Multiscale and multidisciplinary analysis of aging processes in bone
Multiscale and multidisciplinary analysis of aging processes in bone"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The world population is increasingly aging, deeply affecting our society by challenging our healthcare systems and presenting an economic burden, thus turning the spotlight on
aging-related diseases: exempli gratia, osteoporosis, a silent disease until you suddenly break a bone. The increase in bone fracture risk with age is generally associated with a loss of
bone mass and an alteration in the skeletal architecture. However, such changes cannot fully explain increased fragility with age. To successfully tackle age-related bone diseases, it is
paramount to comprehensively understand the fundamental mechanisms responsible for tissue degeneration. Aging mechanisms persist at multiple length scales within the complex hierarchical
bone structure, raising the need for a multiscale and multidisciplinary approach to resolve them. This paper aims to provide an overarching analysis of aging processes in bone and to review
the most prominent outcomes of bone aging. A systematic description of different length scales, highlighting the corresponding techniques adopted at each scale and motivating the need for
combining diverse techniques, is provided to get a comprehensive description of the multi-physics phenomena involved. SIMILAR CONTENT BEING VIEWED BY OTHERS SUB-TRABECULAR STRAIN EVOLUTION
IN HUMAN TRABECULAR BONE Article Open access 14 August 2020 BONE REMODELING: AN OPERATIONAL PROCESS ENSURING SURVIVAL AND BONE MECHANICAL COMPETENCE Article Open access 18 July 2022 MICRO-CT
DATA OF EARLY PHYSIOLOGICAL CANCELLOUS BONE FORMATION IN THE LUMBAR SPINE OF FEMALE C57BL/6 MICE Article Open access 14 May 2021 INTRODUCTION According to the 2019 United Nations data, the
elder population is increasing in almost all countries. Currently, 9% of the world population is over 65 and that percentage will increase up to 16% by 20501. This evolution will profoundly
impact our society, challenging our healthcare systems, and bringing the necessity of redesigning our healthcare services to accommodate the needs of the increasing number of geriatric
patients2 and guaranteeing them a better life quality. Among the several degenerative and chronic pathologies and conditions associated with aging, a prominent role is played by bone and
skeletal diseases. Osteoporosis is a widespread pathological condition that affects more than 200 million people worldwide and is associated with aging3. Osteoporosis and, more generally,
the age-related degradation of bone tissue, causes a significant increase in fracture risk for the elderly. Indeed, age-specific rates of fracture incidence are higher in the oldest age
groups, affecting more than 15% of those aged 95 and above4. From this perspective, more widespread injury-prevention efforts and access to screening and treatment of osteoporosis for older
individuals should help one to reduce the overall burden and improve patient and family lives4. In order to look at bone fragility in a wider perspective5 and to successfully tackle
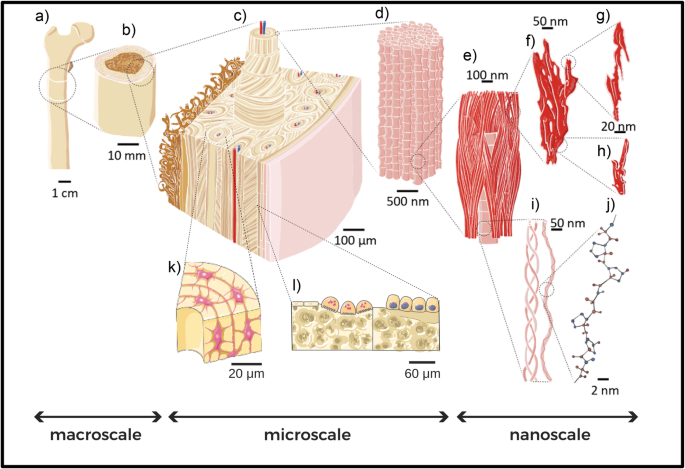
age-related bone diseases, it is crucial to deeply understand the fundamental mechanisms responsible for bone tissue degradation. Indeed, bone is a complex multiscale composite material, in
which organic and inorganic components arrange themselves following specific patterns and structures at different length scales. The inorganic mineral hydroxyapatite crystals (see Fig. 1f, g
and h) and the organic collagen molecules (see Fig. 1i, j) constitute the main building blocks of bone. These fundamentals “bricks” of bone arrange themselves to form mineralized collagen
fibrils (see Fig. 1d). Fibrils form more complex structures going up in scale (in a certain way reminiscent of a fractal geometry), such as fibers and bundles of fibers. The latter are
arranged, by following diverse orientations, depending on the anatomical area, into arrays and lamellae (at submiscroscale). During remodeling, lamellae circumferentially arrange to form
cylindrical features, called osteons (see Fig. 1c), which are interspersed into interstitial lamellae, forming bone extracellular matrix. Inside the complex structure of the bone matrix, a
variety of bone cells live and play a fundamental and active role in bone growth and remodeling (see Fig. 1k, l). All those elements play a role in determining the features and properties of
bone tissue at the meso- and macro-scale (see Fig. 1b and a, respectively). It is clear how a systematic study of the aging processes in bone should embrace this complexity and carefully
take care of the changes that aging causes at each level of bone’s hierarchical structure, pursuing a multiscale approach. Recent works have covered different aspects of bone aging, such as
changes in the nanoscale bone components6, the impact on bone cellular processes with consequences on bone tissue7, and the variation of bone mechanical properties8. In some cases, a
multiscale approach has been adopted7,8,9,10, but keeping the focus on a specific topic related to bone aging7,8, or using a specific technique10. Indeed, the literature scenario on bone
aging is quite ‘fragmented’, with different aspects of bone that have been historically studied by different disciplines, such as medicine, biology, chemistry, physics, engineering, and
material sciences. Each of them applied a variety of experimental and computational techniques (see Fig. 2) to get an insight into the bone properties and to unveil the fundamental
mechanisms involved in bone tissue and its age-driven degradation. Nevertheless, communication among different disciplines, each one speaking its own language, is not always easy. An effort
towards a thorough multidisciplinary approach, combining the results obtained from vastly different experimental and theoretical studies, is broadly instrumental to build a clear picture of
bone aging and to get a profound understanding of aging-related alterations and diseases. For this purpose, in this paper we review the most prominent results regarding all aspects of bone
aging, with a multiscale and multidisciplinary approach, to give the reader a wide view of the topic. (The present work mainly focuses on human bone. Through the dissertation, results coming
from other animal models are reported when necessary to clarify some aspects of bone aging. Most of the reported studies on osteoporosis involving animals, especially species characterized
by short life spans (such as mice) concern ovariectomized specimens or subjects fed with low calcium intake to mimic osteoporotic conditions in humans11,12. Nevertheless, the reader should
keep in mind that bone could be considered a universal natural material, with similar composition among vertebrates and where significant differences across species arise mainly as
architectural variations at the upper micro- and macro-scale, as an adaptation to specific body structures and movement requirements of each animal13. For this reason, several results on
bone aging mechanisms obtained in animals maintain a general validity). Each section deals with a precise length scale (nano, micro, and macroscale, respectively), and each subsection delves
into prominent changes observed in bone structure with age at that scale. Together with the results, each subsection contains details on the most common techniques employed to obtain them.
We combine, in each subsection, results coming from different techniques, giving an idea of how each problem could be tackled in a multidisciplinary way. Open questions and unresolved issues
are pointed-out, with some perspective on possible future research. NANOSCALE STRUCTURE OF BONE At a nanoscale level, bones are made of several components that chemically and physically
interact, imparting bones the mechanical and structural properties they need to sustain bodies, protect organs, and to respond to internal and external stimuli. At this length scale, the
inorganic part is composed of hydroxyapatite (HAP) and water. The organic part is formed by collagen molecules and non-collagenous proteins (NCPs), such as osteopontin, osteocalcin, and
proteoglycans. Hydroxyapatite crystals and collagen molecules organize themselves into a precise structure, the fibril (see Fig. 1). The fibril is composed of collagen molecules aligned
along their long direction, with some gaps, which harbor most of the HAP platelets. A precise periodicity exists in mineralized fibrils, called the D-period, on the scale of ≈67 nm. The
D-period is composed of two parts, the overlap and gap regions; the gap is the space where hydroxyapatite fills most of the interfibrillar voids14. For many years, a linear packing model,
with a simple lateral repeat of the fibril array has been considered for fibrils. More recent studies assume that the 67 nm periodicity, which determines the two-dimensional stacking,
directs the three-dimensional pattern, generating a twisting crystalline structure in fibrils10. The mineralized collagen fibril is typically 50–200 nm in diameter15. Even if collagen and
hydroxyapatite are the prominent bone building blocks at the nanoscale, the role of NCPs should not be underestimated. This group of proteins represents ~10% of bone extracellular matrix
organic components16,17 and is located at the collagen-apatite interface18,19. CHANGES IN HYDROXYAPATITE PLATELET SIZE DURING AGING Bone growth, especially for what concerns the mineral
part, is a process that has been extensively studied: bone mineralization studies have been approached from both the experimental and computational point of views (for some recent examples
see refs. 14,20). Bone mineralization starts from amorphous calcium phosphate deposited between collagen fibril molecules. From the mineralization nuclei, platelets of poorly crystalline
carbonated apatite form both within the collagen fibrils (intra-fibrillar) and along their length (extra-fibrillar)6. In particular, extra-fibrillar HAP is thought to represent 75% of the
mineral present in bone21. The HAP crystals, confined inside and in-between the collagen fibrils, grows and aggregates forming tiny crystals with a platelet shape. Their dimensions are in
the range of tens to hundreds of nanometers, with the long axis usually aligned with the collagen fibrils22,23,24,25. The platelet shape of HAP, in particular its thickness (ranging from 1
to 7 nm in humans), has the advantage of maximizing the surface area, giving bone mineral a high biochemical and physiological reactivity23. This is of primary relevance considering that
bone mineral is the main source of calcium for the entire organism, and this life-essential chemical element should be stored in an efficient way to be biologically available. Besides the
biochemical role, HAP crystal shape and size also play a crucial role in the mechanical properties of bone nano-composites, as shown in previous computational studies which analyzed the
effects of HAP nano-confinement26,27. It has been observed, for example, that the size of HAP crystals can influence the failure behavior at the nanoscale level, especially with respect to
the stress distribution and crack propagation28. This is true not only for HAP crystals alone, but also for the collagen-HAP composite, where the nanoconfined size of HAP platelets enhances
their strength, stiffness, and their capacity to dissipate mechanical energy26, and can influence the mechanical properties of interface between collagen and HAP27. Therefore, understanding
the impact of aging on the size of HAP platelets in bones becomes a crucial issue to deepen our understanding of bone mechanics at the nanoscale level. Whether and how the crystal platelets
change with age is an open and still debated issue. From an experimental point of view, measurements of HAP platelet dimensions have been performed on mouse25,29, bovine23 and human bone
samples22,24,30 using a variety of techniques. The most widespread techniques are X-ray diffraction studies (for an example of data that can be obtained with this method, see Fig. 3a),
Fourier-transform infrared spectroscopy (FTIR)25,31, and electron microscopy24,32. Yet, no consensus about how HAP platelets change in dimensions during aging has been reached, because of
limitations of indirect measurement techniques (i.e., HAP crystal size changes could be a hypothesis to explain a variation in mineralization, but not the only factor that plays a role) and
because of the very limited number of bone samples usually considered in each study. To give an idea to the reader of the state of the art and of the flourishing debate in this experimental
field, we will summarize some significant findings. The experimental work of ref. 29 analyzed femurs from young adult (3-month old), middle-aged (8-month old) and aged (24-month old)
Sprague–Dawley rats through Raman microspectroscopy. From the analysis of Raman spectra they observed a slight increase in crystallinity in old rats compared to young ones, suggesting a more
ordered crystal lattice in the aged group. In addition, they measured a higher value of mineralization (relative amounts of mineral and organic matrix) in the aged samples with respect to
the young ones. Those two aspects, increased mineral-to-matrix ratio and increased crystallinity could be explained by increased sizes of HAP platelets in aged rats29, a conclusion also
reported in the recent review on changes in bone matrix properties with aging by ref. 6. Other authors, instead, found no significant differences in HAP platelets dimensions between young
and old individuals24,33. This observation was reported from the Transmission Electron Microscopy (TEM) bright field analysis performed by Rubin et al. on six samples of human trabecular
bones, three coming from young individuals and three from osteoporotic, aged ones24 (see Fig. 3b). Other authors, instead, observed a slight decrease of the HAP unit-cell volume associated
with age: this is the case of a recent study by Foley et al. based on X-ray diffraction of bone samples coming from human skeletons buried in an old cemetery30. With this technique, the
authors measured both the lattice parameters and the crystallite size, obtained as coherent diffracting domain inferred from the peaks of the X-ray spectra. Nevertheless, the measures of the
crystallite sizes did not show a clear trend: in some bones, for example in the sternum, HAP platelets are larger in the aged sample; in other cases (such as the clavicle), they are shorter
in the _c_-axis direction with respect to the young ones; yet in other bones the size is the same for both the young and aged HAP platelets. The authors pointed out a difficulty that is
common to all the measurements conducted on HAP platelets through X-ray techniques: peaks broadening in X-ray spectra, due to the poorly crystalline nature of bioapatite, complicates the
interpretation of crystallite size, computed as coherently scattering domain size obtained using the Scherrer equation34. Nevertheless, more detailed information has been obtained from X-ray
diffraction about the dimension of the unit cell of bioapatite mineral. Foley et al.30 observed that the unit cell volume of bioapatite shrinks with age, due to a reduction of the _a_ and
_b_ lattice parameters (see Fig. 3c), whereas the _c_ length remains relatively constant with age. This confirms previous results obtained by ref. 22 via X-ray diffraction studies on a wider
bone sample, composed of human iliac crest of 87 individuals aged 0–90 years, that revealed how lattice volume of bone apatite decreases with age. Moreover, it is consistent with the XRD
measurements of ref. 35 that found lower lattice parameters in human adult bone samples compared to fetal ones. This reduction of lattice parameters is caused by chemical changes that occurs
in bioapatite during aging, in particular by the increase in carbonate content due to substitutions in the crystal lattice (for further details see “Increase of carbonate substitutions in
HAP crystal lattice during aging”). Whether and how the variation of lattice parameters, that has been reported thus far, has a direct effect on the dimensions of the HAP nano-platelets
present in bone is still unclear. As mentioned above, consensus on how the aging process affects the dimensions of HAP crystals inside bones has not been reached yet and an experimental
study on a statistically significant number of young and old samples is still missing. X-ray studies have been the most popular technique to investigate crystal properties, thanks to the
ease of the sample preparation process, but they have also shown some limitations in the spectra interpretation due to the low crystallinity of biological apatite. On the other hand,
measurements of single HAP platelets from old and young bones could be performed with transmission electron microscopy (TEM) to highlight the modifications that aging could cause at a single
platelet level. In that case, however, the bottle-neck process is sample preparation, since it requires particular attention in separating the mineral from the organic part of bone, while
avoiding damage to the nano-crystals. INCREASE OF CARBONATE SUBSTITUTIONS IN HAP CRYSTAL LATTICE DURING AGING The studies on the characteristics of hydroxyapatite platelets in bones also
highlight a change in the chemical composition of the mineral during the aging process. The apatite structure is particularly favorable to chemical substitutions inside the crystal lattice.
