Genome-wide screen of mycobacterium tuberculosis-infected macrophages revealed gid/ctlh complex-mediated modulation of bacterial growth
Genome-wide screen of mycobacterium tuberculosis-infected macrophages revealed gid/ctlh complex-mediated modulation of bacterial growth"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The eukaryotic Glucose Induced Degradation/C-Terminal to LisH (GID/CTLH) complex is a highly conserved E3 ubiquitin ligase involved in a broad range of biological processes.
However, a role of this complex in host anti-microbial defenses has not been described. We exploited _Mycobacterium tuberculosis_ (_Mtb_) induced cytotoxicity in macrophages in a FACS based
CRISPR genetic screen to identify host determinants of intracellular _Mtb_ growth restriction. Our screen identified 5 (_GID8_, _YPEL5_, _WDR26_, _UBE2H_, _MAEA_) of the 12 predicted members
of the GID/CTLH complex as determinants of intracellular growth of both _Mtb_ and _Salmonella_ serovar Typhimurium. We show that the anti-microbial properties of the GID/CTLH complex
knockout macrophages are mediated by enhanced GABAergic signaling, activated AMPK, increased autophagic flux and resistance to _Mtb_ induced necrotic cell death. Meanwhile, _Mtb_ isolated
from GID/CTLH knockout macrophages are nutritionally starved and oxidatively stressed. Our study identifies the GID/CTLH complex activity as broadly suppressive of host anti-microbial
responses against intracellular bacterial infections. SIMILAR CONTENT BEING VIEWED BY OTHERS MYCOBACTERIAL DNA-BINDING PROTEIN 1 IS CRITICAL FOR BCG SURVIVAL IN STRESSFUL ENVIRONMENTS AND
SIMULTANEOUSLY REGULATES GENE EXPRESSION Article Open access 29 August 2023 LYSOSOME REPOSITIONING AS AN AUTOPHAGY ESCAPE MECHANISM BY _MYCOBACTERIUM TUBERCULOSIS_ BEIJING STRAIN Article
Open access 22 February 2021 DEPLETION OF ESSENTIAL MYCOBACTERIAL GENE GLMM REDUCES PATHOGEN SURVIVAL AND INDUCES HOST-PROTECTIVE IMMUNE RESPONSES AGAINST TUBERCULOSIS Article Open access 06
August 2024 INTRODUCTION _Mycobacterium tuberculosis_ (_Mtb_) is an intracellular pathogen that causes tuberculosis (TB), the leading cause of global mortality due to a single infectious
disease1. At the onset of infection, _Mtb_ is inhaled into the airways and phagocytosed by resident alveolar macrophages. This initial interaction triggers the recruitment of
monocyte-derived macrophages, which gradually become the more abundant host macrophage population as the infection becomes more established. It is estimated that only 5–10% of _Mtb-_infected
individuals will progress to active TB disease1. Whether the non-progressors remain latently infected, or progress to clear the infection is a subject of ongoing debate2. _Mtb_ has evolved
to survive and grow within the macrophage through the subversion of the anti-microbial properties of the phagocyte. In brief, the bacterium blocks normal phagosomal acidification and
phagosome/lysosome fusion3,4,5,6. Inside the macrophage, the bacterium also relies on access to host fatty acids and cholesterol to fuel its biosynthetic demands7. The outcome of this
interplay is strongly influenced by both the macrophage lineages involved and the development of an acquired immune response. It is now known that lung macrophages comprise two separate
lineages; tissue-resident alveolar macrophages that are fetal stem cell-derived and populate the lung during embryogenesis, and interstitial macrophages that are derived from blood monocytes
and are recruited to the lung upon insult or infection8,9. While resident alveolar macrophages are more permissive to _Mtb_ growth, recruited interstitial macrophages are innately more
hostile to the intracellular bacteria10. Eventually, _Mtb_ parasitization of the macrophage usually ends in the death of the infected phagocyte. However, the route to cell death also plays a
major role in bacterial survival. If the infected macrophage undergoes apoptosis, _Mtb_ will likely be killed, either in the apoptotic body or as a result of efferocytosis11,12. In
contrast, macrophage death via necrosis supports _Mtb_ growth and aids in bacterial spread13,14. One of the most significant determinants of _Mtb_ fate inside macrophages is its ability to
perforate the phagosome by an _ESX-1_ type VII secretion system15. Phagosomal damage releases _Mtb_ effectors into the host cytosol that suppress apoptosis and drive necrosis13,16. Through
the action of _ESX-1_, _Mtb_ also inhibits macrophage anti-microbial defenses such as autophagy and inflammasome-mediated production of interleukin-1β (IL-1β)17,18. The route to macrophage
cell death is clearly important to _Mtb_’s success as a pathogen. Two groups have conducted genome-wide CRISPR/Cas9 screens targeting _Mtb_-induced macrophage cell death pathways. Zhang and
colleagues screened RAW264.7 cells and identified multiple genes in the type 1 interferon signaling pathway that, when disrupted, conferred protection against _Mtb_-induced cell death19.
Similarly, Lai et al.20 performed a whole genome screen on THP1 cells infected with _M. bovis_ BCG and also identified hits in the type 1 interferon pathway as having protective effects on
infected cells. Both of these screens used the diminished death of infected cells to select for genes that, when deleted, provided the cells with some degree of protection. In this study, we
used a similar CRISPR screening approach but emphasized a different selection pressure. We hypothesized that improved cell survival could also be a result of mutations that restrict
bacterial growth with the potential to identify novel host pathways of bacterial control. We performed a genome-wide CRISPR/Cas9 screen on primary Hoxb8 conditionally immortalized murine
bone marrow macrophages and identified the mammalian GID/CTLH complex as a strong determinant of intracellular growth of _Mtb_ in macrophages. We show that knockout of individual components
of the GID/CTLH complex is strongly restrictive to the intracellular growth of _Mtb_ and _S_. Typhimurium. Mechanistically, knockout of the GID/CTLH complex promotes anti-microbial responses
in macrophages through an enhanced autophagic response, increased metabolic resilience, and resistance to cell death. GID/CTLH knockout macrophages are also anti-inflammatory and, at the
same time, restrict intracellular _Mtb_ access to essential nutrients. Our work demonstrates that the GID/CTLH complex activity broadly suppresses the macrophage’s anti-microbial responses
and, therefore, represents a tractable target for host-directed therapies (HDTs) in TB control strategies. RESULTS FACS-BASED MACROPHAGE SURVIVAL CRISPR SCREEN TO IDENTIFY INTRACELLULAR
DETERMINANTS OF _MTB_ REPLICATION Infection of macrophages with virulent _Mtb_ strains induces necrotic cell death13,14,21, a virulence mechanism that enables the bacteria to escape the
phagosome and spread. To identify genes that contribute to improved macrophage survival after _Mtb_ infection, we developed a flow cytometry-activated sorting (FACS)-based CRISPR screen in
murine macrophages derived from estradiol responsive Hoxb8 Cas9+ conditionally immortalized myeloid precursors22 using cytotoxicity as a selection outcome, anticipating that restriction of
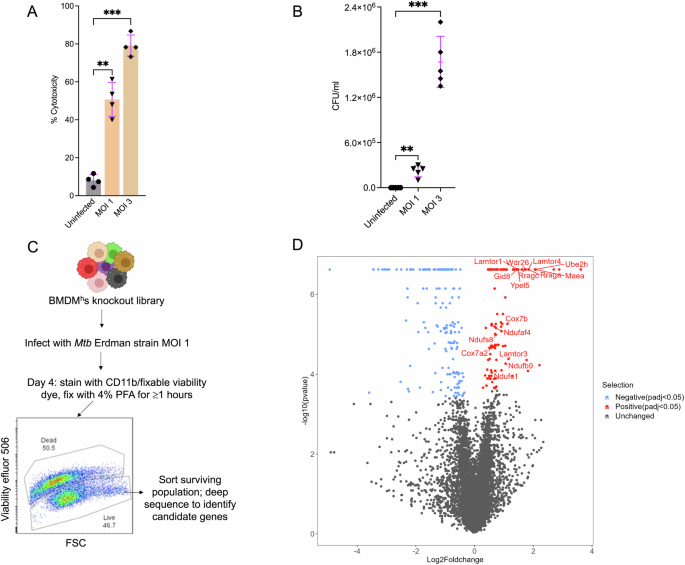
_Mtb_ growth would enhance cell survival. We first optimized _Mtb_ cytotoxicity in Hoxb8 bone marrow-derived macrophages (BMDMhs) at different multiplicity of infections (MOIs). Infection of
BMDMhs with the virulent _Mtb_ Erdman strain induced ~50% macrophage cytotoxicity at MOI 1 and >75% cytotoxicity at MOI 3 after 4 days of infection, as measured by the lactate
dehydrogenase (LDH) release assay (Fig. 1A). Similar levels of cytotoxicity were evident when we analyzed the infected macrophages by flow cytometry after staining with live dead viability
dye (Fig. S1A). As further evidence of bacteria release from dying macrophages, an MOI-dependent increase in _Mtb_ colony forming units (CFUs) was observed in supernatants from the infected
macrophage cultures (Fig. 1B). We, therefore, chose a 4-day infection with the _Mtb_ Erdman strain at MOI 1 as a selection pressure for our CRISPR screen as it resulted in sufficient
proportions of surviving macrophages (Fig. S1A) which can be flow-sorted for downstream library preparation and sequencing. We then transduced Hoxb8 Cas9+ myeloid progenitors with the Brie
knockout lentiviral single guide RNA (sgRNA) library23 that targets ~19,674 mouse protein-coding genes with four sgRNAs/gene. BMDMhs knockout libraries were differentiated from the Hoxb8
Cas9+ myeloid progenitor library (Fig. S1B) and infected with the _Mtb_ Erdman strain at MOI 1 for 4 days. Surviving macrophages based on live dead staining were flow sorted and sequenced to
identify candidate genes (Fig. 1C). We carried out the screen in three independent replicates and identified hits by comparing sgRNA read counts in the sample groups to the input
unperturbed BMDMh library using the MAGeCK-VISPR pipeline (Fig. S2)24. We identified 259 genes whose knockout modulated BMDMhs responses to _Mtb_ infection based on a false discovery rate of
<0.05 (Fig. 1D, Supplementary Data 1). 104 genes were significantly enriched (conferred relative protection to _Mtb_-induced cytotoxicity), while 155 genes were significantly depleted.
