Collective buoyancy-driven dynamics in swarming enzymatic nanomotors
Collective buoyancy-driven dynamics in swarming enzymatic nanomotors"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Enzymatic nanomotors harvest kinetic energy through the catalysis of chemical fuels. When a drop containing nanomotors is placed in a fuel-rich environment, they assemble into
ordered groups and exhibit intriguing collective behaviour akin to the bioconvection of aerobic microorganismal suspensions. This collective behaviour presents numerous advantages compared
to individual nanomotors, including expanded coverage and prolonged propulsion duration. However, the physical mechanisms underlying the collective motion have yet to be fully elucidated.
Our study investigates the formation of enzymatic swarms using experimental analysis and computational modelling. We show that the directional movement of enzymatic nanomotor swarms is due
to their solutal buoyancy. We investigate various factors that impact the movement of nanomotor swarms, such as particle concentration, fuel concentration, fuel viscosity, and vertical
confinement. We examine the effects of these factors on swarm self-organization to gain a deeper understanding. In addition, the urease catalysis reaction produces ammonia and carbon
dioxide, accelerating the directional movement of active swarms in urea compared with passive ones in the same conditions. The numerical analysis agrees with the experimental findings. Our
findings are crucial for the potential biomedical applications of enzymatic nanomotor swarms, ranging from enhanced diffusion in bio-fluids and targeted delivery to cancer therapy. SIMILAR
CONTENT BEING VIEWED BY OTHERS LOCOMOTION AND DISAGGREGATION CONTROL OF PARAMAGNETIC NANOCLUSTERS USING WIRELESS ELECTROMAGNETIC FIELDS FOR ENHANCED TARGETED DRUG DELIVERY Article Open
access 23 July 2021 CHEMICALLY-POWERED SWIMMING AND DIFFUSION IN THE MICROSCOPIC WORLD Article 27 May 2021 ION-EXCHANGE ENABLED SYNTHETIC SWARM Article 11 January 2021 INTRODUCTION
Collective behaviour is widespread in nature. While individual units of a group obey simple rules, they present complex and intriguing collective behaviour when assembling into highly
ordered structures1. Living organisms use distributed or swarm intelligence to accomplish sophisticated tasks to survive. Examples range from collective cell migration2, honeybees adapting
to repeated shaking to maintain mechanical stability of the swarm3, to emperor penguins packing in a huddle in a highly coordinated manner to survive cold winter4. Multiple synthetic
swarming systems have been developed with inspiration from nature including: (1) applying one or multiple external forces, such as magnetic fields5,6,7,8, light9,10, ultrasound11,12,
electric fields13,14, (2) utilizing chemicals as signals15,16,17, (3) combining biological microswimmers, such as sperm cells and algae, into artificial moieties as a hybrid
integration18,19,20, (4) exploiting DNA base-pair interactions21,22. These well-designed swarms show many advantages compared to single-unit functionalities, like enhanced coverage and fluid
mixing, intelligent multitasking, collective chemotaxis and perception, and environmental adaptation. Micro/nanomotors (MNMs) are synthetic active devices achieving self-propulsion through
converting various types of energy into mechanical motion23,24. Earlier works on enzyme-powered MNMs have demonstrated the motion of single particles25,26,27 and small clusters28, as well as
proof-of-concept studies in drug delivery29,30,31,32,33 and sensing34,35. Nonetheless, recent reports have shifted focus to the collective motion of these particles. Recently, Hortelao et
al.36 reported the emergent swarming behaviour of enzymatic nanomotors. The urease-powered nanomotors show collective migration in urea, demonstrating the ability to swim across complex
paths compared to the inactive nanomotors. Furthermore, the active collective dynamics, combined with advanced imaging technologies, position them as promising tools in the field of
biomedicine. For example, swarms of radio-labelled nanobots have shown an eightfold increase in tumour penetration and approximately a 90% reduction in tumour size during radionuclide
therapy37. Swarms of catalase-powered nanobots overcome and disrupt mucus layer, resulting in a 60-fold increase in mucus barrier penetration, through in vitro and ex vivo validation38.
Hyaluronidase and urease nanomotor swarms work synergistically for enhanced diffusion in viscous media, such as synovial fluid, paving the way for treating joint injuries39. Similarly,
collagenase-powered MNMs40,41 and urease-powered iron oxide nanomotor swarms42 were exploited to disrupt collagen fibres, serving as a model of the extracellular medium. This disruption
facilitates cell spheroids penetration and enhances the delivery efficiency of a second swarm of nanomotors by 10-fold. Although enzyme-powered MNMs have primarily demonstrated their
potential in biomedical applications, the mechanisms underlying the emergent collective behaviour remain to be clarified. Inspired by nature, the intriguing collective phenomenon bears a
resemblance to bioconvection. Bioconvection is a self-organized and self-sustained vortex motion that arises naturally in suspensions of microorganisms43. It visually resembles the
Rayleigh–Bénard convection in fluid heated from below44. The bioconvection emerges due to the unstable density gradients resulting from the accumulation of buoyant microorganisms45. Each
microorganism plays a pivotal role in driving accumulation and fluid flow. Certain gravitactic algae or aerotactic bacteria exhibit upward swimming. In the presence of an upper surface, they
form a thin boundary layer of microorganism-rich heavier fluid, which becomes unstable, leading to the formation of falling plumes46. In synthetic MNMs, buoyancy-driven convection has been
employed for directional motility and cargo delivery. One approach utilizes incident light to generate convective flow through the photothermal effect. This convective flow can drive TiO2
micromotors to aggregate and form clusters47,48, or enable magnetic colloidal collectives to drift using fluidic currents49. Another approach involves enzymes fixed on a surface, which
catalyze fuels, inducing density variations between the reactants and products of chemical reactions. For instance, urease-attached macroscale sheets exhibit clockwise or counterclockwise
rotation via a solutal buoyancy mechanism arising from enzyme pumps50. Additionally, urease attached to a lipid membrane induces fluid flow and the directional transport of tracer
particles51. These studies underscore the significant implications of convective flow on the collective movement of micro/nanoparticles. Here, the buoyancy-driven convective dynamics of
collective enzymatic nanomotors arises from the density difference between the product-rich particulate and the denser environment. We attach enzymes onto the surface of nanoparticles, which
move dynamically with the fluid flow. We describe the emergent collective behaviour of enzymatic nanomotor in three dimensions (3D) to unveil the underlying mechanisms. We model a swarm as
light particulates immersed in a denser fluid environment. The particulate swarm moves upward, creating a convective flow in a closed fuel-filled space. Control factors for directional and
collective mobility of enzymatic nanomotors, such as particle and fuel concentration and media viscosity, are investigated. In 3D space, convective flows develop complex patterns such as
vortices. To further validate our findings, we vertically confine the system to two dimensions, where convective flow is constrained to a plane, inhibiting the possible flow configurations.