The most abundant substitution is carbonate (CO3)−2, which constitutes as much as 5–9 wt% of bone HAP36. In particular, there are two main types of substitutions: type A, that consist of
substitutions of carbonate ions (CO3)−2 for hydroxyl ions (OH)−, and type B, that consist of substitutions of carbonate ions (CO3)−2 for phosphate ions (PO4)−3. In both cases, the charge is
not balanced, and vacancies are introduced in the hydroxyl and calcium sites, to provide local charge compensation and increase the stability37. Further details about the chemistry and
physics of the substitutions, their energetics, and how they modify the chemical bonds inside the crystal lattice can be found in previous theoretical studies based on quantum mechanics
models38,39 and chemical studies on synthetic carbonated apatites40,41. Several experimental studies have shown that the number of such defects in the mineral lattice increases with age and
in the case of bone diseases such as osteoporosis29,33,40,42. Akkus et al.29, comparing femurs from Sprague-Dawley rats of different age groups, reported an incidence of carbonate
substitutions in the aged group that was 17% greater than that measured in the young group. The study of ref. 33, instead, focused on the comparison between osteoporotic and non-osteoporotic
bones, choosing samples coming from osteoporotic patients who had suffered fragility fractures at the femoral neck, and consequently required hip replacement surgery (thus considering
particularly compromised bone tissue), and from healthy donors as control group. Based on XRD and FTIR analysis, the authors observed an 11% increase of the carbonate to phosphate ratio for
the fractured osteoporotic material compared to the non-fractured one33. In addition, the review by ref. 42 described the reported trend of increasing carbonate to phosphate ratio with age
in several animal models such as C57BL/6 mice43 and baboons44. Nevertheless, also on this crucial point, a broad consensus has not been reached about the precise impact of aging on the
chemical composition of bone mineral. The recent FTIR measurements by ref. 30 suggest, instead, a decrease of carbonate content with age. Systematic compositional analysis to quantify the
carbon content of each sample should clarify the issue and help resolve whether carbonate content varies with age. However, from the experimental side, the main obstacle to this
straightforward approach is the difficulty of distinguishing between carbon compounds residing in the organic component of bone and carbonate in the apatite crystal structure30. As discussed
above, the presence of carbonate ions (CO3)−2 in the structure of hydroxyapatite modifies its lattice parameters and plays an essential role in regulating bone mineral morphology,
decreasing the atomic order, coherent domain size, and surface energy45. These chemical changes have a significant impact on the mechanical properties and response of the bone mineral.
Furthermore, the change in mechanical properties at the single crystal level should be considered as a possible cofactor contributing to the overall decrease in bone toughness observed at
higher length scales with aging and osteoporosis. A recent study on synthetic apatites with different carbonate levels has experimentally determined apatite elastic properties, using in situ
hydrostatic pressure loading and synchrotron X-ray diffraction. The measurements revealed that the increase of carbonate levels reduces the bulk modulus and the elastic strain ratio, likely
as a result of decreased bond strength due to (CO3)−2 substitutions46. These recent experimental results are of particular interest from the perspective of studying the variations in
mechanical properties of bone nano components during aging. For this reason, the role of carbonate substitutions occurring in bone hydroxyapatite during aging has been investigated also from
a numerical point of view, through molecular dynamics (MD) simulations. Deymier et al.45 studied the effect of an increasing percentage of B-type carbonate substitutions (see Fig. 3c). They
show an almost linear decrease in elastic moduli as a function of increasing carbonate concentration. Such studies are of primary importance to investigate, in a systematic and quantitative
way, the effects that the increased number of carbonate substitutions have on the mechanical properties of bone. The controlled environment of simulations allows the variation of a single
parameter at a time (in this case the carbonate substitutions percentage), disentangling effects that are difficult to be studied independently in experiments, and making it possible to
perform direct mechanical testing at nanometer scale. It is important to note that, thus far, comparison with experimental results has been done with artificial apatites, that can be
synthesized controlling the level of carbonate substitutions. From an experimental point of view, it would be of extreme interest to obtain results similar to Wingender et al46, but on
biological bone samples characterized by different levels of carbonate substitutions. Of course, to achieve this goal, some technical difficulties need be overcome, regarding sample
preparations and the above mentioned problem of precise quantification of the carbonate levels. ENZYMATIC AND NON-ENZYMATIC CROSS-LINKING IN BONE At the nanoscale, collagen cross-links,
bonds that form within and between collagen molecules, are critical for modulating bone’s material properties. We distinguish between two different types of collagen cross-links: enzymatic
and non-enzymatic ones. Enzymatic collagen cross-links form intermolecular covalent bonds between specific amino acids of tropocollagen helices. To form a mature and stable enzymatic
cross-link, two stages are required. Initially, as the collagen molecules are released from the cell, the lysyl oxidase enzymatically modifies the lysine on specific sites. These lysine
products (allysine, hydroxylysine and hydroxyallysine) link two collagen molecules together (such as hydroxylysino-norleucine (HLNL) and dihydroxylysino-norleucine (DHLNL)), in their
immature divalent form. Reacting with different amino acids, these immature cross-links can further mature into a trivalent (or tetra-valent) form to connect two to three collagen molecules
(such as pyridinoline (PYD) and deoxypyridinoline (DPD))(see Fig. 3d)47. Non-enzymatic collagen cross-links, instead, initiate from the glycation process and are named advanced glycation
end-products (AGEs). From the chemical point of view, many different AGEs exist, such as vesperlysine, pentosidine, carboxymethyl-lysine, glucosepane, imidazolone, and furosine; nevertheless
experimental studies on the effect of AGEs in bone tissue are based on quantitative measurements of just two AGE markers: pentosidine (the most widespread) and carboxymethyl-lysine (CML),
that are easy to be accurately measured6. Some enzymatic cross-links have shown changes in their quantities during aging. For example, histidinohydroxylysinonorleucine (HHL) has a rapid
increase at birth followed by a gradual increase with aging48, and a recent study shows that there is a decline of immature enzymatic crosslinks during aging49. However, overall, the
collagen cross-links formed by enzymatic processes do not appreciably change in number and properties with aging, are very stable6,50 and when they reach the mature, trivalent form,
contribute to improve bone fracture toughness51. On the other side, significant amounts of AGEs accumulate in proteins with long half-lives, such as collagen, during aging52. More
specifically, cross-links formed by non-enzymatic processes, measured through pentosidine content, show a significant increase in bone matrix with age50. Despite the well-established
accumulation of AGEs in proteins contained in tissue of individuals affected by age-related chronic diseases, it is not yet clear whether AGEs are a cause or a consequence of aging52,53.
Even the impact that AGEs have on collagen mechanical properties has not been fully understood. While enzymatic cross-links form in very specific locations at the ends of the collagen
molecules, non-enzymatic ones form in the middle of collagen fibers6. This could reduce collagen fiber deformation plasticity, as observed in in vitro ribation/glycation models54, making the
tissue more brittle and contributing to reduced bone strength and toughness6. This is in agreement with some experimental studies on the role of collagen cross-links on mineralized fibril
mechanical properties, conducted with small- and wide-angle X-ray scattering (SAXS and WAXS)55. The study of ref. 55, carried out on a group of patients with osteoarthritis, revealed an
enhancement in the collagen scattering with age, that could be explained by an increased level of cross-links, responsible for collagen fibril stiffening. Experimental mechanical tests
performed on denatured bone samples, instead, indicate that the stiffness of the collagen network decreases with age, irrespective of the increased AGEs accumulation50 and that bone collagen
connectivity does not correlate with pentosidine content56. The experiments performed by refs. 50,56 are based on pentosidine measurments to quantify AGEs, and this could be a significant
limitation since it represents only a possible biomarker of non-enzymatic cross-links, it is unclear whether the amount of pentosidine is proportionally related to the amount of the other
advanced glycation end products50, and pentosidine content measured in aged human bone are small compared to the concentration of all of the covalent, enzymatic crosslinks found in bone57.
Regarding the impact of age on collagen mechanobiology, recent studies also reported how the accumulation of AGE cross-links could promote the force transfer through the collagen backbone,
instead of through friction between collagens (sliding), forcing the collagen to lose its ability to dissipate energy, and resulting in abrupt failures58. The introduction of AGE cross-links
also leads to randomly located stress concentrations, due to their spontaneous formation sites, and might interfere with some naturally designed fracture mechanisms, for instance, the
sacrifice of the weak bonds59, which diminishes the toughness of collagen fibril. The mechanical implications of changes in collagen cross-links during aging deserve further investigation,
to clarify the role of non-enzymatic cross-links. Undoubtedly, nanoscale collagen cross-links (both enzymatic and non-enzymatic) can play a role in influencing the mechanical properties and
biological processes of bone even at higher length scales. At the cellular level, AGEs have been observed to interfere with integrin-mediated attachment of osteoblasts (cells specialized in
bone construction) to a type-I collagen matrix60. Again, zooming out from the nanoscale upwards, it is interesting to notice how the formation of mature enzymatic collagen cross-links
effectively turns the individual tropocollagen molecules into a large interconnected polymer structure47, which changes its plasticity when AGEs accumulate with age. This has a direct effect
on mechanical properties, since connectivity and plasticity influence the fracture behavior of the collagen network61. Besides a direct impact on collagen mechanical properties, collagen
cross-links modifications with aging could also affect collagen degradation, influencing the bone remodeling process. A recent work of ref. 62 investigates how the changes undergone by
collagen due to aging influence its degradation by matrix metalloproteinases (MMPs), a process that plays a crucial role in osteoporosis. The authors reported how aged collagen fibers
(characterized by an increase in AGEs and mineral deposits) show a significantly reduced degradation with respect to the native fibrillar collagen. These observations suggest how aging
modifications of collagen could block the degradation by either masking appropriate cleavage sites or by preventing the protease-generated partial unfolding because of increased stiffness. A
promising approach that could help in elucidating the direct effects of collagen cross-links on the mechanical properties of bone at the nanoscale is the one employed by ref. 47. The
authors have implemented a coarse grain numerical model of collagen fibrils, to study the impact of enzymatic cross-link maturation from childhood to adulthood. It would be of extreme
interest to apply the same approach to non-enzymatic cross-links, to characterize the effect of AGEs accumulation on nanoscale mechanical properties in silico. An excellent starting point
for building in silico simulations of collagen is the recent online tool ColBuilder63, that include full-atom models of cross-linked collagen fibrils for 20 different species, obtained
integrating the low-resolution structure of collagen fibril available from X-ray fiber diffraction with high-resolution structures of short collagen-like peptides from X-ray crystallography
and mass spectrometry data64. REDISTRIBUTION OF WATER IN BONE DURING AGING Despite the popular notion of bone as an extremely solid and compact material, water is a fundamental part of bone
tissue and represents 10% of bone’s relative composition30. At the nanoscale, inside the composite structure of bone, water molecules are present at different locations with a variety of
biological, chemical, physical, and mechanical functions. In particular, water occupies free spaces in bone tissue or is bounded to collagen molecules or HAP mineral. The aging process has
an impact on bone water, not only reducing its overall content in aged bone (dropping to 5% of bone’s relative composition), but also subjecting it to a redistribution inside bone tissue.
