Engineered lactobacilli display anti-biofilm and growth suppressing activities against pseudomonas aeruginosa
Engineered lactobacilli display anti-biofilm and growth suppressing activities against pseudomonas aeruginosa"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Biofilms are an emerging target for new therapeutics in the effort to address the continued increase in resistance and tolerance to traditional antimicrobials. In particular, the
distinct nature of the biofilm growth state often means that traditional antimcirobials, developed to combat planktonic cells, are ineffective. Biofilm treatments are designed to both reduce
pathogen load at an infection site and decrease the development of resistance by rendering the embedded organisms more susceptible to treatment at lower antimicrobial concentrations. In
this work, we developed a new antimicrobial treatment modality using engineered lactic acid bacteria (LAB). We first characterized the natural capacity of two lactobacilli, _L. plantarum_
and _L. rhamnosus_, to inhibit _P. aeruginosa_ growth, biofilm formation, and biofilm viability, which we found to be dependent upon the low pH generated during culture of the LAB. We
further engineered these LAB to secrete enzymes known to degrade _P. aeruginosa_ biofilms and show that our best performing engineered LAB, secreting a pathogen-derived enzyme (PelAh),
degrades up to 85% of _P. aeruginosa_ biofilm. SIMILAR CONTENT BEING VIEWED BY OTHERS MECHANISMS OF ANTIMICROBIAL RESISTANCE IN BIOFILMS Article Open access 01 October 2024 THE RIBONUCLEASE
PNPASE IS A KEY REGULATOR OF BIOFILM FORMATION IN _LISTERIA MONOCYTOGENES_ AND AFFECTS INVASION OF HOST CELLS Article Open access 07 June 2023 SELECTIVE INHIBITION OF THE AMYLOID MATRIX OF
_ESCHERICHIA COLI_ BIOFILMS BY A BIFUNCTIONAL MICROBIAL METABOLITE Article Open access 19 October 2023 INTRODUCTION As an important virulence factor for pathogenic microbes, biofilms are
associated with an expanding array of pathologies, including various airway, gastrointestinal, and ocular infections, endocarditis, periodontitis, osteomyelitis, cystitis, and chronic
wounds1,2,3,4,5,6,7. Biofilms represent a distinct growth state, morphologically distinguished by bacteria residing within a self-produced matrix of extracellular polymeric substances (EPS),
that may include proteins, extracellular DNA (eDNA), polysaccharides, and lipids8. Within the biofilm, isogenic cells exhibit phenotypic diversity that is driven by the discrete
microenvironments created by metabolite, ion, gas, and antimicrobial gradients within the biofilm. Biomedically, this phenotypic diversity manifests as distinct tolerances or resistances to
traditional antimicrobials as well as the host immune system9,10. Additionally, biofilms stabilize surface colonization and are frequently less susceptible to traditional methods of surface
decontamination, exacerbating the recalcitrance to treatment. Thus, clearance of mature biofilms is an essential component for the successful resolution of numerous infections, especially
those that are chronic or recurrent in nature. Invasive burn wounds and chronic wounds, or wounds that fail to progress through the later stages of the normal healing process, are commonly
contaminated or colonized by a multitude of biofilm-forming organisms. Standard treatments for these wound types include nanocrystalline silver, silver sulphadiazine, iodine, or topical
antibiotics. However, these treatments are often ineffective at reducing wound infection, add unnecessary expense, and/or inhibit the healing process11,12,13,14. Further, extensive use of
these treatments has bred a large population of multi-drug-resistant microbes for which new treatments that target both planktonic and biofilm cells are necessary. A widespread biofilm
targeting strategy is the enzymatic degradation of biofilm polymer(s) to decrease surface adhesion and return the entrained bacteria to a more treatable phenotype15,16,17,18,19,20,21. Rapid
advancement in synthetic biology and probiotic therapies have led to interest in developing engineered bacteriotherapies or live biotherapeutic products. These “smart”, bacteria-based
therapeutic delivery vectors provide sustained delivery of the therapeutic and dynamically respond to environmental signals, while retaining their innate probiotic qualities22,23,24,25.
Recent examples of bacteriotherapies include the delivery of enzymes, antimicrobials, metabolites, or anti-inflammatory proteins to combat metabolic deficiencies, tumors, inflammation,
biofilms, and infections22,26,27,28,29,30,31,32. In this study, we construct and assess the utility of genetically engineered probiotic bacteria as anti-biofilm and antimicrobial agents
against the common wound pathogen _Pseudomonas aeruginosa_. We selected lactic acid bacteria (LAB) as the chassis strains for the bacteriotherapy due to their broad-spectrum antimicrobial
and wound healing capacities, genetic tractability, and well-characterized expression systems for the production and secretion of heterologous proteins. Furthermore, Several LAB have been
shown to impair the growth of drug-resistant _P. aeruginosa_ clinical isolates33. More specifically, _Lactobacillus plantarum_ and _Lactobacillus rhamnosus_, the species used in this work,
enhance the outcome of mouse _P. aeruginosa_ infection models, increase epithelial migration, and are equally as effective as current treatments when applied to human burn wounds34,35,36,37.
