Treatment of diabetic mice with the sglt2 inhibitor ta-1887 antagonizes diabetic cachexia and decreases mortality
Treatment of diabetic mice with the sglt2 inhibitor ta-1887 antagonizes diabetic cachexia and decreases mortality"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A favorable effect of an inhibitor of the sodium–glucose cotransporter 2 (SGLT2i) on mortality of diabetic patients was recently reported, although mechanisms underlying that effect
remained unclear. Here, we examine SGLT2i effects on survival of diabetic mice and assess factors underlying these outcomes. To examine SGLT2i treatment effects in a model of severe
diabetes, we fed genetically diabetic _db/db_ mice a high-fat diet and then assessed outcomes including diabetic complications between SGLT2i TA-1887-treated and control mice. We also
compare effects of SGLT2i TA-1887 with those of lowering blood glucose levels via insulin treatment. Untreated _db/db_ mice showed remarkable weight loss, or cachexia, while TA-1887-treated
mice did not but rather continued to gain weight at later time points and decreased mortality. TA-1887 treatment prevented pancreatic beta cell death, enhanced preservation of beta cell mass
and endogenous insulin secretion, and increased insulin sensitivity. Moreover, TA-1887 treatment attenuated inflammation, oxidative stress, and cellular senescence, especially in visceral
white adipose tissue, and antagonized endothelial dysfunction. Insulin treatment of _db/db_ mice also prevented weight loss and antagonized inflammation and oxidative stress. However,
insulin treatment had less potent effects on survival and prevention of cellular senescence and endothelial dysfunction than did TA-1887 treatment. SGLT2i treatment prevents diabetic
cachexia and death by preserving function of beta cells and insulin target organs and attenuating complications. SGLT2i treatment may be a promising therapeutic strategy for type 2 diabetes
patients with morbid obesity and severe insulin resistance. SIMILAR CONTENT BEING VIEWED BY OTHERS IMEGLIMIN MITIGATES THE ACCUMULATION OF DYSFUNCTIONAL MITOCHONDRIA TO RESTORE INSULIN
SECRETION AND SUPPRESS APOPTOSIS OF PANCREATIC Β-CELLS FROM _DB/DB_ MICE Article Open access 14 March 2024 CARDIOPROTECTIVE EFFECTS OF SHORT-TERM EMPAGLIFLOZIN TREATMENT IN DB/DB MICE
Article Open access 12 November 2020 THADA INHIBITION IN MICE PROTECTS AGAINST TYPE 2 DIABETES MELLITUS BY IMPROVING PANCREATIC Β-CELL FUNCTION AND PRESERVING Β-CELL MASS Article Open access
23 February 2023 INTRODUCTION Type 2 diabetes incidence is increasing worldwide and is a primary cause of death. Along with hypertension and dyslipidemia, type 2 diabetes is an important
risk factor for cardiovascular disease and is accompanied by microvascular complications; thus prevention of macrovascular and microvascular complications is a critical issue in diabetes
treatment.1, 2 Large-scale studies have been carried out relevant to prevention of microvascular complications, but thus far, only a few trials of antidiabetic agents have demonstrated
improvement of cardiovascular events and decreased mortality.3,4,5,6 The UK Prospective Diabetes Study Group showed that metformin treatment of overweight patients decreased diabetes-related
mortality, while intensive blood-glucose control through antidiabetic agents, including insulin, did not significantly reduce cardiovascular events but tended to decrease myocardial
infarction, which included non-fatal and fatal myocardial infarction and sudden death.3, 4 However, in a 10-year post-interventional follow-up (UKPDS 80), post-trial risk reductions emerged
in the intensive therapy group for diabetes-related death, myocardial infarction and death from any cause, suggesting that improvements in controlling blood glucose levels are crucial to
manage cardiovascular events and decrease mortality. Relevant to this need, the EMPA-REG OUTCOME trial showed a significant effect of the sodium–glucose cotransporter 2 inhibitor (SGLT2i)
empagliflozin in antagonizing death from cardiovascular causes or death from any cause in patients with type 2 diabetes at high cardiovascular risk.7 In this trial, however, there were no
significant between-group differences in rates of myocardial infarction or stroke. Interpretations of this outcome vary, but some propose that factors other than those that decrease blood
glucose levels contribute to decreased mortality from cardiovascular or other causes.8,9,10 Here, to test effects of an SGLT2i in a severe diabetic mouse model, we employed genetically
diabetic _db/db_ mice fed a high-fat diet (HF). We compared treatment outcomes including diabetic complications between mice treated with the SGLT2i TA-1887 and untreated controls and also
assessed outcomes following insulin treatment. We confirm that SGLT2i treatment has beneficial effects in improving diabetic outcomes relative to insulin treatment and discuss mechanisms
potentially underlying these effects. RESULTS TA-1887 TREATMENT DECREASES MORTALITY IN SEVERELY DIABETIC MICE For analysis, we used TA-1887, an SGLT2i with selectivity for SGLT2 versus
SGLT1, similar to canagliflozin.11, 12 To determine treatment effects, we evaluated _db/db_ mice (also known as _Lepr_ −/− mice) fed a HF diet as a model of severe diabetes and treated them
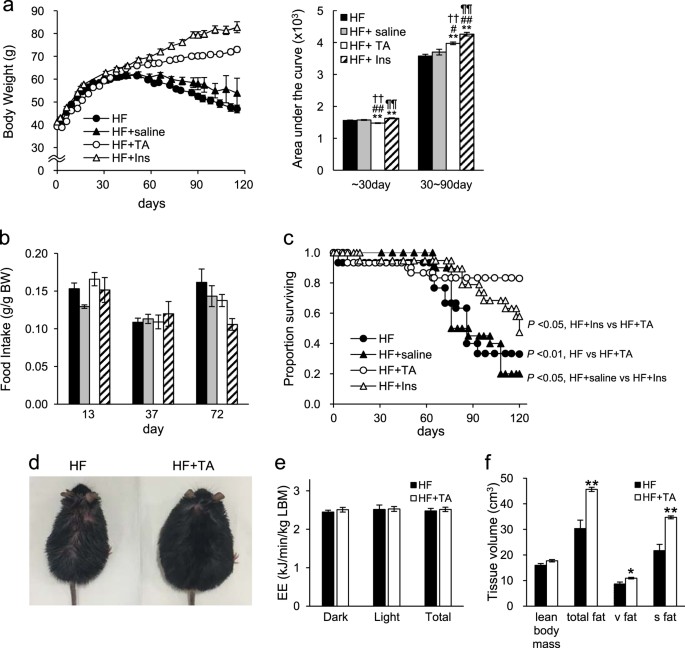
with or without TA-1887. As reported by others,13, 14 in 1st month body weight of TA-1887-treated mice decreased relative to that of untreated mice (Fig. 1a). However, after a month,
untreated mice showed first a slow increase in body weight and then a decline, whereas body weight of TA-1887-treated mice remained greater overall than that of untreated animals (Fig. 1a).