The highest-scoring protective hits belonged to the TOR signaling pathway (Fig. 1D, Supplementary Data 1, 2), which has already been implicated in macrophage control of _Mtb_25. All the
enriched 10 genes in the TOR pathway (_RRAGC_, _LAMTOR1_, _RRAGA_, _TSC1_, _FLCN_, _TSC2_, _LAMTOR4_, _LAMTOR3_, _TBC1D7_, _RPS6KA1_) are part of the mTOR signaling cascade which plays a
crucial role in mammalian cell growth and nutrient signaling26. These hits are consistent with the literature that mTOR inhibition is known to activate autophagy, a host response that
restricts intracellular _Mtb_ growth in macrophages27. Our hits also included a number of genes and pathways that could negatively impact _Mtb_ growth (Fig. 1D, Supplementary Data 2), such
as the mitochondrial complex 1 (_NUBPL_, _TMEM126B_, _NDUFAF4_, _NDUFS8_, _ACAD9_, _NDUFB9_, _NDUFC1_), iron-sulfur cluster binding, protein dephosphorylation, regulation of GTPases activity
and lysosomal functions. Mitochondrial complex 1 is a target of metformin, a promising HDT against _Mtb_28 while disruption of iron homeostasis in macrophages by knockout of the iron–sulfur
clusters could be restrictive to _Mtb_ growth by limiting the bacteria’s access to iron29. We also identified 5 of the 12 predicted members of the highly conserved mammalian multi-subunit
GID/CTLH complex30,31 (_MAEA, WDR26_, _UBE2H_, _YPEL5_, _GID8_) (Fig. 1D, Supplementary Data 1) as potential determinants of macrophage resistance to _Mtb_ driven cell death. Interestingly,
2 of these hits (_WDR26_, _YPEL5_) were previously identified in a related CRISPR screen in THP1 macrophages using _M. bovis_ BCG20. KNOCKOUT OF THE GID/CTLH COMPLEX IN MACROPHAGES RESULTS
IN STRONG INTRACELLULAR BACTERIA GROWTH RESTRICTION AND CONFERS HIGH-LEVEL RESISTANCE TO _MTB_-INDUCED CELL CYTOTOXICITY The GID/CTLH complex was first identified in yeast as a multi-subunit
E3 ligase that targets surplus gluconeogenic enzymes for proteasomal degradation when glucose-starved cells are re-supplied with the substrate32,33. In mammals, the complex appears to
fulfill other biological functions like cell proliferation and survival, cell migration and adhesion, erythropoiesis, and neurodegenerative diseases34. In immunity, some members of the
complex (_RANBP9_) have been implicated in antigen processing, efferocytosis, and anti-inflammatory activities35,36. We generated de novo knockouts in Hoxb8 Cas9+ myeloid progenitors for the
five GID/CTLH hits identified in our screen (_MAEA, WDR26_, _UBE2H_, _YPEL5_, _GID8_), which represent ~50% of the predicted functional members of the complex in humans and mice30. We
targeted each gene with 2 sgRNAs and were able to achieve >85% knockout efficiencies (Supplementary Data 3) as analyzed by the Inference of CRISPR Edits (ICE) tool37. Similar levels of
protein knockout were also confirmed by western blot analysis for all five genes in BMDMhs differentiated from the Hoxb8 Cas9+ mutants (Fig. S3). We then infected the GID/CTLH knockout
BMDMhs (_GID8__−/−_, _YPEL5__−/−_, _WDR26__−/−_, _UBE2H__−/−_, _MAEA__−/−__)_ differentiated from the Hoxb8 Cas9+ mutants with the _Mtb_ Erdman strain at MOI 0.4 for 4 days to determine the
intracellular growth replication rates of _Mtb_ in these macrophages. An MOI of 0.4 was chosen to minimize macrophage cell death on the day of plating CFUs in accordance with prior
optimizations (Fig. 1B, S1A). A strong _Mtb_ growth restriction was observed in all the five GID/CTLH mutants as compared to macrophages transduced with a non-targeting scramble sgRNA
control (Fig. 2A). The growth restriction phenotypes were not due to differences in bacteria uptake or phagocytosis defects as there were no significant differences when we plated CFUs 3
hours post infection (Fig. 2A). Similar intracellular growth restriction phenotypes were observed when we infected selected mutants (_MAEA__−/−_, _GID8__−/−_, _WDR26__−/−_) with the _Mtb_
Erdman-Lux reporter strain and monitored bacterial growth by luciferase expression (Fig. 2B). We also analyzed the growth restriction phenotypes of the GID/CTLH knockout BMDMhs using another
intracellular bacterium, _Salmonella enterica_ serovar Typhimurium (_S_. Typhimurium), in the gentamicin protection assay38. _MAEA__−/−_ and _GID8__−/−_ knockout BMDMhs were infected with
the wild-type _S_. Typhimurium ATCC 14028s strain at MOI 10, and CFUs were plated 4 and 18 hours post infection. Again, we observed a strong intracellular growth restriction of _S_.
Typhimurium in these mutant macrophages, compared to scramble sgRNAs (Fig. S4A). The growth restriction phenotypes were also not due to bacterial uptake differences as similar bacteria
numbers were evident in scramble and mutant macrophages infected with the isogenic BFP-expressing _S_. Typhimurium strain, _phoN_::BFP39 for 1-hour, as analyzed by confocal microscopy (Fig.
S4B, C). To confirm that intracellular bacterial growth restriction phenotypes of GID/CTLH knockout in macrophages were conserved in human cells, we generated _GID8_ and _MAEA_ knockouts in
primary human monocyte-derived macrophages (hMDMs) by directly electroporating CRISPR ribonucleoproteins (CRISPR RNPs) in monocytes40. We targeted each gene with at least 2 sgRNAs and were
able to achieve 50–70% knockout efficiencies with some sgRNAs, specifically sgRNA 2 for _hGID8__−/−_ and sgRNA 1 for _hMAEA__−/−_ (Fig. S5A, Supplementary Data 3). More importantly, we
observed _Mtb_ growth restriction levels that were dependent on the degree of protein knockout in these hMDM mutants by both CFUs and luciferase readouts (Fig. S5B, C). These data
illustrated the functional conservation of the GID/CTLH complex in mediating intracellular growth restriction of bacteria in both mice and humans. To assess GID/CTLH knockout BMDMhs ability
to resist _Mtb_-induced cell death, which was a key feature of our CRISPR screening strategy, we analyzed the mutant macrophages cell death kinetics upon infection with the _Mtb_ Erdman
strain at MOI 1 by microscopy 4- and 5-days post infection. _Mtb_ infection induced macrophage death that resulted in about half of the cells appearing necrotic by day 4 in scrambled sgRNA
macrophages (Fig. 2C), which is consistent with LDH and flow cytometry-based quantifications (Figs. 1A and S1A). Almost all the cells in the scramble control appeared necrotic by day 5 (Fig.
2C). However, GID/CTLH knockout macrophages were highly resistant to cell death with 100% survival rates on days 4 and 5. Together, these data suggested that the knockout of the GID/CTLH
complex does not just restrict the intracellular growth of _Mtb_ in macrophages but also inhibits necrotic cell death programs triggered by the bacteria. We investigated whether knockout of
other members of the GID/CTLH complex that were not identified in the primary screen also impacted _Mtb_ growth in BMDMhs by generating _RANBP9__−/−_ and MKLN1−/− knockouts (Fig. S6A,
Supplementary Data 3) and assessing bacterial growth upon infection with the _Mtb_ Erdman-Lux reporter strain at MOI 0.5. We observed significant _Mtb_ growth restriction in _RANBP9__−/−_
and _MKLN1__−/−_ knockout BMDMhs 4 days post-infection when compared to scramble sgRNA (Fig. S6B). _RANBP9__−/−_ and _MKLN1__−/−_ BMDMhs also displayed increased resistance to _Mtb_ induced
cell death when infected with the _Mtb_ wild-type Erdman strain at MOI 1 for 4 days (Fig. S6C). The levels of _Mtb_ growth restriction and resistance to cell death in _RANBP9__−/−_ and
_MKLN1__−/−_ BMDMhs were lower than the GID/CTLH members identified in the screen (Fig. 2). The design of the screen clearly favored those members of the complex which, upon knockout,
resulted in strongest anti-bacterial phenotypes. _MTB_-INFECTED GID/CTLH KNOCKOUT MACROPHAGES ARE METABOLICALLY RESILIENT AND MORE GLYCOLYTICALLY ACTIVE In yeast, the GID/CTLH complex is
directly involved in glucose metabolism by regulating gluconeogenesis32. Even though some studies have shown that the complex is dispensable in the regulation of carbohydrate metabolism in
human cells30, others have identified glycolytic enzymes L‐lactate dehydrogenase A chain (LDHA) and pyruvate kinase M1/2 (PKM) as direct targets of GID/CTLH ubiquitination41. In fact,
depletion of the GID/CTLH complex member _RMND5A_ reduces polyubiquitination of LDHA and PKM and renders the cells more glycolytic41. Moreover, the GID/CTLH complex (member _RMND5A_) appears
to regulate cell energy homeostasis by negatively modulating the activity of the AMP-activated protein kinase (AMPK)42. We therefore profiled the metabolic state of a selected GID/CTLH
knockout BMDMhs (_GID8__−/−__, MAEA__−/−_ and _WDR26__−/−_) in uninfected or _Mtb_ infected conditions. We chose to characterize these 3 mutants further because, amongst the GID/CTLH complex
members, _GID8_ or _TWA1_ is a scaffold that holds the complex together, _MAEA_ forms part of the catalytic core30, and _WDR26_ was identified in a previous related screen20 and is also
predicted to be central to the core structure of the complex30. We first checked the relative mRNA expression of the glycolytic enzyme hexokinase 2 (_Hk2_) and the gluconeogenic enzyme
fructose-1,6-bisphosphatase 1 (_FBP1_) in these GID/CTLH knockout BMDMhs. We observed a significant increase in mRNA expression of _HK2_ in uninfected GID/CTLH knockout BMDMhs as compared to