The convective dynamics resemble bioconvection and provide insights into the mechanisms of collective behaviour observed in enzymatic nanomotors. RESULTS AND DISCUSSION In our study, we view
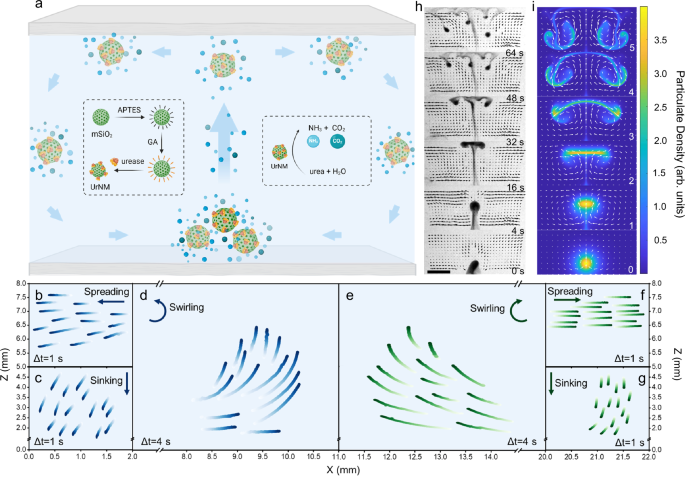
the collective behaviour of enzymatic nanomotors from the side or from the top. The enzymatic nanomotors are based on mesoporous silica nanoparticles with urease attached (UrNMs). Detailed
characterization can be found in the Supplementary Materials (Fig. S1) and the Methods section. The molecularly unbalanced distribution of enzymes generates net motion for single
nanomotors52,53 in urea, Fig. S2. From the side, upon introducing a drop of particulate in a fuel-filled chamber, the drop shows upward motion against gravity, generating a convective flow
within the closed space, Fig. 1a. During an ascending stage, there are two counter-rotating vortices within a droplet. A characteristic hydrodynamic flow pattern is displayed in Fig. S3. As
the particulate reaches the upper boundary, it spreads to balance the mean upward force, forming a layer of unstable particle-rich fluid. The layer then sinks in the form of falling plumes.
Trajectory tracking of UrNMs in a 22 × 8 × 1.6 mm (length × height × width) chamber shows the spreading, sinking, and swirling stages of the convective dynamics, Fig. 1b–g. These
trajectories on the left and right are not perfectly symmetric due to experimental limitations. The upward movement of a nanomotor swarm is due to buoyancy arising from the density
difference between the reaction product-rich particulate and the media with fuel. We state that individual nanomotors perform urease catalysis reaction and generate ammonia and carbon
dioxide, making the particulate lighter. Since the temperature change during chemical reactions is not obvious (Fig. S4), we rule out heat effect on the upward movement. In addition, the
analysis of enzymatic activity and the upward velocity of UrNMs in urea at physiological temperature (37 °C) shows no significant difference compared to room temperature (25 °C), Fig. S5a–c.
We performed computational modelling based on two-fluid hydrodynamics and compared the computational results to the experiments; a good qualitative agreement is obtained, see Fig. 1h, i. To
verify the universality of our mechanisms, we synthesized catalase-powered nanomotors (CatNMs) and observed the convective dynamics of these enzymatic nanomotors in hydrogen peroxide (H2O2)
(Fig. S6 and video S1). Notably, the instant chemical reaction of CatNMs in H2O2 results in a burst of oxygen bubbles, which drives the CatNMs to move upward against gravity within one
second. We expect that this buoyancy-driven mechanism will also apply to other asymmetric motors, such as Janus motors. Previous studies in our group have verified the difference between the
Janus structure and the patchy-like structure. The motion of a single out-of-equilibrium particle arises from the asymmetric distribution of ions, which generates an ionic gradient54. A
theoretical study has shown that Janus particles exhibit higher velocities compared to patchy-like motors55. Additionally, it has been reported that micron-sized hollow urease motors present
a 3D motion at a single particle level56, thus it is expected that large populations of these particles will also show collective motion. However, if the fabrication of Janus structures
involves heavier materials like platinum or gold, sedimentation may neglect buoyancy57,58,59, impeding the upward collective movement. That suggests that the buoyancy-driven mechanism could
be universal for various types of motors across different length scales, provided that the gravitational effects would not suppress the buoyancy-driven motion. CONTROLLING COLLECTIVE
BEHAVIOUR OF URNMS Collective behaviour can be viewed from the side. We studied the influence of three main control factors, UrNM concentration, urea concentration, and viscosity mediated by
hyaluronic acid (HA) concentration, on the collective behaviour. As illustrated in Fig. 2a, there are three stages of the collective behaviour of enzymatic nanomotors, i.e., ascending (1, 2
and 3), spreading (2 and 3), and sinking (3). When a swarm of nanomotors seeded in the bottom of the chamber that is filled with fuel, they show directional mobility against gravity. Under
different conditions, they display various forms of collective behaviour and velocity. Figure 2b shows the _z_-component of the particulate centre of mass as a function of time within 32 s.
The velocity difference can be deducted from Fig. S7 showing that for higher UrNM concentrations, particulates reach a lower _z_ position in 5 s. As the UrNM concentration increases to 20
mg/mL, the majority of the nanomotor swarms cannot get to the upper boundary due to gravity (Fig. 2b, c and video S2). The velocity field was analyzed by front-tracking the particulate based
on custom Python code. As expected, compared with passive nanoparticles (MSNPs), active nanomotors show enhanced upward speeds, Fig. S8 and video S3. We assume that enzymatic catalysis of
urea produces microbubbles60 and the product, ammonia, makes this particulate less dense. Although the product quantity may be larger with higher UrNM concentration, the density of
particulate increases as well when we increase the concentration of nanoparticles. We state that there should be a competition between the two opposite conditions, after which the effect of
increased particulate density takes the lead, and the upward particulate velocity decreases with the increased UrNM concentration. Buoyancy, the main driving force, is strongly influenced by
fuel concentration. Figure 2d, e show that the upward speeds increase with the fuel concentration. One can clearly observe the upward motion of particulates at concentrations of 150 mM urea
and above. However, in the presence of 100 mM urea concentration, particulate almost stays at the seeding point, and there is no difference between the upward motion of active swarms and
passive particulates. We argue that this is because in low urea concentration, density difference resulting in a buoyancy force is not sufficient to lift the particulate. To distinguish the
behaviours between active nanomotors and passive nanoparticles, we introduce phenol red, a pH indicator, into the urea solution. The chemical reactions that occur during the collective
movement of active nanomotors lead to a pH change in the surrounding solution, resulting in a colour shift from light yellow to pink, video S4 and Fig. S9. This colour change indicates the
location of the active UrNMs. For comparison, passive nanoparticles were tested in the same condition (10 mg/mL, 200 mM urea). They expand along the bottom plane at time 0, and the pH of
their surrounding solution remains unchanged. We added hyaluronic acid into the fuel to change the media viscosity observing that the upward speeds of particulate decreases with the increase
in concentration of hyaluronic acid, Fig. 2f, g. As it was shown above, active swarms show enhanced speed compared to passive particulates in viscous media. When the concentration of
hyaluronic acid increases to 3 mg/mL, both active swarms and passive particulates remain at the seeding point because higher viscosities inhibit fluid convection. We conducted particulate
velocity analysis at elevated heights in the middle of the chamber. In Fig. 2h, active particles move slightly faster in the middle of their paths and decrease their speeds when approaching
the upper boundary in different groups, while passive particles keep decreasing their speeds (Fig. S10). For instance, a particulate of 5 mg/mL UrNMs moves upward at 1.74 ± 0.09 mm/s at 4 mm
height, 1.93 ± 0.14 mm/s at 5 mm height, and 1.70 ± 0.08 mm/s at 7 mm height, while a particulate of the same concentration of passive nanoparticles moves at 0.96 ± 0.03 mm/s at 4 mm
height, 0.68 ± 0.02 mm/s at 5 mm height, and 0.47 ± 0.02 mm/s at 7 mm height. The acceleration process of active particulate could be due to the density changes caused by chemical reaction
products. Additionally, during the spreading stage, active nanomotors form a thin boundary layer of particle-rich fluid that continues to spread until it meets the side boundary. In
contrast, passive particles form a less stable boundary layer, leading to the formation of larger falling plumes earlier, causing them to sink before reaching the side boundary (see videos
S2 and S3). We assume this occurs because the products of chemical reactions make the active particles less dense, and the faster upward movement of active nanomotors creates a more dynamic
environment with increased fluid flow, making it less likely to form large falling plumes. PRODUCTS OF URNMS CATALYSIS REACTION ACCELERATE THE DIRECTIONAL MOVEMENT Urease catalyzes the
decomposition of urea into ammonia (NH3) and carbon dioxide (CO2). On the one hand, NH3 is highly soluble in water due to the formation of hydrogen bonds with water molecules. This
interaction results in a smaller density of the solution61,62. On the other hand, the released NH3 dissolves in water, resulting in an alkaline solution (Fig. 3a) and promoting CO2 to
dissolve. Under proper fuel concentration, the formation of NH3 and CO2 microbubbles can be observed60. However, in acidic buffers, such as acetate buffer (pH = 4.6, Fig. 3b), CO2 may exist
because the abundant hydrogen ions inhibit the dissolution of CO2 and the ionization of carbonic acid. The main reaction rate constants for CO2 and NH3 in phosphate buffer saline (PBS)
buffer and acetate buffer are presented in table S1. In PBS buffer, the rate constant for CO2 dissolution63 in basic solutions (k1 = 1.21 × 104 M−1s−1) is much higher than the reverse rate
constant (k−1 = 4.0 × 10−4 s−1). In acetate buffer, ammonia dissolves in acidic solutions. The rate constant for CO2 dissolution in water (k4 = 0.037 s−1) is much smaller than the reverse
constant of HCO3- combining with H+ (k−4 = 1.24 × 105 M−1 s−1). We conducted experiments to verify the existence of NH3 and CO2. In Fig. 3c, cover papers were pre-dipped in phenol red
solutions. Upon adding urease or UrNMs into urea solution, NH3 is produced and volatilizes until it dissolves in the cover paper that contains phenol red, the colour change of which from
light yellow to pink indicates the presence of NH3. The production of CO2 can be observed in acetate buffer, which maintains an acidic environment during the urease catalysis reaction, Fig.