As a comparison, mice treated with insulin showed continued weight gain, an effect not seen in saline-injected controls (Fig. 1a). Although TA-1887 or insulin treatment increased body
weight, insulin-treated mice showed enhanced weight gain relative to TA-1887 animals (Fig. 1a). We observed no difference in food intake between TA-1887 and insulin-treated groups (Fig. 1b),
suggesting that body weight differences between groups could be due to differences in lipid accumulation. _db/db_ mice fed a HF diet normally survive for only 3–5 months, while those fed
normal chow live approximately 10 months.15, 16 Either TA-1887 or insulin treatment significantly increased survival of _db/db_ mice fed a HF diet, although survival rates of TA-1887-treated
mice were significantly greater (Fig. 1c). Following the death of treated or untreated mice, we performed X-ray computed tomography (CT) scanning and necropsy (Supplementary Table 3). Our
investigation included evaluation of potential brain hemorrhage, cerebral infarction, vascular calcification, vascular obstruction, and myocardial infarction. We also searched for cancerous
masses in lung, liver, stomach, intestine, and kidney. We found none of these pathologies (data not shown) and were therefore unable to determine the cause of death of any of these mice.
Most untreated mice, however, lost more than 10% of body weight before death, an outcome rarely seen in TA-1887-treated or insulin-treated mice (Supplementary Table 3). These observations
suggest that untreated mice die from events associated with diabetic cachexia, a condition not suffered by treated mice. TA-1887 TREATMENT DOES NOT ALTER ENERGY EXPENDITURE IN _DB/DB_ MICE
FED A HF DIET By approximately day 80 of drug treatment, untreated mice showed thinning coat fur (Fig. 1d), while TA-1887-treated mice appeared healthy but severely obese. Given differences
in body weight between groups, we measured energy expenditure after 9 weeks of drug treatment in TA-1887-treated and untreated groups by indirect calorimetry and observed no differences
between groups (based on lean body mass) (Fig. 1e). To analyze potential changes in tissue composition accompanying weight changes, we performed CT scanning after 3 months of drug treatment.
Adipose tissue volume in TA-1887-treated mice significantly increased relative to untreated controls, particularly in subcutaneous adipose tissue, although lean body mass was comparable
between groups (Fig. 1f). TA-1887 ANTAGONIZES HYPERGLYCEMIA AND INCREASES ENDOGENOUS INSULIN SECRETION We next evaluated blood glucose levels of _db/db_ mice fed a HF diet. TA-1887 treatment
over a 60-day period markedly reduced blood glucose levels (Fig. 2a). Plasma insulin levels in untreated mice decreased over time but tended to increase in TA-1887-treated mice (Fig. 2b).
Immunohistochemical insulin staining in pancreatic tissue revealed an increased volume of pancreatic beta cells (Fig. 2c). Furthermore, real-time polymerase chain reaction (PCR) analysis of
genes in pancreas indicated increased levels of the insulin transcripts INS1 and INS2 in TA-1887-treated relative to untreated controls (Fig. 2d). Moreover, to assess pancreatic beta cell
death, which could be associated with changes in endogenous insulin secretion, we performed double immunostaining of insulin and the apoptosis marker cleaved (active) caspase 3 in pancreatic
tissues. Insulin staining increased but that of cleaved (active) caspase 3 decreased in pancreatic islets of TA-1887-treated relative to untreated mice, suggesting that blocking beta cell
death preserves beta cell mass (Fig. 2e). TA-1887 TREATMENT ENHANCES INSULIN SENSITIVITY Next we assessed insulin sensitivity using an intraperitoneal insulin tolerance test (IPITT).
TA-1887-treated _db/db_ mice fed a HF diet showed significantly lower blood glucose levels than did untreated controls, indicating improved insulin sensitivity (Fig. 2f). To identify target
organs underlying this effect, we evaluated gene expression by real-time PCR. Expression of glycolytic genes increased in epididymal white adipose tissue (eWAT), inguinal WAT (iWAT),
gastrocnemius muscle (MG) and soleus muscle (MS) following TA-1887 treatment relative to untreated controls (Fig. 2g). However, expression of glycolytic genes in liver or brown adipose
tissue (BAT) was comparable between groups (Fig. 2g and Supplementary Fig. 1a). TA-1887 ATTENUATES SYSTEMIC AND TISSUE INFLAMMATION AND REDUCES LEVELS OF SENESCENCE MARKERS IN SEVERELY
DIABETIC MICE To indentify factors underlying improved insulin sensitivity and glucose utilization, we evaluated levels of transcripts encoding inflammatory mediators in eWAT, iWAT, MG and
MS from _db/db_ mice treated and untreated with TA-1887.17, 18 Expression of several inflammatory mediators such as IL-6, IL-1b, MCP1, CD68 and mmp12 decreased, particularly in eWAT and
iWAT, in TA-1887-treated mice relative to untreated controls, and some reduction in inflammatory markers was seen in MG and MS (Fig. 3a). Levels of inflammatory transcripts were partially
reduced in liver (but not in BAT) (Supplementary Fig. 1b). In terms of systemic inflammation, TA-1887 treatment reduced plasma IL-6 levels significantly relative to untreated controls (Fig.