We add to this body of evidence, showing that _L. plantarum_ WCFS1 and _L. rhamnosus_ GG (LGG) are effective inhibitors of planktonic growth, biofilm formation, and the viability of
biofilm-embedded cells (biofilm viability) of the burn wound isolate _P. aeruginosa_ PA14 (PA14). We further increase the usefulness of _L. plantarum_ and LGG by engineering them to secrete
enzymes known to degrade PA14 biofilms and demonstrate the efficacy of this design for degradation of mature PA14 biofilms. RESULTS _L. PLANTARUM_ AND LGG INHIBIT PA14 GROWTH IN A
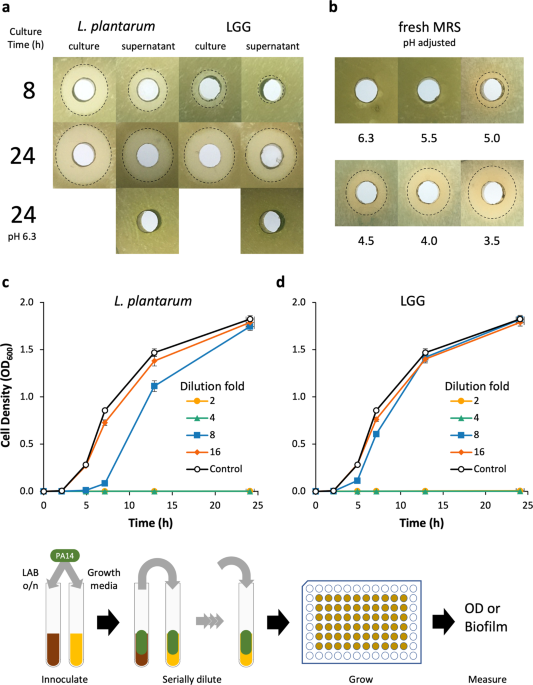
PH-DEPENDENT MANNER The feasibility of _L. plantarum_ and LGG as therapeutic vectors was first analyzed by characterizing their innate capacity to inhibit PA14 growth using an agar-well
diffusion assay and dilutions of LAB cultures in a modified MIC assay. The agar-well diffusion assay was used to determine the aeration and duration of LAB culture that maximally inhibited
PA14 growth. When _L. plantarum_ cultures were grown aerobically in a test tube or flask, growth inhibition of PA14 moderately increased (Supplementary Fig. 1). However, culture aeration had
no impact on LGG growth inhibition of PA14. Early phase _L. plantarum_ and LGG cultures (grown ≤4 h) and supernatants failed to inhibit PA14 growth, while late-stage (≥8 h) cultures and
supernatants of both organisms inhibited PA14 growth (Fig. 1a, Supplementary Fig. 1). The 24 h cultures of _L. plantarum_ were marginally more inhibitory than those of LGG; yet the
supernatants of both LAB exhibited similar growth inhibition against PA14. Generally, we found that PA14 growth inhibition increased with LAB culture duration and cultures were more
inhibitory than cell-free supernatants. The pH of 24 h supernatants was 3.8–3.9 and when we adjusted their pH back to the starting pH of 6.3, we observed no growth inhibition. To determine
if the inhibitory activity was due to pH alone or a factor that was active at low pH, we adjusted the pH of fresh MRS down to that of spent media and evaluated its inhibitory activity
against PA14. Decreasing the medium pH increased growth inhibition and when adjusted to pH 3.5, the medium had similar inhibitory activity as that of a 24 h LAB culture of pH 3.8–3.9. We
also used a modified minimum inhibitory concentration (MIC) assay to more quantitatively evaluate the inhibition of PA14 growth by _L. plantarum_ and LGG supernatants over time and determine
the relative quantity of spent supernatant necessary for bioactivity. Supernatants from 24 h cultures of _L. plantarum_ and LGG diluted up to 25% (i.e., 4× dilution) of the culture volume
completely inhibited PA14 growth (Fig. 1c, d). _L. plantarum_ supernatant diluted 8× still retained some inhibitory activity, while 8× dilution of LGG supernatants had no inhibitory activity
relative to growth medium alone. A dilution of 16×, or greater, of either LAB culture supernatant failed to inhibit PA14 growth. _L. PLANTARUM_ AND LGG INHIBIT PA14 BIOFILM FORMATION AND
VIABILITY Having characterized _L. plantarum_ and LGG inhibition of planktonic PA14 cells, we also analyzed the impact _L. plantarum_ and LGG supernatants had on PA14 biofilm formation and
biofilm viability (i.e., viability of cells in the biofilm matrix). We used the modified MIC assay workflow to evaluate the inhibition of PA14 biofilm formation. The supernatants from _L.
plantarum_ and LGG cultures inhibited PA14 biofilm formation in a concentration-dependent manner (Fig. 2a). Only at dilutions greater than 16× did biofilm form at detectable levels. The MRS
media control also inhibited PA14 biofilm formation, but only when undiluted or diluted 2-fold. LAB cell-free supernatants also inhibited the viability of PA14 cells embedded within
biofilms, as assessed by XTT dye assay38 (Fig. 2b). _L. plantarum_ and LGG supernatants diluted by 8× or less were able to inhibit PA14 biofilm viability such that no viable cells could be
detected relative to the control. When we plotted the viability against the pH of LAB culture dilutions, we found that the transition to viable biofilms correlates with the increase in pH
caused by dilution into PBS (Fig. 2c). Fresh MRS buffered to pH 5 and below completely inhibited biofilm viability—a finding in agreement with our previous finding that this medium has an
innate capacity to inhibit PA14 growth when adjusted to a lower pH (Fig. 1b). Interestingly, MRS adjusted to pH 6.25 and 5.5 also inhibited PA14 biofilm viability more than the diluted LAB
cultures of a similar pH, indicating innate anti-PA14 biofilm activity in the MRS. However, we found the major driver of decreased biofilm viability to be low pH. Generally, PA14 biofilms
treated with solutions with a pH ≤ 4.5 were nonviable, while solutions with a pH ≥ 5.2 were viable. We also found that for a given pH, undiluted spent LAB supernatant is more inhibitory
compared to that diluted in PBS. _L. PLANTARUM_ SECRETED MATRIX-DEGRADING ENZYMES DISRUPT MATURE PA14 BIOFILMS _P. aeruginosa_ biofilms are predominantly composed of an array of
polysaccharides (Alg or alginate, Psl, Pel), and eDNA, and the specific composition is dependent upon the genetic background and environment39. The strain _P. aeruginosa_ PA14, a burn wound
isolate, does not contain a functional operon to produce Psl, and does not produce alginate as a biofilm component40,41. Instead, PA14 produces biofilms predominantly composed of Pel
polysaccharide and eDNA42. _P. aeruginosa_ biofilms containing these components were previously shown to be sensitive to enzymatic degradation by solutions containing DNase, cellulase, or
native glycoside hydrolases produced by _P. aeruginosa_ to release biofilm cells and transition to planktonic growth16,43,44. We constructed broad host range LAB expression vectors for
secretion of the cellulase EngZ, a processive endoglucanse from the Gram-positive _Clostridium cellulovorans_; NucA, a thermostable nuclease from _Staphylococcus aureus_, a pathogen found to
infect similar sites as _P. aeruginosa_; and PelAh (the hydrolase domain of PelA) a native glycoside hydrolase from _P. aeruginosa_ that hydrolyzes the Pel polysaccharide. We validated the
expression and secretion of the biofilm degrading enzymes from _L. plantarum_ and LGG using SDS–PAGE of induced culture supernatants and enzyme activity assays. LAB cultures that contained
NucA, EngZ, or PelAh expression vectors had protein bands and/or enzymatic activity in the filtered supernatants, which indicates successful secretion of the intended enzymes. Specifically,
the supernatants of NucA-expressing and PelAh-expressing LAB contained protein bands of the appropriate size (Fig. 3a) but we saw no visible band for EngZ. The larger molecular weight of
EngZ compared to NucA and PelAh puts it in a region where numerous other protein bands in the gel make it difficult to resolve individual proteins, so we also checked for enzymatic activity.
We confirmed that the supernatants of LAB secreting EngZ had CMCase activity (Fig. 3b), whereas the supernatants of LAB secreting NucA had DNase activity (Fig. 3c). We tested the ability of
LAB cultures expressing and secreting NucA, EngZ, or PelAh, as well as their cell-free (filtered) supernatants, to degrade mature PA14 biofilms (Fig. 3d, Supplementary Fig. 2). We chose to
induce the cultures in BHI to decouple the growth and inhibition of biofilm formation that we previously characterized in MRS, from the biofilm degradation capacity of the secreted enzymes.