scramble (Fig. S7A). _Mtb_ infection maintained a higher expression of _HK2_ in GID/CTLH knockout BMDMhs 4 hours post infection as compared to scramble, but these responses resolved after
24 hours of infection (Fig. S7A). This is consistent with a biphasic metabolic profile of macrophage responses to _Mtb_ infection, which is characterized by an early upregulation of
glycolytic genes in the first 4–8 hours of infection that diminishes 24–48 hours later43. We did not, however, observe any differences in _FBP1_ expression in uninfected or _Mtb_ infected
GID/CTLH knockout BMDMhs (Fig. S7A). These results suggest that GID/CTLH knockout in BMDMhs does not impact gluconeogenesis (at least at the transcript level) but programs the cells to a
more glycolytic state, which is consistent with previous observations41. To further confirm these phenotypes, we analyzed the metabolic status of GID/CTLH knockout BMDMhs by Seahorse
extracellular flux analyses using the Agilent Mito and Glycolysis Stress Test kits. Compared to scramble sgRNA, uninfected GID/CTLH knockout BMDMhs displayed increased basal oxygen
consumption rates (OCRs) and reduced spare respiratory capacity (SRC) (Fig. S7B, C) which suggested impacted mitochondrial activities and oxidative phosphorylation as a consequence of
GID/CTLH knockout. In agreement with our qPCR data (Fig. S7A), we also observed increased glycolytic flux in uninfected GID/CTLH knockout BMDMhs as evidenced by high basal extracellular
acidification rates (ECAR) and spare glycolytic capacity (SGC) (Fig. S7D). Under basal conditions, _Mtb_ infection of wild type macrophages increases glycolytic rates that are required to
control bacterial replication44 while at the same time decelerates mitochondrial respiration45. Indeed, when we infected scramble macrophages with _Mtb_ for 24 hours, SRC rates collapsed to
almost baseline levels (Fig. 3A, B) compared to uninfected cells (Fig. S7B, C). However, _Mtb_ infected GID/CTLH knockout macrophages were more resilient as they maintained significantly
higher basal OCRs and SRCs as compared to scramble sgRNA (Figs. 3A, B, S7C). Glycolytic rates in _Mtb_ infected GID/CTLH knockout BMDMhs were also significantly higher as compared to
scramble sgRNA (Fig. 3C, D) albeit at an even higher rate than the uninfected (Fig. S7D). Together, our qPCR and extracellular flux analysis data demonstrated that knockout of the GID/CTLH
complex in macrophages impairs mitochondrial respiration and proportionally increases flux through glycolysis. However, GID/CTLH knockout BMDMhs are able to withstand an _Mtb_-induced
bioenergetic shutdown45 despite displaying reduced spare mitochondrial capacity in uninfected conditions. Our metabolic analyses also pointed to a state of energy stress in GID/CTLH knockout
BMDMhs, which could be regulated, in part, by AMPK, a master regulator of cell energy homeostasis46. Given that AMPK can be modulated (negatively) by the GID/CTLH complex42, we also checked
AMPK activity in uninfected and _Mtb_-infected GID/CTLH knockout macrophages by monitoring the levels of total and activated (phosphorylated) AMPK in western blot analyses. Both uninfected
and _Mtb_ infected GID/CTLH knockout macrophages displayed reduced expression of total AMPK (Figs. 3E and S8A–C), which could possibly explain reduced SRC rates of these mutants at least in
uninfected states (Fig. S7B). We, however, observed increased AMPK phosphorylation in both uninfected and _Mtb_-infected GID/CTLH knockout macrophages (Figs. 3E, F and S8A, B), and the
P-AMPK/AMPK ratios were significantly higher in the mutants (Fig. 3G). DUAL RNA-SEQUENCING IDENTIFIES GABAERGIC SIGNALING AND AUTOPHAGY AS MAJOR HOST DETERMINANTS OF _MTB_ RESTRICTION IN
GID/CTLH KNOCKOUT BMDMHS To further identify effectors that mediate _Mtb_ growth restriction in GID/CTLH knockout BMDMhs, we performed dual RNA-sequencing (dual RNA-seq) of _Mtb_-infected
knockout macrophages to capture both host and bacterial transcriptomes47. Scramble sgRNAs or GID/CTLH knockout BMDMhs (_GID8__−/−__, MAEA__−/−__, WDR26__−/−_) were infected with the _Mtb_
smyc’::mCherry strain for 4 days. On day 4, we flow-sorted infected macrophages based on mCherry positivity and extracted RNA for dual RNA-seq47. Principal component analysis (PCA) of the
macrophage transcriptomes revealed unique clustering of _GID8__−/−__, MAEA__−/−_ and _WDR26__−/−_ BMDMhs which, despite being slightly distant from each other, clustered close together in
the first principal component as compared to scramble sgRNA (Fig. 4A). Using an adjusted _p_ value of <0.05 and an absolute log2 fold change of > 0.5, we identified 1911 upregulated
and 918 downregulated genes in _GID8__−/−_ macrophages, 2106 upregulated and 940 downregulated genes in _MAEA__−/−_ macrophages and 2890 upregulated and 1845 downregulated genes in
_WDR26__−/−_ macrophages (Supplementary Data 4). Due to the similar separation of _GID8__−/−__, MAEA__−/−_ and _WDR26__−/−_ in our PCA analysis (Fig. 4A), we generated a Venn diagram to
identify differentially expressed (DE) genes in common between the three mutant macrophage populations. Indeed, the majority of DE genes (1267 upregulated, 421 downregulated) were present in
the _GID8__−/−__, MAEA__−/−_ and _WDR26__−/−_ _Mtb_ infected BMDMhs (Fig. 4B, C, Supplementary Data 4). Among the highly upregulated genes in the commonly DE gene set were those involved in
macrophage effector functions that are known to control _Mtb_ replication, such as the NADPH oxidase (_NOX3_) and autophagy (_ATG9B, TRIM2, TRIM7, and TRIM16_) (Supplementary Data 4).
Ferroportin 1 (_SLC40A1_), the only known membrane protein to transport iron out of the cells48, was also among the most significantly upregulated genes in _GID8__−/−__, MAEA__−/−_ and
_WDR26__−/−_ _Mtb_ infected BMDMhs (Supplementary Data 4). This suggested that GID/CTLH knockout in BMDMhs could restrict _Mtb_ growth by nutrient (iron) limitation, enhancing autophagy and
increasing the production of reactive oxygen species (ROS). Given that the role of the GID/CTLH complex in macrophage anti-microbial functions is mostly unknown, we used our RNA-seq results
to check the expression status (at transcript level) of the 12 GID/CTLH members, at least in wild-type (scramble) _Mtb_ infected macrophages. We detected transcripts for all 12 members of
the GID/CTLH complex, which suggests active expression of the entire complex in myeloid cells (Fig. S9A). To gain more insights into biological pathways that could mediate _Mtb_ growth
restriction in GID/CTLH knockout macrophages, we performed pathway enrichment analysis49 of the _GID8__−/−__, MAEA__−/−_ and _WDR26__−/−_ commonly DE genes (Supplementary Data 4). In the
upregulated gene set, we identified 60 biological processes (BP), 30 cellular components (CC), and 27 molecular functions (MF), which were enriched based on an adjusted _p_ value of <0.05
(Fig. 4D, Supplementary Data 5). Amongst the commonly downregulated DE genes, 332 BP, 16 CC, and 44 MF were enriched (Fig. S9B, Supplementary Data 5). Of the top enriched BPs in the
commonly upregulated genes (Fig. 4D), the most enriched pathways included those involved in Ca2+ and G protein receptor signaling, effector processes that are known to play important roles
in macrophage responses against _Mtb_50,51. Of note, γ-Aminobutyric acid (GABA) and neurotransmitter transport were, similarly, amongst the most enriched pathways in the GID/CTLH knockout
upregulated genes (Fig. 4D, Supplementary Data 5). Within the GID/CTLH complex, only one member (Muskelin, _MKLN1_) has been shown to be involved in GABA receptor trafficking and
internalization52. We, however, identified six genes in the GABAergic signaling pathway (_GABBR1_, _GABRB2_, _GABARAPL1_, _SLC32A1_, _SLC6A13_, _SLC6A12_) which were upregulated in all the
three _Mtb_ infected GID/CTLH knockout mutants; _GID8__−/−_, _MAEA__−/−_ and _WDR26__−/−_ (Supplementary Data 4 and 5). In fact, _GABBR1_ and _GABRB2_ were amongst the most significantly
upregulated genes in the GID/CTLH knockout macrophages with up to 350-fold upregulation as compared to scramble sgRNA (Supplementary Data 4). GABAergic signaling has recently been linked to
intracellular macrophage control of _Mtb_ and _S_. Typhimurium. Treatment of macrophages with GABA or GABAergic drugs enhances the anti-microbial properties of macrophages against
intracellular bacterial infections by promoting phagosome maturation and autophagy53. Moreover, the apparent GABAergic protective responses in BMDM macrophages were driven, in significant
part, by an increase in Ca2+ signaling53. Taken together, our pathway enrichment data suggested that GID/CTLH knockout in BMDMhs increases GABA and Ca2+ signaling, which may, similarly,
promote macrophage control of _Mtb_ by promoting autophagy53. A further consequence of GABA treatment in _Mtb_ infected BMDMs and in infected mice lungs in vivo was the inhibition of
pro-inflammatory markers such as Tnf and IL-653. Similarly, pro-inflammatory pathways were the most significantly enriched pathways in our GID/CTLH commonly downregulated genes (Fig. S9B,
Supplementary Data 5). These included pathways involved in IL-1β production, antigen presentation, toll receptor signaling, and cellular responses to Tnf. Our further analysis of the RNA-seq
data did indeed show significant downregulation of IL-1β and Tnf transcripts in all the GID/CTLH knockout macrophages infected with _Mtb_ on day 4 (Fig. S9C). Consistent with the known role