3b), suggesting that levels of inflammatory mediators decrease throughout the body. We also performed immunostaining for Mac-3, a surface glycoprotein that serves as a macrophage marker, to
assess macrophage infiltration of eWAT and iWAT. Mice treated with TA-1887 showed decreased Mac-3 staining in both eWAT and iWAT, suggesting that reduced tissue inflammation contributes to
reduced plasma IL-6 levels (Supplementary Fig. 2a). Cellular senescence is associated with inflammation and marked by expression of genes such as p21 and p16Ink4a, effectors of cellular
aging.19, 20 Thus, we assessed p21 and p16Ink4a expression in eWAT, iWAT, MG and MS in treated and untreated mice. TA-1887 treatment remarkably decreased levels of p16INK4a transcripts in
eWAT, where expression of multiple inflammatory mediators was most markedly decreased, although p16INK4a expression was comparable in treated versus untreated animals in other tissues, and
p21 expression was similar in all tissues analyzed (Fig. 3c and Supplementary Fig. 1c). Moreover, accumulation of senescent cells in eWAT, iWAT, and MG was confirmed by assessing
senescence-associated beta-galactosidase activity using SPiDER beta-Gal staining.21 Beta-Gal staining decreased in eWAT from TA-1887-treated relative to untreated mice, but was equivalent in
iWAT and MG from treated and untreated mice (Fig. 3d). These findings suggest that TA-1887 antagonizes cellular senescence in specific cell types. TA-1887 ALLEVIATES OXIDATIVE STRESS IN
_DB/DB_ MICE FED A HF DIET Oxidative stress impacts insulin resistance and senescence.22, 23 Thus we asked whether TA-1887 treatment modulated oxidative stress, as marked by 8-OHdG
expression.24 TA-1887-treated _db/db_ mice fed a HF diet showed decreased levels of urinary 8-OHdG relative to untreated mice, suggestive of decreased systemic oxidative stress (Fig. 4a). We
then undertook immunostaining to detect 8-OHdG in eWAT, iWAT, and MG. TA-1887 treatment reduced 8-OHdG expression relative to untreated controls in all three tissues (Fig. 4b). Analysis of
those tissues plus MS also showed that transcripts encoding the antioxidative enzymes Mn-SOD and catalase increased in samples from TA-1887-treated relative to untreated mice (Fig. 4c).
Although we observed no difference in 8-OHdG immnostaining in BAT and liver from treated and untreated mice, catalase expression increased in liver of TA-1887-treated relative to untreated
mice (Supplementary Fig. 1d). Increased expression of antioxidative enzymes, particularly in eWAT and iWAT, suggests that these factors may mediate reduced oxidative stress seen in response
to TA-1887 treatment. TA-1887 IMPROVES ENDOTHELIAL FUNCTION IN _DB/DB_ MICE FED A HIGH FAT-DIET We next asked what effect TA-1887 treatment had on cardiovascular function of _db/db_ mice fed
a HF diet. To do so, we evaluated endothelium-dependent relaxation in response to acetylcholine25, 26 with or without drug. TA-1887-treated mice showed a slight relaxation response to
acetylcholine, while that response was absent in the aorta of untreated mice (Fig. 4d). To address underlying mechanisms, we assessed expression of mRNAs encoding senescence markers or
antioxidative enzymes in aorta tissue but observed no differences between groups (Fig. 4f, g). We then examined expression of genes encoding vascular inflammatory markers. Levels of
transcripts encoding intracellular adhesion molecule-1 (Icam-1) decreased in TA-1887-treated relative to untreated groups, while those of vascular cell adhesion molecule-1 (Vcam-1) were
comparable between groups (Fig. 4e). INSULIN TREATMENT INCREASES FAT VOLUME BUT PRESERVES PANCREATIC BETA CELL FUNCTION AND ENHANCES GLUCOSE UTILIZATION Given that insulin treatment of
_db/db_ mice fed a high fat-diet antagonizes diabetic cachexia and mortality (Fig. 1), we asked how insulin exerts this effect. CT scanning of insulin-treated mice revealed increased amounts
of subcutaneous fat relative to untreated _db/db_ mice fed a high fat-diet (Fig. 5a). Pancreatic tissue of insulin-treated mice also showed upregulated expression of INS1 and INS2 mRNAs
relative to controls (Fig. 5b). Moreover, immunohistochemistry confirmed that pancreatic beta cell volume increased in insulin-treated mice, indicative of preserved pancreatic beta cell
function (Fig. 5c). In addition, double immunostaining of insulin and active caspase 3 showed increased insulin staining but decreased staining of active caspase 3 in pancreatic islets of
insulin-treated relative to untreated control mice, indicating that insulin treatment prevents beta cell death (Fig. 5d). Mice treated with insulin showed markedly increased plasma insulin
levels and reduced blood glucose levels compared with untreated controls (Fig. 5e, f). Finally, some tissues (namely, eWAT, iWAT, MG and MS) of insulin-treated mice showed enhanced
expression of mRNAs encoding glycolytic enzymes, suggesting that glucose utilization is enhanced in these organs (Fig. 5g). INSULIN TREATMENT HAS DIVERSE EFFECTS ON TARGET ORGAN GENE
EXPRESSION AND PATHOLOGICAL STATE We next evaluated gene expression and related pathological changes in peripheral insulin-sensitive organs of mice in the presence or absence of insulin
treatment. Relevant to inflammatory markers, we observed that plasma IL-6 levels decreased in insulin-treated compared to untreated mice (Fig. 5h). In eWAT and iWAT, levels of mRNAs encoding
inflammatory mediators decreased in insulin-treated relative to control mice, although levels were comparable between groups in MG and MS (Fig. 5i). Moreover, immunostaining for Mac-3
revealed reduced macrophage infiltration into eWAT and iWAT of insulin-treated mice relative to untreated controls (Supplementary Fig. 2b). In terms of oxidative stress, insulin-treated mice
showed decreased urinary 8-OHdG levels relative to untreated mice and weaker immnohistochemical staining of 8-OHdG in eWAT, iWAT and MG relative to untreated controls (Fig. 6a). Moreover,
expression of transcripts encoding the antioxidative enzyme Mn-SOD increased in eWAT, iWAT, and MG tissues of insulin-treated mice (Fig. 6b). Finally, relevant to senescence markers, we
found that insulin-treated mice showed increased expression of p21 mRNA in eWAT, iWAT, MG (_p_ = 0.06) and MS, and of p16INK4a mRNA in iWAT and MG (_p_ = 0.07) relative to untreated mice
(Fig. 6c). Consistent with these results, senescence-associated beta-Gal activity increased in eWAT, iWAT and MG from insulin-treated compared with untreated mice (Fig. 6d). ENDOTHELIAL
DYSFUNCTION IS EXACERBATED BY INSULIN TREATMENT IN _DB/DB_ MICE FED A HF DIET Finally, to assess effects of insulin on cardiovascular outcomes, we evaluated endothelial function in
insulin-treated and control _db/db_ mice fed a HF diet. Aorta tissue of insulin-treated mice did not exhibit any relaxation response to acetylcholine but rather showed an increased
contractile response relative to the untreated group (Fig. 6e). Expression of mRNAs encoding p21 and p16 (Fig. 6g) and MnSOD and catalase (Fig. 6h) was comparable in aorta of insulin-treated
and untreated groups; however, levels of Vcam-1 transcripts increased in aorta of insulin-treated relative to untreated groups, whereas Icam-1 levels were equivalent in both groups (Fig.