Cultures and supernatants of _L. plantarum_ expressing PelAh were highly effective at biofilm degradation, resulting in 80% and 85% reduction in biofilm biomass, respectively (Fig. 3d). _L.
plantarum_ PelAh supernatants maintained similar percent biofilm degradation even at 8-fold dilution (Supplementary Fig. 3). However, EngZ and NucA expressing cultures and supernatants were
ineffective at degrading PA14 biofilms. When we applied induced LGG cultures expressing PelAh to PA14 biofilms, there was a considerable increase in biofilm biomass, even when the
broad-spectrum Gram-negative antimicrobial polymyxin B was added to the culture to selectively inhibit additional PA14 growth (Supplementary Fig. 2). We observed less biofilm when LGG
supernatants were added to PA14 biofilms. Much of the biofilm was evident on the bottom of the well when LGG cultures were added to PA14 biofilms, but not with LGG supernatants or untreated
PA14 biofilms. Though LGG PelAh supernatants did degrade PA14 biofilms, they required polymyxin B to inhibit PA14 for noticeable degradation relative to PA14 biofilms incubated with _L.
plantarum_ supernatants containing the empty vector control. No biofilm was present when LGG was cultured in wells that did not contain PA14 biofilms, suggesting that PA14 may aid in LGG
surface adhesion. CULTURE PH DETERMINES EFFECTIVENESS OF ENGINEERED _L. PLANTARUM_ ANTI-BIOFILM ACTIVITY Having established the significance of pH for PA14 growth and viability when treated
with LAB cultures and supernatants, and knowing the optimal pH for NucA and EngZ are 9─10 and ~745,46, respectively, we postulated that we could enhance NucA-mediated and EngZ-mediated
biofilm degradation by modulating the pH-induced LAB supernatants. However, increasing culture pH did not significantly enhance biofilm degradation by NucA or EngZ relative to the control
(Fig. 4a). We found no biofilm degradation by any of the enzymes when supernatants were buffered to pH 4.0 or pH 9.0. Interestingly, but unsurprisingly, formation of biofilm biomass was
dramatically enhanced at pH 7.0 for all supernatants, although PelAh was still effective at lowering biofilm biomass by 40% relative to the control. NucA also moderately decreased PA14
biofilm biomass at pH 7.0, but the difference was not found to be significant (_p_ > 0.05). While performing the biofilm degradation assay, it became apparent that the large increase in
crystal violet (CV) staining at pH 7.0 and 9.0 was due to formation of additional PA14 biofilm. We illustrated this additional biofilm formation in test tubes (Fig. 4b). Mature biofilm
treated with empty vector supernatant had a single biofilm at the air–solid–liquid interface at the original height of the culture volume during biofilm formation. When the control
supernatant solution was buffered to pH 7.0, and added to the mature biofilm, a second biofilm formed at the height of the control supernatant, accounting for the higher biofilm biomass
detected in the microplate assay. This second biofilm was not present when the mature biofilm was incubated with PBS, which reveals that PA14 can utilize residual nutrients in the _L.
plantarum_ supernatant to form additional biofilm. The unmodified and pH 7.0 buffered PelAh supernatants degraded the mature biofilm, however, some minimal new biofilm was formed when the pH
was adjusted to pH 7.0. DISCUSSION LAB effect their antimicrobial activity though a variety of mechanisms, including the production of antimicrobial proteins/peptides, inhibitory
metabolites, and organic acids. While _L. plantarum_ WCFS1 produces three bacteriocins (plantaricins A, EF, and JK), all of which act against a relatively narrow range of physiologically
similar Gram-positive bacteria47,48, and _L. rhamnosus_ GG produces an array of antimicrobial peptides, with varying degrees of activity against both Gram-positive and Gram-negative
bacteria49, we found the distinguishing inhibitory factor of LAB supernatants and cultures against PA14 growth to be low pH. The inhibitory activity of both _L. plantarum_ and LGG inversely
correlated to decreasing pH and was abolished if the LAB supernatant was buffered to a more neutral pH. As heterofermentors, we expect both lactobacilli to produce lactic acid and acetic
acid as fermentation products50,51. Though both of these acids inhibit the growth of Gram-negative pathogens like _P. aeruginosa_52,53,54, PA14 growth inhibtion was indistinguishable from
growth medium buffered to an equivalent pH range, indicating that the identity of the acid was not very important. The low pH of the LAB supernatants was also important for decreasing
biofilm formation and biofilm viability, and was a major factor in the success of the degradation of biofilm by engineered LAB. Buffering the supernatants of LAB cultures directly or by
dilution in PBS to more neutral pH resulted in maintenance of biofilm viability and the capacity to form new or more biofilms. Indeed, other investigations into multispecies bacterial
communities have revealed that pH is a major determinant of success and failure within the community, and can generate a competitive advantage that results in elimination of acid-intolerant
species55. As a topical wound therapy, maintenance of the low pH would be beneficial for pathogen load reduction and clearance. Dilute organic acid solutions (e.g. acetic acid) have been
used as a first-aid measure to stave off infection, yet we found that LAB cultures were more inhibitory than their acidic supernatants alone. Valdez et al. also found that _L. plantarum_
cultures are more inhibitory to _P. aeruginosa_ than the supernatants alone56. _L. plantarum_ is known to remain viable at pH values below those found following 24 h culture in MRS (pH <
4), which would explain why cultures are better at inhibiting PA14 compared to supernatants; _L. plantarum_ can continue to acidify, and increase inhibitory activity, by utilizing residual
nutrients in the media or from the LB agar plates onto which they were applied. This illustrates a major advantage to the use of LAB cultures over acidified solutions or supernatants. LAB
cultures can continue to acidify their environment when given additional nutrients, thus maintaining an antimicrobial environment. Similarly, LAB cultures could conceivably continue to
consume fermentable substrates in the wound bed, competing with pathogens for nutrients, and counteracting the return to neutral pH by the natural buffering capacity of the blood57. In fact,
the skin normally maintains an acidic pH (4.0–5.5), and the normal wound healing processes—including decreased metalloprotease activity, oxygenation, epithelial migration, and