of the GID/CTLH complex in cell proliferation34, pathways related to DNA replication were also among the most enriched in the downregulated gene set (Fig. S9B). We also observed a
significant downregulation of pathways related to cell death programs and apoptosis signaling (Supplementary Data 5) in GID/CTLH knockout BMDMhs, which would possibly explain the increased
resistance of these macrophages to _Mtb_-induced cytotoxicity (Fig. 2C). NUTRITIONAL STRESS IS THE MAIN TRANSCRIPTOME SIGNATURE OF INTRACELLULAR _MTB_ IN GID/CTLH KNOCKOUT BMDMHS To assess
the impact of host stressors on _Mtb_, we analyzed parallel transcriptomes of intracellular _Mtb_ (Fig. 4A). Using an adjusted _p_ value of <0.05 and an absolute log2 fold change of
>0.3, we identified 168 _Mtb_ genes (87 upregulated, 81 downregulated) in _GID8__−/−_ BMDMhs, 40 _Mtb_ genes (13 upregulated, 27 downregulated) in _MAEA__−/−_ BMDMhs and 46 _Mtb_ genes
(22 genes upregulated, 26 downregulated) in _WDR26__−/−_ BMDMhs which were DE (Fig. 5A, Supplementary Data 6). Interestingly, clusters of upregulated _Mtb_ genes in these GID/CTLH knockout
BMDMhs were readily categorized into essential _Mtb_ biological processes such as iron scavenging, cholesterol breakdown, fatty acid oxidation, amino acid metabolism and stress responses
(Fig. 5B). _Mtb_ utilizes Fe3+ iron specific siderophores, mycobactins and carboxymycobactins, to scavenge and acquire iron from the host for its nutritional requirements. _Mtb_ mycobactins
are organized into a cluster of _Mbt_ (_MbtA_-_MbtJ_) and _Mbt-2_ (_MbtK_-_MbtN)_ genes54. We identified five genes in the _Mbt_ cluster (_MbtB_, _MbtI_, _MbtD_, _MbtE, MbtF_) which were
upregulated in _Mtb_ transcriptomes isolated from GID/CTLH knockout BMDMhs (Fig. 5B, Supplementary Data 6). A positive regulator of _Mbt_ operonic gene expression, _HupB_55, was also
upregulated in all the three _Mtb_ transcriptomes isolated from GID/CTLH knockout macrophages (Fig. 5B, Supplementary Data 6). These data suggested that GID/CTLH knockout macrophages, in
part, limit intracellular _Mtb_ growth by reducing the bacteria’s access to iron. This is consistent with our host transcriptome data which showed a significant upregulation of the iron
efflux transporter (_SLC40A1_) in GID/CTLH knockout macrophages (Supplementary Data 4). The data is also consistent with our previous findings, which have shown that iron limitation is one
of the prominent stresses experienced by _Mtb_ in vivo in lung interstitial macrophages, which are less permissive to _Mtb_ growth47 and that similar _Mtb-_restrictive phenotypes can be
reproduced in human macrophages using iron chelating chemical inhibitors29. _Mtb_ nutrient limitation in GID/CTLH knockout macrophages was not limited to iron as we also observed an
upregulation of genes involved in beta-oxidation of fatty acids (_FadE9_, _FadD5_, _FadE22_, _FadE23_)56, cholesterol breakdown (_HsaD_)57 and several members of the _Mce1_ operon (_Mce1A_,
_Mce1B_, _Mce1F_, _Mce1C_, _yrbE1A_, _IprK_), which is required for _Mtb_ fatty acid import58,59. Our previous work has demonstrated that limiting _Mtb_’s access to iron can upregulate
cholesterol metabolism genes to re-balance impaired metabolic fluxes that arise due to diminished activity of iron-dependent metabolic pathways29. Even though upregulation of _Mtb_ lipid
metabolism genes in GID/CTLH knockout macrophages could, indeed, be a compensatory mechanism to iron limitation29, _Mtb_ nutrient restriction in these macrophages appears to be more
pervasive as the bacteria also upregulates genes involved in tryptophan biosynthesis pathways (_TrpE_, _TrpA_) (Fig. 5B, Supplementary Data 6). _Mtb_ scavenges tryptophan from its host
macrophage, however, upon immune activation, the macrophage turns on its tryptophan degradation pathway, and the bacteria respond by upregulation of its tryptophan biosynthetic genes60,61.
GID/CTLH knockout macrophages also appear to induce other non-nutritional stresses to intracellular _Mtb_ as evidenced by the upregulation of genes required to enhance survival at low pH in
the phagosome (_Rv1264_)62. _Mtb_ DNA damage and repair genes (_DnaN_, _DnaB_, _gyrB_, _recA_) (Fig. 5B) were also upregulated, which is indicative of increased oxidative stress as we have
previously observed in _Mtb_ isolated from growth-restrictive interstitial macrophages in vivo47. Interestingly, _Mtb_ in GID/CTLH knockout macrophages downregulated major virulence genes,
including those belonging to the _ESX-1_ type VII secretion system (Fig. 5B). We identified 4 genes in the _ESX-1_ operon (_espA_, _esxB_, _esxH_, _espF_) which were significantly
downregulated in _Mtb_ isolated from GID/CTLH knockout macrophages. A further three genes (_WhiB1, WhiB3, WhiB6_) belonging to the _WhiB-_like family of transcription factors that play key
roles in _Mycobacteria_ virulence and antibiotic resistance63 were also significantly downregulated (Fig. 5B, Supplementary Data 6). Of note, _WhiB6_ is one of the main regulators of _ESX-1_
gene expression in _Mtb_64,65. Central to the activity of all _WhiB_ transcription factors are highly conserved cysteine-bound iron-sulfur clusters that can act as reductive sinks to host
nitric oxide or ROS produced during macrophage activation63. It has been demonstrated that disruption of iron-sulfur clusters in _WhiB_ transcription factors either by iron limitation or
excessive nitrosative stress can trigger a significant reprogramming of gene expression in _Mtb_66. Downregulation of _Mtb WhiB_ genes in GID/CTLH knockout macrophages could also, therefore,
be a result of iron-limiting conditions in these mutant phagocytes, which in the case of _WhiB6_ could trigger a feed-forward effect to downregulate downstream _ESX-1_ genes. As a
consequence of _ESX-1_ downregulation, _Mtb_ resident in GID/CTLH knockout macrophages may be attenuated and possibly trapped in phagosomes, given the role of this secretion system in _Mtb_
virulence and phagosomal escape15. Several toxin-antitoxin (TA) genes were also downregulated in _Mtb_ isolated from GID/CTLH knockout macrophages (Fig. 5B, Supplementary Data 6). Despite a
reductive evolution in the _Mtb_ genome, TA systems have been maintained to support _Mtb_ replication, virulence and stress adaptation67. Reduced expression of _Mtb_ TA genes in GID/CTLH
knockout macrophages could thus be a further indication of shutdown in most bacterial virulence processes in parallel with the observed growth restriction phenotypes (Figs. 2A, B). GID/CTLH
KNOCKOUT MACROPHAGES DISPLAY INCREASED GABAERGIC AND CA2+ SIGNALING We followed up on our dual RNA-seq data (Fig. 4) to experimentally validate some of the host effectors that mediate _Mtb_
growth restriction in GID/CTLH knockout BMDMhs. We first checked the levels of GABA signaling in mutant macrophages by staining the cells for total endogenous GABA with an anti-GABA
antibody. Murine BMDMs and lung macrophages express functional GABA in resting states53, and we assessed levels of GABA in uninfected scramble BMDMhs by confocal microscopy (Fig. S10A). All
the three uninfected GID/CTLH knockout BMDMhs had significantly higher levels of GABA as compared to scramble sgRNA (Figs. S10A and 6B). It was previously shown that _Mtb_ infection of
mouse BMDMs in vitro and in vivo significantly decreases GABA levels53. In agreement with these findings, we observed a marked reduction of GABA expression in _Mtb-_infected scramble BMDMhs
(Fig. 6A). In contrast, _Mtb_ infection significantly amplified GABA levels in GID/CTLH knockout BMDMhs to even greater levels than uninfected cells (Fig. 6A, B). Increased GABAergic
signaling can positively modulate intracellular Ca2+ mobilization68. Moreover, Ca2+ signaling and transport were over-represented in GID/CTLH knockout BMDMhs commonly upregulated genes (Fig.
4D). We, therefore, checked intracellular Ca2+ mobilization in GID/CTLH knockout BMDMhs by loading the cells with a cell-permeable fluorogenic calcium-binding dye (Fluoforte®) followed by
quantification of emitted fluorescence post stimulation with a Ca2+ mobilization agonist, adenosine triphosphate (ATP). Compared to scramble sgRNA BMDMhs, uninfected GID/CTLH knockout BMDMhs
displayed increased intracellular calcium mobilization as evidenced by significantly higher normalized fluorescence 5 minutes after stimulation with ATP (Fig. S10B). In common with GABA
signaling, _Mtb_ infection significantly reduced Ca2+ mobilization in scramble BMDMhs but amplified the responses in GID/CTLH knockout macrophages (Fig. S10B). These functional data
corroborate our host dual RNA-seq data sets, which showed a specific enrichment of GABAergic and Ca2+ signaling pathways in _Mtb_-infected GID/CTLH knockout macrophages (Fig. 4D,
Supplementary Data 5). GID/CTLH KNOCKOUT MACROPHAGES ARE MORE AUTOPHAGIC AND ANTI-INFLAMMATORY Increased GABA signaling in macrophages enhances anti-microbial responses by promoting
autophagy53. GABARAPL1, one of the upregulated GABA type A receptor in GID/CTLH knockout BMDMhs (Supplementary Data 4) is a homolog of the autophagy related protein 8 (_ATG8_)69. Genes
involved in the autophagic process (_ATG9B, TRIM2, TRIM7_, and _TRIM16)_ were also among the most significantly upregulated in _Mtb_ infected GID/CTLH knockout BMDMhs (Supplementary Data 4).