3b. CO2 bubbles produced by UrNMs reacting with urea dissolving in acetate buffer can be observed on the wall of a cuvette (video S5). We filled cuvettes with 300 mM urea solutions that were
dissolved either in PBS buffer or in acetate buffer. Then UrNMs or urease solutions were added to the cuvettes, respectively. Video S5 shows clearly the convective flow from the turbidity
while for smaller urease molecules, the solutions remain transparent. In addition, the produced NH3 and CO2 in acetate buffer can be directly detected by a gas sensor, an optoelectronic
analysis equipment that is able to accurately detect low-concentration gases at the ppm level, as shown in Fig. 3d, e. The enzymatic activity of UrNMs in urea solutions in both PBS buffer
(Fig. 3f, S11) and acetate buffer (Fig. S12) was examined. In PBS buffer, the specific enzymatic activity of UrNMs increases from 4.08 ± 0.02 U/mg in 50 mM urea solutions to 4.82 ± 0.41 U/mg
in 300 mM urea solutions. In acetate buffer, the specific enzymatic activity of UrNMs is slightly weaker, with 1.94 ± 0.05 U/mg in 50 mM urea solutions and 3.36 ± 0.45 U/mg in 300 mM urea
solutions. This is because the known optimum pH for urease catalytic activity is around 7 ~ 864. We also examined the enzymatic activity of UrNMs in urine. Figure S13 shows that the
enzymatic activity of urease decreases from 5.25 ± 0.06 U/mg to 3.56 ± 0.15 U/mg in simulated urine65, and to 3.57 ± 0.20 U/mg in real urine. Although there is a significant decrease in
enzymatic activity in urine for 30 min, the activity of UrNMs is still relatively high, verifying the potential of UrNMs for in vivo applications. The above results indicate that the urease
catalysis reaction produces dissolved NH3 and CO2 in PBS buffer and dissolved NH3 and CO2 gas in acetate buffer. Therefore, upon quantifying the upward velocity of the UrNMs particulate, the
results indicate a faster upward movement, from 1.01 ± 0.04 mm/s in PBS buffer to 1.14 ± 0.04 mm/s in acetate buffer, as shown in Fig. S14. These findings validate our assumption that the
chemical products result in a higher density difference between the particulate and the media with fuel, leading to an accelerated movement. VERTICAL CONFINEMENT SHAPES COLLECTIVE BEHAVIOUR
Since buoyancy is the primary force that drives the self-organization of active particulates, we studied the influence of vertical confinement on their collective behaviour. As shown in Fig.
4, microfluidic chips with three different heights (1.6 mm, 0.5 mm, and 0.25 mm) were designed and filled with urea in the vertically confined chamber. Then active UrNMs were introduced and
entered the chamber from the side by capillary force. In Fig. 4a and video S6, these active UrNMs swarms exhibit collective movement in the chamber of 1.6 mm height. The density maps,
observed from the top, show that the swarms aggregate, coarsen, and change their patterns over time. Particle image velocimetry (PIV) also confirms that the fluid flow is initially faster
when the nanomotors are injected into the chamber. Fig. S15–S17 show the PIV results at 25 s time intervals in confinement with different heights. After 50 s, nanomotors keep moving and
collective behaviour is still transient. After 100 s, the fluid flow keeps a relatively high speed, 1.5 μm/s on average. However, the fluid flow direction remains the same according to the
arrows. As a comparison, without fuel UrNMs sink to the bottom in a confined chamber and expand along the bottom plane, Fig. S18. The convective flow is also weaker than that caused by UrNMs
with fuel, Fig. S19–21. When the vertical confinement is changed to 0.5 mm, the movement of active UrNMs becomes localized. In Fig. 4b, the density map shows that the pattern of UrNMs only
slightly changes over time. The PIV reveals that fluid flow velocity decreases compared to larger height values. After 50 s, the swarms barely move. When the height is further reduced to
0.25 mm, the swarms’ movement is hindered, as displayed by the unchanged shape of swarms over time and the decreased velocity of fluid flow in PIV, Fig. 4c. Active UrNMs in PBS solutions
also show decreased velocity when the chamber height decreases (Fig. S21). However, compared with the active UrNMs in fuel, there are no significant differences. We also analyzed the swarm
dynamics by pixel intensity distribution. A time-lapse sequence of snapshots at 12 s time intervals from video recordings is selected. As shown in Fig. S22, in a 1.6 mm-high chamber, the
pixel intensity of active UrNMs in fuel is broadly distributed in the region of interest (ROI) in the initial 60 seconds, and gradually changes to narrowly distributed in 2 min. However, for
the 0.5 mm-high chamber and the 0.25 mm-high chamber, pixel intensities are monodispersed in the ROI within the time durations. As a comparison, the pixel intensities of active UrNMs in PBS
solutions are highly monodispersed in the three different chambers, Fig. S23. These results indicate that the vertical confinement controls the swarms by affecting fluid convective flows
and provide insight into the buoyancy-driven collective behaviour of nanomotors. COMPUTATIONAL MODELLING SHOWS SIMILARITY WITH EXPERIMENTS Our starting point is two-fluid hydrodynamics66.
One fluid is a solvent with the kinematic viscosity _η_, flow velocity V, solvent pressure _p_, and solvent density _ρ_0. Second fluid is the particulate with the volume density _ρ_,
coarse-grained particulate velocity U, and pressure _P_ = _qρ_, and the factor _q_ depends on the temperature (as for gases). We describe the dynamics by the simplified Navier-Stokes Eq.