6f). DISCUSSION Here we have conducted parallel investigations of TA-1887 and insulin on diabetic complications in _db/db_ mice fed a HF diet. We show overall that both TA-1887 and insulin
decrease inflammation and oxidative stress, and preserve function of pancreatic beta cells and insulin target organs in these mice. Moreover, we find that in some cases TA-1887 may have more
potent effects on endothelial function, cellular senescence and survival (see Supplementary Table 2 and Figs. 3–5). We observe that TA-1887 treatment of _db/db_ mice fed a HF diet enabled
mice to gain body weight over time, preventing a cachectic state brought on by severe diabetes and decreasing mortality relative to untreated controls. Nonetheless, TA-1887-treated mice were
obese and showed increased visceral and subcutaneous WAT. Exacerbation of obesity, particularly an increased visceral WAT, generally induces invasion of inflammatory cells, such as
macrophages, and initiates adipose tissue inflammation followed by insulin resistance and aggravation of hyperglycemia.27, 28 Expression of inflammatory markers and macrophage infiltration,
however, decreased in adipose tissue of TA-1887-treated mice compared with controls, and TA-1887-treated mice demonstrated increased insulin sensitivity and enhanced glucose utilization.
Visceral adipose tissue of TA-1887-treated mice also showed decreased expression of the senescence marker p16INK4a relative to untreated mice. TA-1887-treated mice also showed decreased
oxidative stress, which impacts insulin resistance and senescence, as indicated by marker analysis of urine and tissues, potentially due to increased expression of the antioxidative enzymes
MnSOD and catalase. Insulin secretion and enhanced insulin sensitivity is critical to avoid pathological weight loss and to store energy in adipose tissue.29 However, sustained hyperglycemia
that accompanies severe obesity exhausts pancreatic beta cells, inducing their apoptosis and decreasing insulin secretion.30, 31 While untreated _db/db_ mice fed a HF diet showed
significantly decreased endogenous insulin levels (Fig. 2b), plasma insulin levels in TA-1887-treated mice were relatively stable, as was beta cell mass, possibly due to loss of
glucotoxicity via increased urinary glucose excretion. Insulin-treated diabetic mice also showed preserved pancreatic beta cell function (Fig. 5b, c). However, it is noteworthy that plasma
insulin levels were 10-fold higher in insulin-relative to TA-1887-treated mice (compare values in Fig. 2b to those shown in Fig. 5f). Insulin signaling not only mediates glucose uptake and
serves as a growth signal but is also involved in aging.32, 33 Accordingly, insulin-treated mice are exposed to higher levels of insulin than are TA-1887-treated mice, potentially
accelerating cellular and tissue senescence. Hyperglycemia itself induces senescence through reactive oxygen species (ROS) production and advanced glycation end products.34,35,36
Hyperglycemia also induces macrophage infiltration in some organ tissues and escalates inflammatory conditions.37 Inflammatory mediators also induce senescence, and senescent cells produce
senescence-associated secretory phenotypes factors, which initiate and propagate similar phenotypes in other cells.38 Insulin-treated mice showed reduced blood glucose and attenuated
inflammation and oxidative stress but increased expression of senescence markers in all tissues analyzed, suggesting, that in this case, hyperinsulinemia (which was 10-fold higher than that
seen in the TA-1887-treated group) is primarily responsible for senescence. High insulin concentrations can also activate the insulin-like growth factor-1 (IGF-1) receptor,39 and
insulin/IGF-1 signaling induces ROS and promotes cellular senescence via the ROS-p53 pathway.40, 41 Insulin /IGF-1 signaling also reportedly promotes senescence phenotypes in the absence of
inflammation or oxidative stress via several mechanisms. Among these are the p53-p21 pathway via PI3K,42 increased p53 stabilization and activation through SIRT1 inhibition,43 and ERK
activation, which also upregulates p53 and promotes its stability and activity.44, 45 By contrast, TA-1887-treated mice did not show increased expression of senescence markers and in fact
exhibited decreased p16INK4a expression in visceral WAT. TA-1887 treatment also decreased blood glucose levels, inflammation and oxidative stress. We conclude that maintenance of appropriate
blood glucose and insulin levels may antagonize senescence. Previous reports suggest that adipose tissue is important in terms of survival.46,47,48 Decreased insulin/IGF-1 signaling in
adipose tissue extends lifespan in _Drosophila_ and mice,46, 47 and subsequent activation of the forkhead transcription factor (FOXO) may underlie longevity.46, 48 Interestingly, in eWAT and
iWAT of TA-1887-treated mice, we observed increased expression of forkhead targets,49 such as genes that encode the antioxidative enzymes MnSOD and catalase, and relief of oxidative stress
(Fig. 4a–c). Hyperglycemia reportedly promotes vascular inflammation and endothelial dysfunction and contributes to vascular disease.50 Although TA-1887 or insulin treatment ameliorated
hyperglycemia in diabetic mice, only TA-1887 attenuated endothelial dysfunction (Fig. 4d, 6e). Hyperinsulinemia-induced excess insulin activity caused by insulin administration promotes
vascular inflammation by producing proinflammatory cytokines in vascular smooth muscle cells.51 It is also noteworthy that TA-1887 treatment decreased levels of Icam-1, but not of Vcam-1,
while insulin treatment had the opposite effect, increasing Vcam-1 but not Icam-1 levels (Fig. 4e, 6f). Others have reported that high glucose stimulation upregulates Icam-1 but not Vcam-1
expression.52, 53 Furthermore, insulin stimulation reportedly promotes both Vcam-1 and Icam-1 expression in endothelial cells,54 supporting the idea that regulation of these factors differs.