angiogenesis—are correlated with acidic pH58,59. Conversely, elevated wound pH is often associated with chronic wounds57. Thus, LAB-mediated acidification should create an inhospitable
environment for acid-intolerant pathogens and is not expected to have a negative impact on the normal wound healing process. Interestingly, we found that even though the two lactobacilli
investigated in this study—_L. plantarum_ and LGG—inhibited PA14 viability by lowering the culture pH, their outcomes were divergent when applied to biofilms. Though _L. plantarum_ secreting
PelAh degraded PA14 biofilms, LGG secreting PelAh increased biofilm biomass, which we found to be the result of two factors. First, the considerable increase in biofilm biomass seen on the
bottom of the culture well following the addition of LGG culture to PA14 biofilms (Supplementary Fig. 2) was dependent upon the presence of LGG culture and an extant PA14 biofilm matrix,
suggesting LGG adheres to PA14 cells or EPS. While LGG is known to produce adherent biofilms, it is not known to do this when grown on MRS60, which we found as well. Second, we found that
the addition of LGG supernatants also increased biofilm biomass and resulted in a second layer of PA14 biofilm, such as that shown in Fig. 4b, unless the antimicrobial polymyxin B was added
to the culture. This was possible because LGG grew less well in BHI, and the culture pH at the end of induction was 6.7−7.0, and thus the pH was not low enough to inhibit PA14 growth and
biofilm formation. This indicates that a combined antimicrobial–LGG–PelAh therapy could be effective, and it remains entirely possible that the increased adhesion of LGG could enhance its
antimicrobial effect by maintaining close proximity to the pathogen—both of which may be worthy of future investigations. After engineering the lactobacilli to secrete a series of biofilm
degrading enzymes, we found that only PelAh secreted by _L. plantarum_ was effective at degrading PA14 biofilms. Surprisingly, DNAse and EngZ secreted by _L. plantarum_ were unable to
appreciably degrade PA14 biofilms even though previous investigations have shown the efficacy of enzymes of these classes to be effective anti-biofilm agents against this strain43,44. We
verified the secretion and activity of NucA and EngZ in the LAB supernatant and optimized the supernatant pH for the activity of these enzymes, and still found no significant benefit. The
activity of these enzymes at elevated pH may be masked by the additional growth of PA14 biofilm at the elevated pH at which these enzymes are most active. However, EngZ exhibits ~60%
activity even at pH 4.0, and we still saw no impact on PA14 biofilm degradation45. Previous work has shown that cellulase extracts from _Trichoderma viride_ or _Aspergillus niger_ can
degrade PA14 biofilms42,44. However, the biofilm degrading capacity was not attributed to any single enzyme or endoglucanase activity and the exact composition of the extract is unknown.
Further, activity on Pel is likely due to substrate promiscuity, which is often enzyme-dependent. Thus, EngZ may not have the same range of relaxed substrate specificity as _T. viride_ or
_A. niger_ cellulases. The differences in our observations compared to that in literature could also be due to differences is assay conditions. Specifically, PA14 biofilms degradation by
DNase was shown in flow cells, where DNA is known to play an integral role in the structure of biofilm stalks at the solid–liquid interface when under flow42,61. DNA may not play the same
role in static batch cultures where the biofilm forms at the air–solid–liquid interface. Additionally, the DNA present in flow cell biofilms only plays an important adhesive role in early
stage attachment43, and may not play a critical role in maintenance of mature biofilms. DNA also contributes to a plethora of interesting phenotypes in the biofilm, including chelating
cations, inducing antibiotic resistance, promoting inflammation, and aiding extracellular electron transport, all of which are important metrics by which to test this therapy in the
future62,63,64,65. Through the development of this bacteriotherapy for the disruption of PA14 biofilm, we learned potentially important design rules for engineered bacteriotherapies that
target pathogenic biofilms. First, selection of an appropriate organism as the chassis to engineer for the bacteriotherapy is essential. Specifically, the chassis organism’s ability to
inhibit pathogen growth, biofilm formation, and it’s impact on mature biofilms, using the assays described in this work, can determine whether the organism will act as an effective
bacteriotherapy. We found that prevention of additional biofilm formation and pathogen growth is a key to the degradation process. Either the inhibitory activity of low pH, found with _L.
plantarum_ cultures, or the addition of the inhibitory antibiotic polymyxin B, used with LGG cultures, was necessary for biofilm degradation. The polymyxin B biosynthetic pathway has been
cloned and expressed heterologously, which could provide an effective strategy for generating an inhibitory environment independent of pH and might be necessary for in vivo application,
where the wound bed pH can fluctuate66. Additionally, the selection of the appropriate enzyme for biofilm degradation is equally important. We found that PA14-derived PelAh was most
effective at degrading PA14 biofilm, and that other enzymatic activities thought to be effective at degrading PA14 biofilms were ineffective in our assay. Frequently, genes have been
identified within the genome of biofilm-forming organisms that function to degrade the biofilm and release the embedded cells. However, their expression is often suppressed during the
biofilm growth phase to ensure biofilm integrity. Therefore, we propose that the enzymes for biofilm degradation should be sourced from the pathogen itself, as these native enzymes were
“designed” to degrade the EPS polymers. Such observations are consistent with previous studies15,16. Still, the activity of the enzyme derived from the pathogen may not be effective under
the applied conditions, as we found with the pH 4 condition. Thus, the application must strike a balance between pathogen growth inhibition, enzyme activity, and bacteriotherapy
culture/environmental pH, which we found to be the pH 5.0–5.3 range of induced _L. plantarum_ cultures (Fig. 5). The pH working range of the treatment could be further extended through the
addition of an antimicrobial such as polymyxin B, which then removes the upper pH constraint set by pathogen growth inhibition. As current antimicrobial treatments decrease in efficacy, the
development of novel treatments is essential for effectively treating recalcitrant infections. Traditional small molecule screening for antimicrobials has all but ceased due to the high cost