(1), coupled to the reaction-advection equation for the concentration of chemical fuel _c_, Eq. (2), and a mass transport equation for the particulate density, Eq. (3): $${\rho
}_{0}\left({\partial }_{t}{{{\bf{v}}}}+{{{\bf{v}}}}\nabla {{{\bf{v}}}}\right)=\eta {\nabla }^{2}{{{\bf{v}}}}-\nabla p-{{{{\bf{z}}}}}_{0}\rho \left(g\alpha -\epsilon c\right)$$ (1)
$${\partial }_{t}c+\nabla \cdot \left({{{\bf{v}}}}c\right)={D}_{c}{\nabla }^{2}c-\gamma \rho c$$ (2) $${\partial }_{t}\rho+\nabla \cdot \left({{{\bf{v}}}}\rho \right)=\left(q{\nabla
}^{2}\rho+\alpha g{\partial }_{z}\rho \right)/{\kappa }_{1}$$ (3) where \({{{{\boldsymbol{z}}}}}_{0}\)_ρєc_ is the volume buoyancy force due to gas generation, \({{{{\boldsymbol{z}}}}}_{0}\)
is the unit vector in the _z_-direction, the gas is produced due to the reaction between fuel _c_ and particulate _ρ_ with the reaction rate _γ_. Other parameters: fuel diffusion _D__c_,
gravity acceleration _g_, relative particulate/solvent density contrast _α_, _є_ is the relative buoyancy coefficient that depends on the density of reaction products, and _κ_1 is the
normalized drag coefficient. The details of model derivation are presented in Supplementary Note 1. Equations (1)–(3) were solved by the finite difference method using Matlab. We considered
a two-dimensional rectangular integration domain (corresponding to the size view) with periodic boundary conditions in the _x_-direction and non-slip conditions in the _z_-direction. The
primary difference with models of enzyme-generated solutal buoyancy mechanisms considered in ref. 67. is that the enzyme distribution is not fixed but dynamically updated by the
reaction-generated flow. When buoyancy is not sufficient to counterbalance the gravity of particulates, like in the cases of high concentration of particles and low concentration of fuel,
the particulate is not able to rise to the top plane and sink to the bottom after seeding, Fig. 5a, left panel. On the contrary, in the cases of low concentration of particles and high
concentration of fuel, particulates rise and spread along the top plane, then descend, experiencing a similar process as in the experiment, Fig. 5a, right panel, and video S7. In
simulations, the volume density _ρ_ changes from 1 to 4, chemical fuel _c_ ranges from 0.6-1.2, and kinematic viscosity _η_ varies from 0.1-1.0 to simulate different concentrations of
particles, fuel, and HA, respectively. In Fig. 5b–d, frames at dimensionless time 2.8 are chosen from computer videos for different parameters. Figure 5b shows that in the same time frame,
particulate with smaller density _ρ_ enters the sinking stage, while particulate with larger _ρ_ is still in the ascending or spreading stage, indicating that lighter particulates move
faster. This observation agrees with the experimental results and can be further verified by Fig. 5e. The mean velocity of particulate during upward movement decreases with the increase of
density _ρ_. In Fig. 5c, particulate settles to the bottom when chemical fuel concentration _c_ is low (_c_ = 0.6). Increasing the _c_ value (_c_ = 0.8) triggers particulate’s upward
movement, yet it settles before reaching the top plane. Only relatively high fuel concentrations force the particulate to go through the three stages, and its upward speed increases with the
increase of _c_ value. In Fig. 5f, the gradual increase of the mean particulate velocity with the fuel concentration from simulations agrees with that observed in the experiments. The
effect of viscosity is shown in Fig. 5d, g. Particulate in lower viscosity media enters the sinking stage earlier than for higher viscosity. Computational modelling confirms that the
increased fuel viscosity slows down the particulate motion. COMPUTATIONAL MODELLING OF THE VERTICAL CONFINEMENT EFFECTS We performed computational modelling of the effect of vertical
confinement on collective behaviour. The details are presented in Supplementary Note 2. The model is derived from Eqs. (1)–(3) by height-averaging using the approach like in ref. 68. The
corresponding two-dimensional equations in the _x-y_ plane are solved by the quasi-spectral method in the periodic square domain using Matlab. Parameter _β_ is proportional to the reaction
rate and parameter _ε_~_h_2, where _h_ is the height of the chamber. We adjust the value of these two control parameters to describe the fluid flow slowdown caused by confinement. In Fig.
6a, numerical results show that in vertical confinement, particulate moves dynamically and form aggregates in the centre area of the cell. A similar phenomenon has been observed in
experiment, Fig. 4a. However, when the chamber’s height is reduced, the fluid flow slows, and the reaction rate decreases. As a result, particulate movement becomes more localized, and the
shape formed by a particulate remains almost unchanged within the time durations, as shown in Fig. 6b, c and video S8. Furthermore, there is no significant difference between the swarm
dynamics in two highly confined chambers because fluid convection is inhibited by vertical confinement. In conclusion, we investigated the collective behaviour of enzymatic nanomotors from
the side and from the top. We attribute their collective behaviour to buoyancy-induced convection. When introducing a drop of UrNMs, dispersed in PBS buffer, into a fuel medium (high
concentration of urea dissolved in PBS), the UrNMs exhibit directional upward movement due to buoyancy arising from the density difference between the particulate and the fuel medium. UrNMs
decompose urea and generate carbon dioxide and ammonia, with the latter dissolving in water, further reducing the particulate density and enhancing its upward movement. When reaching the
solid-air interface, UrNMs spread along the interface, form an unstable layer of front, and then sink in the form of finger-like aggregates. The process resembles natural bioconvection in
microorganismal suspensions. Particle concentration, fuel concentration, and viscosity are crucial parameters to control enzymatic collective behaviour. Specifically, increasing particle
concentration, decreasing fuel concentration, or increasing viscosity can decrease the density difference between the particulate and the fuel, impeding the initiation of upward movement and
subsequent convection. This phenomenon explains the settlement of nanoparticles to the bottom when observed under inverted microscopy. Furthermore, the movement of UrNMs in vertical
confinement also serves as a demonstration of buoyancy-induced convection. Confinement hinders fluid convection, indicating that the collective behaviour of enzymatic nanomotors requires
vertical spaces to overcome dissipation. While these control factors are essential for understanding collective behaviour, further studies are needed to investigate how to effectively guide
swarm dynamics. Possible strategies could involve combining external fields or exploiting collective chemotaxis behaviour. We performed computational modelling based on the buoyancy-driven
convection mechanisms; the results align well with experimental findings. In computational modelling, particulate ascends due to buoyancy, spreads upon reaching the top, and consequently
descends because of gravity. Consistent with the experimental observations, an increase in particulate density (_ρ_), a decrease in fuel concentration (_c_), or an increase in fuel viscosity
(_η_) decreases the mean particulate velocity. Computational modelling also agrees with experimental observations for particulate moving in vertical confinement. By adjusting the parameters