Taken together, differential effects of TA-1887 and insulin treatment on endothelial function may be due in part to differences in vascular inflammation caused by hyperinsulinemia as blood
glucose levels improve, an event with consequences for mortality. There is some concern that SGLT2 inhibitors, which activate gluconeogenesis, may induce muscle atrophy.55, 56 Our CT scan
findings showed no reduction in lean body mass but rather the opposite tendency (Fig. 1f). Sano et al. reported that patients with type 2 diabetes treated with a SGLT2 inhibitor exhibit
increased grip strength, indicating that SGLT2i treatment does not necessarily promote muscle weakness, a typical symptom of sarcopenia, but rather strengthens it.57 Long-term use of SGLT2i
could rescue fat and glycogen synthesis and energy storage in skeletal muscle by improving insulin sensitivity and preserving endogenous insulin secretion, an effect that might antagonize
increased lipolysis or muscle catabolism. We also assessed vascular events related to type 2 diabetes and observed no sign of macrovascular events, such as brain hemorrhage, cerebral
infarction or necrotic changes in myocardium (data not shown). Relevant to microvascular events, we evaluated proteinuria, a major complication of diabetes. We observed reduced proteinuria
in both TA-1887-treated and insulin-treated versus untreated mice (Supplementary Fig. 6), supporting the idea that both TA-1887 and insulin treatments antagonize type 2 diabetes. Finally,
there are currently many treatment options for type 2 diabetes, and appropriate selection of therapy individualized to each patient is needed. To date, anti-diabetic agents with a
hypoglycemic effect potent enough to relieve glucotoxicity, improve insulin sensitivity, and preserve endogenous insulin secretion with minimum load on pancreatic beta cells do not exist.
SGLT2i, hence, could present an effective alternative treatment for type 2 diabetes, while potentially associated obesity could be prevented by appropriate dietary management. Further
studies are required to explore this possibility. MATERIALS AND METHODS MATERIALS TA-1887 (3-(4-cyclopropylbenzyl)-4-fluoroindole-_N_-glucoside) was supplied by Mitsubishi Tanabe Pharma
Corporation (Osaka, Japan). ANIMALS Six-week-old male _db/db_ mice were purchased from CLEA Japan Inc (Tokyo, Japan). Mice were maintained in a pathogen-free facility under controlled
environmental conditions and exposed to a 12:12 h light:dark cycle. After 2 weeks of acclimation, mice were fed HF diets (HFD-32; CLEA Japan Inc., Tokyo, Japan) with or without TA-1887
treatment (0.01% w/w in chow). To assess effects of chronic insulin treatment, animals attached to either insulin or normal saline pumps (Alzet, model 2002; DURECT, Cupertino, CA) were
similarly fed and received insulin (3 μg/g/day) or control saline, respectively. Blood glucose levels of insulin-treated mice were adjusted to ~200 mg/dl by additional administration of
long-acting insulin (Insulin Glargine, Sanofi, Gentilly, France). Animal experiments were approved by the institutional review board at Kumamoto University, and all animals received humane
care. INDIRECT CALORIMETRY Energy expenditure was measured using an indirect calorimetry system (MK-5000RQ, Muromachi Kikai Co., Ltd., Tokyo, Japan), as previously reported.58 COMPUTED
TOMOGRAPHY (CT) Mice were anesthetized by intraperitoneal injection of pentobarbital, and adiposity was assessed using an X-ray CT system (La Theta; Aloka Ltd., Tokyo, Japan). SURVIVAL
ANALYSIS Eight-week-old male _db/db_ mice fed a HF diet were assigned to four groups: high-fat (_n_ = 30), high-fat with TA-1887 (_n_ = 30), saline pump (_n_ = 20), and insulin pump (_n_ =
20) groups for survival analysis. Survival was monitored several times a week. Survival curves were plotted using the Kaplan Meier method. INTRAPERITONEAL INSULIN TOLERANCE TEST (IPITT)
After 10 weeks on each diet, mice fasted overnight (14 h) underwent IPITT with a 0.75 U/kg body weight insulin solution. Tail vein blood glucose levels were determined using a STAT STRIP
Xpress 900 monitor (Nova Biomedical Corporation, Waltham, MA). ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) Plasma insulin was assessed using the Morinaga Ultra-Sensitive Mouse/Rat Insulin
ELISA Kit according to the manufacturer’s recommendations (Morinaga Institute of Biological Science, Inc., Yokohama, Japan). Plasma IL-6 concentrations were determined using Mouse IL-6 ELISA
MAX Deluxe Sets (Biolegend, San Diego, CA). 8-hydroxy-2’-deoxyguanosine (8-OHdG) concentrations in urine were measured by ELISA (Nikken Seil, Shizuoka, Japan). QUANTITATIVE REAL-TIME PCR
Total RNA was extracted using TRIzol reagent according to the manufacturer’s protocol. DNase-treated RNA was reverse transcribed using a PrimeScript RT reagent Kit (Takara Bio Inc., Shiga,
Japan). Quantitative real-time PCR was performed using SYBER Premix Ex Taq II (Takara Bio Inc.). Relative transcript abundance was normalized to that of 18 S rRNA levels. Primer sequences
are shown in Supplementary Table 1. IMMUNOSTAINING For all procedures, samples were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin blocks, which were cut into 4-μm sections,
air-dried and then deparaffinized. For immunohistochemistry, after antigen retrieval endogenous peroxidase activity was blocked by treating sections with either 3% H2O2 in Tris-buffered
saline for 10 min, or, in the case of 8-OHdG detection, 0.5% H2O2 in methanol for 30 min. Sections were then blocked with 5% goat serum for 20 min at room temperature (RT) and incubated with
primary antibodies overnight at 4 °C. After PBS washing, sections were treated with secondary antibodies using Histofine Simple Stain MAX-PO (Nichirei Biosciences Inc., Tokyo, Japan) or an
EnVision System-HRP kit (Dako, Carpinteria, CA), according to the manufacturers’ instructions. To detect 8-OHdG, blocking and secondary antibody reactions were carried out using a Histofine
mouse staining kit (Nichirei Biosciences Inc., Tokyo, Japan). Peroxidase activity was visualized by incubation with a 3,3-diaminobenzidine solution. Slides were counterstained with
hematoxylin and mounted. Antibodies used were: anti-insulin (1:100, sc-9168, Santa Cruz Bio, Dallas, TX), anti-8-OHdG (1:20, Nikken Seil, Shizuoka, Japan) and anti-Mac-3 (1:100, BD