Specifically, with aging there is a significant relocation of water from HAP-collagen interface, a loosely bound compartment, to the more tightly bound compartment, in which water molecules
strongly bind to collagen triple helices (see Fig. 3e)6. This has an impact on the mechanical properties of bone nano-composites. Previous computational studies on collagen-HAP
nanocomposites have shown how hydrogen bonds could act as sacrificial bonds, facilitating protein sliding on the mineral surface in a discontinuous way, thus increasing the energy required
for failure. Hence, water at the HAP-collagen interface acts as a “lubricant layer”, promoting the H-bond formation and leading to a general increase in the mechanical properties26. Besides,
it enhances the shearing of collagen over hydroxyapatite, decreasing the direct coupling between collagen and mineral phase and redistributing load among them27. The water tightly bound to
collagen molecules, instead, acts to stabilize the tropocollagen triple helix, stiffening the collagen fibrils. The relocation of water from the HAP-collagen interface to the tightly bound
compartment that occurs with aging could worsen the overall mechanical properties of bone nano composites. Water also plays a role inside the hydroxyapatite mineral itself: hydroxyapatite
and bioapatite are extremely hydrophilic and form complex amorphous hydrated layers on their surfaces46. Davies et al.65 proposed a layered structure with thin apatitic platelets sandwiched
between octacalcium phosphate citrate (OCP-citrate) hydrated layers (again see Fig. 3e) and demonstrated how such a model structure could explain NMR and X-ray data of real bones, such as
the plate-like shape of mineral crystals, the presence of significant quantities of bound water molecules and the relatively high concentration of hydrogen phosphate. It has to been noticed
that the presence of water and citrate layers between the HAP platelets could regulate the mineral crystallinity, preventing the platelets from merging into single larger crystals6. For this
reason, a deeper investigation of the effect of aging on the structural water present in HAP mineral could be of particular interest. NON-COLLAGENOUS PROTEINS IN BONE AND THEIR ROLE IN
AGING Non-collagenous proteins (NCP) make up ~10% of the organic component of bone extracellular matrix, with collagen being the dominant component making up the remaining 90%16,17.
Different types of bone may contain differing amounts of the same NCP; for instance cortical bone contains significantly more osteocalcin compared to trabecular bone, while osteonectin is
present in higher amounts in trabecular bone66. Moreover, bone matrix-associated NCPs are not exclusively unique to bone matrix; for example osteopontin and osteonectin are expressed in a
variety of tissue types67. Recent studies suggest that NCPs in bone matrix are multifunctional and likely behave synergistically to affect cellular processes, such as cell attachment, cell
differentiation, and regulation of hydroxyapatite deposition68. But, NCPs are not only biologically active molecules that contribute to cellular processes; given their sizeable fraction in
bone matrix, they contribute to structural and mechanical properties directly as well. NCPs are localized at the collagen-apatite interface18,19. As a result, they likely contribute to load
transfer at several hierarchical levels in bone matrix6,69. The absence of both osteocalcin and osteopontin in genetically-modified mouse models lacking NCPs osteocalcin and osteopontin, for
example, is associated with reduced fracture toughness of bone70. Overall, while the role of NCPs in bone is increasingly well-understood, we know relatively little about how the quantity
of NCPs changes with age and how age-associated trends impact the matrix quality. Of the limited information that exists on NCP changes with aging, most is concerned with the two predominant
NCPs in the bone matrix, namely osteocalcin (OCN) and osteopontin (OPN). Osteocalcin is the most abundant NCP in bone, making up ~20% of all NCPs in bone matrix71. Secreted by mature
osteoblasts and by osteocytes, it has high affinity to calcium, accelerating nucleation of hydroxyapatite and regulating early stages of bone healing. It also regulates the activity of
osteoclasts and promotes bone resorption, as well as insulin secretion and glucose homeostasis71,72,73,74,75,76,77,78,79,80. There is evidence to suggest that as people age, the level of
circulating osteocalcin in their bodies decreases81,82, leading to a decrease in and altered distribution of OCN found in the bone matrix. This phenomenon has been observed in both men and
women83, as well as in experimental animals such as mice and monkeys81,82. The amount of OCN in bone varies depending on the mean tissue age of the bone, with osteonal bone containing up to
20 times more OCN than interstitial bone. In mice, a lack of osteocalcin has been linked to increased net bone formation, which results in higher trabecular bone volume and periosteal
apposition76. A recent study has also identified a direct link between glycation of OCN with the formation of the AGE pentosidine, and the loss of bone toughness84. Osteopontin inhibits
mineral formation and delays nucleation; osteopontin-deficient mice present larger crystal size and an increased mineral content85. Osteopontin also regulates bone resorption through
osteoclast attachment and migration86,87. Simultaneously it may behave as a mechanical bone matrix “glue” that contributes to intrafibrillar sacrificial bonds, allowing greater stress
relaxation and deformation prior to failure88,89. There is some evidence to suggest that while the quantity of some NCPs, like osteopontin, may not change with age, their composition may
change, potentially affecting matrix properties. Notably, phosphorylation of bone matrix proteins begins to decline in middle age (at age 55-75) and appears to decline by roughly 20% by age
8090. This effect is more pronounced in women90. Higher levels of phosphorylation are associated with higher bone toughness, while decreased phosphorylation with aging alters the negatively
charged OPN molecule and extinguishes an energy dissipation mechanism characterized by OPN unfolding and replacement by calcium ions on the hydroxyapatite surface. In the absence of this
energy dissipation mechanism, bone toughness is diminished, allowing cracks to propagate more easily90. While some recent studies have considered effects of aging on NCPs in bone matrix, the
mechanisms of aging associated with changes to the quantity and quality of NCPs in the bone matrix are still poorly understood (see Table 1 for an overview on known NCPs functions and
variation with age). Future work can provide further insight into the effects of aging on less abundant NCPs; while their low content in bone matrix may diminish their direct impact on
mechanical bone quality over time, indirect effects are still likely at play. Resolving the cause-and-effect matrix of functional triggers and consequences of NCP modifications with aging
may offer new, yet unexplored pathways toward targeted therapies. MICROSCALE STRUCTURE OF BONE At the microscale, we distinguish between the extracellular matrix of bone and the cellular
component of bone. These two components are closely linked, since bone is a living material, subject to the continuous remodeling action of specialized cells. At this length scale, the
single bone fibrils arrange to form bone fibers, in combination with extrafibrillar hydroxyapatite. Each fibril is surrounded by a crust of mineral 20−30 nm thick and packed together and
aligned with other fibrils to form fibers of 3–7 μm in width. Fibers are composed of single bundles of fibrils or by the combination of many bundles10. The organization of bone tissue at the
microscale already reflects functional requirements of cortical and trabecular bone. Cortical bone consists of mineralized collagen fibers organized into 3–7 μm thick lamellae, which are
arranged into concentric structures, thus forming the osteon. The osteon consists of a cylindrical structure of 100–500 μm in diameter crossed, along its length, by the Haversian canal,
through which blood vessels and nerve endings run. Trabecular bone consists of trabeculae of lamellar bone oriented and intertwined with each other to form cavities in which bone marrow is
stored and preserved. The orientation of the trabeculae results from mechanical forces applied on the bone tissue. Inside the bone tissue, there exists a cellular component that consists of
osteoprogenitor cells, osteoblasts, osteocytes, osteoclasts, and bone lining cells. Osteoprogenitor cells, osteoblasts and osteocytes are consecutive stages of the same cell type, the
pluripotent mesenchymal cell that differentiates in osteogenic direction, whereas osteoclasts derive from the monocyte-macrophage lineage. The bone lining cells are quiescent osteoblasts
implicated in the coupling between the resorption and neoformation processes91. Osteoblasts, primarily involved in the synthesis of the bone matrix and its mineralization process, are
polarized cells with high synthetic activity, as shown by the well-developed endoplasmic reticulum, and Golgi apparatus, and numerous secretory vesicles containing pyrophosphatase and
alkaline phosphatase92,93. Alkaline phosphatase, when activated, produces phosphate ions that combine with calcium ions present in the extracellular matrix. In contrast, the role of
pyrophosphatase is not fully defined. Some studies have proposed that activation of this enzyme leads to the release of pyrophosphate from the diphosphonates, thus leading to their
inactivation. In fact, diphosphonates with pyrophosphate groups can combine with hydroxyapatite crystals, inhibiting the mineralization process94. For the mineralization of the bone matrix,
a contribution is also made by molecules directly produced by osteoblasts. Osteonectin and osteocalcin are two of the molecules that contribute to the mineralization process: osteonectin
promotes the nucleation of hydroxyapatite crystals, while osteocalcin inhibits the precipitation of calcium phosphate95. The production of bone matrix and its subsequent mineralization occur
following a precise orientation process: * in a first step, the osteoblasts lay down the bone matrix from the side facing the pre-existing bone matrix; * in a second step, deposition occurs
along the entire circumference of the osteoblasts, thus distancing themselves from surrounding cells; * during the final stages of matrix deposition, the osteoblasts slow down their
metabolic processes by undergoing the transformation into osteocytes, while osteoprogenitor cells give rise to more osteoblasts; * at the end of the bone tissue formation process, the
osteoblasts close to the bone surface terminate their activity, resulting in morphological changes such as reduction of organelles and flattening of the cell, thus transforming into the cell
phenotype of bone lining cells96. Osteoblasts play a critically important role in the bone remodeling processes, by triggering bone matrix resorption through both direct and indirect
mechanisms. In fact, osteoblasts also produce and secrete proteolytic enzymes that possess the ability to break down organic matrix components. One of these enzymes is the collagenase, which
acts by removing the nonmineralized osteoid that lines the surface of the bone, thus allowing the osteoclast cellular component to adhere to the mineralized matrix and to break it down97.
Osteoclasts are multinucleated cells formed by the fusion of mononuclear progenitor cells, which in turn derive from hematopoietic precursors of the granulocyte-macrophage lineage (CFU-GM).
In osteoclasts, the cell domain in direct contact with the bone surface is distinguishable into two zones: the clear zone, site of adhesion to the bone, and the ridged zone, characterized by
the presence of numerous dense cellular extrusions98. Osteoblasts can indirectly regulate the processes of bone remodeling by acting on the differentiation of osteoclasts, the cells
responsible for bone matrix resorption. Osteoblasts secrete the cytokines RANKL (Receptor Activator of Nuclear Factor kB Ligand) and osteoprotegerin (OPG), whose release is balanced to
maintain proper resorption activity by osteoclasts99. The cytokine RANKL, belonging to the superfamily of TNF (Tumor Necrosis Factor), is a protein localized at the membrane level of
osteoblasts and their precursors. RANKL binds its receptor, RANK, which is expressed on the cell membrane of pre-osteoclasts. RANKL-RANK binding triggers the processes of differentiation
toward the osteoclastic phenotype100. The main antagonist for RANKL activity is OPG, also expressed by cells of osteoblastic lineage, which competes with RANKL for binding to the RANK
receptor. The RANKL/OPG ratio plays a key role in the regulation of bone resorption processes, so that ratio fluctuations can lead to the onset of bone pathologies due to a non-physiological
regulation of the bone resorption process101. The osteocytes are cells orchestrating these remodeling processes through biomechanical mechanisms (see “Canalicular network and fluid pressure
acting on osteocytes”). Several computational models proposed in recent years have demonstrated the fundamental role of mechanical loading as a powerful and stable regulator of the
biochemical processes underlying the mechanisms for maintaining bone architecture102. The mechanisms of perception of mechanical load change include deformation changes at the level of bone
tissue, hydrostatic pressure, and flow potentials generated by extracellular fluid. It was initially thought that extracellular flow potentials could be generated by electrokinetic effects
associated with a micropore system connected to the collagen-apatite structure103. Subsequent studies showed that the porosity was attributable precisely to the lacunar channels and that
therefore these channels are the site from which flow potentials start104. Flow potentials are the electrokinetic effects that can regulate the movement of ions such as calcium across cell
membranes. One of the earliest theories of fluid flow proposed that osteocyte activation was due to the flow of extracellular fluid along the dendritic processes of osteocytes. In vitro
experiments demonstrated that osteocytes produce and secrete molecules in response to mechanical stimulation, upregulating the target genes of the Wnt/b-catenin pathway, the anabolic
regulator of bone mass, and downregulating sclerostin, an antagonist of the Wnt signaling pathway105. CHANGES AT THE CELLULAR LEVEL DURING AGING Osteoblasts, osteoclasts, and osteocytes have
a limited lifespan, controlled by the number of replication cycles that decreases with age106, and which is presumably determined by the length of telomeres at the ends of their genes (see
Fig. 4a.I). A telomere is a repetitive length of DNA and associated proteins that provide stability at the ends of chromosomes. However, telomeres decrease in length with each cell division
due to the inability of the cell to fully replicate this region, and upon reaching a critical level of telomere length, cells undergo cellular senescence or apoptosis. In addition, levels of
telomerase, the enzyme that can extend the life cycle, decrease with age107. External factors, such as UV rays and oxidative stress, also contribute to the aging process of bone cells by
accelerating telomere shortening108. Numerous studies over the past decade have sought to elucidate the molecular regulation of telomere length. Of note, it was determined why humans have a
relatively short telomere length of between 5 and 15 kb109, while in mice telomeres are about 50 kb long110, although humans have a much longer lifespan than mice. Evidence is emerging that
a critical variable determining species lifespan is the rate of telomere shortening, rather than their initial length110. Specifically, human telomeres shorten at a rate of ~70 bp per
year111, while mouse telomeres shorten at a rate of 7000 bp per year110, despite relatively abundant telomerase activity in mice112. This significant difference in telomeres shortening rate
likely contributes to the difference in longevity between mice and humans113. Moreover, it has been shown that there is a non-linear shortening trend of telomere length across the human
lifespan114 but similar studies have not been carried out in mouse models. In relation to bone tissue, telomere shortening, or telomerase deficiency, could be useful in monitoring the
biological aging of bone cells. However, considering them as biomarkers to predict osteoporosis and fractures in the clinical setting is still preliminary. Indeed, the relationship between
telomere shortening and uncoupling of bone remodeling processes observed in murine studies has not always been consistent with observational studies in humans115. These discrepancies may be
due to some limitations of clinical studies, such as measuring telomere length in peripheral blood leukocytes rather than osteoblasts. Moreover, osteoporosis is a multifactorial disease, in
which pathophysiological mechanisms other than age-dependent telomere shortening may prevail. For instance, as all cells show changes in gene expression and activity with age, bone cells may
develop the inability to respond to mechano-transduction forces durig aging116. This increased susceptibility to mechanical damage leads to increased apoptosis and altered regulation of
gene expression. The aging process also affects bone mesenchymal stem cells (BMMSCs), reducing their proliferation and causing imbalanced differentiation, that significantly contribute to
age-related bone loss117. Even changes occurring in bone extra-cellular matrix (ECM) at the nanoscale, could have direct consequences on bone cell activity and behavior. For example
AGEs-modification of type-I collagen impairs the integrin-mediated adhesion of osteoblastic cells to the matrix60 and the presence of several NCPs could influence the adhesion between
osteoblasts and osteclasts and ECM67,118. CHANGES IN BONE REMODELING The main cellular change impacting the skeleton is the altered rate of bone remodeling, the process by which osteoclasts
remove existing bone and osteoblasts form new bone. In fact, as the number of osteoblast precursors decreases with age, the amount of bone deposited at each remodeling cycle decreases119.