_β_ and _κ_, corresponding to the reaction rate and the chamber height, respectively, the computational model predicts that vertical confinement shapes the swarms by controlling fluid
convection. The buoyancy-driven convective flow enables the collective movement of enzymatic nanomotors and promotes a more homogeneous particle distribution. In a fuel-rich environment,
collective behaviour occurs naturally due to buoyancy and chemical reactions, without requiring external forces. This buoyancy-driven dynamics can be harnessed to design future protocols for
large tissue and organ volumes, such as the bladder and joints. It allows overcoming the limitations of current cancer treatments, including sedimentation and poor dispersion in small
volumes, thereby facilitating mass transport, accumulation, penetration, and effective diffusivity of individual motors. METHODS SYNTHESIS OF MSNPS-NH2 Mesoporous silica nanoparticles
(MSNPs) serving as chassis for urease-propelled nanomotors were synthesized by the sol-gel procedure according to our previous report36. Briefly, a mixture of TEOA (35 g), Milli-Q water (20
ml), and CTAB (570 mg) was heated to 95 °C under reflux for 30 minutes. TEOS (1.5 ml) was then added dropwise, and the reaction continued for 2 hours. The resulting MSNPs were collected by
centrifugation (2000 × _g_, 5 min) and washed with ethanol, with the process repeated three times. CTAB was removed by refluxing the MSNPs in a methanol (30 ml) and hydrochloric acid (1.8
ml) mixture at 80 °C for 24 hours. Finally, the MSNPs were collected by centrifugation (2000 × _g_, 5 min), washed in ethanol (three times), and their concentration evaluated by dry
weighing. The surface of MSNPs was then modified for further functionalization. Briefly, 20 mg MSNPs in ethanol 99% (Panreac Applichem cat. no. 131086-1214) and 100 μL
3-aminopropyltriethoxysilane (APTES) 99% (Sigma-Aldrich cat. no. 440140) were mixed and placed in an end-to-end shaker at room temperature for 24 h. The resulting nanoparticles were then
collected and washed in ethanol by centrifugation (2000 × _g_, 5 min) four times to remove residual APTES. The collected MSNPs-NH2 nanoparticles were dried for further use. SYNTHESIS OF
URNMS AND CATNMS The prepared MSNPs-NH2 nanoparticles (2.5 mg) were resuspended in 1 mL PBS 1× (Thermo Fisher Scientific cat. no. 70011-036) and activated with 100 μL GA 25 wt%
(Sigma-Aldrich cat. no. G6257) in an end-to-end shaker for 2.5 hours at room temperature. The activated MSNPs-NH2 were then collected and washed four times in PBS 1× by centrifugation (2000
× _g_, 5 min), then resuspended in 1 mL PBS 1× with 3 mg urease from _Canavalia ensiformis_ (Sigma-Aldrich cat. no. U4002), or in 1 mL PBS 1× with 1 mg catalase from bovine liver
(Sigma-Aldrich cat. no. C40). The mixture reacted at room temperature in an end-to-end shaker overnight. The resulting urease-nanomotors (UrNMs)/catalase-nanomotors (CatNMs) were collected
and washed thrice in PBS 1× by centrifugation (2000 × _g_, 5 min). Keep the supernatant of centrifugation for further quantification of the enzyme linkage. Finally, resuspend the collected
nanomotors in PBS 1× (0.5 mL) and store them in the fridge at 4 °C for future use. DLS MEASUREMENTS OF URNMS Malvern Nanosizer (Zetasizer Nano ZSP) was used to measure the diffusion
coefficient of UrNMs across a range of urea concentrations (0, 50, 100, 150, and 300 mM) and the surface charge of MSNPs, MSNPs-NH2, and UrNMs. We analyzed the diffusion coefficient of UrNMs
(20 μg/mL) at each urea concentration and zeta potential values of each type of nanoparticles (20 μg/mL) with three runs per experiment. Nine measurements per type of particle were
performed to obtain statistically relevant data. URNMS CHARACTERIZATION The synthesized MSNPs were characterized by scanning electron microscope (SEM), revealing a uniform particle size
distribution centred at 450 nm (Fig. S1a, b). Amino groups were then grafted on the surface of MSNPs, facilitating further modification of urease on the MSNPs surface by linking GA molecules
between amino groups. The surface modification process was characterized by zeta potential measurements (Fig. S1c). The introduction of amino groups results in a negative surface charge of
MSNPs reversed from -38.7 ± 4.61 mV to a positive surface charge of 27.77 ± 7.9 mV. The subsequent linking of GA molecules and urease is confirmed by particle surface charge changes due to
the presence of abundant aldehyde groups and carboxyl groups, with negative surface charge reverses to -14.5 ± 9.13 mV and -9.02 ± 4.34 mV for GA molecule and urease, respectively. DLS
measurement indicates that the prepared UrNMs show an enhanced diffusion coefficient in elevated urea concentrations (Fig. S1d). OPTICAL VIDEO RECORDING AND NANOPARTICLE TRACKING The
collective behaviour of UrNMs in a vertically confined space was recorded using a Leica DMi8 microscope equipped with a high-speed cooled charge-coupled device (CCD) camera from Hamamatsu
and a 2.5× objective lens. Two pieces of cover glasses were separated by spacers (Silicone isolators from Grace Bio-Labs) with varying heights: 1.6 mm, 0.5 mm, and 0.25 mm. The confined
space was then filled either with PBS or with a 300 mM urea solution in PBS and positioned under the microscope. A drop of UrNMs or MSNPs (3 μL) was added to the liquid-filled chamber, and
videos (25 fps, 2 min) were recorded. Optical videos from the side view were recorded using either a digital camera (Thorlabs, DCC1240M-GL/Thorlabs, CS165CU) equipped with a lens (FUJINON,
HF35HA-1S) or a Leica DFC3000G camera equipped with a 10×/0.3 objective lens. A 22 × 1.6 × 8 mm (length × width × height) chamber was prepared by separating two pieces of cover glasses with
spacers. A drop of UrNMs or MSNPs (3 μL) was added to the liquid-filled chamber and videos (15 fps, 2 min) were recorded. Then these videos were analyzed using a home-designed programme in
Python27. The motion profiles of urease-powered nanomotors were analyzed in a 50 mM urea solution with varying ionic strengths, achieved by adding different concentrations of NaCl (0.05,
0.5, 5, 25, 50 mM). Videos were recorded at 25 fps for 30 seconds using an inverted optical microscope (Leica DMi8) equipped with a Hamamatsu digital camera (C11440) and a 63× water
immersion objective. For each experiment, a drop of urea solution with the specified NaCl concentration was placed on a glass slide, followed by the addition of 3 µL of UrNMs. The glass
slide was then covered with a coverslip. The acquired videos were analyzed using a custom-designed Python software. The mean square displacement (MSD) can be calculated from the extracted
trajectories by the equation MSD(Δt) = 4_D_Δt, where _D_ is the diffusion coefficient. BICINCHONINIC ACID (BCA) ASSAY The amount of urease or catalase linked onto the MSNPs surface was
quantified by BCA analysis (Thermo Fisher Scientific cat. no. 23227), table S2. Bovine serum albumin (BSA) was used as the standard for quantifying the concentration of protein
concentrations. First, a series of BSA concentrations 2000, 1500, 1000, 750, 500, 250, 125, 25, 0 μg/mL were prepared, and BSA solutions and the as-prepared supernatant of UrNMs or CatNMs
samples were added separately into a 96-multi-well plate, 25 μL for each well. Then 200 μL working reagent (light sensitive), made with 50 parts of reagent A and 1 part of reagent B, was
added to each well that has been used, either with the BSA standards or the sample. Next, shake the plate for 30 s to mix the solutions, and incubate the reaction for 30 min at 37 °C.