Biosciences, Franklin Lakes, NJ). For double immunofluorescence of pancreatic tissue, endogenous biotin and peroxidase activity was blocked using a Biotin Blocking System (Dako, Carpinteria,
CA) and 3% H2O2, respectively. Sections were then incubated overnight with anti-active caspase 3 antibody (1:250, Promega Corp., Madison, WI), and staining performed using a Tyramide Signal
Amplification kit (PerkinElmer, Boston, MA). After PBS washing, specimens were incubated with anti-insulin antibody (1:100, sc-9168, Santa Cruz Bio, Dallas, TX) overnight at 4 °C. After PBS
washing, sections were incubated with Alexa Fluor 594-labeled anti-rabbit IgG (1:500, Invitrogen Corp., Carlsbad, CA) and Streptavidin-Fluorescein (1:500, PerkinElmer, Boston, MA) as second
antibodies. Fluorescent imaging was performed after PBS washing. SPIDER BETA-GAL STAINING Tissues (eWAT, iWAT and MG) were placed in O.C.T. Compound (Sakura Finetek USA Inc., Torrance, CA)
in Tissue-Tek Cryomolds (Sakura Finetek USA Inc., Torrance, CA) and flash-frozen in hexane cooled with solid carbon dioxide. Sections (WAT: 15 μm, MG: 6 μm) were cut using a cryostat, and
mounted onto glass slides. They were then fixed in 4% paraformaldehyde for 20 min at RT, washed in PBS, and immersed in 20-μM SPiDER beta-Gal staining solution21 (Dojindo Molecular
Technologies, Inc., Rockville, MD) for 1 h at 37 °C. Imaging was performed after washing with PBS. VASCULAR ENDOTHELIAL FUNCTION After mice were killed, the aorta was removed and measured
for vascular endothelial function. Pressurized aortas were kept in a chamber of warmed (37 °C) and oxygenated (95% air-5% CO2) Krebs solution. Endothelium-dependent relaxation was assessed
by measuring dilatory responses to increasing acetylcholine concentration (10−7–10−4 mol/L) in vessels pre-treated with phenylephrine at 5 × 10−5 mol/L. STATISTICAL ANALYSES Results are
reported as means ± standard error (SEM). Statistical differences were determined using the unpaired two-tailed Student’s _t_-test or Kruskal–Wallis tests with Bonferroni correction for
multiple comparisons. Kaplan–Meier analysis was performed by the log-rank statistic with the Holm’s method to test for significant differences in survival. Statistical significance is
reported as a _P_ value < 0.05 or <0.01. DATA AVAILABILITY All data that support the findings of this study are in this published article and its Supplementary information, or are
available from the corresponding author on reasonable request. REFERENCES * Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2
diabetes (UKPDS 35): prospective observational study. _BMJ_ 321, 405–412 (2000). Article PubMed PubMed Central CAS Google Scholar * Emerging Risk Factors Collaboration. et al. Diabetes
mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. _Lancet_ 375, 2215–2222 (2010). Article CAS Google
Scholar * UK Prospective Diabetes Study (UKPDS). Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in
patients with type 2 diabetes (UKPDS 33). _Lancet_ 352, 837–853 (1998a). Article Google Scholar * UK Prospective Diabetes Study (UKPDS). Group Effect of intensive blood-glucose control
with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). _Lancet_ 352, 854–865 (1998b). Article Google Scholar * Holman, R. R. et al. 10-year follow-up of
intensive glucose control in type 2 diabetes. _N. Engl. J. Med._ 359, 1577–1589 (2008). Article PubMed CAS Google Scholar * Group, A. C. et al. Intensive blood glucose control and
vascular outcomes in patients with type 2 diabetes. _N. Engl. J. Med._ 358, 2560–2572 (2008). Article Google Scholar * Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and
mortality in type 2 diabetes. _N. Engl. J. Med._ 373, 2117–2128 (2015). Article PubMed CAS Google Scholar * Cardoso, C. R., Ferreira, M. T., Leite, N. C. & Salles, G. F. Prognostic
impact of aortic stiffness in high-risk type 2 diabetic patients: the Rio deJaneiro type 2 diabetes cohort study. _Diabetes Care_ 36, 3772–3778 (2013). Article PubMed PubMed Central CAS
Google Scholar * Bakris, G. L. & Molitch, M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. _Diabetes Care_ 37, 867–875 (2014). Article PubMed Google Scholar
* Tikkanen, I. et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. _Diabetes Care_ 38, 420–428 (2015). Article PubMed CAS Google Scholar *
Nomura, S. et al. Novel Indole-N-glucoside, TA-1887 as a sodium glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. _ACS Med Chem. Lett._ 5, 51–55 (2014). Article PubMed
CAS Google Scholar * Kuriyama, C. et al. Analysis of the effect of canagliflozin on renal glucose reabsorption and progression of hyperglycemia in zucker diabetic Fatty rats. _J.
Pharmacol. Exp. Ther._ 351, 423–431 (2014). Article PubMed CAS Google Scholar * Bolinder, J. et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue
distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. _J. Clin. Endocrinol. Metab._ 97, 1020–1031 (2012). Article PubMed CAS Google Scholar
* Suzuki, M. et al. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. _Nutr. Diabetes_ 4,
e125 (2014). Article PubMed PubMed Central CAS Google Scholar * Zhang, H. M. et al. Geldanamycin derivative ameliorates high fat diet-induced renal failure in diabetes. _PLoS One_ 7,
e32746 (2012). Article PubMed PubMed Central CAS Google Scholar * Okada-Iwabu, M. et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. _Nature_ 503,
493–499 (2013). Article PubMed CAS Google Scholar * Hotamisligil, G. S. Inflammation and metabolic disorders. _Nature_ 444, 860–867 (2006). Article PubMed CAS Google Scholar *
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. _J. Clin. Invest._ 116, 1793–1801 (2006). Article PubMed PubMed Central CAS Google Scholar *
Tominaga, K. The emerging role of senescent cells in tissue homeostasis and pathophysiology. _Pathobiol. Aging Age Relat. Dis._ 5, 27743 (2015). Article PubMed CAS Google Scholar *
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. _Nat. Med._ 21, 1424–1435 (2015). Article
PubMed PubMed Central CAS Google Scholar * Doura, T. et al. Detection of LacZ-positive cells in living tissue with single-cell resolution. _Angew. Chem. Int. Ed. Engl._ 55, 9620–9624
(2016). Article PubMed CAS Google Scholar * Ceriello, A. & Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease?