Cell aging in bone tissue is plausibly regulated by modifications of the signal pathway activated by proteins of the Wnt (Wingless-type MMTV integration site) family120. Comparing the
expression levels of all Wnt proteins, their expression is significantly decreased in the bone tissue of aged mice compared with young mice121. In agreement with this, transgenic
overexpression of Wnt 10b has shown to prevent bone loss in aged mice122. Notably, it has been suggested that oxidative stress also contributes to bone cell aging by interfering with the Wnt
pathway, albeit indirectly. Indeed, reactive oxygen species (ROS) activate FoxO (forkhead box proteins), which in turn binds _β_-catenin, minimizing its effective concentration in cells and
thus decreasing osteoblast formation123. In parallel, activation of PPAR-_γ_ (peroxisome proliferator-activated receptor gamma) by ligands, generated by lipid oxidation, can negatively
interfere with the Wnt pathway, contributing to the age-dependent decrease in osteoblast formation124 (see Fig. 4a.II). Overall, these aging-induced cellular changes affect the rate of bone
remodeling, the process by which osteoclasts remove existing bone (resorption) and osteoblasts replace it (formation). In fact, the amount of bone deposited at each remodeling cycle
decreases due to a reduction in the number of osteoblast precursor cells, the number of mesenchymal cells from which they are derived, or a reduction in the lifespan of osteoblasts. There is
also a parallel decrease in hematopoietic cells, which are precursors of osteoclasts. However, since the surface area available for resorption due to bone thinning also decreases, the net
result is in favor of an increased rate of resorption in the bone remodeling process. CANALICULAR NETWORK AND FLUID PRESSURE ACTING ON OSTEOCYTES Bone tissue is able to sense mechanical
stimuli coming from the outside and to adapt itself in response, through the action of bone cells. Osteocytes are embedded in bone matrix, located inside specific micrometer-sized holes
termed lacunae. Dendrites departing from osteocytes cross the bone matrix through narrow tunnels (roughly 300-nm-wide) named canaliculi, to connect with other osteocytes, osteblasts, and
blood vessels125. This spatial organization gives rise to the lacunocanalicular network (LCN) that pervasively crosses bone tissue and within which an interstitial fluid is free to flow. The
fluid moving in LCN inside bone tissue has been proposed to be responsible for passing mechanical signals to osteocytes. Indeed, deformations occurring through the bone matrix are too small
to be directly sensed by bone cells and a further mechanism to communicate those mechanical changes is required. An external loading acts to deform the bone and the LCN network inside it,
inducing a fluid movement through the canaliculi. This fluid flow could be sensed by osteocytes as a drag force sufficiently strong to trigger a mechanoresponse126. This hypothesis has been
tested in the recent work of ref. 127, using controlled in vivo loading experiments on mouse tibiae. The authors used an interesting combination of a circuit theory model to calculate the
fluid flow in realistic LCN network architectures, obtained from detailed microscopy images of bone sections, and in vivo microcomputed tomography (μCT) to directly measure formed and
resorbed bone as a response to mechanical loading. This approach demonstrates a direct spatial correlation between predicted mechanoresponse, based on fluid flow patterns in the actual LCN
architecture, and the measured mechanoresponse127. Since LCN mechanotrasduction plays a key role in activating bone formation, it is of crucial importance to understand how the aging process
affects this function. In a recent study using _μ_CT, the lacunae and canals in fibulae of female mice coming from two different age groups are compared: 5-month old (young adult mice) and
23-month old (old mice). These experiments give some clues of how LCN varies with age: older mice have lower canal volume density and show smaller and more rounded lacunae with respect to
young mice128. Such morphological alterations in LCN with advancing age could potentially affect the ability of osteocytes to properly sense and respond to mechanical stimuli. The effects of
aging on the lacunae, on the osteocytes themselves and on the canaliculi/dendrite connectivity are the object of a recent review125. A significant reduction (around 20%) in the number of
lacunae per bone area is observed with advancing age, accompanied by a reduction in volume and a shift towards more spherical shapes of the lacunae themselves125. Observations on young and
old mice also highlighted an increase in the percentage of empty lacunae (i.e., not occupied by an osteocyte) with aging125. Those lacunae left empty due to osteocyte death are subject to
the micropetrosis phenomenon, being gradually filled with mineral. Furthermore, experiments on human and mouse bone also revealed a clear loss of osteocytes dendricity with increasing age,
leading to a 30% decrease of the number of canaliculi125 (see Fig. 4d). These effects combined together lead to significant changes in canalicular fluid flow with aging, making the process
of communication of mechanical loading less efficient and making the detection of microdamage less accurate. Furthermore, the alterations affecting the lacunocanalicular network could be
associated with a specific pathology, as reported in the recent experimental study by ref. 129. Indeed, comparing bone samples of osteoporotic patients with samples coming from long-term
immobilizated ones and from healthy ones as control, differences in LCN modifications have been reported129. For example, immobilization cases are characterized by lower osteocyte density
accompanied by a higher proportion of empty lacunae, a symptom of increased osteocyte apoptosis with respect to the osteoporosis cases129 (see Fig. 4e). In this way, observing the LCN
changes could also help one to better understand the mechanisms underlying bone degradation in different clinical conditions. CHANGES IN COLLAGEN NETWORK The aging process affects the
collagen behavior not only at the molecular scale but at higher dimensions, impacting the complex hierarchical structure that collagen assumes in bone matrix. Collagen fibrils are assemblies
of collagen molecules connected by covalent cross-links130. In this sense, the collagen structure can be considered to be a networked rope where each element of the array transmits force to
the rest of the array via the lysine-hydroxylysine mediated crosslinks131. Furthermore, collagen fibrils themselves assemble into collagen fibers and then into lamellae and osteons, giving
rise to a complex collagen network. Experimental studies at the beginning of 2000s identified a correlation between age-related changes in bone mechanical properties and collagen network
integrity (see Fig. 4b). For example, the study by ref. 50 on thirty human femur samples coming from donors ranging from 19 to 89 years of age, measured both the mechanical properties of the
bone samples, obtained via three-point bending tests, and of the demineralized bone extracted from the same specimens using an acid solution to remove the mineral phase and focus on
collagen network characteristics. This approach revealed a significant decrease of failure strength, elastic modulus, and work to fracture of collagen network with increasing age.
Furthermore, a significant correlation between mechanical integrity of the collagen network and work to fracture bone have been highlighted. More recently ref. 56 have performed similar
mechanical tests on a wider and heterogeneous donor group composed of fifty-four individuals ranging from 21 to 101 years of age, performing also hydrothermal isometric tension (HIT) testing
on decalcified bone samples. The collagen is heated, until it begins to denature; the melting process, in which its triple helix structure dissociated into a random amorphous coil
conformation, drives the specimen to shrink. In HIT testing the collagen is held under isometric constraint, and the contractile force generated from the melting process can be measured.
With this technique, the authors measure the collagen’s thermal stability, via the denaturation temperature, and network connectivity, measured as maximum rate of isometric tension
generation. These experiments show a clear correlation between transverse fracture resistance of cortical bone and bone collagen network connectivity56. The limitation of these experimental
studies stems from the delicate chemical procedures needed to separate the mineral part of the bone from the collagen phase. Decalcification, for example, can impact the hydration status of
bone collagen, making the measurements less accurate. An interesting approach to more deeply understand the implications of collagen connectivity on its mechanical properties comes from the
recent study of Burla et al.61 that combines rheological experiments with computer simulations of collagen networks. The work focuses on disordered collagen networks, obtained by
polymerizing collagen extracted from different species and various tissue sources at different temperatures, exhibiting a variety of collagen architectures. To consider a more widespread
scenario in terms of connectivity, the authors add telopeptide sequences at the end of the collagen triple helix, to mediate intrafibrillar cross-linking. The authors demonstrate that the
connectivity of collagen network, defined as the mean number of fibers meeting at a junction, is the main determinant of collagen fracture61. This result holds for isotropic collagen
networks that could be found in skin and cartilage, while bone collagen is anisotropic with an ordered and hierarchical structure. Nevertheless, the way in which collagen fibrils and fibers
are connected and interact with each other may have significant implications on bone mechanical properties. THE ROLE OF OSTEONS AND DIFFERENT OSTEON MORPHOTYPES Osteons are tubular
structures, ≈100 μm in diameter, formed by aligned mineralized collagen lamellae that organize themselves into concentric layers. Osteons play a crucial role in deflecting and twisting
cracks at the microscale, providing bone with extrinsic toughening mechanisms9, which contribute to toughness enhancement. When observed using circularly polarized light microscopy (CPL),
two kinds of osteons can be detected, according to the collagen fiber orientation: “bright” ones, composed of oblique (±45∘) oriented collagen fibers and “dark” ones, composed of collagen
fibers parallel with the osteon axis (see Fig. 4c). Recent studies revealed that these two classes of osteons not only differ in collagen fiber orientation, but also show differences in
terms of composition and mechanical properties. “Dark” osteons have smaller and rounder lacunae, higher mineral-to-matrix ratio and carbonate-to-phosphate ratio, and higher elastic modulus
with respect to “bright” ones132. Furthermore, it has been observed, through finite-element models (FEM) simulations and mechanical tests on 3D printed osteon-inspired samples, that
mechanically “dark” osteons perform better under tension, while “bright” oseons perform better under compression133. Very interestingly, some of the characteristics of the osteons with
parallel fibers ("dark”) are similar to the ones typically observed in aged bone. Yet they have been observed inside the bone of the same middle-aged individual, revealing a coexistence
of these two kinds of osteons that play different mechanical roles inside the tissue: “dark” ones lead to higher stiffness and hardness while “bright” ones provide ductility and energy
dissipation132. It would be of interest to study whether and how the number and spatial distribution of “dark” and “bright” osteons change with age, for example applying polarized light
microscopy to a set of bone samples similar to the ones considered by ref. 129. MACROSCALE STRUCTURE OF BONE At the mesoscale, lamellae organize themselves into two distinct structures with
different morphological and mechanical properties: the cortical and the trabecular bone. The cortical bone is a compact structure, usually found in the outer part of bones, formed by
lamellae that regularly organize to form osteons around Haversian canals. The cortical bone is then composed of several osteons with interstitial lamellae around them (probably reminiscent
of previous osteons), to form a compact and dense structure with high strength and stiffness. Trabecular bone, despite having the same chemical composition as cortical bone, has a completely
different structure. It shows a spongy and porous structure, formed by small beams called trabeculae (composed of different layers of lamellae) that form a complex network rich in cavities.
Trabecular bone is generally found in the inner part of bones and, due to the multiple orientations that the trabeculae can assume, it is particularly efficient in redistributing stresses.