and uncertainty of success. Engineered bacteriotherapies provide an alternative strategy for developing antimicrobials, with specific component parts (organism, enzyme, intended pathogen)
that can be intentionally modified to address the challenges of particular infections. Though we present promising data for the ability to target _P. aeruginosa_ PA14 biofilms using an
engineered bacteriotherapy, further validation of this system is required in vivo. Expanding the pathogen targets, host infection sites, and adding additional functionalities, such as the
production of specific antimicrobials, will better validate this system as an effective treatment alternative to existing therapies. METHODS BACTERIAL GROWTH AND TRANSFORMATION All strains
used in this study are listed in Supplementary Table 1. _E. coli_ and _P. aeruginosa_ were grown in LB broth and plated on LB agar, unless stated otherwise. Erythromycin and ampicillin were
added to _E. coli_ cultures at 200 or 100 µg/mL, respectively. Lactobacilli were grown in De Man, Rogosa, and Sharpe (MRS; RPI Corp) broth and plated on MRS agar (1.5% w/v) plates unless
stated otherwise. Erythromycin was added to lactobacillus cultures at 5 µg/mL when necessary. All cultures were grown at 37 °C; _E. coli and L. plantarum_ cultures were grown shaking (250
rpm) and LGG was grown statically, unless stated otherwise. _E. coli_ transformation was performed using MES or TSS competent cells. _L. plantarum_ WCFS1 transformation was performed using a
method derived from Aukrust and Blom67. LGG transformation was performed using the method described in De Keersmaecker et al.68. LAB ANTIMICROBIAL PLATE ASSAY Overnight cultures of LAB were
diluted 1000× into 10 mL fresh media and 1 mL aliquots were removed from the culture at the designated times. Following aliquot removal from LAB culture, cells were pelleted at 4000 × _g_
for 15 min and the resulting supernatant was filtered through 0.22 µm PES filter and frozen at ─20 °C until the following day. The following day, overnight cultures of _P. aeruginosa_ were
diluted 100× in LB broth and 100 µL was plated on the surface of LB agar. Agar wells were excised from the agar plate and 200 µL of fresh lactobacilli culture or filtered culture supernatant
was added to each well. Plates were incubated at 37 °C overnight and inhibition was evaluated qualitatively by inhibition of pathogen growth. PLASMID CONSTRUCTION All vectors used and
constructed in this study are listed in Supplementary Table 2. All DNA oligos were ordered from Eurofins Genomics or GENEWIZ and sequences are given in Supplementary Table 3. _E. coli_ TG1
was modified by knockout of _endA_ to improve transformation efficiency and plasmid quality of pSIP411-derived vectors. DH5α was used to propagate pSIP401-derived plasmids. Gene knockout and
verification of _endA_ in _E. coli_ TG1 was performed using λRed recombineering69 using primers 17−20. Variants of the pSIP401 and pSIP411 plasmids with inserts containing the Lp_3050
secretion signal, 6× histidine tag, thrombin cleavage site, and multiple cloning site (MCS) were constructed using primers 1–5 (Supplementary Table 3). The inserts for these constructs were
generated by overlap extension PCR. The product and vectors (pSIP401 and pSIP411) were digested with _Bgl_II and _Pml_I to construct pTCC200 and pTCC210. The _nucA_ gene was amplified from
genomic DNA prepared from _S. aureus_ UAMS1 using primers 6 and 7. The _engZ_ gene was amplified from genomic DNA of _C. cellulovorans_ (purchased from DSMZ) using primers 8 and 9. Amplified
DNA fragments containing _nucA_ or _engZ_ were digested with _Sal_I and _Pml_I for insertion into the same digested pTCC200. The gene for PelAh was amplified from genomic DNA prepared from
PA14 using primers 10 and 11. Plasmid pTCC210 was amplified using primers 12 and 13 and combined with the PelAh fragment using NEBuilder® HiFi DNA Assembly. All enzymes were purchased from
New England Biolabs (NEB, Ipswich, MA). All cloning inserts were amplified using Phusion® DNA polymerase. All inserts in modified plasmids were verified by colony PCR and sequenced by
Eurofins Genomics LLC (Louisville, KY) or Genewiz, Inc. (Cambridge, MA) using primers 14, 15, and 16. LIQUID CULTURE BIOFILM FORMATION _P. aeruginosa_ PA14 biofilms were grown by diluting a
24 h culture 200× into salt-free LB (sfLB; 10 g/L tryptone, 5 g/L yeast extract). For biofilm inhibition studies, PA14 was also diluted 200× into the supernatants from 24 h cultures of _L.
plantarum_ and LGG, and this culture was subsequently serially diluted with a new sfLB PA14 subculture to maintain consistent cell density. The new PA14 cultures were dispensed in 150 μL
aliquots into wells of white Lumitrac high-bind 96-well microplates (Greiner Bio-One, Monroe, NC). Wells at the plate edge were filled with water and only interior wells were used for
biofilm formation. Microplate lids were sealed with parafilm and incubated for 24 h at 30 °C without shaking. Biofilms biomass was then quantified or incubated further to measure treatment
efficacy. BIOFILM QUANTIFICATION The biofilm biomass was measured by staining adherent cells with CV. Wash steps were performed using low pipette flow rates to prevent removal of adherent
cells. Biofilms grown in liquid cultures as described above were washed 2× with 250 µL PBS to remove non-adherent cells. 250 µL of aqueous 0.1% CV was added to each well, and plates were
incubated for 15 min. Following incubation, plates were inverted and washed 4× with 300 µL phosphate-buffered saline (PBS; 8 g/L NaCl, 0.2 g/L KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). Plates were
dried at 37 °C. De-staining was performed by addition of 300 µL of 4:1 ethanol:acetone solution. After 15–20 min, 200 µL of the solubilized CV solution was transferred to a new 96-well
microplate, and the absorbance at 570 nm wavelength was measured (Spectramax® M3, Molecular Devices, San Jose, CA). ENZYME INDUCTION AND SECRETION VALIDATION Protein expression in _L.
plantarum_ and LGG was performed by growing overnight cultures in MRS and sub-culturing to OD600 of 0.05 in BHI supplemented with 0.5% (w/v) glucose. Cultures were grown until OD600 0.2–0.3,
pelleted, and induced by resuspending in fresh 2× BHI 0.5% glucose with 200 ng/mL IP-673. Induced cultures were grown for ~5 h at 30 °C. For induced supernatants, cells were pelleted by
centrifugation at 4500 × _g_ for 10 min, and the supernatant was filtered through a 0.22 µm PES filter. Polymyxin B (100 µg/mL, RPI Corp) was added to LAB cultures and supernatants when
stated. For SDS–PAGE, 10 µL of 4× loading buffer was added to 30 µL LAB supernatant and heated at 95 °C for 5 min. 20 µL of the processed supernatant was loaded per well on a 4–12% Bis–Tris