Moreover, in both uninfected and _Mtb_ infected conditions, GID/CTLH knockout BMDMhs display significantly increased AMPK phosphorylation (Figs. 3E–G and S8A, B). AMPK activates catabolic
processes like autophagy as part of the energy stress response in cell-starvation conditions46. These observations suggested that GID/CTLH knockout BMDMhs may be more autophagic as part of
their anti-microbial responses. We therefore checked autophagic flux in GID/CTLH knockout BMDMhs by (1) immunoblotting to monitor the turnover of the microtubule-associated protein light
chain 3 (LC3) from its LC3I to LC3II version (2) confocal imaging of anti-LC3 stained cells to quantify total LC3 puncta per individual cell and LC3 colocalizing with fluorescent _Mtb_. We
observed significantly increased autophagic flux in _Mtb_ infected GID/CTLH knockout BMDMhs as evidenced by high LC3I to LC3II turnover ratios when compared to scramble BMDMhs (Figs. 7A, B,
and S11A, B). The magnitude of LC3I to LC3II turnover proportionally increased based on the bacterial burden at different MOIs (Figs. 7A, B and S11A, B). Some GID/CTLH knockout BMDMhs
(_MAEA__−/−_) displayed increased LC3I to LC3II turnover even in uninfected conditions (Figs. 7A, B, and S11A, B). Furthermore, _Mtb_-infected GID/CTLH knockout BMDMhs displayed
significantly higher numbers of total LC3 puncta per cell as compared to scramble when we analyzed the anti-LC3 stained cells by confocal microscopy (Fig. S11C, D). More importantly, GFP
expressing _Mtb_ significantly colocalized with LC3 in GID/CTLH knockout BMDMhs (Fig. S11C, E) when compared to scramble sgRNA, which indicated increased autophagic targeting of _Mtb_ in
these mutant macrophages. Even though knockout of the GID/CTLH member, _MAEA_, has been shown to impair autophagy70 in hematopoietic stem cells, our data supports the contention that
increased autophagy is one of the main drivers of _Mtb_ restriction in GID/CTLH knockout macrophages. _Mtb_ infected macrophages with increased GABA expression also diminish the expression
of pro-inflammatory markers53. We checked the expression of pro-inflammatory markers (IL-1β and type 1 interferons (IFN-β)) in GID/CTLH knockout macrophages during the early stages of _Mtb_
infection. We already observed a significant downregulation of pro-inflammatory markers including Tnf and IL-1β in GID/CTLH knockout macrophages in our RNA-seq data, collected 4 days post
infection (Fig. S9). We concentrated our further analysis of the expression of IFN-β and IL-1β in the first 24 hours of infection. qPCR analysis showed that _Mtb_ infection caused a
significant upregulation of IFN-β mRNA in scramble macrophages 4 hours post infection, but this response diminished 24 hours post infection (Fig. 7C). In contrast, IFN-β mRNA expression was
completely suppressed in GID/CTLH knockout macrophages at both time points, and this was confirmed by ELISA quantification of IFN-β in cell supernatants 24 hours post infection (Fig. 7C).
mRNA levels of IL-1β significantly increased in GID/CTLH knockout macrophages during the early 4 hours of infection, but this quickly resolved after 24 hours of infection to non-significant
levels (Fig. S12A). This contrasted with depleted levels of IL-1β mRNA 4 days post infection in our RNA-seq data (Fig. S9B, C). We were, however, able to confirm by ELISA that the reduction
of IL-1β mRNA expression at 24 hours corresponds with significantly lower levels of released IL-1β in culture supernatants as compared to scramble sgRNA (Fig. S12A). Western blot analysis
demonstrated that both the pro and mature versions of IL-1β were equally impacted (Fig. S12B), suggesting that the anti-IL-1β properties of GID/CTLH knockout could be occurring upstream of
IL-1β caspase mediated processing. These data provide further evidence that GID/CTLH knockout impairs the production of pro-inflammatory markers in _Mtb_-infected macrophages. DISCUSSION In
this report, we have employed a high throughput CRISPR genetic screen in primary macrophages to identify host effectors which, upon perturbation, improve the control of intracellular
replication of _Mtb_. In the screen, pathways known previously to interfere with _Mtb_ growth when perturbed, such as mTOR signaling and the mitochondrial OXPHOs, were specifically enriched.
However, we also identified a novel role for the mammalian GID/CTLH complex in macrophage anti-microbial properties and found that its presence and activity correlated inversely with
intracellular _Mtb_ replication. The GID/CTLH complex is an evolutionarily conserved member of the E3 ubiquitin-proteasome system, which was initially identified in yeast as a regulator of
gluconeogenesis32 but is more functionally diverse in higher eukaryotes34. With up to 12 functional members of the complex identified in mice and humans30,34, the catalytic core of the
complex is formed by the 2 RING proteins MAEA and RMND5A, while GID8 (TWA1) is a predicted scaffold that holds the complex together30. The biological significance of the complex in mammals
is still being investigated34. However, it is ubiquitously expressed in most mammalian cells30, which suggests its activities may have evolved to fulfill diverse functions. As we show in
this study, all 12 members of the GID/CTLH complex appear to be actively transcribed in macrophages. Individual knockout of >50% of the GID/CTLH complex members in murine and human
macrophages renders these phagocytes hostile to the intracellular replication of both _Mtb_ and _S_. Typhimurium. It has been suggested that the GID/CTLH complex is involved in regulating
cell proliferation, death, and survival pathways34. SiRNA-mediated knockdown of the GID/CTLH complex member RANBP9 made the cells more resistant to programmed cell death71,72. Indeed,
knockout of the GID/CTLH complex in macrophages also rendered these phagocytes more resistant to _Mtb_ induced necrotic cell death, a favorable host outcome, which can limit bacterial spread
and virulence11,14. Moreover, GID/CTLH knockout in macrophages reprogrammed these cells to a more glycolytic state with increased activation of AMPK in line with previous observations41,42.
Our results also identified additional anti-microbial responses upregulated in GID/CTLH complex knockout macrophages, such as the NADPH oxidase, autophagy, GPCR signaling, iron efflux,
GABAergic signaling, and intracellular Ca2+ mobilization. GABAergic signaling has only recently been implicated in macrophage control of intracellular bacterial pathogens by promoting
autophagy and inhibiting the production of pro-inflammatory cytokines53. Our results independently corroborate these findings as we observe increased GABA levels in GID/CTLH knockout BMDMhs,
which is accompanied by enhanced autophagy and elevated anti-inflammatory properties. Dual RNA-seq also revealed that _Mtb_ residing in GID/CTLH knockout macrophages displayed
transcriptional signatures consistent with nutritional stress as characterized by the upregulation of iron scavenger pathways, cholesterol breakdown, amino acid metabolism, fatty acid
import, and oxidation. There is an increased interest in the incorporation of HDTs in anti-TB drug regimens as a possible means of shortening the duration of treatment28,51,73,74. In theory,
HDTs have the potential to (1) enhance host immune responses (2) promote macrophage control of intracellular _Mtb_ (3) reduce non-productive and tissue injurious inflammation to improve
lung function, and (4) minimize host stresses that lead to induction of _Mtb_ drug tolerance74. As we report, knockout of the GID/CTLH complex in macrophages induces a broad range of anti-TB
responses and results in anti-inflammatory host cells, which suggests it would make a tractable target for HDTs against _Mtb_. Several members of this complex are upregulated in a variety
of cancers, and targeting this complex for cancer therapeutics is an area of current interest34,75,76,77. As efforts continue to identify novel HDTs against TB1,51,74,78, the GID/CTLH
complex offers an attractive target, and discovery efforts could benefit from the emerging therapeutic pursuit of the complex in the cancer field. METHODS ETHICS STATEMENT The protocols used
in this study have been reviewed and approved by the Institutional Biosafety Committee (protocol #16291-3) and the Institutional Animal Care and Use Committee (protocol # 2011-0086) at
Cornell University. BACTERIAL STRAINS The parent _Mtb_ Erdman _strain_ (ATCC 35801, PDIM positive) was used in all CFU experiments. Fluorescent _Mtb_ reporters in the Erdman background;
_Mtb_ Erdman smyc’::mCherry and _Mtb_ Erdman hsp60::GFP have been described previously79,80. The _Mtb_ Erdman-Lux strain was generated by transformation with the pMV306G13+Lux plasmid81.
_Mtb_ was grown to log phase at 37 °C in MiddleBrook 7H9 broth supplemented with 10% oleic acid/albumin/dextrose/catalase (OADC Enrichment; Becton, Dickinson and Company), 0.2% glycerol, and
0.05% tyloxapol (Sigma-Aldrich). Reporter strains were maintained in the presence of an appropriate antibiotic; _Mtb_ Erdman-Lux _Mtb_ strain: 25μg/ml kanamycin, _Mtb_ Erdman hsp60::GFP:
25μg/ml kanamycin and 50 μg/ml hygromycin, _Mtb_ Erdman smyc’::mCherry: 50 μg/ml hygromycin. For _Salmonella_ experiments, wild-type _S_. Typhimurium (CA32; ATCC 14028s) and the BFP
expression strain _S_. Typhimurium CA4705 phoN::BFP39 were used. _Salmonella_ strains were propagated at 37 °C in Luria-Bertani (LB) broth. MAMMALIAN CELLS AND CULTURE Murine Hoxb8-Estradiol
(ER) responsive Cas9+ eGFP conditionally immortalized myeloid progenitor cells were generated as previously described22. Hoxb8-ER cells were maintained in RPMI (Corning®) supplemented with
10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 20 ng/ml murine GM-CSF (PeproTech), 0.5 μM β-estradiol, 10 mM HEPEs and 1% penicillin/streptomycin. To obtain BMDMhs
from Hoxb8-ER myeloid progenitors, cells were rinsed twice with 1× PBS to completely remove β-estradiol and resuspended in BMDM differentiation media; DMEM (Corning®) supplemented with 10%
FBS, 15% L-cell conditioned media, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1% penicillin/streptomycin at 37 °C for 6–7 days. Cells were maintained at a density of ~0.5 × 106 cells/ml
during differentiation. The HEK-293FT cell line was purchased from Invitrogen and cultured in DMEM supplemented with 10% FBS, 1% non-essential amino acids, 2 mM L-glutamine, 1 mM sodium
pyruvate, and 1% penicillin/streptomycin. Human monocytes were commercially obtained from the University of Nebraska Medical Center Elutriation Core and differentiated to HMDMs in DMEM
medium with 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 1% penicillin/streptomycin and 10% pooled human serum (Sera Care). INFECTION OF MACROPHAGES WITH _MTB_ BMDMhs were infected
with _Mtb_ as previously described82. Briefly, log phase bacteria were pelleted, resuspended in basal uptake buffer (25 mM dextrose, 0.5% bovine serum albumin, 0.1% gelatin, 1 mM CaCl2, 0.5
mM MgCl2 in PBS) and syringed with a tuberculin syringe for at least 20 times. Bacteria were then resuspended in antibiotic free macrophage media before infection at specified MOIs. CFU
QUANTIFICATION OF INTRACELLULAR BACTERIA Confluent macrophage monolayers (human and murine) were infected with _Mtb_ at an appropriate MOI. After 3–4 hours, extracellular bacteria were
removed by washing with fresh macrophage media at least three times. At indicated time points, macrophages were lysed with 0.01% SDS in water for 15 minutes and serially diluted in 0.05%
Tween-80 in 1× PBS. Lysates were plated on 7H10 OADC agar plates and incubated at 37 °C for CFU counting 3–4 weeks later. CLONING OF SGRNAS, LENTIVIRAL PRODUCTION, AND TRANSDUCTION sgRNAs
for CRISPR-mediated gene knockouts (Supplementary Data 3) were selected from the Brie murine knockout library23 or ordered in predesigned form from IDT for human CRISPR RNP targets. For
lentiviral delivery of sgRNAs, oligo pairs were annealed and cloned into the pLentiGuide-Puro (Addgene #52963). sgRNA inserts were confirmed by sequencing. To generate lentivirus particles
for transduction, pLentiGuide-Puro plasmids with sgRNAs of interest were co-transfected with Invitrogen packaging plasmids (pLP1, pLP2, and pLP/VSVG) in 293FT cells using the Lipofectamine
3000 lentiviral production protocol according to manufacturer’s guidelines. To transduce Hoxb8 Cas9+ eGFP progenitors, 2.5 × 105 cells in 12-well plates were spin-infected at 1000 × _g_ for
90 minutes at 32 °C in the presence of 10 µg/ml protamine sulfate. 48 hours post-transduction, 8 µg/ml puromycin was added and maintained for 4 days. After the 4-day selection period,
puromycin-resistant cells were briefly expanded for 1–2 days and frozen for future experiments. To quantify CRISPR editing efficiencies, total genomic DNA was prepared from cell pellets
using the Qiagen DNeasy Blood and Tissue kit from which genomic sites surrounding the sgRNA target sites were PCR amplified using the Invitrogen Platinum _Taq_ polymerase. Population-level
editing efficiencies were estimated using ICE37 and western blot analysis of differentiated BMDMhs. CRISPR SCREEN KNOCKOUT LIBRARY PREPARATION The murine Brie knockout library was obtained
from Addgene (#73633)23. The library (in pLentiGuide-Puro background) contains 78,637 sgRNAs targeting 19,674 genes (four sgRNAs/gene) with an additional 1000 non-targeting sgRNA controls.
