, 1341–1351 (2012). Article CAS Google Scholar * Lambertini, M. et al. Dose‐dense adjuvant chemotherapy in HER2‐positive early breast cancer patients before and after the introduction
of trastuzumab: exploratory analysis of the GIM2 trial. _Int. J. Cancer_ 147, 160–169 (2020). Article CAS Google Scholar * Regan, M. M. et al. Absolute benefit of adjuvant endocrine
therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. _J. Clin. Oncol._ 34, 2221–2231
(2016). Article CAS Google Scholar * Schafer, J. L. & Olsen, M. K. Multiple imputation for multivariate missing-data problems: a data analyst’s perspective. _Multivar. Behav. Res._
33, 545–571 (1998). Article CAS Google Scholar * Bonetti, M. & Gelber, R. D. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data.
_Stat. Med._ 19, 2595–2609 (2000). Article CAS Google Scholar * Grambsch, P. M. & Therneau, T. M. Proportional hazards tests and diagnostics based on weighted residuals. _Biometrika_
81, 515–526 (1994). Article Google Scholar * Bonetti, M., Zahrieh, D., Cole, B. F. & Gelber, R. D. A small sample study of the STEPP approach to assessing treatment-covariate
interactions in survival data. _Stat. Med._ 28, 1255–1268 (2009). Article Google Scholar * Puglisi, F. et al. Metadata record for the article: composite risk and benefit from adjuvant
dose-dense chemotherapy in hormone receptor positive breast cancer. https://doi.org/10.6084/m9.figshare.14547321 (2021). Download references ACKNOWLEDGEMENTS The GIM group received financial
support for trial conduct from Bristol-Myers Squibb and Pharmacia. The funders were not involved in the study design, collection, analysis, interpretation of the data, the writing of this
article, or the decision to submit it for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine (DAME), University of Udine, Udine, Italy Fabio Puglisi &
Lorenzo Gerratana * Department of Medical Oncology, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano (PN), Italy Fabio Puglisi & Lorenzo Gerratana * Department of Internal
Medicine and Medical Specialties (DiMI), School of Medicine, University of Genoa, Genova, Italy Matteo Lambertini & Lucia Del Mastro * Department of Medical Oncology, U.O.C. Clinica di
Oncologia Medica, IRCCS Ospedale Policlinico San Martino, Genova, Italy Matteo Lambertini * Clinical Epidemiology Unit, IRCCS Ospedale Policlinico San Martino, Genova, Italy Marcello Ceppi
& Luca Boni * Day Hospital Oncologico Multidisciplinare, Istituto di Candiolo, FPO-IRCCS, Candiolo, Italy Filippo Montemurro * Department of Oncology, ASU FC University Hospital, Udine,
Italy Stefania Russo * Oncologia Medica 2, IRCCS Ospedale Policlinico San Martino, Genova, Italy Claudia Bighin * Department of Breast Oncology, Istituto Nazionale Tumori IRCCS “Fondazione
G. Pascale”, Napoli, Italy Michelino De Laurentiis * Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy Mario Giuliano & Sabino De Placido *
AUSL-IRCCS Reggio Emilia, Reggio Emilia, Italy Giancarlo Bisagni * Breast Unit Ospedale S Anna, Citta’ della Salute e della Scienza di Torino, Torino, Italy Antonio Durando * Breast Unit,
ASL Citta’ di Torino, Torino, Italy Anna Turletti * Breast Unit, Department of Medical Oncology AO S. Croce e Carle Ospedale di Insegnamento, Cuneo, Italy Ornella Garrone * Medical Oncology,
S.Orsola University Hospital, Bologna, Italy Andrea Ardizzoni * Department of Medical Oncology, Sandro Pertini Hospital and S. Eugenio Hospital, ASL Roma2, Roma, Italy Teresa Gamucci *
Medical Oncology, Azienda Ospedaliera S Giuseppe Moscati, Avellino, Italy Giuseppe Colantuoni * Clinical Trial Unit, Istituto Nazionale Tumori IRCCS “Fondazione G. Pascale”, Napoli, Italy
Adriano Gravina * Department of Medical Oncology, Istituto Regina Elena per lo Studio e la Cura dei Tumori, Roma, Italy Francesco Cognetti * UO Breast Unit, IRCCS Ospedale Policlinico San
Martino, Genova, Italy Lucia Del Mastro Authors * Fabio Puglisi View author publications You can also search for this author inPubMed Google Scholar * Lorenzo Gerratana View author
publications You can also search for this author inPubMed Google Scholar * Matteo Lambertini View author publications You can also search for this author inPubMed Google Scholar * Marcello
Ceppi View author publications You can also search for this author inPubMed Google Scholar * Luca Boni View author publications You can also search for this author inPubMed Google Scholar *
Filippo Montemurro View author publications You can also search for this author inPubMed Google Scholar * Stefania Russo View author publications You can also search for this author inPubMed
Google Scholar * Claudia Bighin View author publications You can also search for this author inPubMed Google Scholar * Michelino De Laurentiis View author publications You can also search
for this author inPubMed Google Scholar * Mario Giuliano View author publications You can also search for this author inPubMed Google Scholar * Giancarlo Bisagni View author publications You
can also search for this author inPubMed Google Scholar * Antonio Durando View author publications You can also search for this author inPubMed Google Scholar * Anna Turletti View author
publications You can also search for this author inPubMed Google Scholar * Ornella Garrone View author publications You can also search for this author inPubMed Google Scholar * Andrea
Ardizzoni View author publications You can also search for this author inPubMed Google Scholar * Teresa Gamucci View author publications You can also search for this author inPubMed Google
Scholar * Giuseppe Colantuoni View author publications You can also search for this author inPubMed Google Scholar * Adriano Gravina View author publications You can also search for this
author inPubMed Google Scholar * Sabino De Placido View author publications You can also search for this author inPubMed Google Scholar * Francesco Cognetti View author publications You can
also search for this author inPubMed Google Scholar * Lucia Del Mastro View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS F.P., C.M., B.L.,
and L.D.M. were responsible for the conception and design; F.P., L.G., M.L., C.M., B.L., and L.D.M. were responsible for the acquisition and analysis; all authors were responsible for the
interpretation of the data and approval and editing of the final manuscript. CORRESPONDING AUTHOR Correspondence to Fabio Puglisi. ETHICS DECLARATIONS COMPETING INTERESTS F.P. reports
research grant/funding from AstraZeneca, Eisai, personal fees from AstraZeneca, Roche, Amgen, Lilly, Novartis, Pfizer, outside the submitted work; L.G. reports non-financial support from
Menarini Silicon Biosystems, personal fees from Lilly, outside the submitted work; M.L. reports personal fees from Roche, Takeda, Theramex, Lilly, Pfizer, outside the submitted work; F.M.