Afterward, the absorbance of both BSA solutions and the samples after the reaction was measured at a wavelength of 562 nm. By comparing the protein quantity remaining in the supernatant to
the initial amount of protein added and the standard concentrations of BSA, the amount of attached enzyme can be quantified. ENZYMATIC ACTIVITY MEASUREMENT Urease activity was detected
before and after being linked on the surface of MSNPs and was compared in PBS and acetate buffer. 0.025 mM Phenol red (Sigma-Aldrich cat. no. 114529) was added to different concentrations of
urea solutions (0, 50, 100, 200, and 300 mM). The solvent of these solutions could be either PBS or acetate buffer. 2 μL PBS solution or UrNMs or urease (130 μg/mL) were added in a 96-well
plate separately, followed by the addition of urea solutions. The 96-well plate was immediately placed in a multimode microplate reader (BioTek Synergy HTX). The absorbance changes of phenol
red were measured in real time at a wavelength of 560 nm for 60 min for measurements in PBS buffer and 100 min for acetate buffer. The incubation was performed at room temperature with a
minimum measurement interval of 30 seconds, and the sample was orbitally shaken at a minimal frequency. ESTIMATION OF SPECIFIC ENZYMATIC ACTIVITY The specific enzymatic activity was
determined by calculating the slope of the enzymatic activity curve. According to the Beer-Lambert law, $$A={klc}$$ where _A_ is the absorbance, _k_ is the molar attenuation coefficient of
phenol red, and _l_ is the path length of 0.5 cm, the concentration changes _c_ of phenol red per minute were computed. Subsequently, the specific enzymatic activity was derived based on the
quantity of enzyme utilized. GAS DETECTION The generated CO2 and NH3 were identified using a gas detector (Dräger X-am 7000). In a glass bottle filled with urea dissolved in PBS (10 mL, 200
mM) or acetate buffer (10 mL, 200 mM), UrNMs (2.5 mg) were added, and the caps were securely fastened to prevent gas release. After 30 min, the bottle caps were removed, and the probe of
the gas detector was placed over the solutions to record the generated gases. VIDEOS ANALYSIS To investigate the dynamics of swarms over time, the recorded videos were analyzed by pixel
intensity distribution and density maps. For pixel intensity distribution, snapshots of videos were captured at 12-second intervals. Subsequently, a region of interest (ROI) measuring 300
pixels by 300 pixels was selected, and the pixel intensity distribution within the ROI was analyzed using ImageJ software. To perform density map analysis, the videos were initially
processed to remove the background using ImageJ software. Then 40-second segments were extracted from these videos. The cumulative pixel intensity of these segments, consisting of 1000
frames each, was computed and visualized using the turbo colormap. PARTICLE IMAGE VELOCIMETRY (PIV) The PIV of recorded videos was conducted by a custom Python code based on the OpenPIV
library. The consecutive frames of videos within desired time intervals were extracted and then loaded into the code OpenPIV, with an interrogation window size of 32 × 32 pixels
(width×height), an overlap of 16 × 16 pixels (horizontal×vertical), and a frame rate of 3.33 fps. The results were then reloaded into the Python code to adjust the arrow size and display
particle velocities in colour bars. DATA AVAILABILITY All data supporting the findings are available within the article and the Supplementary Information. The raw data generated in this
study have been deposited in the Figshare database https://doi.org/10.6084/m9.figshare.27134523.v169. CODE AVAILABILITY The custom scripts used for computational analysis in this study are
available on GitHub at https://github.com/SC357/Convective_Dynamics70. The code is provided under the MIT License. Additional information can be found in the repository’s README file and can
also be requested from the corresponding author. REFERENCES * Wang, H. & Pumera, M. Coordinated behaviors of artificial micro/nanomachines: From mutual interactions to interactions with
the environment. _Chem. Soc. Rev._ 49, 3211–3230 (2020). Article PubMed Google Scholar * Shim, G., Devenport, D. & Cohen, D. J. Overriding native cell coordination enhances external
programming of collective cell migration. _Proc. Natl Acad. Sci. USA._ 118, e2101352118 (2021). Article PubMed PubMed Central CAS Google Scholar * Peleg, O., Peters, J. M., Salcedo, M.
K. & Mahadevan, L. Collective mechanical adaptation of honeybee swarms. _Nat. Phys._ 14, 1193–1198 (2018). Article CAS Google Scholar * Zitterbart, D. P., Wienecke, B., Butler, J. P.
& Fabry, B. Coordinated movements prevent jamming in an emperor penguin huddle. _PLoS One_ 6, e20260 (2011). Article ADS PubMed PubMed Central CAS Google Scholar * Yang, M. et al.
Swarming magnetic nanorobots bio-interfaced by heparinoid-polymer brushes for in vivo safe synergistic thrombolysis. _Sci. Adv._ 9, eadk7251 (2023). Article PubMed PubMed Central CAS
Google Scholar * Yu, J. et al. Active generation and magnetic actuation of microrobotic swarms in bio-fluids. _Nat. Commun._ 10, 5631–5642 (2019). Article ADS PubMed PubMed Central CAS
Google Scholar * Xie, H. et al. Reconfigurable magnetic microrobot swarm: multimode transformation, locomotion, and manipulation. _Sci. Robot._ 4, eaav8006 (2019). Article PubMed Google
Scholar * Bente, K. et al. Selective actuation and tomographic imaging of swarming magnetite nanoparticles. _ACS Appl. Mater. Interfaces_ 4, 6752–6759 (2021). CAS Google Scholar * Chen,
M. et al. Programmable dynamic shapes with a swarm of light-powered colloidal motors. _Angew. Chem. Int. Ed._ 60, 16674–16679 (2021). Article ADS CAS Google Scholar * Aubret, A.,
Youssef, M., Sacanna, S. & Palacci, J. Targeted assembly and synchronization of self-spinning microgears. _Nat. Phy._ 14, 1114–1118 (2018). Article CAS Google Scholar * Xu, T. et al.
Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. _J. Am. Chem. Soc._ 137, 2163–2166 (2015). Article PubMed CAS Google Scholar *
Tang, S. et al. Structure-dependent optical modulation of propulsion and collective behavior of acoustic/light-driven hybrid microbowls. _Adv. Funct. Mater._ 29, 1809003–1809009 (2019).
Article ADS Google Scholar * Leunissen, M. E., Vutukuri, H. R. & Van Blaaderen, A. Directing colloidal self-assembly with biaxial electric fields. _Adv. Mater._ 21, 3116–3120 (2009).
Article CAS Google Scholar * Ma, F., Wang, S., Wu, D. T. & Wu, N. Electric-field-induced assembly and propulsion of chiral colloidal clusters. _Proc. Natl Acad. Sci. USA._ 112,
6307–6312 (2015). Article ADS PubMed PubMed Central CAS Google Scholar * Duan, W., Liu, R. & Sen, A. Transition between collective behaviors of micromotors in response to different
stimuli. _J. Am. Chem. Soc._ 135, 1280–1283 (2013). Article PubMed CAS Google Scholar * Gentile, K., Somasundar, A., Bhide, A. & Sen, A. Chemically powered synthetic “living”
systems. _Chem_ 6, 2174–2185 (2020). Article CAS Google Scholar * Ziepke, A., Maryshev, I., Aranson, I. S. & Frey, E. Multi-scale organization in communicating active matter. _Nat.
Commun_. 13, 6727–6736 (2022). * Zhang, F. et al. Extremophile-based biohybrid micromotors for biomedical operations in harsh acidic environments. _Sci. Adv._ 8, eade645 (2022). Article ADS
Google Scholar * Chen, H. et al. An engineered bacteria-hybrid microrobot with the magnetothermal bioswitch for remotely collective perception and imaging-guided cancer treatment. _ACS
Nano_ 16, 6118–6133 (2022). Article PubMed CAS Google Scholar * Zhuang, J. & Sitti, M. Chemotaxis of bio-hybrid multiple bacteria-driven microswimmers. _Sci. Rep._ 6, 32135–32144
(2016). Article ADS PubMed PubMed Central CAS Google Scholar * Akter, M. et al. Cooperative cargo transportation by a swarm of molecular machines. _Sci. Robot._ 7, eabm0677 (2022).