The library was amplified using the Moffat’s lab CRISPR knockout library amplification protocol83. To generate lentivirus, 293FT cells were transfected as described above, scaling up tissue
culture flasks depending on the amount of virus required to achieve desired library coverage. ~180 million Cas9 eGFP Hoxb8 progenitor cells were then transduced with the lentiviral library
in the presence of 8 µg/ml polybrene at an MOI of ~0.3 (8 12-well plates, 2 × 106 cells/well, >700× library coverage) to ensure that only a single gene was targeted in every cell. 48
hours post-transduction, cells were selected with 8 µg/ml puromycin for 4 days and frozen in multiple aliquots of >40 million cells (>500× library coverage). Before screening, each
aliquot was thawed and allowed to recover in Hoxb8 media for 3–4 days. Cells were then rinsed in 1× PBS at least 2 times before transfer into BMDM differentiation media to generate BMDMhs
knockout libraries. _MTB_ INFECTION OF BMDMH KNOCKOUT LIBRARY, ANTIBODY STAINING, AND FLOW SORTING The BMDMh knockout library was infected with the _Mtb_ Erdman strain at MOI 1 for 4 days.
The screen was carried out in three independent replicates with ~200 million cells per replicate (2 T-300 flasks). On day 4, all the cells were harvested by initially pretreating cell
monolayers with Accutase for 5–10 minutes at room temperature, followed by scrapping in cold 1× PBS. Harvested cells were stained with the live/dead viability dye eFlour 506 (Invitrogen;
1:1000) and the PE anti-mouse/human CD11b antibody (Biolegend; 1:500) for 30 minutes at 4 °C. Stained cells were fixed in paraformaldehyde (PFA) for >1 hour before FACS of cells in the
live gate. FACS was carried out on a Sony MA900 sorter, and a minimum of 10 million cells were sorted per replicate to achieve a >150× library coverage. DNA EXTRACTION, BARCODE
AMPLIFICATION, SEQUENCING AND ANALYSIS Genomic DNA was extracted from the sorted cells as well as the unperturbed input library at full coverage (>300× library coverage) using a modified
salt precipitation protocol as described previously84. Briefly, for 3 × 107−5 × 107 frozen cell pellets (scaled down proportionally for lower cell numbers), 6 ml of NK Lysis Buffer (50 mM
Tris, 50 mM EDTA, 1% SDS, pH 8) and 30 μl of 20 mg/ml Proteinase K (Qiagen) were added to the cells in a 15 ml falcon tube and incubated at 55 °C overnight to lyse the cells and de-crosslink
PFA fixation. Next day, 30 μl of 10 mg/ml RNAse A diluted in NK lysis buffer was added to the lysed sample and incubated at 37 °C for 30 minutes. Samples were chilled on ice before the
addition of 7.5 mM ice-cold ammonium acetate to precipitate proteins. After a brief vortex, insoluble protein fractions were pelleted by centrifugation at ≥4000 × _g_ for 10 minutes. The
supernatant was transferred to a new 15 ml falcon tube and DNA was precipitated with Isopropanol. Precipitated DNA was washed three times with 70% ethanol and airdried. Dried pellets were
dissolved in sterile nuclease-free water and DNA concentration was measured on a Nanodrop 1000 (Thermo Scientific). Amplification of sgRNAs cassettes from the extracted DNA was performed
using Illumina compatible primers from IDT as described previously23 with some minor modifications. PCR amplification was carried out using KAPA HIFI Hotstart PCR (Roche) with the following
reaction mixture in a 50 μl volume: 10 μL 5× reaction buffer, 1.5 μL dNTP, 1.5 μL P5 primer mix, 10 μM, 1.5 μL of P7 primer 10 μM, 2.5 μl DMSO, 0.5 μL polymerase, up to 2 μg of genomic DNA
or 10 ng of plasmid DNA, up to 50 μL with water. PCR cycling conditions were as per manufactures protocol. Target PCR products were gel extracted using the Qiagen gel extraction kit and
re-purified with the GeneJET PCR Purification kit (Thermo) before sequencing on an Illumina NextSeq500. Fastq files were mapped to the sgRNA library Index using MAGeCK-VISPR v0.5.6, which
allows for automated trimming of adaptors and identification of sgRNA length24. Ranked sgRNA and gene hits were similarly obtained with the MAGeCK-VISPR workflow using Robust Rank
Aggregation. GO TERM ENRICHMENT GO term enrichment was carried out in R using the enrichGO function of clusterProfiler v4.12.249. Enriched BP, CC, or MF were filtered based on an adjusted
_p_ value of <0.05. In some cases, top enriched terms were visualized by tree plots of related GO clusters SEAHORSE FLUX ANALYSES Extracellular flux analyses to measure OCRs and ECARs
were performed using the Seahorse-8 XFp flux analyzer (Agilent). BMDMhs were plated at a density of 1 × 105 cells/well in eight-well Seahorse plates overnight. Cells were then infected with
_Mtb_ Erdman strain at MOI 1 or left uninfected for another 24 hours. 1 hour before the assay, BMDMh media was replaced with Seahorse base medium (without phenol red and sodium bicarbonate,
with 5 mM HEPES) and placed at 37 °C in a non-CO2 incubator. For the Mito stress test to measure OCR, 10 mM glucose, 2 mM L-glutamine, and 1 mM sodium pyruvate were added to the Seahorse
base medium, while only glutamine was added for the Glycolysis stress test to measure ECARs. OCR and ECAR measurements (normalized to the number of seeded cells per well) were performed in
line with specific kit instructions by sequential injection of oligomycin (2.5 μM), FCCP, fluoro-carbonyl cyanide phenylhydrazone (1.5 μM), rotenone/antimycin A (0.5 μM) for the Mito stress
assay and glucose (10 mM), oligomycin (2.5 μM), 2-Deoxy-D-glucose (50 mM) for the Glycolysis stress assay. Three measurements were taken under basal conditions and after each drug injection.
WESTERN BLOT ANALYSIS Cells were washed in ice-cold 1× PBS and lysed with RIPA buffer (Thermo Fisher) containing protease inhibitors (Roche) for 30 minutes at 4 °C with gentle agitation.
The Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Fisher) was used to measure the concentration of protein lysates. Equal amounts of protein (30 μg) were separated by SDS PAGE on a 4–20%
gradient gel and transferred to a nitrocellulose membrane. The membrane was blocked for non-specific binding by incubating with 5% non-fat milk in PBST (0.1% tween in 1× PBS) for at least 30
minutes. Blots were washed and incubated with primary antibodies diluted in 2% non-fat milk in PBST at 4 °C overnight. The following primary antibodies were used in western blot analyses.
Anti-AMPKα Mab (1:1000, Cell signaling Technology), anti-Phospho-AMPKα Mab (1:500, Cell signaling Technology), anti-β Actin (1:1000, Cell Signaling Technology), anti-LC3B (1:1000, Cell
Signaling Technology), anti-GID8 (1:1000, Proteintech), anti-YPEL5 (1:1000, Proteintech), anti-WDR26 (1:300, Bioss), anti-UBE2H (1:1000, Proteintech), anti-MAEA (1 μg/ml, R&D Systems),
anti-MKLN1 (1:250, Proteintech), anti-RANBP9 (1:1000, Proteintech) anti-IL-1β (1:1000, Cell Signaling Technology). Secondary antibodies used were horseradish peroxidase-conjugated polyclonal
donkey anti-sheep IgG (1:1000, R&D Systems) and anti-rabbit/mouse StarBright Blue 700 (1:2500, Biorad). Blots were developed by the SuperSignal™ West Atto Ultimate Sensitivity Substrate
(Thermo Fisher) or directly imaged in case of fluorescent secondary antibodies. Images were acquired on a ChemiDoc MP imaging system (Biorad). Western blot band intensities were quantified
in ImageJ. DUAL RNA-SEQUENCING OF BMDMH _MTB_ INFECTED MACROPHAGES Dual RNA-sequencing of _Mtb_ infected BMDMhs was carried out as described previously47,85 with modifications as described
below. Briefly, BMDMhs were infected with the _Mtb_ Erdman smyc’::mCherry reporter strain at MOI 0.5. Four days post infection, cells were harvested and stained with the live/dead viability
dye eFlour 506 (Invitrogen; 1:1000) and the PE anti-mouse/human CD11b antibody (Biolegend; 1:500) for 30 minutes at 4 °C. Live mCherry Cd11b positive cells were sorted on a Sony MA900
sorter. 250,000 _Mtb_ infected sorted macrophages were spun down, resuspended in 150 μl sorter buffer, and transferred to a tube containing 600 μl Trizol. Samples were mixed and incubated at
room temperature for 5 minutes followed by centrifugation at ~12,000 × _g_ for 20 minutes to pellet the bacteria. 600 μl of Trizol supernatant (host RNA) was then transferred to a new tube.