The common soil hypothesis revisited. _Arterioscler. Thromb. Vasc. Biol._ 24, 816–823 (2004). Article PubMed CAS Google Scholar * Salmon, A. B. Beyond diabetes: does obesity-induced
oxidative stress drive the aging process? _Antioxidants (Basel_) 5, 24 (2016). * Valavanidis, A., Vlachogianni, T. & Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical
biomarker of oxidative stress and carcinogenesis. _J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev._ 27, 120–139 (2009). Article PubMed CAS Google Scholar * Hodgson, J. M.
& Marshall, J. J. Direct vasoconstriction and endothelium-dependent vasodilation. Mechanisms of acetylcholine effects on coronary flow and arterial diameter in patients with nonstenotic
coronary arteries. _Circulation_ 79, 1043–1051 (1989). Article PubMed CAS Google Scholar * Matsubara, J. et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves
endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. _J. Am. Coll. Cardiol._ 59, 265–276 (2012). Article PubMed CAS Google Scholar *
Stienstra, R. et al. Inflammasome is a central player in the induction of obesity and insulin resistance. _Proc. Natl. Acad. Sci. USA_ 108, 15324–15329 (2011). Article PubMed PubMed
Central Google Scholar * Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. _J. Clin. Invest._ 116,
1494–1505 (2006). Article PubMed PubMed Central CAS Google Scholar * Raz, I., Eldor, R., Cernea, S. & Shafrir, E. Diabetes: insulin resistance and derangements in lipid metabolism.
Cure through intervention in fat transport and storage. _Diabetes Metab. Res. Rev._ 21, 3–14 (2005). Article PubMed CAS Google Scholar * Wajchenberg, B. L. beta-cell failure in diabetes
and preservation by clinical treatment. _Endocr. Rev._ 28, 187–218 (2007). Article PubMed CAS Google Scholar * Guillausseau, P. J. et al. Abnormalities in insulin secretion in type 2
diabetes mellitus. _Diabetes Metab._ 34, S43–48 (2008). Article PubMed CAS Google Scholar * Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like
gene that regulates longevity and diapause in Caenorhabditis elegans. _Science_ 277, 942–946 (1997). Article PubMed CAS Google Scholar * Clancy, D. J. et al. Extension of life-span by
loss of CHICO, a Drosophila insulin receptor substrate protein. _Science_ 292, 104–106 (2001). Article PubMed CAS Google Scholar * Blazer, S. et al. High glucose-induced replicative
senescence: point of no return and effect of telomerase. _Biochem. Biophys. Res. Commun._ 296, 93–101 (2002). Article PubMed CAS Google Scholar * Ksiazek, K., Passos, J. F., Olijslagers,
S. & von Zglinicki, T. Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. _Biochem. Biophys. Res. Commun._ 366,
793–799 (2008). Article PubMed CAS Google Scholar * Liu, J. et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via
activation of endoplasmic reticulum stress-dependent p21 signaling. _Cell. Signal._ 26, 110–121 (2014). Article PubMed CAS Google Scholar * Niu, S. et al. Broad infiltration of
macrophages leads to a proinflammatory state in streptozotocin-induced hyperglycemic mice. _J. Immunol._ 197, 3293–3301 (2016). Article PubMed CAS Google Scholar * Nelson, G. et al. A
senescent cell bystander effect: senescence-induced senescence. _Aging Cell._ 11, 345–349 (2012). Article PubMed CAS Google Scholar * Boucher, J., Tseng, Y. H. & Kahn, C. R. Insulin
and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. _J. Biol. Chem._ 285, 17235–17245 (2010). Article
PubMed PubMed Central CAS Google Scholar * Katic, M. & Kahn, C. R. The role of insulin and IGF-1 signaling in longevity. _Cell. Mol. Life Sci._ 62, 320–343 (2005). Article PubMed
CAS Google Scholar * Handayaningsih, A. E. et al. IGF-I enhances cellular senescence via the reactive oxygen species-p53 pathway. _Biochem. Biophys. Res. Commun._ 425, 478–484 (2012).
Article PubMed CAS Google Scholar * Clark, M. A., Perks, C. M., Winters, Z. E. & Holly, J. M. DNA damage uncouples the mitogenic response to IGF-I in MCF-7 malignant breast cancer
cells by switching the roles of PI3 kinase and p21WAF1/Cip1. _Int. J. Cancer_ 116, 506–513 (2005). Article PubMed CAS Google Scholar * Tran, D. et al. Insulin-like growth factor-1
regulates the SIRT1-p53 pathway in cellular senescence. _Aging Cell_ 13, 669–678 (2014). Article PubMed PubMed Central CAS Google Scholar * Nguyen, T. T., Sheppard, A. M., Kaye, P. L.
& Noakes, P. G. IGF-I and insulin activate mitogen-activated protein kinase via the type 1 IGF receptor in mouse embryonic stem cells. _Reproduction_ 134, 41–49 (2007). Article PubMed
CAS Google Scholar * Cagnol, S. & Chambard, J. C. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. _FEBS J._ 277, 2–21 (2010). Article
PubMed CAS Google Scholar * Hwangbo, D. S. et al. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. _Nature_ 429, 562–566 (2004). Article PubMed
CAS Google Scholar * Bluher, M., Kahn, B. B. & Kahn, C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. _Science_ 299, 572–574 (2003). Article PubMed
CAS Google Scholar * Giannakou, M. E. et al. Long-lived Drosophila with overexpressed dFOXO in adult fat body. _Science_ 305, 361 (2004). Article PubMed CAS Google Scholar * Ponugoti,
B., Dong, G. & Graves, D. T. Role of forkhead transcription factors in diabetes-induced oxidative stress. _Exp. Diabetes Res._ 2012, 939751 (2012). Article PubMed PubMed Central CAS
Google Scholar * Bakker, W., Eringa, E. C., Sipkema, P. & van Hinsbergh, V. W. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity.
_Cell. Tissue Res._ 335, 165–189 (2009). Article PubMed CAS Google Scholar * Sato, Y. et al. Increased expression of CCAAT/enhancer binding protein-beta and -delta and monocyte
chemoattractant protein-1 genes in aortas from hyperinsulinaemic rats. _Diabetologia_ 50, 481–489 (2007). Article PubMed CAS Google Scholar * Park, C. W. et al. High glucose-induced
intercellular adhesion molecule-1 (ICAM-1) expression through an osmotic effect in rat mesangial cells is PKC-NF-kappa B-dependent. _Diabetologia_ 43, 1544–1553 (2000). Article PubMed CAS
Google Scholar * Baumgartner-Parzer, S. M. et al. Modulation by high glucose of adhesion molecule expression in cultured endothelial cells. _Diabetologia_ 38, 1367–1370 (1995). Article
PubMed CAS Google Scholar * Li, G. et al. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells.