Article PubMed CAS Google Scholar * Keya, J. J. et al. DNA-assisted swarm control in a biomolecular motor system. _Nat. Commun._ 9, 453–460 (2018). Article ADS PubMed PubMed Central
Google Scholar * Chen, S. et al. Dual-source powered nanomotor with integrated functions for cancer photo-theranostics. _Biomaterials_ 288, 121744–121753 (2022). Article PubMed CAS
Google Scholar * Chen, S. et al. Active nanomotors surpass passive nanomedicines: current progress and challenges. _J. Mater. Chem. B_ 10, 7099–7107 (2022). Article PubMed CAS Google
Scholar * Ma, X. et al. Enzyme-powered hollow mesoporous Janus nanomotors. _Nano Lett._ 15, 7043–7050 (2015). Article ADS PubMed Google Scholar * Ma, X., Hortelao, A. C., Miguel-López,
A. & Sánchez, S. Bubble-free propulsion of ultrasmall tubular nanojets powered by biocatalytic reactions. _J. Am. Chem. Soc._ 138, 13782–13785 (2016). Article PubMed PubMed Central
CAS Google Scholar * Arqué, X. et al. Intrinsic enzymatic properties modulate the self-propulsion of micromotors. _Nat. Commun._ 10, 2826–2837 (2019). Article ADS PubMed PubMed Central
Google Scholar * Ma, X. & Sanchez, S. A bio-catalytically driven Janus mesoporous silica cluster motor with magnetic guidance. _Chem. Commun._ 51, 5467–5470 (2015). Article CAS
Google Scholar * Hortelão, A. C., Patiño, T., Perez-Jiménez, A., Blanco, À. & Sánchez, S. Enzyme-powered nanobots enhance anticancer drug delivery. _Adv. Funct. Mater._ 28,
1705086–1705095 (2018). Article Google Scholar * Llopis-Lorente, A. et al. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. _ACS Nano_ 13,
12171–12183 (2019). Article PubMed CAS Google Scholar * Hortelao, A. C., Carrascosa, R., Murillo-Cremaes, N., Patino, T. & Sánchez, S. Targeting 3D bladder cancer spheroids with
urease-powered nanomotors. _ACS Nano_ 13, 429–439 (2019). Article PubMed CAS Google Scholar * Tang, S. et al. Enzyme-powered Janus platelet cell robots for active and targeted drug
delivery. _Sci. Robot._ 5, eaba6137 (2020). Article PubMed Google Scholar * Choi, H., Cho, S. H. & Hahn, S. K. Urease-powered polydopamine nanomotors for intravesical therapy of
bladder diseases. _ACS Nano_ 14, 6683–6692 (2020). Article PubMed CAS Google Scholar * Patino, T. et al. Self-sensing enzyme-powered micromotors equipped with pH-responsive DNA
nanoswitches. _Nano Lett._ 19, 3440–3447 (2019). Article ADS PubMed CAS Google Scholar * Liu, X. et al. Urease-powered micromotors with spatially selective distribution of enzymes for
capturing and sensing exosomes. _ACS Nano_ 17, 24343–24354 (2023). Article PubMed CAS Google Scholar * Hortelao, A. C. et al. Swarming behavior and in vivo monitoring of enzymatic
nanomotors within the bladder. _Sci. Robot._ 6, eabd2823 (2021). Article PubMed Google Scholar * Simó, C. et al. Urease-powered nanobots for radionuclide bladder cancer therapy. _Nat.
Nanotechnol._ 19, 554–564 (2024). Article ADS PubMed PubMed Central Google Scholar * Serra-Casablancas, M. et al. Catalase-powered nanobots for overcoming the mucus barrier. _ACS Nano_
18, 16701–16714 (2024). Article PubMed PubMed Central CAS Google Scholar * Ruiz-González, N. et al. Swarms of enzyme-powered nanomotors enhance the diffusion of macromolecules in
viscous media. _Small_ 20, 2309387–2309403 (2024). Article Google Scholar * Ramos-Docampo, M. A., Wang, N., Pendlmayr, S. & Städler, B. Self-propelled collagenase-powered
nano/micromotors. _ACS Appl. Nano Mater._ 5, 14622–14629 (2022). Article CAS Google Scholar * Ramos-Docampo, M. A. et al. Microswimmers with heat delivery capacity for 3D cell spheroid
penetration. _ACS Nano_ 13, 12192–12205 (2019). Article PubMed CAS Google Scholar * Fraire, J. C. et al. Light-triggered mechanical disruption of extracellular barriers by swarms of
enzyme-powered nanomotors for enhanced delivery. _ACS Nano_ 17, 7180–7193 (2023). Article PubMed PubMed Central CAS Google Scholar * Kessler, J. O. & Hill, N. A. The growth of
bioconvection patterns in a uniform suspension of gyrotactic micro-organisms. _J. Fluid Mech._ 195, 223–237 (1988). Article ADS MathSciNet PubMed Google Scholar * Bodenschatz, E.,
Pesch, W. & Ahlers, G. Recent developments in Rayleigh-Bénard Convection. _Annu. Rev. Fluid Mech._ 32, 709–778 (2000). Article ADS Google Scholar * Bees, M. A. Advances in
bioconvection. _Annu. Rev. Fluid Mech._ 52, 449–476 (2020). Article ADS MathSciNet Google Scholar * Metcalfe, A. M. & Pedley, T. J. Falling plumes in bacterial bioconvection. _J.
Fluid Mech._ 445, 121–149 (2001). Article ADS Google Scholar * Zhang, J. et al. Light-powered, fuel-Free oscillation, migration, and reversible manipulation of multiple cargo types by
micromotor swarms. _ACS Nano_ 17, 251–262 (2023). Article PubMed CAS Google Scholar * Sun, M. et al. Bioinspired self-assembled colloidal collectives drifting in three dimensions
underwater. _Sci. Adv._ 9, eadj4201 (2023). Article ADS PubMed PubMed Central Google Scholar * Kumar, B. V. V. S. P., Patil, A. J. & Mann, S. Enzyme-powered motility in buoyant
organoclay/DNA protocells. _Nat. Chem._ 10, 1154–1163 (2018). Article ADS PubMed CAS Google Scholar * Song, J., Shklyaev, O. E., Sapre, A., Balazs, A. C. & Sen, A. Self‐propelling
macroscale sheets powered by enzyme pumps. _Angew. Chem. Int. Ed._ 63, e202311556 (2023). Article Google Scholar * Sapre, A. et al. Enzyme catalysis causes fluid flow, motility, and
directional transport on supported lipid bilayers. _ACS Appl. Mater. Interfaces_ 16, 9380–9387 (2024). Article PubMed CAS Google Scholar * Patiño, T. et al. Influence of enzyme quantity
and distribution on the self-propulsion of non-Janus urease-powered micromotors. _J. Am. Chem. Soc._ 140, 7896–7903 (2018). Article PubMed Google Scholar * Patiño, T., Llacer-Wintle, J.,
Pujals, S., Albertazzi, L. & Sánchez, S. Unveiling protein corona formation around self-propelled enzyme nanomotors by nanoscopy. _Nanoscale_ 16, 2904–2912 (2023). Article Google
Scholar * De Corato, M. et al. Self-propulsion of active colloids via ion release: theory and experiments. _Phys. Rev. Lett._ 124, 108001–108006 (2020). Article ADS PubMed Google Scholar
* De Corato, M., Pagonabarraga, I., Abdelmohsen, L. K. E. A., Sánchez, S. & Arroyo, M. Spontaneous polarization and locomotion of an active particle with surface-mobile enzymes. _Phys.
Rev. Fluids_ 5, 122001–122011 (2020). Article ADS Google Scholar * Arqué, X. et al. Ionic species affect the self-propulsion of urease-powered micromotors. _Research_ 2020, 1–14 (2020).
* Simmchen, J. et al. Topographical pathways guide chemical microswimmers. _Nat. Commun._ 7, 10598–10606 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Katuri, J.,
Uspal, W. E., Popescu, M. N. & Sánchez, S. Inferring non-equilibrium interactions from tracer response near confined active Janus particles. _Sci. Adv._ 7, eabd0719 (2021). Article ADS
PubMed PubMed Central CAS Google Scholar * Katuri, J., Caballero, D., Voituriez, R., Samitier, J. & Sanchez, S. Directed flow of micromotors through alignment interactions with
micropatterned ratchets. _ACS Nano_ 12, 7282–7291 (2018). Article PubMed CAS Google Scholar * Feng, Y. et al. Self-adaptive enzyme-powered micromotors with switchable propulsion
mechanism and motion directionality. _Appl. Phys. Rev._ 8, 011406–011413 (2021). Article ADS CAS Google Scholar * Hales, J. M. & Drewes, D. R. Solubility of ammonia in water at low
concentrations. _Atmos. Environ._ 13, 1133–1147 (1979). Article ADS CAS Google Scholar * Gottwald, F. et al. Physical properties of ammonia solutions. Ammonium nitrate-ammonia-water and
urea-ammonia-water. _Ind. Eng. Chem._ 44, 910–913 (1952). Article Google Scholar * Wang, X., Conway, W., Burns, R., McCann, N. & Maeder, M. _Phys. Chem. A_ 114, 1734–1740 (2010).