In a single bone there is a combination of these two tissues, with the cortical bone forming 80% of bone mass and the trabecular bone the remaining 20%. Nevertheless, due to its composition,
the trabecular bone has a higher surface to volume ratio and is much more active from the metabolic point of view, being subject to more frequent remodeling. Mechanically, there is a
combination of two different materials, the cortical bone with high strength and stiffness, able to resist high compressive and tensile loads, and the trabecular bone, more flexible and
tough, able to absorb large amounts of energy. Considering a transversal section of a long bone, such as a human femur, proceeding from the external towards the internal part, one finds: *
The _periosteum_, a dense and thin membranous layer, mostly made of elastic fibrous material also containing blood vessels and nerves. In the inner part, it accommodates bone cell
progenitors and osteoblasts. * The _intracortical area_, the central part of cortical bone, with osteons and interstitial lamellae around them. The intracortical area is crossed by the
lacunocanalicular network, with osteocytes inside it, and by Harvesian canals that allow the passage of blood vessels and nerves. * The _endosteum_, a thin membrane that surrounds the
medullary cavity. It consists of a layer of flattened osteoprogenitor cells and collagenous fibers. * The _trabecular part_, which occupies the inner part of long bones. Between the holes of
the spongy tissue, _bone marrow_ is accomodated. Bone marrow is a semi-solid tissue formed by hematopoietic cells, marrow adipose tissue, and supportive stromal cells. At this length scale,
bone tissue could be observed in a direct way with imaging techniques such as Computed Tomography (CT) and Dual X-ray absorptiometry (DXA), and experimentally characterized through
mechanical testing (e.g., tensile, compressive, three point bending tests). CHANGES IN BONE MINERAL DENSITY (BMD) The most evident change that could be macroscopically detected in bones with
aging, is the loss of bone mass. The estimation of bone “quantity” is of daily use in clinical practice, where it is usually measured as bone mineral density (BMD). The measure of the BMD
is performed via Dual-X Ray Photon Absorptiometry (DXA), a technique developed in the mid-1980s, that uses highly collimated low-energy X-ray beams passing through soft tissues and bony
parts of the body, captured by a detector. In this way it is possible to obtain an image from the exiting beam, where the intensity of the image is inversely related to the density of the
traversed body parts134. Thanks to the low dose of radiation needed and the non-invasiveness of the procedure, BMD became very popular as predictor of bone resistance to load and fracture
and is today the gold diagnostic standard for osteoporosis diagnosis. Population studies showed how in humans BMD increases during childhood and reaches a maximum value around 30 years of
age both in men and women. After the peak BMD starts to gradually decline in men with advancing age. In women, instead, there is a more rapid decline of bone mass associated with menopause,
that becomes more gradual with further aging. Despite its spread, BMD has some intrinsic limitations. First of all, it depends on the anatomical location of the sample134 and on the
instrument calibration based on standard phantoms of known density that should be carefully chosen135. Moreover, BMD is an areal measurement, expressed in grams per square centimeter and
because of its two-dimensional nature it cannot capture bone three-dimensional microarchitecture and distinguish between the cortical and trabecular tissue. For this reason, a new
morphological parameter has been introduced to gain information on bone microarchitecture, the trabecular bone score (TBS). TBS is obtained from DXA, as BMD, and it is a textural measurement
based on the experimental variograms of projected gray-level DXA images136. As seen in Fig. 5a, TBS is able to distinguish among patients with similar BMD, unveiling skeletal microstructure
features often associated with aging or pathology137,138. The research of appropriate and highly predictive tools to assess bone quantity and quality at the macroscale level and to estimate
fracture risks in clinical practice is a wide and continuously evolving field. Together with BMD and TBS, officially accepted in clinical practice, researchers proposed other instruments,
for example the FRAX, a computer-based algorithm developed by the World Health Organization Collaborating Centre for Metabolic Bone Diseases that calculates fracture probability from readily
obtained clinical risk factors139 and the BSI (Bone Strain Index), obtained from a complementary tool, based on Finite Element (FE) models, that assesses the resistance of bone to simulated
compressive loads to improve the DXA accuracy140. Nevertheless, it is important to understand that each of those measurements on macroscopic bone features is not able to accurately predict
fracture risk and could not reveal all the subtle changes occurring with age and disease in such a complex tissue as bone. It will be necessary to make a combined use of them and even to
make an effort to connect those macroscopic changes with more fundamental processes occurring at lower length scales. CHANGES IN MORPHOLOGICAL PARAMETERS MEASURED WITH ΜCT Widely used in
bone research is the microcomputed tomography technique (μCT), an X-ray-based imaging tool that allows the production of high-resolution three-dimensional (3D) images composed of
two-dimensional “slices” of a target specimen. With that technique, it is possible to measure features of bone architecture in the μm-mm scale, obtaining more detailed and quantitative
information on cortical and trabecular tissue and their morphological variation with aging. In Table 2 we show some of the most significant morphological parameters that have been measured
for bone tissue at the meso and macroscale, together with their variation with age as observed in previous experimental works on both humans and animals. Indeed, numerous studies have shown
that mouse models, particularly the C57BL/6J strain, share with humans such features as ovarian follicle decline, irregular cycle, and steroid hormone fluctuations that influence bone.
Therefore, they have been widely used in research on mechanisms responsible for aging morphological changes in the skeleton due to their small size and short lifespan (average of about 2
years). In male and female mice, the volume of trabecular bone in the long bones begins to decrease in parallel with a reduction in cortical thickness (Ct.Th) after about 6 months of age,
with a higher degree of reduction in females141 (see Fig. 5c). μCT analysis of femurs showed the ratio between trabecular volume and total volume (BV/TV) decline by 63.8% in male mice and
80.8% in females from 6 to 22 months of age. In correlation with BV/TV reductions, parallel decreases in trabecular number (Tb.N) of 12.1% and 40.3%, respectively, were observed. Likewise,
trabecular BMD was reduced by 27.4% in males and 41% in females. As for trabecular bone of tibia, BV/TV was decreased by 60.4% in males and 85.6% in females, associated with Tb.N decline of
24.6% and 47.6%, respectively. Trabecular bone of the spine showed similar reductions in BV/TV, accompanied by 19.1% Tb.N reduction in males and 42.5% in females141. The decline in BV/TV is
most attributable to a decrease in the number of trabeculae, which may be accompanied by an increase in trabecular thickness, particularly in long bones. This may be due to an increase in
the average thickness of the remaining trabeculae as the thinner ones are removed. However, trabecular thickness has been shown to increase due to a compensatory mechanism by the remaining
trabeculae, which support increased mechanical loads as the thinner ones are reabsorbed142. It is still unclear whether, in the face of endocortical resorption and decline in material
properties with age, periosteal expansion continues with aging in the mid-diaphyseal regions to maintain flexural strength of whole bone. In fact, some studies observed that there was no
substantial increase in the cross-sectional area of cortical bone despite the continued loss of trabecular bone volume142. The variation of bone morphological parameters with age has also
been studied in human bone samples, confirming many trends observed in animal models. Loss of trabecular bone mass in humans starts from the third decade of life and in women is accelerated
with menopause; in elderly individuals cortical bone (Ct.BMD) loss and increased cortical bone porosity (Ct.Po) are also observed. The main modifications induced by age in the trabecular
tissue are the decrease of the trabecular number (Tb.N), thickness (Tb.Th), and connectivity (measured as connectivity density ConnD). In those processes, sex-specific differences have been
observed, showing how the loss of trabecular bone mass in women is mainly due to the reduction of the number of trabeculae, while men lose bone mass prominently because trabecular thinning
occurs. Furthermore osteoporotic patients show lower bone volume fraction (BV/TV) with respect to old normal individuals, with osteoporotic women showing even lower values of BV/TV with
respect to osteoporotic men42. It has to been noticed how results on Tb.Th trends with aging differ among mice and humans, showing a thickening of the remaining trabeculae in mice and a
thinning of the trabeculae in humans. Further investigation is needed to clarify this issue, to reveal different mechanisms involved in the two species. Going up in scale, μCT images on
human cadaveric bone samples allowed the measurement of the morphological properties of the Harvesian structure present in cortical bone. Those measurements revealed how the Haversian canal
(HC) diameter increases with aging while the osteon wall thickness (OWT) decreases significantly, with a direct impact on the bone’s ability to deflect cracks143. For the sake of brevity, we
only highlight the trend that each morphological parameter follows during aging, without further details on the numerical values typically assumed. The interested reader can find in the
recent review by van der Bergh et al.144 a collection of updated numerical values of bone structure parameters coming from HR-pQCT for female and male human subjects and, when available,
their variation with aging. CHANGES TO MECHANICAL PROPERTIES The idea of characterizing bone tissue at the macroscale, using load-displacement curves obtained from mechanical tests, as usual
in an engineering study of a material, goes back to the ’70s145,146. Already from the first studies, an overall worsening in terms of mechanical properties with aging is revealed146. This
trend has then been confirmed in later experimental works147,148 (see Fig. 5d). In the following, we briefly review the main variations that aging causes to bone tissue properties. Cortical
and trabecular bones are usually tested independently, since their different structures imbue them with different mechanical properties, hence different loading curves8. Results have been
summarized in Table 3. REDUCTION OF ENERGY ABSORPTION WITH AGING The quantity of energy that a material sample is able to absorb gives an idea of material toughness and tells us something
about the material’s ability to respond to external mechanical loads and adapt to them, prior to failure. More in detail, it is possible to estimate the work to fracture (W_f_) of a sample,
defined as the work per unit area to break into two pieces an unnotched specimen loaded in bending or tension. From an experimental point of view, W_f_ is measured by computing the area
under the load/displacement curve divided by twice the area of the fracture surface149. The first measurements of W_f_, performed on mm-sized cortical bone samples extracted from human
donors of different age, already showed a decline of absorbed energy with advancing age. In particular, each decade of life seems to bring a decline of W_f_ of 8.7% of its value at 35
years150. Subsequent studies not only confirmed the decrease of work to fracture with increasing age, but also underlined differences between energy absorption in the preyield and postyield
regimes. Indeed, the total elastic energy absorbed in preyield deformation (W_f__c_) is almost constant for young and middle aged bone samples, showing a decline of almost 30% for elderly
ones. The energy absorbed during postyield deformation (W_f__p_), instead, decreases continuously with advancing age and correlates with the progressive deterioration of collagen networks’
mechanical properties50. Even in bovine samples tested under compression in the transverse and longitudinal directions, the subtended area of the stress-strain curves is significantly
smaller for the adult specimen with respect to the young one, underlying the greater ability of young bone to absorb energy and to deform plastically without fracturing151. For trabecular
bone, instead, the primary macroscopic mechanisms for energy absorption are fracture and buckling of trabeculae, allowing reduction of the stresses transmitted to the cortical tissue and the
delay of the complete bone failure145. It is clear, therefore, how a decrease in the number of trabeculae (see paragraph “Changes in morphological parameters measured with µCT”) with aging
has a significant impact on the decrease of the absorbed energy. Nevertheless, directly comparing the work to fracture data coming from different studies could be difficult because there is
a dependence on the sample dimensions that should be taken into account. For this reason many recent works used the crack initiation and growth to asses bone sample toughness (see subsection
“Decline of crack initiation- and crack growth-toughness with age”). DECLINE OF CRACK INITIATION- AND CRACK GROWTH-TOUGHNESS WITH AGE Mechanical tests performed on real bone samples show
how fracture onset and propagation change with age both in cortical and trabecular bone tissue. Nalla et al.152 measured crack propagation in the longitudinal direction (parallel to osteons
axis) of human cortical bones taken from donors from 34 to 99 years of age. The authors reported a significant decrease of crack initiation toughness with age (reducing in value by 40% from
40 to 100 years of age) and an evident decline of crack-growth toughness, that becomes almost negligible in the oldest samples. Furthermore, based on integrated use of synchrotron X-ray
computed tomography (SRCT), a direct observation of the crack propagation and microstructure is possible (see Fig. 5b). This technique highlights how the number and size of crack bridges
(uncracked portion of tissue separating crack fronts) significantly decrease with age, depriving bone of a crucial extrinsic toughening mechanism152. Similar results have been recently
confirmed by nanoindentation tests performed by Yadav et al.143 on young and aged cadaver bones. They found that even in the transverse direction (perpendicular to osteons axis) both crack
initiation and crack growth toughness are significantly lower in the aged group143. In this transverse direction osteons play a fundamental role in toughening the tissue deflecting the
cracks9. Yadav et al.143 reported an increase in Harvesian canal diameters and a decrease in osteons wall thickness with age. These features weaken the osteon crack deflection ability,
making the cracks initiate and grow more easily. In the trabecular tissue, instead, the crack initiation and propagation are less explored issues, due to high shape complexity. A recent work
of ref. 153 applied cyclic compressive loading to human trabecular bone samples using contrast agents to directly observe the location and distribution of microscopic cracks and
accumulation of submicroscopic cracks. Interestingly, the study revealed how damage distributes and how samples with thinner “rod-like” trabeculae, transversely oriented to applied loads,
show enhanced fatigue resistance153. That approach of “coloring” damaged, microcracked regions was used to obtain insights into the mechanisms involved in fatigue resistance, but could be
potentially expanded and exploited to study the variation of trabecular bone damage patterns with aging. REDUCTION IN TENSILE ULTIMATE STRAIN WITH AGE In addition to the ability to absorb
energy and resist crack initiation and propagation, bone tissue also loses plasticity and ability to deform with advancing age. From a mechanical point of view, this property could be
assessed by measuring the tensile ultimate strain, defined as the strain value corresponding to the peak of the stress-strain curve. Tensile ultimate strain gives a measure of the maximum
deformation that a material is able to sustain prior to failure. Morgan et al.8 reported a decline of tensile ultimate strain for human cortical bone of approximately 10% per decade, from a
high of 5% strain at age 20–30 years to a low of less than 1% strain above age 80 years. This is an indicator of how bone becomes macroscopically less ductile and more brittle with aging,
favouring the onset of fractures. Interestingly, in-situ SAXS measurements performed on human bone samples during tensile tests, have shown that for a given strain applied to the bone
tissue, the strain carried by the collagen fibrils is significantly less (by some 25%), in aged bone compared to young147. This behavior is even more evident in diabetic bone in mice, where
the ultimate strain of collagen fibrils is reduced by 40% respect to healthy mice154. Those facts suggest a key role of collagen fibrils in decreasing overall bone ultimate strain, becoming
less deformable, probably due to the increasing presence of AGEs cross-links (see “Enzymatic and non-enzymatic cross linking in bone”)147,154. REMARKS AND PERSPECTIVES “There is plenty of
room at the bottom” said the Nobel Prize physicist Richard Feynman showing a pin to his audience. With that famous quote he was explaining how behind a common object there is still a lot to
uncover going down in scale, reaching atoms and subatomic particles and considering the fundamental interactions among them155. In a certain way, we aim to do the same with bone tissue
research and treatment strategies for bone aging. This peculiar biological tissue has been the subject of many research works since decades, attracting the interest of various fields,
ranging from medicine and biology to engineering and physics. Each discipline borrowed its techniques to unveil some aspects of bone structure, characteristic of the material and biological
process involved in bone formation and remodeling. As emerging from the previous sections, the aging process affects the bone tissue in many different ways, with repercussions at multiple
length scales. Indeed, changes in the chemical structures (i.e., mineralization and chemical defects in hydroxyapatite) and molecular bonds (i.e., collagen cross-links) of bone components
occur at the nanoscale, modifying the fundamental building blocks that constitute bone hierarchical structure. Besides, bone cell activity is significantly modified by aging. In particular,
the number of osteoblasts precursors decreases, with a significant impact on bone remodeling process, especially on new bone formation, since the amount of bone deposited at each remodeling
cycle decreases. Then, aging brings distinct changes to bone tissues, as observed at the micro- and macro- length scales with different imaging techniques, with a direct and measurable
impact also on bone mechanical properties, which tend to decline with advancing age. Even if each of those aspects has been investigated separately, through experimental or computational
techniques, reaching in certain cases a good agreement among different studies and exhaustive explanations, there is still room for further scientific exploration. At the nanoscale level,
how hydroxyapatite crystals change in dimensions and composition with age, how collagen cross-links vary and how non-collagenous properties behave with aging are open questions, still
awaiting a definitive answer. Moreover, the investigation of links, connections and relationships among different aspects of bone aging occurring at different length scales could be
particularly important. There is still a lot to unveil about how changes occurring at the molecular and cellular levels influence the macroscopic behavior of bone, in a direct or indirect
way. Clarifying and quantifying how modifications of bone building blocks at the nanoscale translate to bone tissue properties is an extremely interesting area of research with many gaps in
knowledge yet to be filled. This challenge requires a truly multidisciplinary approach, with the interpenetration of different skills and the joint use of different techniques. Even if
challenging, that approach would be of high interest for a profound understanding of the fundamental mechanisms involved in bone aging, paving the way for the development of better
diagnostics, and more efficient and targeted therapies for age-related bone diseases. REFERENCES * United Nations, Global issue: ageing. https://www.un.org/en/global-issues/ageing. * Heede,
K., Bouckaert, N. & Voorde, C. The impact of an ageing population on the required hospital capacity: results from forecast analysis on administrative data. _Eur. Geriatr. Med._ 10,
697–705 (2019). Article PubMed Google Scholar * Kanis, J. A. et al. Scope: a scorecard for osteoporosis in europe. _Arch. Osteoporos._ 8, 1–63 (2013). Article Google Scholar * Wu, A.-M.