Fresh 400 μl of Trizol was added to the tube containing the bacterial pellet together with 150 μl of zirconia beads. Bead beating was performed on a FastPrep-24™ bead beater (MP
biomedicals) in 2 cycles of 1 minute, resting 2 minutes on ice between each cycle. 40% of host RNA (~240 μl) was then added back to the original bead-containing tube, which allows for a good
proportion capture of both host and bacteria transcripts by getting rid of some of the host RNA47,85. Chloroform was added (200 µL of chloroform for 1 mL of Trizol) to the tubes which were
mixed thoroughly and spun at 12,000 × _g_ for 15 minutes at 4 °C. Total RNA was purified from the aqueous phase using the Zymo RNA clean and concentrator-5 kit according to manufactures
instructions. Ribosomal RNA (rRNA) depletion in the RNA samples was performed using the QIAseq FastSelect pan-bacterial and H/M/R kits in the duo-depletion mode. Libraries were prepared from
rRNA-depleted samples using the NEBNext Ultra II Directional RNA Library Prep Kit (New England Biolabs) and sequenced on the Novaseq 6000 S4 (Illumina). Paired-end sequenced reads were
mapped to the mouse reference genome (GRCm39) and the _Mtb_ H37Rv genome (ASM19595v2) using STAR-2.7.10b. Raw counts were obtained using HTSeq v2.02 with GRCm39 and ASM19595v2 annotations.
Normalization of read counts and differential expression analysis were performed in R using DESeq2 v1.44.0 and APEGLM v1.26.1 for log fold change estimation as described previously47,85.
Genes with <10 raw counts were excluded from downstream analyzes. Pathway enrichment was performed on DE genes using clusterProfiler as described in the prior section. INTRACELLULAR CA2+
MOBILIZATION ASSAY BMDMhs were seeded in 96-well black clear bottom plates at a density of 1 × 105 cells/well and incubated at 37 °C in 5% CO2 overnight. The next day, macrophage media was
discarded and replaced with the Fluoforte® dye (Enzo Life) in 100 μl Hank’s balanced salt solution (HBSS) in the presence of a dye efflux inhibitor. After 1 hour of incubation (45 minutes at
37 °C and 15 minutes at room temperature), 20 μl of 6 μM ATP was added to each well for a final concentration of 1 μM. Fluoforte® fluorescence (excitation 490 nM/emission 525 nM) was
quantified at time 0 (baseline) and after 5 minutes on an Envision plate reader (PerkinElmer). Normalized relative fluorescence units (ΔRFU) were calculated for each well using the following
formula: ΔRFU = (Fluorescence taken after 5 minutes of ATP stimulation−Baseline fluorescence taken at the start of the assay)/Baseline fluorescence taken at the start of the assay.
IMMUNOFLUORESCENCE AND CONFOCAL MICROSCOPY Macrophage monolayers in Ibidi eight-well chambers were infected with _Mtb_ at an appropriate MOI as described in prior sections. At specified time
intervals, cells were fixed with 4% PFA for >1 hour and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) for 15 minutes. Permeabilized cells were blocked with 3% bovine serum albumin
(BSA) for 1 hour and incubated with primary antibody diluted in 3% BSA at 4 °C overnight. Next day, cells were washed with 1× PBS three times and incubated with secondary antibodies for 1
hour at room temperature. Primary antibodies used were anti-GABA (1:400, Sigma-Aldrich) and anti-LC3B (1:1000, Cell Signaling Technology). The Alexa Fluor™ 647 goat anti-rabbit secondary
antibody (1:500, Thermo Scientific) was used. After mounting, images were acquired using a Leica SP5 confocal microscope. Z-stacks were re-constructed in ImageJ, from which mean fluorescence
intensities (MFI) for individual cells or colocalizations were performed. CRISPR RNP DELIVERY IN HUMAN MONOCYTE-DERIVED MACROPHAGES Isolated human monocytes were left in culture for 24
hours before transfection. On the day of transfection, cells were harvested, washed with 1× PBS, and resuspended in P3 Primary Cell Nucleofection Buffer (Lonza) at a concentration of 5 × 106
cells in 20 μl for each electroporation. Equal volumes of 100 μM Alt-R crRNA and Alt-tracrRNA (IDT) were annealed to form sgRNA duplexes by incubation at 95 °C for 5 minutes followed by
cooling to room temperature for >15 minutes. To form CRISPR RNPs, annealed sgRNA duplexes were mixed with IDT Alt-R S.p. Cas9 Nuclease V3 at a 3:1 molar ratio and incubated for at least
20 minutes at room temperature. For nucleofection, 4 μl of CRISPR RNP was mixed with the 20 μl cell suspension in P3 buffer and transferred into the supplied nucleofector cassette strip. The
strip was inserted into the Lonza 4D-Nucleofector and nucleofected with the Buffer P3, CM-137 conditions as previously described40. After nucleofection, cells were transferred into Petri
dishes and allowed to complete differentiation to HMDMs for 5–6 days. Knockout efficiencies were confirmed in HMDMs by ICE analysis and western blot. QUANTITATIVE RT-PCR Total RNA was
isolated from uninfected or _Mtb-_infected BMDMhs using Trizol alongside the Zymo RNA clean and concentrator-5 kit according to manufactures’ instructions. cDNA was synthesized from ~1 μg of
extracted RNA using an iScript cDNA synthesis kit (BioRad). RT-PCR was performed for IFN-β and IL-1β on the Applied Biosystems PRISM 7500 qPCR machine. Beta 2 microglobulin was used as a
reference housekeeping gene. Predesigned qPCR primers were purchased from IDT. PCR reactions were carried out using iTaq Universal SYBR Green Supermix (BioRad). ELISA BMDMhs in 96-well
plates were infected with _Mtb_ at an appropriate MOI for 24 hours. Cell culture supernatants were collected from infected cells and assayed for IFN-β and IL-1β using the IFN-β and IL-1β
R&D ELISA kits according to manufactures’ instructions. GENERAL STATISTICS Basic statistical tests were carried out in GraphPad Prism 10. Comparisons between more than two groups were
analyzed using one-way or two ANOVA alongside Dunnett’s multiple comparison test. _P_ values < 0.05 were considered statistically significant. REPORTING SUMMARY Further information on
research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The dual RNA-seq data sets supporting the conclusions of this article are
available in GEO: GSE267063. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE267063. Source data of plotted graphs and uncropped versions of blots are provided as a Source Data file.
Source data are provided with this paper. REFERENCES * WHO. Global tuberculosis report 2023. World Health Organisation, Geneva, Switzerland (2023). * Behr, M. A., Edelstein, P. H. &
Ramakrishnan, L. Rethinking the burden of latent tuberculosis to reprioritize research. _Nat. Microbiol._ 9, 1157–1158 (2024). Article PubMed CAS Google Scholar * Sturgill-Koszycki, S.
et al. Lack of acidification in _Mycobacterium phagosomes_ produced by exclusion of the vesicular proton-ATPase. _Science_ 263, 678–681 (1994). Article ADS PubMed CAS Google Scholar *
Upadhyay, S., Mittal, E. & Philips, J. A. Tuberculosis and the art of macrophage manipulation. _Pathog. Dis._ 76, fty037 (2018). Article PubMed PubMed Central Google Scholar * Huang,
L., Nazarova, E. V. & Russell, D. G. _Mycobacterium tuberculosis_: Bacterial fitness within the host macrophage. _Microbiol. Spectr._ 7, 10.1128 (2019). Article Google Scholar *
Russell, D. G. et al. _Mycobacterium tuberculosis_ wears what it eats. _Cell Host Microbe_ 8, 68–76 (2010). Article PubMed PubMed Central CAS Google Scholar * Wilburn, K. M., Fieweger,
R. A. & VanderVen, B. C. Cholesterol and fatty acids grease the wheels of _Mycobacterium tuberculosis_ pathogenesis. _Pathog. Dis._ 76, fty021 (2018). Article PubMed PubMed Central
Google Scholar * Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. _J. Exp. Med._ 210,
1977–1992 (2013). Article PubMed PubMed Central CAS Google Scholar * Guilliams, M. & Scott, C. L. Does niche competition determine the origin of tissue-resident macrophages? _Nat.