Article CAS Google Scholar * Estiu, G. & Merz, K. M. Catalyzed decomposition of urea. Molecular dynamics simulations of the binding of urea to urease. _Biochemistry_ 45, 4429–4443
(2006). Article PubMed CAS Google Scholar * Sarigul, N., Korkmaz, F. & Kurultak, İ. A new artificial urine protocol to better imitate human urine. _Sci. Rep._ 9, 20159–20169 (2019).
Article ADS PubMed PubMed Central CAS Google Scholar * Ishii, M. & Mishima, K. Two-fluid model and hydrodynamic constitutive relations. _Nucl. Eng. Des._ 82, 107–126 (1984).
Article CAS Google Scholar * Valdez, L., Shum, H., Ortiz-Rivera, I., Balazs, A. C. & Sen, A. Solutal and thermal buoyancy effects in self-powered phosphatase micropumps. _Soft Matter_
13, 2800–2807 (2017). Article ADS PubMed CAS Google Scholar * Aranson, I. S. & Sapozhnikov, M. V. Theory of pattern formation of metallic microparticles in poorly conducting
liquids. _Phys. Rev. Lett._ 92, 234301–234304 (2004). Article ADS PubMed CAS Google Scholar * Chen, S. et al. Collective buoyancy-driven dynamics of enzymatic nanomotors. _Figshare_,
https://doi.org/10.6084/m9.figshare.27134523.v1 (2024). * Chen, S. et al. Collective buoyancy-driven dynamics of enzymatic nanomotors. _Github_, https://doi.org/10.5281/zenodo.13842706
(2024). Download references ACKNOWLEDGEMENTS The research leading to these results has received funding from the grants PID2021-128417OB-I00 and PDC2022-133753-I00 funded by MCIN/AEI/
10.13039/501100011033 and, by “ERDF A way of making Europe” and European Union Next Generation EU, (Bots4BB and BOJOS projects) (S.S.). This project has also received funding from the
European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 866348, iNanoSwarms) (S.S.). The IBEC team wishes to thank the
CERCA programme of the Generalitat de Catalunya, the Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya through the project 2021 SGR
01606, and the “Centro de Excelencia Severo Ochoa”, funded by Agencia Estatal de Investigación (CEX2018-000789-S). We thank Santiago Marco Colás and Eduardo Caballero Saldivar for their
technical support. S.S. and S.C. acknowledge the Predoctoral AGAUR-FI Joan Oró grant (2023 FI-1 00654) funded by “Secretaria d’Universitats i Recerca del Departament de Recerca i
Universitats de la Generalitat de Catalunya” and by European Social Fund Plus. The research of I.S.A. was supported by the NSF award PHY-2140010. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Institute for Bioengineering of Catalonia (IBEC), The Barcelona Institute for Science and Technology (BIST), Baldiri i Reixac 10-12, Barcelona, 08028, Spain Shuqin Chen, Xander Peetroons,
Anna C. Bakenecker, Florencia Lezcano & Samuel Sánchez * Faculty of Physics, University of Barcelona, Martí i Franquès 1, Barcelona, Spain Shuqin Chen * Department of Biomedical
Engineering, The Pennsylvania State University, University Park, PA, 16802, USA Igor S. Aranson * Department of Chemistry, The Pennsylvania State University, University Park, PA, 16802, USA
Igor S. Aranson * Department of Mathematics, The Pennsylvania State University, University Park, PA, 16802, USA Igor S. Aranson * Catalan Institute for Research and Advanced Studies (ICREA),
Passeig Lluís Companys 23, Barcelona, Spain Samuel Sánchez Authors * Shuqin Chen View author publications You can also search for this author inPubMed Google Scholar * Xander Peetroons View
author publications You can also search for this author inPubMed Google Scholar * Anna C. Bakenecker View author publications You can also search for this author inPubMed Google Scholar *
Florencia Lezcano View author publications You can also search for this author inPubMed Google Scholar * Igor S. Aranson View author publications You can also search for this author inPubMed
Google Scholar * Samuel Sánchez View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.C. synthesized and characterized the nanomotors,
performed the motion and velocity analysis, analyzed the data, and wrote the paper. X.P. developed Python scripts for the upward velocity analysis and developed Matlab scripts for the
computational modelling. A.C.B. designed the side view setup and contributed to the simulation work. F.L. analyzed the centre of mass tracking and contributed to the data processing. I.S.A.
conceived the initial idea, developed the computational modelling, and edited the paper. S.S. conceived the initial idea, supervised the project, and edited the paper. CORRESPONDING AUTHORS
Correspondence to Igor S. Aranson or Samuel Sánchez. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Communications_ thanks Jianguo Guan, Akira Kakugo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPORTING MOVIE 1 THE UNIVERSALITY OF THE MECHANISM SUPPORTING MOVIE 2 CONTROL FACTORS INFLUENCE ENZYMATIC COLLECTIVE BEHAVIOUR
SUPPORTING MOVIE 3 CONTROL FACTORS INFLUENCE THE MOTION OF MSNPS SUPPORTING MOVIE 4 THE PH CHANGE OF THE COLLECTIVE DYNAMICS SUPPORTING MOVIE 5 BUBBLES PRODUCED BY ENZYMATIC CATALYSIS
REACTION SUPPORTING MOVIE 6 VERTICAL CONFINEMENT SHAPES COLLECTIVE BEHAVIOUR SUPPORTING MOVIE 7 COMPUTATIONAL MODELLING OF THE CONTROL FACTORS SUPPORTING MOVIE 8 COMPUTATIONAL MODELLING OF
THE VERTICAL CONFINEMENT EFFECTS TRANSPARENT PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, S., Peetroons, X., Bakenecker, A.C. _et al._ Collective buoyancy-driven dynamics in swarming enzymatic nanomotors. _Nat Commun_ 15,
9315 (2024). https://doi.org/10.1038/s41467-024-53664-w Download citation * Received: 29 February 2024 * Accepted: 15 October 2024 * Published: 29 October 2024 * DOI:
https://doi.org/10.1038/s41467-024-53664-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Have you picked the right executor or trustee? - estate planning - wil...3. KNOW WHEN TO USE — AND AVOID — CORPORATE TRUSTEES Speaking of corporate trustees, there’s healthy debate over whethe...
Adults at highest covid risk least likely to get treatmentMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Foreign investors still prefer france to uk or germany, study findsDO YOU RECEIVE THE CONNEXION'S FREE WEEKDAY NEWSLETTER? Sign up here A new study has found that France is the most ...
NWFA | Teams secure finals berths | The Examiner | Launceston, TASPredictably, Turners Beach made short work of West Ulverstone with a goal-scoring spree that left the Lions completely n...
The emerging role of protein l-lactylation in metabolic regulation and cell signallingABSTRACT l-Lactate has emerged as a crucial metabolic intermediate, moving beyond its traditional view as a mere waste p...
Latests News
Collective buoyancy-driven dynamics in swarming enzymatic nanomotorsABSTRACT Enzymatic nanomotors harvest kinetic energy through the catalysis of chemical fuels. When a drop containing nan...
Breakdancer raygun announces retirement following ‘really upsetting’ backlash at paris olympicsShe’s hanging up her dancing shoes. Breakdancer Raygun announced she is retiring after the “really upsetting” backlash s...
Charge 23/01/2012Charge 23/01/2012 Confira a charge do Jornal Notícias do dia de domingo (22/01/2012)https://ndmais.com.br/opiniao/charge...
The revised ap african american studies: what's been changed and whyThe College Board, which runs the Advanced Placement course program, has released a revised framework for its AP African...
Porcelain veneers: a preliminary reviewYou have full access to this article via your institution. Download PDF ARTICLE PDF Authors * J S Clyde View author publ...