et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the global burden of disease study 2019. _Lancet
Healthy Longev._ 2, 580–592 (2021). Article Google Scholar * Acevedo, C., Stadelmann, V. A., Pioletti, D. P., Alliston, T. & Ritchie, R. O. Fatigue as the missing link between bone
fragility and fracture. _Nat. Biomed. Eng._ 2, 62–71 (2018). Article PubMed Google Scholar * Burr, D. B. Changes in bone matrix properties with aging. _Bone_ 120, 85–93 (2019). Article
CAS PubMed Google Scholar * García-Aznar, J. M., Nasello, G., Hervas-Raluy, S., Pérez, M. Á. & Gómez-Benito, M. J. Multiscale modeling of bone tissue mechanobiology. _Bone_ 151,
116032 (2021). Article PubMed Google Scholar * Morgan, E. F., Unnikrisnan, G. U. & Hussein, A. I. Bone mechanical properties in healthy and diseased states. _Annu. Rev. Biomed. Eng._
20, 119–143 (2018). Article CAS PubMed PubMed Central Google Scholar * Huang, W. et al. Multiscale toughening mechanisms in biological materials and bioinspired designs. _Adv. Mater._
31, 1901561 (2019). Article CAS Google Scholar * Kwon, Y. & Clumpner, B. Multiscale modeling of human bone. _Multiscale Multidiscip. Model Exp. Des._ 1, 133–143 (2018). Article
Google Scholar * Wancket, L. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. _Vet. Pathol._ 52, 842–850 (2015). Article CAS
PubMed Google Scholar * Reinwald, S. & Burr, D. Review of nonprimate, large animal models for osteoporosis research. _J. Bone Miner. Res._ 23, 1353–1368 (2008). Article PubMed PubMed
Central Google Scholar * Amson, E. & Bibi, F. Differing effects of size and lifestyle on bone structure in mammals. _BMC Biol._ 19, 87 (2021). Article CAS PubMed PubMed Central
Google Scholar * Milazzo, M., David, A., Jung, G. S., Danti, S. & Buehler, M. J. Molecular origin of viscoelasticity in mineralized collagen fibrils. _Biomater. Sci._ 9, 3390–3400
(2021). Article CAS PubMed PubMed Central Google Scholar * Wagermaier, W., Fratzl, P. Collagen. In: Matyjaszewski, K., Möller, M. (eds.) _Polymer Science: A Comprehensive Reference_,
pp. 35–55. Elsevier, Amsterdam (2012). * Sroga, G.E., Karim, L., Colón, W., Vashishth, D. Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and
proteomics methodology. Molecular & Cellular Proteomics 10 (9) (2011). * Herring, G., Ashton, B. & Chipperfield, A. The isolation of soluble proteins, glycoproteins, and
proteoglycans from bone. _Prep. Biochem._ 4, 179–200 (1974). CAS PubMed Google Scholar * Nikel, O., Laurencin, D., McCallum, S. A., Gundberg, C. M. & Vashishth, D. Nmr investigation
of the role of osteocalcin and osteopontin at the organic–inorganic interface in bone. _Langmuir_ 29, 13873–13882 (2013). Article CAS PubMed PubMed Central Google Scholar * Wang, Z.,
Vashishth, D. & Picu, R. Bone toughening through stress-induced non-collagenous protein denaturation. _Biomech. Model. Mechanobiol._ 17, 1093–1106 (2018). Article CAS PubMed Google
Scholar * Bonucci, E. Bone mineralization. _Front. Biosci.-Landmark_ 17, 100–128 (2012). Article CAS Google Scholar * Nair, A. K., Gautieri, A. & Buehler, M. J. Role of
intrafibrillar collagen mineralization in defining the compressive properties of nascent bone. _Biomacromolecules_ 15, 2494–2500 (2014). Article CAS PubMed Google Scholar * Handschin, R.
& Stern, W. Crystallographic lattice refinement of human bone. _Calcif. tissue Int._ 51, 111–120 (1992). Article CAS PubMed Google Scholar * Eppell, S. J., Tong, W., Katz, J. L.,
Kuhn, L. & Glimcher, M. J. Shape and size of isolated bone mineralites measured using atomic force microscopy. _J. Orthop. Res._ 19, 1027–1034 (2001). Article CAS PubMed Google
Scholar * Rubin, M. A. et al. Tem analysis of the nanostructure of normal and osteoporotic human trabecular bone. _Bone_ 33, 270–282 (2003). Article PubMed Google Scholar * Turunen, M.
J. et al. Bone mineral crystal size and organization vary across mature rat bone cortex. _J. Struct. Biol._ 195, 337–344 (2016). Article CAS PubMed Google Scholar * Libonati, F., Nair,
A. K., Vergani, L. & Buehler, M. J. Mechanics of collagen–hydroxyapatite model nanocomposites. _Mech. Res. Commun._ 58, 17–23 (2014). Article Google Scholar * Qin, Z., Gautieri, A.,
Nair, A. K., Inbar, H. & Buehler, M. J. Thickness of hydroxyapatite nanocrystal controls mechanical properties of the collagen–hydroxyapatite interface. _Langmuir_ 28, 1982–1992 (2012).
Article CAS PubMed Google Scholar * Libonati, F., Nair, A. K., Vergani, L. & Buehler, M. J. Fracture mechanics of hydroxyapatite single crystals under geometric confinement. _J.
Mech. Behav. Biomed. Mater._ 20, 184–191 (2013). Article CAS PubMed Google Scholar * Akkus, O., Adar, F. & Schaffler, M. B. Age-related changes in physicochemical properties of
mineral crystals are related to impaired mechanical function of cortical bone. _Bone_ 34, 443–453 (2004). Article CAS PubMed Google Scholar * Foley, B., Greiner, M., McGlynn, G. &
Schmahl, W. W. Anatomical variation of human bone bioapatite crystallography. _Crystals_ 10, 859 (2020). Article CAS Google Scholar * Mathavan, N., Turunen, M. J., Tägil, M. &
Isaksson, H. Characterising bone material composition and structure in the ovariectomized (ovx) rat model of osteoporosis. _Calcif. Tissue Int._ 97, 134–144 (2015). Article CAS PubMed
Google Scholar * Vallet-Regi, M. & González-Calbet, J. M. Calcium phosphates as substitution of bone tissues. _Prog. Solid State Chem._ 32, 1–31 (2004). Article CAS Google Scholar *
Greenwood, C. et al. Towards new material biomarkers for fracture risk. _Bone_ 93, 55–63 (2016). Article CAS PubMed Google Scholar * Warren, B.E. X-Ray Diffraction. Courier Corporation,
North Chelmsford, Massachusetts, USA (1990). * Meneghini, C., Dalconi, M. C., Nuzzo, S., Mobilio, S. & Wenk, R. H. Rietveld refinement on x-ray diffraction patterns of bioapatite in
human fetal bones. _Biophys. J._ 84, 2021–2029 (2003). Article CAS PubMed PubMed Central Google Scholar * Von Euw, S. et al. Bone mineral: new insights into its chemical composition.
_Sci. Rep._ 9, 1–11 (2019). Google Scholar * Astala, R. & Stott, M. First principles investigation of mineral component of bone: Co3 substitutions in hydroxyapatite. _Chem. Mater._ 17,
4125–4133 (2005). Article CAS Google Scholar * Peroos, S., Du, Z. & Leeuw, N. H. A computer modelling study of the uptake, structure and distribution of carbonate defects in
hydroxy-apatite. _Biomaterials_ 27, 2150–2161 (2006). Article CAS PubMed Google Scholar * Ren, F., Lu, X. & Leng, Y. Ab initio simulation on the crystal structure and elastic
properties of carbonated apatite. _J. Mech. Behav. Biomed. Mater._ 26, 59–67 (2013). Article CAS PubMed Google Scholar * Arnold, E.L., Keeble, D.S., Evans, J., Greenwood, C., Rogers,
K.D. Investigating pair distribution function use in analysis of nanocrystalline hydroxyapatite and carbonate-substituted hydroxyapatite. Acta Crystallogr. Sect. C Struct. Chem. 78 (5)
(2022). * Yasar, O. F. et al. The carbonate and sodium environments in precipitated and biomimetic calcium hydroxy-carbonate apatite contrasted with bone mineral: Structural insights from
solid-state nmr. _J. Phys. Chem. C._ 125, 10572–10592 (2021). Article CAS Google Scholar * Boskey, A. L. & Imbert, L. Bone quality changes associated with aging and disease: a review.
_Ann. N. Y. Acad. Sci._ 1410, 93–106 (2017). Article PubMed PubMed Central Google Scholar * Raghavan, M., Sahar, N. D., Kohn, D. H. & Morris, M. D. Age-specific profiles of
tissue-level composition and mechanical properties in murine cortical bone. _Bone_ 50, 942–953 (2012). Article PubMed PubMed Central Google Scholar * Burket, J. et al. Microstructure and
nanomechanical properties in osteons relate to tissue and animal age. _J. Biomech._ 44, 277–284 (2011). Article PubMed Google Scholar * Deymier, A. C. et al. Protein-free formation of
bone-like apatite: New insights into the key role of carbonation. _Biomaterials_ 127, 75–88 (2017). Article CAS PubMed PubMed Central Google Scholar * Wingender, B. et al. Carbonate
substitution significantly affects the structure and mechanics of carbonated apatites. _Acta Biomater._ 122, 377–386 (2021). Article CAS PubMed Google Scholar * Depalle, B. et al. The
different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen. _Bone_ 110, 107–114 (2018). Article CAS
PubMed Google Scholar * Gaar, J., Naffa, R. & Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. _Org. Chem. Front._ 7,
2789–2814 (2020). Article CAS Google Scholar * Berteau, J.-P. et al. Ratio between mature and immature enzymatic cross-links correlates with post-yield cortical bone behavior: an insight
into greenstick fractures of the child fibula. _Bone_ 79, 190–195 (2015). Article CAS PubMed Google Scholar * Wang, X., Shen, X., Li, X. & Agrawal, C. M. Age-related changes in the
collagen network and toughness of bone. _Bone_ 31, 1–7 (2002). Article PubMed Google Scholar * Gauthier, R. et al. Relationships between human cortical bone toughness and collagen
cross-links on paired anatomical locations. _Bone_ 112, 202–211 (2018). Article PubMed Google Scholar * Baynes, J. W. The role of ages in aging: causation or correlation. _Exp. Gerontol._
36, 1527–1537 (2001). Article CAS PubMed Google Scholar * Chaudhuri, J. et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and
causality. _Cell Metab._ 28, 337–352 (2018). Article CAS PubMed PubMed Central Google Scholar * Willett, T. L., Sutty, S., Gaspar, A., Avery, N. & Grynpas, M. In vitro non-enzymatic
ribation reduces post-yield strain accommodation in cortical bone. _Bone_ 52, 611–622 (2013). Article PubMed Google Scholar * Giannini, C. et al. Scanning SAXS–WAXS microscopy on
osteoarthritis-affected bone–an age-related study. _J. Appl. Crystallogr._ 47, 110–117 (2014). Article CAS Google Scholar * Willett, T. L., Dapaah, D. Y., Uppuganti, S., Granke, M. &
Nyman, J. S. Bone collagen network integrity and transverse fracture toughness of human cortical bone. _Bone_ 120, 187–193 (2019). Article CAS PubMed Google Scholar * Saito, M., Fujii,
K., Soshi, S. & Tanaka, T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of
femoral neck fracture. _Osteoporos. Int._ 17, 986–995 (2006). Article CAS PubMed Google Scholar * Kamml, J., Ke, C.-Y., Acevedo, C. & Kammer, D. S. The influence of ages and
enzymatic cross-links on the mechanical properties of collagen fibrils. _J. Mech. Behav. Biomed. Mater._ 143, 105870 (2023). Article CAS PubMed Google Scholar * Rennekamp, B. et al.