gradient gel. The gel was run in 1× MES running buffer at 120 V for ~1.5 h and developed using silver stain (Pierce Biotechnology Inc., Rockford, IL). Cellulase activity was evaluated by
aliquoting 5 µL of induced cell supernatants on 1.0% agar plates containing 0.5% carboxymethyl cellulose (CMC) and incubated overnight at 37 °C. Plates were subsequently incubated with 0.5%
Congo Red (CR) for 10 min. Residual CR was removed by de-staining with 1 M NaCl. DNase activity was assessed by aliquoting 5 µL of induced cell supernatants on 1.0% agar plates containing
0.2% DNA and incubated overnight at 37 °C. Plates were then treated with 1 N HCl to precipitate residual DNA. ENZYMATIC DEGRADATION OF PA14 BIOFILMS BY ENGINEERED LAB Efficacy of enzymatic
treatment was determined by growing biofilms as described above. The supernatant and nonadherent solids of biofilm cultures were aspirated using a multichannel pipette. 250 µL of induced LAB
culture was aliquoted per well, and biofilm microplates were placed on a rocker at room temperature for 15 h. LAB cultures were aspirated from wells and the plates were washed twice with
250 µL of sterile DPBS (2.67 mM KCl, 136.9 mM NaCl, 1.47 mM KH2PO4, 8.10 mM Na2HPO4). Remaining biofilm was fixed to plates by drying plates overnight in a 37 °C incubator and quantified
using the CV method described previously. BIOFILM VIABILITY ASSAY PA14 biofilms were grown as described above. Filtered LAB supernatants from 24 h LAB cultures were generated as described in
the agar-well diffusion assay, and then serially diluted 2× into PBS pH 7.4. The supernatant from the biofilm cultures was removed and 250 µL of diluted LAB supernatants or buffered MRS
control was added to each well and the plates were incubated at 37 °C for 24 h. The supernatant was then removed and the plates were washed 2× with PBS to remove nonadherent cells. 200 µL of
LB and 100 µL of XTT solution (0.4 mg/mL XTT, Amresco; 50 µM menadione, Alfa Aesar) were added to each well and plates were incubated at 37 °C for 3 h. Microplates were centrifuged at 3000
× _g_ for 10 min and 200 µL of solution was aliquoted into a fresh microplate. The absorbance at 475 nm was taken to determine viability and this value is reported in the corresponding
figures. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data that support the
findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Wu, Y. K., Cheng, N. C. & Cheng, C. M. Biofilms in chronic wounds: pathogenesis
and diagnosis. _Trends Biotechnol._ https://doi.org/10.1016/j.tibtech.2018.10.011 (2019). * Delcaru, C. et al. Microbial biofilms in urinary tract infections and prostatitis: etiology,
pathogenicity, and combating strategies. _Pathogens_ https://doi.org/10.3390/pathogens5040065 (2016). * Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the
natural environment to infectious diseases. _Nat. Rev. Microbiol._ https://doi.org/10.1038/nrmicro821 (2004). * von Rosenvinge, E. C., O’May, G. A., Macfarlane, S., Macfarlane, G. T. &
Shirtliff, M. E. Microbial biofilms and gastrointestinal diseases. _Pathog. Dis._ https://doi.org/10.1111/2049-632X.12020 (2013). * Kavanagh, N. et al. _Staphylococcal osteomyelitis_:
disease progression, treatment challenges, and future directions. _Clin. Microbiol. Rev._ https://doi.org/10.1128/CMR.00084-17 (2018). * Bispo, P. J. M., Haas, W. & Gilmore, M. S.
Biofilms in infections of the eye. _Pathogens_ https://doi.org/10.3390/pathogens4010111 (2015). * Schaudinn, C., Gorur, A., Keller, D., Sedghizadeh, P. P. & Costerton, J. W.
Periodontitis: an archetypical biofilm disease. _J. Am. Dent. Assoc_. https://doi.org/10.14219/jada.archive.2009.0307 (2009). * Flemming, H.-C. & Wingender, J. The biofilm matrix. _Nat.
Rev. Microbiol._ 8, 623–633 (2010). Article CAS Google Scholar * Gilbert, P., Maira-Litran, T., McBain, A. J., Rickard, A. H. & Whyte, F. W. The physiology and collective
recalcitrance of microbial biofilm communities. _Adv. Microb. Physiol._ https://doi.org/10.1016/S0065-2911(02)46005-5 (2002). * Domenech, M., Ramos-Sevillano, E., García, E., Moscoso, M.
& Yuste, J. Biofilm formation avoids complement immunity and phagocytosis of _Streptococcus pneumoniae_. _Infect. Immun_. https://doi.org/10.1128/IAI.00491-13 (2013). * Toussaint, J. et
al. Topical antibiotic ointment versus silver-containing foam dressing for second-degree burns in swine. _Acad. Emerg. Med_. https://doi.org/10.1111/acem.12723 (2015). * Innes, M. E., Umraw,
N., Fish, J. S., Gomez, M. & Cartotto, R. C. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. _Burns_
https://doi.org/10.1016/S0305-4179(01)00015-8 (2001). * Poon, V. K. M. & Burd, A. In vitro cytotoxity of silver: implication for clinical wound care. _Burns_
https://doi.org/10.1016/j.burns.2003.09.030 (2004). * Michaels, J. A. et al. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous
leg ulcers (VULCAN trial). _Br. J. Surg_. https://doi.org/10.1002/bjs.6786 (2009). * Pestrak, M. J. et al. Treatment with the pseudomonas aeruginosa glycoside hydrolase PslG combats wound
infection by improving antibiotic efficacy and host innate immune activity. _Antimicrob. Agents Chemother_. https://doi.org/10.1128/AAC.00234-19 (2019). * Baker, P. et al. Exopolysaccharide
biosynthetic glycoside hydrolases can be utilized to disrupt and prevent _Pseudomonas aeruginosa_ biofilms. _Sci. Adv._ 2, e1501632 (2016). Article Google Scholar * Frederiksen, B.,
Pressler, T., Hansen, A., Koch, C. & Høiby, N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. _Acta Paediatr._ 95, 1070–1074
(2006). Article Google Scholar * Johansen, C., Falholt, P. & Gram, L. Enzymatic removal and disinfection of bacterial biofilms. _Appl. Environ. Microbiol._ 63, 3724–3728 (1997).
Article CAS Google Scholar * Lu, T. K. & Collins, J. J. Dispersing biofilms with engineered enzymatic bacteriophage. _Proc. Natl Acad. Sci. USA_ 104, 11197–11202 (2007). Article CAS
Google Scholar * Lister, J. L. & Horswill, A. R. _Staphylococcus aureus_ biofilms: recent developments in biofilm dispersal. _Front. Cell. Infect. Microbiol_.
https://doi.org/10.3389/fcimb.2014.00178 (2014). * Fleming, D. & Rumbaugh, K. Approaches to dispersing medical biofilms. _Microorganisms_ https://doi.org/10.3390/microorganisms5020015
(2017). * Bober, J. R., Beisel, C. L. & Nair, N. U. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. _Annu. Rev.
Biomed. Eng_. https://doi.org/10.1146/annurev-bioeng-062117-121019 (2018). * Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and therapeutic applications. _Nat. Rev.
Microbiol._ https://doi.org/10.1038/nrmicro.2017.172 (2018). * Mays, Z. J. & Nair, N. U. Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics.