reports personal fees from Roche, Lilly, Novartis, Pfizer, Pierre Fabre, Daiichi Sankyo, outside the submitted work; C.B. reports research grant/funding from Roche, Novartis, personal fees
from Roche, Novartis, Lilly, outside the submitted work; M.D.L. reports personal fees from Novartis, Pfizer, AstraZeneca, Roche, Pierre Fabre, Amgen, Lilly, MSD, Celgene, Eisai, outside the
submitted work; M.G. reports reports non-financial support from Roche, Pfizer, Novartis, Amgen, personal fees from Novartis, Lilly, Pfizer, Eisai, Celgene, AstraZeneca, Roche, outside the
submitted work; A.T. reports personal fees from Eisai, Roche, Pfizer, outside the submitted work; O.G. reports personal fees from Pfizer, Novartis, Eisai, Lilly, non-financial support from
Roche, outside the submitted work; T.G. reports non-financial support from Pfizer, Novartis, Roche, Lilly, personal fees from Eisai, Pfizer, Novartis, Roche, Lilly, outside the submitted
work; G.T. research grant/funding from Roche, Novartis, personal fees from Roche, Novartis, outside the submitted work; A.G. reports non-financial support from Pfizer, personal fees from
Istituto Gentili, outside the submitted work; S.D.P. reports personal fees from GSK, Novartis, Roche, Celgene, AstraZeneca, Amgen, Teva,Eisai, Pfizer, Lilly, outside the submitted work; C.F.
reports non-financial support from Novartis, Roche, MSD, BMS, personal fees from ABBOTT, CELGENE, GSK, Roche, Bayer, Novartis, Amgen, Pfizer, AstraZeneca, EISAI, Vifor Pharma, Merck—Serono,
Boheringer Ingelheim, MSD, BMS, Takeda, Astellas Oncology, Lilly, Genomic Health, outside the submitted work; L.D.M. reports non-financial support from Roche, Celgene, Pfizer, personal fees
from Genomic Health, Pfizer, Seattle Genetics, Pierre Fabre, Lilly, Roche, Novartis, MSD, Eisai, Ipsen, Takeda, outside the submitted work. The remaining authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY DATA 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Puglisi, F., Gerratana, L., Lambertini, M. _et al._ Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone
receptor-positive breast cancer. _npj Breast Cancer_ 7, 82 (2021). https://doi.org/10.1038/s41523-021-00286-w Download citation * Received: 20 August 2020 * Accepted: 27 May 2021 *
Published: 28 June 2021 * DOI: https://doi.org/10.1038/s41523-021-00286-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Network pharmacology suggests biochemical rationale for treating COVID-19 symptoms with a Traditional Chinese MedicineChinese herbal formulas including the lung-cleaning and toxicity-excluding (LCTE) soup have played an important role in ...
Smoking, drinking and eating: public health should not be all about the individualDiseases linked to smoking tobacco, a lack of exercise, drinking alcohol and eating unhealthily are on the rise, even th...
Rock minerals - gardening australiaJosh Byrne JOSH BYRNE: One thing I'm always banging on about is rock minerals.... _Some rock minerals... a little b...
Aless_Vinogradinka_, он продал 49% акций, осталось 35%. Он не участвует в оперативном управлении, но является акционером, поэ...
Just a moment...NOTÍCIAS DO HOSPÍCIO PULOU O MURO E SAIU ANDANDO Enquanto Carla Zambelli mete o pé, Bananinha apresenta-se como o novo e...
Latests News
Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancerABSTRACT The GIM2 phase III trial demonstrated the benefit of dose-dense chemotherapy in node-positive early breast canc...
The public image of cross-infection controlARTICLE PDF Authors * J Bowden View author publications You can also search for this author inPubMed Google Scholar * C ...
The great 'back to the future' web hoaxNews that July 5, 2010 was the day Marty McFly and the 'Doc' travel to in _Back to the Future II_ spread like ...
Classroom design can boost primary pupils’ progress by 16%Peter Barrett received funding for this work from IBI Nightingale and the Engineering and Physical Sciences Research Cou...
Terrorism: are we ignoring the biggest threat?Who poses a greater terrorism threat to Americans—Islamic radicals, or right-wing hate groups? asked STEVE COLL in _NEWY...