Collagen breaks at weak sacrificial bonds taming its mechanoradicals. _Nat. Commun._ 14, 2075 (2023). Article CAS PubMed PubMed Central Google Scholar * McCarthy, A. D., Uemura, T.,
Etcheverry, S. B. & Cortizo, A. M. Advanced glycation endproducts interfere with integrin-mediated osteoblastic attachment to a type-i collagen matrix. _Int. J. Biochem. Cell Biol._ 36,
840–848 (2004). Article CAS PubMed Google Scholar * Burla, F. et al. Connectivity and plasticity determine collagen network fracture. _Proc. Natl Acad. Sci._ 117, 8326–8334 (2020).
Article CAS PubMed PubMed Central Google Scholar * Panwar, P. et al. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. _Matrix Biol._ 65,
30–44 (2018). Article CAS PubMed Google Scholar * Agnieszka Obarska-Kosinska, A.Ü. Benedikt Rennekamp, Gräter, F. ColBuilder. https://colbuilder.h-its.org/. * Obarska-Kosinska, A.,
Rennekamp, B., Ünal, A. & Gräter, F. Colbuilder: a server to build collagen fibril models. _Biophys. J._ 120, 3544–3549 (2021). Article CAS PubMed PubMed Central Google Scholar *
Davies, E. et al. Citrate bridges between mineral platelets in bone. _Proc. Natl Acad. Sci._ 111, 1354–1363 (2014). Article Google Scholar * Ninomiya, J. T. et al. Heterogeneity of human
bone. _J. Bone Miner. Res._ 5, 933–938 (1990). Article CAS PubMed Google Scholar * Carvalho, M. S., Cabral, J. M., Silva, C. L. & Vashishth, D. Bone matrix non-collagenous proteins
in tissue engineering: creating new bone by mimicking the extracellular matrix. _Polymers_ 13, 1095 (2021). Article CAS PubMed PubMed Central Google Scholar * Boskey, A. L.
Noncollagenous matrix proteins and their role in mineralization. _Bone Miner._ 6, 111–123 (1989). Article CAS PubMed Google Scholar * Katsamenis, O. L., Chong, H. M., Andriotis, O. G.
& Thurner, P. J. Load-bearing in cortical bone microstructure: Selective stiffening and heterogeneous strain distribution at the lamellar level. _J. Mech. Behav. Biomed. Mater._ 17,
152–165 (2013). Article CAS PubMed Google Scholar * Poundarik, A. A. et al. Dilatational band formation in bone. _Proc. Natl Acad. Sci._ 109, 19178–19183 (2012). Article CAS PubMed
PubMed Central Google Scholar * Hauschka, P. V., Lian, J. B., Cole, D. & Gundberg, C. M. Osteocalcin and matrix gla protein: vitamin k-dependent proteins in bone. _Physiol. Rev._ 69,
990–1047 (1989). Article CAS PubMed Google Scholar * Poser, J. W., Esch, F. S., Ling, N. C. & Price, P. A. Isolation and sequence of the vitamin k-dependent protein from human bone.
undercarboxylation of the first glutamic acid residue. _J. Biol. Chem._ 255, 8685–8691 (1980). Article CAS PubMed Google Scholar * Calvo, M. S., Eyre, D. R. & Gundberg, C. M.
Molecular basis and clinical application of biological markers of bone turnover. _Endocr. Rev._ 17, 333–368 (1996). CAS PubMed Google Scholar * Gundberg, C. M. Biochemical markers of bone
formation. _Clin. Lab. Med._ 20, 489–502 (2000). Article CAS PubMed Google Scholar * Hauschka, P. V. & Reid, M. L. Timed appearance of a calcium-binding protein containing
_γ_-carboxyglutamic acid in developing chick bone. _Dev. Biol._ 65, 426–434 (1978). Article CAS PubMed Google Scholar * Ducy, P. et al. Increased bone formation in osteocalcin-deficient
mice. _Nature_ 382, 448–452 (1996). Article CAS PubMed Google Scholar * Rammelt, S. et al. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. _J. Biomed.
Mater._ 73, 284–294 (2005). Article Google Scholar * Chenu, C. et al. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human
osteoclast-like cells. _J. Cell Biol._ 127, 1149–1158 (1994). Article CAS PubMed Google Scholar * Bodine, P. & Komm, B. Evidence that conditionally immortalized human osteoblasts
express an osteocalcin receptor. _Bone_ 25, 535–543 (1999). Article CAS PubMed Google Scholar * Glowacki, J. & Lian, J. B. Impaired recruitment and differentiation of osteoclast
progenitors by osteocalcin-deplete bone implants. _Cell Differ._ 21, 247–254 (1987). Article CAS PubMed Google Scholar * Khrimian, L., Obri, A. & Karsenty, G. Modulation of cognition
and anxiety-like behavior by bone remodeling. _Mol. Metab._ 6, 1610–1615 (2017). Article CAS PubMed PubMed Central Google Scholar * Mera, P. et al. Osteocalcin signaling in myofibers
is necessary and sufficient for optimum adaptation to exercise. _Cell Metab._ 23, 1078–1092 (2016). Article CAS PubMed PubMed Central Google Scholar * Ingram, R. T. et al. Age-and
gender-related changes in the distribution of osteocalcin in the extracellular matrix of normal male and female bone. possible involvement of osteocalcin in bone remodeling. _J. Clin.
Investig._ 93, 989–997 (1994). Article CAS PubMed PubMed Central Google Scholar * Bailey, S., Poundarik, A. A., Sroga, G. E. & Vashishth, D. Structural role of osteocalcin and its
modification in bone fracture. _Appl. Phys. Rev._ 10, 011410 (2023). Article CAS PubMed PubMed Central Google Scholar * Hunter, G. K., Hauschka, P. V., Poole, R. A., Rosenberg, L. C.
& Goldberg, H. A. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. _Biochem. J._ 317, 59–64 (1996). Article CAS PubMed PubMed Central Google
Scholar * Denhardt, D. T. & Guo, X. Osteopontin: a protein with diverse functions. _FASEB J._ 7, 1475–1482 (1993). Article CAS PubMed Google Scholar * Denhardt, D. T. & Noda, M.
Osteopontin expression and function: role in bone remodeling. _J. Cell. Biochem._ 72, 92–102 (1998). Article PubMed Google Scholar * Fantner, G. E. et al. Sacrificial bonds and hidden
length dissipate energy as mineralized fibrils separate during bone fracture. _Nat. Mater._ 4, 612–616 (2005). Article CAS PubMed Google Scholar * Bertassoni, L. E. & Swain, M. V.
The contribution of proteoglycans to the mechanical behavior of mineralized tissues. _J. Mech. Behav. Biomed. Mater._ 38, 91–104 (2014). Article CAS PubMed Google Scholar * Sroga, G. E.
& Vashishth, D. Phosphorylation of extracellular bone matrix proteins and its contribution to bone fragility. _J. Bone Miner. Res._ 33, 2214–2229 (2018). Article CAS PubMed Google
Scholar * Everts, V. et al. The bone lining cell: its role in cleaning howship’s lacunae and initiating bone formation. _J. Bone Miner. Res._ 17, 77–90 (2002). Article CAS PubMed Google
Scholar * Capulli, M., Paone, R. & Rucci, N. Osteoblast and osteocyte: games without frontiers. _Arch. Biochem. Biophys._ 561, 3–12 (2014). Article CAS PubMed Google Scholar *
Blair, H. C. et al. Osteoblast differentiation and bone matrix formation in vivo and in vitro. _Tissue Eng. Part B Rev._ 23, 268–280 (2017). Article CAS PubMed PubMed Central Google
Scholar * Michigami, T. & Ozono, K. Roles of phosphate in skeleton. _Front. Endocrinol._ 10, 180 (2019). Article Google Scholar * Lin, X., Patil, S., Gao, Y.-G. & Qian, A. The
bone extracellular matrix in bone formation and regeneration. _Front. Pharmacol._ 11, 757 (2020). Article CAS PubMed PubMed Central Google Scholar * Matic, I. et al. Quiescent bone
lining cells are a major source of osteoblasts during adulthood. _Stem Cells_ 34, 2930–2942 (2016). Article CAS PubMed Google Scholar * Uenaka, M. et al. Osteoblast-derived vesicles
induce a switch from bone-formation to bone-resorption in vivo. _Nat. Commun._ 13, 1066 (2022). Article CAS PubMed PubMed Central Google Scholar * Teitelbaum, S. L. Bone resorption by
osteoclasts. _Science_ 289, 1504–1508 (2000). Article CAS PubMed Google Scholar * Kearns, A. E., Khosla, S. & Kostenuik, P. J. Receptor activator of nuclear factor _κ_b ligand and
osteoprotegerin regulation of bone remodeling in health and disease. _Endocr. Rev._ 29, 155–192 (2008). Article CAS PubMed Google Scholar * Lam, J. et al. Crystal structure of the
trance/rankl cytokine reveals determinants of receptor-ligand specificity. _J. Clin. Investig._ 108, 971–979 (2001). Article CAS PubMed PubMed Central Google Scholar * Hofbauer, L. C.
& Heufelder, A. E. Role of receptor activator of nuclear factor-_κ_b ligand and osteoprotegerin in bone cell biology. _J. Mol. Med._ 79, 243–253 (2001). Article CAS PubMed Google
Scholar * Klein-Nulend, J., Bakker, A. D., Bacabac, R. G., Vatsa, A. & Weinbaum, S. Mechanosensation and transduction in osteocytes. _Bone_ 54, 182–190 (2013). Article CAS PubMed
Google Scholar * Starkebaum, W., Pollack, S. & Korostoff, E. Midroelectrode studies of stress-generated potentials in four-point bending of bone. _J. Biomed. Mater. Res._ 13, 729–751
(1979). Article CAS PubMed Google Scholar * Zhang, D., Weinbaum, S. & Cowin, S. C. Electrical signal transmission in a bone cell network: the influence of a discrete gap junction.
_Ann. Biomed. Eng._ 26, 644–659 (1998). Article CAS PubMed Google Scholar * Li, X. et al. Sclerostin binds to lrp5/6 and antagonizes canonical wnt signaling. _J. Biol. Chem._ 280,
19883–19887 (2005). Article CAS PubMed Google Scholar * Martin, G. M. et al. Replicative life-span of cultivated human cells. _Lab. Investig._ 23, 86–92 (1970). CAS PubMed Google
Scholar * Calado, R. T. Telomeres and marrow failure. _ASH Educ. Program Book_ 2009, 338–343 (2009). Google Scholar * Muller, M. Cellular senescence: molecular mechanisms, in vivo
significance, and redox considerations. _Antioxid. Redox Signal._ 11, 59–98 (2009). Article CAS PubMed Google Scholar * Kong, C. M., Lee, X. W. & Wang, X. Telomere shortening in
human diseases. _FEBS J._ 280, 3180–3193 (2013). Article CAS PubMed Google Scholar * Vera, E., Jesus, B. B., Foronda, M., Flores, J. M. & Blasco, M. A. The rate of increase of short
telomeres predicts longevity in mammals. _Cell Rep._ 2, 732–737 (2012). Article CAS PubMed Google Scholar * Canela, A., Vera, E., Klatt, P. & Blasco, M. A. High-throughput telomere
length quantification by fish and its application to human population studies. _Proc. Natl Acad. Sci._ 104, 5300–5305 (2007). Article CAS PubMed PubMed Central Google Scholar * Blasco,
M. A. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. _EMBO J._ 24, 1095–1103 (2005). Article CAS PubMed PubMed Central Google Scholar *
Whittemore, K., Vera, E., Martínez-Nevado, E., Sanpera, C. & Blasco, M. A. Telomere shortening rate predicts species life span. _Proc. Natl Acad. Sci._ 116, 15122–15127 (2019). Article
CAS PubMed PubMed Central Google Scholar * Ye, Q. et al. Telomere length and chronological age across the human lifespan: a systematic review and meta-analysis of 414 study samples
including 743,019 individuals. Ageing Res. Rev., 102031 (2023). * Wong, S. K., Ima-Nirwana, S. & Chin, K.-Y. Can telomere length predict bone health? a review of current evidence. _Bosn.