_Endocrinology_ 150, 3475–3482 (2009). Article PubMed PubMed Central CAS Google Scholar * Kuo, T., Harris, C. A. & Wang, J. C. Metabolic functions of glucocorticoid receptor in
skeletal muscle. _Mol. Cell. Endocrinol._ 380, 79–88 (2013). Article PubMed PubMed Central CAS Google Scholar * Sandri, M. Autophagy in skeletal muscle. _FEBS Lett._ 584, 1411–1416
(2010). Article PubMed CAS Google Scholar * Sano, M., Meguro S., Kawai T., Suzuki Y. Increased grip strength with sodium-glucose cotransporter 2 inhibitors. _J. Diabetes_ 8, 736–737
(2016). * Kouyama, R. et al. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. _Endocrinology_ 146,
3481–3489 (2005). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Ms. K.Tabu, S. Iwaki, K. Kamada, N. Shirai and M.Nakata (all of the Department of
Molecular Genetics, Kumamoto University) for technical assistance. This work was financially supported in part by Mitsubishi Tanabe Pharma Corporation. TA-1887 was provided by Mitsubishi
Tanabe Pharma Corporation. AUTHOR INFORMATION Author notes * Taichi Sugizaki and Shunshun Zhu authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Molecular
Genetics, Graduate School of Medical Sciences, Institute of Resource Development and Analysis, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto, 860-8556, Japan Taichi Sugizaki, Shunshun
Zhu, Ge Guo, Akiko Matsumoto, Jiabin Zhao, Motoyoshi Endo, Haruki Horiguchi, Jun Morinaga, Zhe Tian, Tsuyoshi Kadomatsu, Keishi Miyata & Yuichi Oike * Department of Immunology, Allergy
and Vascular Medicine, Graduate School of Medical Sciences, Institute of Resource Development and Analysis, Kumamoto University, 1-1-1 Honjo,Chuo-ku, Kumamoto, 860-8556, Japan Taichi
Sugizaki & Keishi Miyata * Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku,
Tokyo, 160-8582, Japan Taichi Sugizaki & Hiroshi Itoh Authors * Taichi Sugizaki View author publications You can also search for this author inPubMed Google Scholar * Shunshun Zhu View
author publications You can also search for this author inPubMed Google Scholar * Ge Guo View author publications You can also search for this author inPubMed Google Scholar * Akiko
Matsumoto View author publications You can also search for this author inPubMed Google Scholar * Jiabin Zhao View author publications You can also search for this author inPubMed Google
Scholar * Motoyoshi Endo View author publications You can also search for this author inPubMed Google Scholar * Haruki Horiguchi View author publications You can also search for this author
inPubMed Google Scholar * Jun Morinaga View author publications You can also search for this author inPubMed Google Scholar * Zhe Tian View author publications You can also search for this
author inPubMed Google Scholar * Tsuyoshi Kadomatsu View author publications You can also search for this author inPubMed Google Scholar * Keishi Miyata View author publications You can also
search for this author inPubMed Google Scholar * Hiroshi Itoh View author publications You can also search for this author inPubMed Google Scholar * Yuichi Oike View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.S. and S.Z. carried out experiments, analyzed data, discussed data, and wrote the manuscript. G.G. and A.M. conducted
experiments, analyzed data and discussed the data. J.Z., M.E., H.H., Z.T., J.M., T.K., K.M. conducted experiments and discussed the data. H.I. analyzed and discussed data and reviewed and
edited the manuscript. Y.O. planned and supervised the study, analyzed data and wrote the manuscript. All authors contributed to revising the manuscript and approved the final version.
CORRESPONDING AUTHORS Correspondence to Taichi Sugizaki or Yuichi Oike. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing financial interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Sugizaki, T., Zhu, S., Guo, G. _et al._ Treatment of diabetic mice with the SGLT2 inhibitor TA-1887 antagonizes diabetic cachexia and decreases mortality. _npj
Aging Mech Dis_ 3, 12 (2017). https://doi.org/10.1038/s41514-017-0012-0 Download citation * Received: 13 January 2017 * Revised: 13 August 2017 * Accepted: 16 August 2017 * Published: 08
September 2017 * DOI: https://doi.org/10.1038/s41514-017-0012-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Dwp change for people on benefits born within three-year period rolled outThe Department for Work and Pensions is launching a new initiative to support benefits claimants born in specific years....
Ryanair passengers praise 'perfect' cabin bag with 'lots of pockets' - YorkshireLiveWhat's OnRyanair passengers praise 'perfect' cabin bag with 'lots of pockets'The VANKEV Backpack is the ideal travel com...
Moyes in tribute to victims and emergency services after liverpool parade crashTHE EVERTON BOSS BECAME THE LATEST FIGUREHEAD TO EXPRESS SOLIDARITY WITH THOSE AFFECTED BY THE CITY CENTRE INCIDENT 11:3...
Foreign office issues fresh warnings to brits heading to turkeyThe UK Foreign Office has today issued a fresh caution to Brits planning to visit Turkey, advising extra care when using...
Karine jean-pierre roasted over ‘orwellian’ tweet touting ‘0% inflation’EXPLORE MORE President Biden’s truth-averse top spokesperson was ridiculed after she touted “0% inflation in July” follo...
Latests News
Treatment of diabetic mice with the sglt2 inhibitor ta-1887 antagonizes diabetic cachexia and decreases mortalityABSTRACT A favorable effect of an inhibitor of the sodium–glucose cotransporter 2 (SGLT2i) on mortality of diabetic pati...
Teacher to carry olympic torch : compton educator, nominated by one of her eighth-grade students, is first in southland to be chosen for the honor.Runners from Los Angeles will soon get the Olympic flame started on its journey to the Summer Games in Atlanta. And in t...
Long Beach Press-Telegram: Local News, Sports, Things to DoHow Trump’s trade war is already effecting California’s portsChinese goods account for 40% of the imports at the Port of...
Lucifer season 5: what is tom ellis's favourite episode of lucifer?“We were doing all of these big dance numbers and it was fantastic. Everyone was really buzzing, when we were doing that...
Epic california snowpack is now the deepest it's been in decadesDrought-weary California is entering February with deeper snowpack than it has seen in four decades, reflecting a health...