_Curr. Opin. Biotechnol._ https://doi.org/10.1016/j.copbio.2018.01.028 (2018). * Waller, M. C., Bober, J. R., Nair, N. U. & Beisel, C. L. Toward a genetic tool development pipeline for
host-associated bacteria. _Curr. Opin. Microbiol._ https://doi.org/10.1016/j.mib.2017.05.006 (2017). * Martín, R. et al. Effects in the use of a genetically engineered strain of _Lactococcus
lactis_ delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. _Hum. Vaccines Immunother_. https://doi.org/10.4161/hv.28549 (2014). * Hwang, I. Y. et al. Engineered
probiotic _Escherichia coli_ can eliminate and prevent _Pseudomonas aeruginosa_ gut infection in animal models. _Nat. Commun_. https://doi.org/10.1038/ncomms15028 (2017). * Isabella, V. M.
et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. _Nat. Biotechnol_. https://doi.org/10.1038/nbt.4222 (2018). * Gurbatri, C. R. et
al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. _Sci. Transl. Med_. https://doi.org/10.1126/scitranslmed.aax0876 (2020). * Han, W. et al. Improvement
of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. _Inflamm. Bowel Dis_. https://doi.org/10.1097/01.mib.0000235101.09231.9e (2006). * del Carmen, S.
et al. Genetically engineered immunomodulatory _Streptococcus thermophilus_ strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. _Appl. Environ. Microbiol_.
https://doi.org/10.1128/AEM.03296-13 (2014). * Vedantam, G. et al. An engineered synthetic biologic protects against _Clostridium difficile_ infection. _Front. Microbiol._
https://doi.org/10.3389/fmicb.2018.02080 (2018). Article PubMed PubMed Central Google Scholar * Jamalifar, H. et al. Antimicrobial activity of different _Lactobacillus_ species against
multi-drug resistant clinical isolates of _Pseudomonas aeruginosa_. _Iran. J. Microbiol._ 3, 21–25 (2011). CAS PubMed PubMed Central Google Scholar * Peral, M. C., Huaman Martinez, M. A.
& Valdez, J. C. Bacteriotherapy with _Lactobacillus plantarum_ in burns. _Int. Wound J._ 6, 73–81 (2009). Article Google Scholar * Khailova, L., Baird, C. H., Rush, A. A., McNamee, E.
N. & Wischmeyer, P. E. _Lactobacillus rhamnosus_ GG improves outcome in experimental _Pseudomonas aeruginosa_ pneumonia: potential role of regulatory T cells. _Shock_
https://doi.org/10.1097/SHK.0000000000000066 (2013). * Mohammedsaeed, W., Cruickshank, S., McBain, A. J. & O’Neill, C. A. _Lactobacillus rhamnosus_ GG lysate increases
re-epithelialization of keratinocyte scratch assays by promoting migration. _Sci. Rep_. https://doi.org/10.1038/srep16147 (2015). * Argenta, A., Satish, L., Gallo, P., Liu, F. & Kathju,
S. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. _PLoS ONE_ 11, e0165294 (2016). Article Google Scholar * Sabaeifard,
P., Abdi-Ali, A., Soudi, M. R. & Dinarvand, R. Optimization of tetrazolium salt assay for _Pseudomonas aeruginosa_ biofilm using microtiter plate method. _J. Microbiol. Methods_
https://doi.org/10.1016/j.mimet.2014.07.024 (2014). * Branda, S. S., Vik, S., Friedman, L. & Kolter, R. Biofilms: the matrix revisited. _Trends Microbiol._ 13, 20–26 (2005). Article CAS
Google Scholar * Lee, D. G. et al. Genomic analysis reveals that _Pseudomonas aeruginosa_ virulence is combinatorial. _Genome Biol._ 7, R90 (2006). Article Google Scholar * Wozniak, D.
J. et al. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 _Pseudomonas aeruginosa_ biofilms. _Proc. Natl Acad. Sci. USA_ 100, 7907–7912
(2003). Article CAS Google Scholar * Jennings, L. K. et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the _Pseudomonas aeruginosa_ biofilm matrix. _Proc.
Natl Acad. Sci. USA_ 112, 11353–11358 (2015). Article CAS Google Scholar * Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. Extracellular DNA required for
bacterial biofilm formation. _Science (80-.)._ 295, 1487–1487 (2002). Article CAS Google Scholar * Friedman, L. & Kolter, R. Genes involved in matrix formation in _Pseudomonas
aeruginosa_ PA14 biofilms. _Mol. Microbiol._ 51, 675–690 (2004). Article CAS Google Scholar * Jeon, S. D., Yu, K. O., Kim, S. W. & Han, S. O. The processive endoglucanase EngZ is
active in crystalline cellulose degradation as a cellulosomal subunit of _Clostridium cellulovorans_. _New Biotechnol._ 29, 365–371 (2012). Article CAS Google Scholar * Cuatrecasas, P.,
Fuchs, S. & Anfinsen, C. B. Catalytic properties and specificity of the extracellular nuclease of _Staphylococcus aureus_. _J. Biol. Chem._ 242, 1541–1547 (1967). CAS PubMed Google
Scholar * Anderssen, E. L., Diep, D. B., Nes, I. F., Eijsink, V. G. H. & Nissen-Meyer, J. Antagonistic activity of _Lactobacillus plantarum_ C11: two new two-peptide bacteriocins,
plantaricins EF and JK, and the induction factor plantaricin A. _Appl. Environ. Microbiol_. https://doi.org/10.1139/m93-178 (1998). * Diep, D. B., Straume, D., Kjos, M., Torres, C. &
Nes, I. F. An overview of the mosaic bacteriocin pln loci from _Lactobacillus plantarum_. _Peptides_ https://doi.org/10.1016/j.peptides.2009.05.014 (2009). * Lu, R. et al. Isolation,
identification, and characterization of small bioactive peptides from _Lactobacillus_ GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. _J.
Pediatr. Gastroenterol. Nutr_. https://doi.org/10.1097/MPG.0b013e3181924d1e (2009). * Zalán, Z., Hudáček, J., Štětina, J., Chumchalová, J. & Halász, A. Production of organic acids by
_Lactobacillus_ strains in three different media. _Eur. Food Res. Technol_. https://doi.org/10.1007/s00217-009-1179-9 (2010). * Silva, M., Jacobus, N. V., Deneke, C. & Gorbach, S. L.
Antimicrobial substance from a human _Lactobacillus_ strain. _Antimicrob. Agents Chemother_. https://doi.org/10.1128/AAC.31.8.1231 (1987). * Alakomi, H. L. et al. Lactic acid permeabilizes
Gram-negative bacteria by disrupting the outer membrane. _Appl. Environ. Microbiol_. https://doi.org/10.1128/AEM.66.5.2001-2005.2000 (2000). * Ryssel, H. et al. The antimicrobial effect of
acetic acid—an alternative to common local antiseptics? _Burns_ https://doi.org/10.1016/j.burns.2008.11.009 (2009). * De Keersmaecker, S. C. J. et al. Strong antimicrobial activity of
_Lactobacillus rhamnosus_ GG against _Salmonella typhimurium_ is due to accumulation of lactic acid. _FEMS Microbiol. Lett_. https://doi.org/10.1111/j.1574-6968.2006.00250.x (2006). *
Ratzke, C. & Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. _PLoS Biol_. https://doi.org/10.1371/journal.pbio.2004248 (2018). * Valdéz, J. C.,
Peral, M. C., Rachid, M., Santana, M. & Perdigón, G. Interference of _Lactobacillus plantarum_ with _Pseudomonas aeruginosa_ in vitro and in infected burns: the potential use of
probiotics in wound treatment. _Clin. Microbiol. Infect._ 11, 472–479 (2005). Article Google Scholar * Gethin, G. The significance of surface pH in chronic wounds. _Wounds UK_ 3, 52–56
(2007). Google Scholar * Greener, B., Hughes, A. A., Bannister, N. P. & Douglass, J. Proteases and pH in chronic wounds. _J. Wound Care_ https://doi.org/10.12968/jowc.2005.14.2.26739
(2005). * Nagoba, B. S., Suryawanshi, N. M., Wadher, B. & Selkar, S. Acidic environment and wound healing: a review. _Wounds_ 27, 5–11 (2015). Google Scholar * Lebeer, S., Verhoeven, T.