J. Basic Med. Sci._ 20, 423 (2020). CAS PubMed PubMed Central Google Scholar * Chen, J.-H., Liu, C., You, L. & Simmons, C. A. Boning up on Wolff’s law: mechanical regulation of the
cells that make and maintain bone. _J. Biomech._ 43, 108–118 (2010). Article PubMed Google Scholar * Ma, Y. et al. Autophagy controls mesenchymal stem cell properties and senescence
during bone aging. _Aging Cell_ 17, 12709 (2018). Article Google Scholar * Morgan, S., Poundarik, A. A. & Vashishth, D. Do non-collagenous proteins affect skeletal mechanical
properties? _Calcif.Tissue Int._ 97, 281–291 (2015). Article CAS PubMed PubMed Central Google Scholar * Szulc, P. & Seeman, E. Thinking inside and outside the envelopes of bone:
dedicated to pdd. _Osteoporos. Int._ 20, 1281–1288 (2009). Article CAS PubMed Google Scholar * Williams, B. O. & Insogna, K. L. Where Wnts went: the exploding field of lrp5 and lrp6
signaling in bone. _J. Bone Miner. Res._ 24, 171–178 (2009). Article CAS PubMed Google Scholar * Rauner, M., Sipos, W. & Pietschmann, P. Age-dependent wnt gene expression in bone and
during the course of osteoblast differentiation. _Age_ 30, 273–282 (2008). Article PubMed PubMed Central Google Scholar * Bennett, C. N. et al. Regulation of osteoblastogenesis and bone
mass by wnt10b. _Proc. Natl Acad. Sci._ 102, 3324–3329 (2005). Article CAS PubMed PubMed Central Google Scholar * Almeida, M., Han, L., Martin-Millan, M., O’Brien, C. A. &
Manolagas, S. C. Oxidative stress antagonizes wnt signaling in osteoblast precursors by diverting _β_-catenin from t cell factor-to forkhead box o-mediated transcription. _J. Biol. Chem._
282, 27298–27305 (2007). Article CAS PubMed Google Scholar * Manolagas, S. C. & Parfitt, A. M. What old means to bone. _Trends Endocrinol. Metab._ 21, 369–374 (2010). Article CAS
PubMed PubMed Central Google Scholar * Tiede-Lewis, L. M. & Dallas, S. L. Changes in the osteocyte lacunocanalicular network with aging. _Bone_ 122, 101–113 (2019). Article PubMed
PubMed Central Google Scholar * Weinbaum, S., Cowin, S. C. & Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. _J. Biomech._
27, 339–360 (1994). Article CAS PubMed Google Scholar * Tol, A. F. et al. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. _Proc.
Natl Acad. Sci._ 117, 32251–32259 (2020). Article PubMed PubMed Central Google Scholar * Hemmatian, H. et al. Age-related changes in female mouse cortical bone microporosity. _Bone_ 113,
1–8 (2018). Article PubMed Google Scholar * Rolvien, T. et al. Long-term immobilization in elderly females causes a specific pattern of cortical bone and osteocyte deterioration
different from postmenopausal osteoporosis. _J. Bone Miner. Res._ 35, 1343–1351 (2020). Article CAS PubMed Google Scholar * Gautieri, A., Vesentini, S., Redaelli, A. & Ballarini, R.
Modeling and measuring visco-elastic properties: from collagen molecules to collagen fibrils. _Int. J. Non-Linear Mech._ 56, 25–33 (2013). Soft Matter: a nonlinear continuum mechanics
perspective. Article Google Scholar * Orgel, J. P., Irving, T. C., Miller, A. & Wess, T. J. Microfibrillar structure of type i collagen in situ. _Proc. Natl Acad. Sci._ 103, 9001–9005
(2006). Article CAS PubMed PubMed Central Google Scholar * Stockhausen, K. E. et al. Collagen fiber orientation is coupled with specific nano-compositional patterns in dark and bright
osteons modulating their biomechanical properties. _ACS Nano_ 15, 455–467 (2021). Article CAS PubMed Google Scholar * Grezzana, G. et al. Probing the role of bone lamellar patterns
through collagen microarchitecture mapping, numerical modeling, and 3d-printing. _Adv. Eng. Mater._ 22, 2000387 (2020). Article CAS Google Scholar * Chun, K.J. Bone densitometry. In:
_Seminars in Nuclear Medicine_, Vol. 41, 220–228 Elsevier, (2011). * Knowles, N. K., Reeves, J. M. & Ferreira, L. M. Quantitative computed tomography (qct) derived bone mineral density
(bmd) in finite element studies: a review of the literature. _J. Exp. Orthop._ 3, 1–16 (2016). Article Google Scholar * Mirzaali, M. J. et al. Determinants of bone damage: An ex-vivo study
on porcine vertebrae. _PLoS One_ 13, 0202210 (2018). Article Google Scholar * Silva, B. C. et al. Trabecular bone score: a noninvasive analytical method based upon the dxa image. _J. Bone
Miner. Res._ 29, 518–530 (2014). Article PubMed Google Scholar * Shevroja, E., Cafarelli, F. P., Guglielmi, G. & Hans, D. Dxa parameters, trabecular bone score (tbs) and bone mineral
density (bmd), in fracture risk prediction in endocrine-mediated secondary osteoporosis. _Endocrine_ 74, 20–28 (2021). Article CAS PubMed PubMed Central Google Scholar * Kanis, J. A.,
McCloskey, E., Johansson, H., Oden, A. & Leslie, W. D. Frax® with and without bone mineral density. _Calcif. tissue Int._ 90, 1–13 (2012). Article CAS PubMed Google Scholar *
Colombo, C. et al. A new finite element based parameter to predict bone fracture. _PLoS One_ 14, 0225905 (2019). Article Google Scholar * Shim, J., Iwaya, C., Ambrose, C. G., Suzuki, A.
& Iwata, J. Micro-computed tomography assessment of bone structure in aging mice. _Sci. Rep._ 12, 8117 (2022). Article CAS PubMed PubMed Central Google Scholar * Glatt, V., Canalis,
E., Stadmeyer, L. & Bouxsein, M. L. Age-related changes in trabecular architecture differ in female and male c57bl/6j mice. _J. Bone Miner. Res._ 22, 1197–1207 (2007). Article PubMed
Google Scholar * Yadav, R. N. et al. Effect of ageing on microstructure and fracture behavior of cortical bone as determined by experiment and extended finite element method (xfem). _Med.
Eng. Phys._ 93, 100–112 (2021). Article PubMed Google Scholar * Bergh, J. et al. The clinical application of high-resolution peripheral computed tomography (hr-pqct) in adults: state of
the art and future directions. _Osteoporos. Int._ 32, 1465–1485 (2021). Article PubMed PubMed Central Google Scholar * Hayes, W. C. & Carter, D. R. Postyield behavior of subchondral
trabecular bone. _J. Biomed. Mater. Res._ 10, 537–544 (1976). Article CAS PubMed Google Scholar * Evans, F. G. Mechanical properties and histology of cortical bone from younger and older
men. _Anat. Rec._ 185, 1–11 (1976). Article CAS PubMed Google Scholar * Zimmermann, E. A. et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple
length scales. _Proc. Natl Acad. Sci._ 108, 14416–14421 (2011). Article CAS PubMed PubMed Central Google Scholar * Zimmermann, E. A. & Ritchie, R. O. Bone as a structural material.
_Adv. Healthc. Mater._ 4, 1287–1304 (2015). Article CAS PubMed Google Scholar * Ritchie, R. et al. Measurement of the toughness of bone: a tutorial with special reference to small animal
studies. _Bone_ 43, 798–812 (2008). Article CAS PubMed PubMed Central Google Scholar * Zioupos, P. & Currey, J. Changes in the stiffness, strength, and toughness of human cortical
bone with age. _Bone_ 22, 57–66 (1998). Article CAS PubMed Google Scholar * Manilay, Z., Novitskaya, E., Sadovnikov, E. & McKittrick, J. A comparative study of young and mature
bovine cortical bone. _Acta Biomater._ 9, 5280–5288 (2013). Article CAS PubMed Google Scholar * Nalla, R. K., Kruzic, J. J., Kinney, J. H. & Ritchie, R. O. Effect of aging on the
toughness of human cortical bone: evaluation by r-curves. _Bone_ 35, 1240–1246 (2004). Article CAS PubMed Google Scholar * Torres, A. M. et al. Bone-inspired microarchitectures achieve
enhanced fatigue life. _Proc. Natl Acad. Sci._ 116, 24457–24462 (2019). Article CAS PubMed PubMed Central Google Scholar * Acevedo, C. et al. Contributions of material properties and
structure to increased bone fragility for a given bone mass in the ucd-t2dm rat model of type 2 diabetes. _J. Bone Miner. Res._ 33, 1066–1075 (2018). Article CAS PubMed Google Scholar *
Feynman, R.P. Plenty of room at the bottom. In: _Proc._ _APS Annual Meeting_ (1959). * Mirzaali, M. J. et al. Mechanical properties of cortical bone and their relationships with age, gender,
composition and microindentation properties in the elderly. _Bone_ 93, 196–211 (2016). Article PubMed Google Scholar * Kafantari, H., Kounadi, E., Fatouros, M., Milonakis, M. &
Tzaphlidou, M. Structural alterations in rat skin and bone collagen fibrils induced by ovariectomy. _Bone_ 26, 349–353 (2000). Article CAS PubMed Google Scholar * Milovanovic, P. et al.
Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. _ACS Nano_ 7, 7542–7551 (2013). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by Fondazione Cariplo, under the Young Researcher grant initiative [grant n.2020-3615]. A.T. would like to acknowledge the funding support from the
National Science Foundation (CMMI 2145759) and the National Institute of Health (1R56AG075690-01A1, 1R01AG084715, 5U01HL146188). We would like to thank Professor Robert O. Ritchie for the
insightful discussions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Nano Science and Technology@PoliMi, Istituto Italiano di Tecnologia, Via Rubattino 81, Milano, 20134, Italy
Linda Ravazzano & Flavia Libonati * Department of Precision and Regenerative Medicine and Ionian Area (DiMePRe-J), University of Bari Aldo Moro, Piazza Giulio Cesare 11, Bari, 70124,
Italy Graziana Colaianni & Maria Grano * School of Mechanical, Aerospace, and Manufacturing Engineering, University of Connecticut, 191 Auditorium Road, Unit 3139, Storrs, 06269, CT, USA
Anna Tarakanova & Yu-Bai Xiao * Department of Biomedical Engineering, University of Connecticut, 260 Glenbrook Road, Unit 3247, CT, 06269, Storrs, USA Anna Tarakanova * Department of
Mechanical, Energy, Management and Transport Engineering - DIME, University of Genova, Via all’Opera Pia 15, Genova, 16145, Italy Flavia Libonati Authors * Linda Ravazzano View author
publications You can also search for this author inPubMed Google Scholar * Graziana Colaianni View author publications You can also search for this author inPubMed Google Scholar * Anna
Tarakanova View author publications You can also search for this author inPubMed Google Scholar * Yu-Bai Xiao View author publications You can also search for this author inPubMed Google
Scholar * Maria Grano View author publications You can also search for this author inPubMed Google Scholar * Flavia Libonati View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS F.L. and L.R. conceptualized the work. L.R. drafted the whole manuscript and arranged the figures. G.C. contributed to “Microscale structure of bone”,
in particular writing subsection “Changes at the cellular level during aging” and “Changes in bone remodeling”. A.T. wrote subsection “Non-collagenous proteins in bone and their role in
aging”. Y.X. contributed to writing subsection “Enzymatic and non-enzymatic cross-linking in bone”. M.G., A.T. and F.L. critically reviewed the manuscript. All authors read and agreed to the
published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Flavia Libonati. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ravazzano, L., Colaianni, G., Tarakanova, A.
_et al._ Multiscale and multidisciplinary analysis of aging processes in bone. _npj Aging_ 10, 28 (2024). https://doi.org/10.1038/s41514-024-00156-2 Download citation * Received: 12 December
2023 * Accepted: 07 May 2024 * Published: 15 June 2024 * DOI: https://doi.org/10.1038/s41514-024-00156-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Some of the most beautiful chocolate in the city, and Champagne at CompartésFood Some of the most beautiful chocolate in the city, and Champagne at Compartés Compartés creates some of the most bea...
Barack obama praises mlb decision to pull all-star game out of georgiaEXPLORE MORE Former President Obama has gone to bat for Major League Baseball, issuing a rare tweet Saturday praising th...
Celebrity bear hunt star lottie moss' life including feud with sisterLOTTIE MOSS IS ONE OF THE 12 CELEBRITIES TAKING PART IN BEAR GRYLLS' NEW NETFLIX SERIES, CELEBRITY BEAR HUNT, AND A...
Melania trump and ivanka trump slammed for ditching face masksBoth Melania Trump, 50, and Ivanka Trump, 38, are well known for their expensive wardrobes and for making headlines with...
Everything about the bottomnavigationbar in flutterThis blog post covers 5 advanced use cases of BottomNavigationBar which enhances the user experience. When there are mul...
Latests News
Multiscale and multidisciplinary analysis of aging processes in boneABSTRACT The world population is increasingly aging, deeply affecting our society by challenging our healthcare systems ...
World News | Latest International News | Global World News | World Breaking Headlines Today | Republic WorldNews / World News World News All Europe News Africa News Americas News Australia & New Zealand UK News China News Russia...
Page Not FoundPage Not Found Sorry, this page has not been found.Back to home...
Help older adults in your community with tax preparationEvery tax season, AARP Foundation Tax-Aide volunteers serve their communities by helping their neighbors complete and fi...
‘rt-pcr testing labs in kashmir overwhelmed’: div admin asks cmos to rationalize covid-19 samplingAmid the prevailing surge in COVID-19 cases, Divisional administration in Kashmir has asked the Chief Medical Officers i...