L. A., Vélez, M. P., Vanderleyden, J. & De Keersmaecker, S. C. J. Impact of environmental and genetic factors on biofilm formation by the probiotic strain _Lactobacillus rhamnosus_ GG.
_Appl. Environ. Microbiol_. https://doi.org/10.1128/AEM.01393-07 (2007). * Allesen-Holm, M. et al. A characterization of DNA release in _Pseudomonas aeruginosa_ cultures and biofilms. _Mol.
Microbiol_. https://doi.org/10.1111/j.1365-2958.2005.05008.x (2006). * Mulcahy, H., Charron-Mazenod, L. & Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance
in _Pseudomonas aeruginosa_ biofilms. _PLoS Pathog_. https://doi.org/10.1371/journal.ppat.1000213 (2008). * Chiang, W. C. et al. Extracellular DNA shields against aminoglycosides in
_Pseudomonas aeruginosa_ biofilms. _Antimicrob. Agents Chemother_. https://doi.org/10.1128/AAC.00001-13 (2013). * Fuxman Bass, J. I. et al. Extracellular DNA: a major proinflammatory
component of _Pseudomonas aeruginosa_ biofilms. _J. Immunol._ https://doi.org/10.4049/jimmunol.0901640 (2010). Article PubMed Google Scholar * Das, T. et al. Phenazine virulence factor
binding to extracellular DNA is important for _Pseudomonas aeruginosa_ biofilm formation. _Sci. Rep_. https://doi.org/10.1038/srep08398 (2015). * Kim, S. Y., Park, S. Y., Choi, S. K. &
Park, S. H. Biosynthesis of polymyxins B, E, and P using genetically engineered polymyxin synthetases in the surrogate host _Bacillus subtilis_. _J. Microbiol. Biotechnol_.
https://doi.org/10.4014/jmb.1505.05036 (2015). * Aukrust, T. & Blom, H. Transformation of _Lactobacillus_ strains used in meat and vegetable fermentations. _Food Res. Int._ 25, 253–261
(1992). Article CAS Google Scholar * De Keersmaecker, S. C. J. et al. Flow cytometric testing of green fluorescent protein-tagged _Lactobacillus rhamnosus_ GG for response to defensins.
_Appl. Environ. Microbiol_. https://doi.org/10.1128/AEM.02605-05 (2006). * Datsenko, K.A. & Wanner, B. L. One-step inactivation of chromosomal genes in _Escherichia coli_ K-12 using PCR
products. _Proc. Natl Acad. Sci. USA_ 97, 6640–6645 (2000). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Dr. David R. Snydman (Tufts Medical
Center, Boston, MA), Dr. Abraham L. Sonenshein (Tufts University School of Medicine, Boston, MA), Dr. Michiel Kleerebezem (Wageningen University & Research, Wageningen, Netherlands), Dr.
Roberto Kolter (Harvard Medical School, Boston, MA), Dr. Ann Hochschild (Harvard Medical School, Boston, MA), Dr. Huimin Zhao (University of Illinois, Urbana, IL), Dr. Geir Mathiesen
(Norwegian University of Life Sciences, Ås, Norway), and Dr. Jan Peter van Pijkeren (University of Wisconsin, Madison, WI) for graciously providing strains and/or plasmids required to
complete this work. This work was financially supported by grant number DP2HD091798 of the National Institutes of Health and a Tufts Collaborates! grant from Tufts University. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemical & Biological Engineering, Tufts University, Medford, MA, USA Todd C. Chappell & Nikhil U. Nair Authors * Todd C.
Chappell View author publications You can also search for this author inPubMed Google Scholar * Nikhil U. Nair View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Project was conceived by N.U.N. and experiments were designed by N.U.N. and T.C.C. Laboratory work and data analysis was performed by T.C.C. Manuscript was written by
T.C.C. and edited by N.U.N. CORRESPONDING AUTHOR Correspondence to Nikhil U. Nair. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL FILE REPORTING
SUMMARY CHECKLIST RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Chappell, T.C., Nair, N.U. Engineered lactobacilli display anti-biofilm and growth suppressing activities against _Pseudomonas aeruginosa_. _npj Biofilms Microbiomes_ 6, 48
(2020). https://doi.org/10.1038/s41522-020-00156-6 Download citation * Received: 08 April 2020 * Accepted: 07 October 2020 * Published: 30 October 2020 * DOI:
https://doi.org/10.1038/s41522-020-00156-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Very young investors are up double-digit percentages on 10 stocksYoung investors are taking their share of lumps in the S&P 500. But they're still enjoying some stock winners, ...
Bigg boss 10: it's bani vs gaurav inside the house; housemates also target the two!Gaurav Chopra and VJ Bani Following Rahul’s unanticipated eviction, it seems that the dynamics inside the house have bee...
Like father, like son: prince charles takes over prince philip's ro...The Prince of Wales took to the wheel during a visit to the Royal College of Art today and was pictured turning a large ...
Gloria estefan says emilio estefan was 'lost at sea' for 10 days while he escaped cubaEmilio Estefan went to great lengths to escape Cuba and its dictatorship. On Thursday's episode of _Red Table Talk:...
Tour bus crash - Los Angeles TimesFeb. 4, 2013 7:21 AM PT Eight people were killed and more than 30 injured when a tour bus crashed on a narrow mountain r...
Latests News
Engineered lactobacilli display anti-biofilm and growth suppressing activities against pseudomonas aeruginosaABSTRACT Biofilms are an emerging target for new therapeutics in the effort to address the continued increase in resista...
Nine killed in north nigeria road accidentAbuja [Nigeria], July 7 (ANI/Xinhua): Nine people were killed and 12 others injured in a road accident involving multipl...
The growing numbers and problems of women on skid rowAt a time when she least expected it, Lynda Gray found herself torn from a life of comfort and stability and thrown into...
Eczema: three easy self-care tipsEczema is a common skin condition which causes the skin to become itchy, sore, red, cracked and inflamed. The condition ...
How the devastating 1918 flu pandemic helped advance us women’s rightsWhen disaster strikes, it can change the fabric of a society – often through the sheer loss of human life. The 2004 Indi...