Rev. Immunol._ 17, 451–460 (2017). Article PubMed CAS Google Scholar * Huang, L., Nazarova, E. V., Tan, S., Liu, Y. & Russell, D. G. Growth of _Mycobacterium tuberculosis_ in vivo
segregates with host macrophage metabolism and ontogeny. _J. Exp. Med._ 215, 1135–1152 (2018). Article PubMed PubMed Central CAS Google Scholar * Behar, S. M. et al. Apoptosis is an
innate defense function of macrophages against _Mycobacterium tuberculosis_. _Mucosal Immunol._ 4, 279–287 (2011). Article PubMed PubMed Central CAS Google Scholar * Martin, C. J. et
al. Efferocytosis is an innate antibacterial mechanism. _Cell Host Microbe_ 12, 289–300 (2012). Article PubMed PubMed Central CAS Google Scholar * Sun, J. et al. The tuberculosis
necrotizing toxin kills macrophages by hydrolyzing NAD. _Nat. Struct. Mol. Biol._ 22, 672–678 (2015). Article ADS PubMed PubMed Central CAS Google Scholar * Chen, M., Gan, H. &
Remold, H. G. A mechanism of virulence: virulent _Mycobacterium tuberculosis_ strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in
macrophages leading to necrosis. _J. Immunol._ 176, 3707–3716 (2006). Article PubMed CAS Google Scholar * van der Wel, N. et al. _M. tuberculosis_ and _M. leprae_ translocate from the
phagolysosome to the cytosol in myeloid cells. _Cell_ 129, 1287–1298 (2007). Article PubMed Google Scholar * Simeone, R. et al. Phagosomal rupture by _Mycobacterium tuberculosis_ results
in toxicity and host cell death. _PLoS Pathog._ 8, e1002507 (2012). Article PubMed PubMed Central CAS Google Scholar * Romagnoli, A. et al. ESX-1 dependent impairment of autophagic flux
by _Mycobacterium tuberculosis_ in human dendritic cells. _Autophagy_ 8, 1357–1370 (2012). Article PubMed PubMed Central CAS Google Scholar * Shah, S. et al. Cutting edge:
_Mycobacterium tuberculosis_ but not nonvirulent _Mycobacteria_ inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. _J. Immunol._ 191, 3514–3518
(2013). Article PubMed CAS Google Scholar * Zhang, L., Jiang, X., Pfau, D., Ling, Y. & Nathan, C. F. Type I interferon signaling mediates _Mycobacterium tuberculosis_-induced
macrophage death. _J. Exp. Med._ 218, e20200887 (2021). Article PubMed CAS Google Scholar * Lai, Y. et al. Illuminating host-mycobacterial interactions with genome-wide CRISPR knockout
and CRISPRi screens. _Cell Syst._ 11, 239–251.e237 (2020). Article PubMed CAS Google Scholar * Mahamed, D. et al. Intracellular growth of _Mycobacterium tuberculosis_ after macrophage
cell death leads to serial killing of host cells. _Elife_ 6, e22028 (2017). Article PubMed PubMed Central Google Scholar * Kiritsy, M. C. et al. Mitochondrial respiration contributes to
the interferon gamma response in antigen-presenting cells. _Elife_ 10, e65109 (2021). Article PubMed PubMed Central CAS Google Scholar * Doench, J. G. et al. Optimized sgRNA design to
maximize activity and minimize off-target effects of CRISPR-Cas9. _Nat. Biotechnol._ 34, 184–191 (2016). Article PubMed PubMed Central CAS Google Scholar * Wang, B. et al. Integrative
analysis of pooled CRISPR genetic screens using MAGeCKFlute. _Nat. Protoc._ 14, 756–780 (2019). Article PubMed PubMed Central CAS Google Scholar * Singh, P. & Subbian, S. Harnessing
the mTOR pathway for tuberculosis treatment. _Front. Microbiol._ 9, 70 (2018). Article PubMed PubMed Central CAS Google Scholar * Kim, J. & Guan, K.-L. mTOR as a central hub of
nutrient signalling and cell growth. _Nat. Cell Biol._ 21, 63–71 (2019). Article PubMed CAS Google Scholar * Gutierrez, M. G. et al. Autophagy is a defense mechanism inhibiting BCG and
_Mycobacterium tuberculosis_ survival in infected macrophages. _Cell_ 119, 753–766 (2004). Article PubMed CAS Google Scholar * Singhal, A. et al. Metformin as adjunct antituberculosis
therapy. _Sci. Transl. Med._ 6, 263ra159 (2014). Article PubMed Google Scholar * Theriault, M. E. et al. Iron limitation in _M. tuberculosis_ has broad impact on central carbon
metabolism. _Commun. Biol._ 5, 685 (2022). Article PubMed PubMed Central CAS Google Scholar * Lampert, F. et al. The multi-subunit GID/CTLH E3 ubiquitin ligase promotes cell
proliferation and targets the transcription factor Hbp1 for degradation. _eLife_ 7, e35528 (2018). Article PubMed PubMed Central Google Scholar * Kobayashi, N. et al. RanBPM, Muskelin,
p48EMLP, p44CTLH, and the armadillo-repeat proteins ARMC8alpha and ARMC8beta are components of the CTLH complex. _Gene_ 396, 236–247 (2007). Article PubMed CAS Google Scholar * Santt, O.
et al. The yeast GID complex, a novel ubiquitin ligase (E3) involved in the regulation of carbohydrate metabolism. _Mol. Biol. Cell_ 19, 3323–3333 (2008). Article PubMed PubMed Central
CAS Google Scholar * Chen, S. J., Wu, X., Wadas, B., Oh, J. H. & Varshavsky, A. An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. _Science_ 355,
eaal3655 (2017). Article PubMed PubMed Central Google Scholar * Maitland, M. E. R., Lajoie, G. A., Shaw, G. S. & Schild-Poulter, C. Structural and functional insights into GID/CTLH
E3 ligase complexes. _Int. J. Mol. Sci._ 23, 5863 (2022). Article PubMed PubMed Central CAS Google Scholar * Wang, L. et al. The Ran-binding protein RanBPM can depress the NF-κB pathway
by interacting with TRAF6. _Mol. Cell Biochem._ 359, 83–94 (2012). Article PubMed CAS Google Scholar * Subramanian, M. et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and
antigen cross-presentation in vivo. _J. Clin. Invest._ 124, 1296–1308 (2014). Article PubMed PubMed Central CAS Google Scholar * Conant, D. et al. Inference of CRISPR edits from Sanger
trace data. _CRISPR J._ 5, 123–130 (2022). Article PubMed CAS Google Scholar * Wu, J. et al. High-throughput assay to phenotype _Salmonella_ enterica Typhimurium association, invasion,
and replication in macrophages. _J. Vis. Exp._ e51759 (2014). * Eade, C. R. et al. _Salmonella_ pathogenicity Island 1 Is expressed in the chicken intestine and promotes bacterial
proliferation. _Infect. Immun._ 87, e00503–e00518 (2019). Article PubMed CAS Google Scholar * Freund, E. C. et al. Efficient gene knockout in primary human and murine myeloid cells by
non-viral delivery of CRISPR-Cas9. _J. Exp. Med._ 217, e20191692 (2020). Article PubMed PubMed Central CAS Google Scholar * Maitland, M. E. R., Kuljanin, M., Wang, X., Lajoie, G. A.
& Schild-Poulter, C. Proteomic analysis of ubiquitination substrates reveals a CTLH E3 ligase complex-dependent regulation of glycolysis. _FASEB J._ 35, e21825 (2021). Article PubMed
CAS Google Scholar * Liu, H. et al. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. _Autophagy_ 16, 1618–1634 (2020). Article PubMed CAS Google
Scholar * Shi, L., Jiang, Q., Bushkin, Y., Subbian, S. & Tyagi, S. Biphasic dynamics of macrophage immunometabolism during _Mycobacterium tuberculosis_ infection. _mBio_ 10, e02550–18
(2019). Article PubMed PubMed Central CAS Google Scholar * Gleeson, L. E. et al. Cutting edge: _Mycobacterium tuberculosis_ induces aerobic glycolysis in human alveolar macrophages that
is required for control of intracellular bacillary replication. _J. Immunol._ 196, 2444–2449 (2016). Article PubMed CAS Google Scholar * Cumming, B. M., Addicott, K. W., Adamson, J. H.
& Steyn, A. J. _Mycobacterium tuberculosis_ induces decelerated bioenergetic metabolism in human macrophages. _Elife_ 7, e39169 (2018). Article PubMed PubMed Central Google Scholar *
Garcia, D. & Shaw, R. J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. _Mol. Cell_ 66, 789–800 (2017). Article PubMed PubMed Central CAS Google
Scholar * Pisu, D., Huang, L., Grenier, J. K. & Russell, D. G. Dual RNA-seq of Mtb-infected macrophages in vivo reveals ontologically distinct host-pathogen interactions. _Cell Rep._
30, 335–350.e334 (2020). Article PubMed PubMed Central CAS Google Scholar * Donovan, A. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. _Cell Metab._ 1,
191–200 (2005). Article PubMed CAS Google Scholar * Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. _Innovation_ 2, 100141 (2021). PubMed
PubMed Central CAS Google Scholar * Malik, Z. A., Denning, G. M. & Kusner, D. J. Inhibition of Ca(2+) signaling by _Mycobacterium tuberculosis_ is associated with reduced
phagosome-lysosome fusion and increased survival within human macrophages. _J. Exp. Med._ 191, 287–302 (2000). Article PubMed PubMed Central CAS Google Scholar * Stanley, S. A. et al.
Identification of host-targeted small molecules that restrict intracellular _Mycobacterium tuberculosis_ growth. _PLoS Pathog._ 10, e1003946 (2014). Article PubMed PubMed Central Google
Scholar * Heisler, F. F. et al. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. _Neuron_ 70, 66–81 (2011). Article PubMed PubMed Central
CAS Google Scholar * Kim, J. K. et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. _Nat. Commun._ 9, 4184 (2018). Article
ADS PubMed PubMed Central Google Scholar * Krithika, R. et al. A genetic locus required for iron acquisition in _Mycobacterium tuberculosis_. _Proc. Natl Acad. Sci. USA_ 103, 2069–2074
(2006). Article ADS PubMed PubMed Central CAS Google Scholar * Pandey, S. D. et al. Iron-regulated protein HupB of _Mycobacterium tuberculosis_ positively regulates siderophore
biosynthesis and is essential for growth in macrophages. _J. Bacteriol._ 196, 1853–1865 (2014). Article PubMed PubMed Central Google Scholar * Wipperman, M. F., Yang, M., Thomas, S. T.
& Sampson, N. S. Shrinking the FadE proteome of _Mycobacterium tuberculosis_: insights into cholesterol metabolism through identification of an α2β2 heterotetrameric acyl coenzyme A
dehydrogenase family. _J. Bacteriol._ 195, 4331–4341 (2013). Article PubMed PubMed Central CAS Google Scholar * Lack, N. A. et al. Characterization of a carbon-carbon hydrolase from
_Mycobacterium tuberculosis_ involved in cholesterol metabolism. _J. Biol. Chem._ 285, 434–443 (2010). Article PubMed CAS Google Scholar * Forrellad, M. A. et al. Role of the Mce1
transporter in the lipid homeostasis of _Mycobacterium tuberculosis_. _Tuberculosis_ 94, 170–177 (2014). Article PubMed CAS Google Scholar * Nazarova, E. V. et al. Rv3723/LucA
coordinates fatty acid and cholesterol uptake in _Mycobacterium tuberculosis_. _Elife_ 6, e26969 (2017). Article PubMed PubMed Central Google Scholar * Lott, J. S. The tryptophan
biosynthetic pathway is essential for _Mycobacterium tuberculosis_ to cause disease. _Biochem. Soc. Trans._ 48, 2029–2037 (2020). Article PubMed PubMed Central CAS Google Scholar *
Zhang, Y. J. et al. Tryptophan biosynthesis protects _Mycobacteria_ from CD4 T-cell-mediated killing. _Cell_ 155, 1296–1308 (2013). Article PubMed PubMed Central CAS Google Scholar *
Tews, I. et al. The structure of a pH-sensing Mycobacterial adenylyl cyclase holoenzyme. _Science_ 308, 1020–1023 (2005). Article ADS PubMed CAS Google Scholar * Bush, M. J. The
actinobacterial WhiB-like (Wbl) family of transcription factors. _Mol. Microbiol._ 110, 663–676 (2018). Article PubMed PubMed Central CAS Google Scholar * Chen, Z. et al. Mycobacterial
WhiB6 differentially regulates ESX-1 and the Dos regulon to modulate granuloma formation and virulence in Zebrafish. _Cell Rep._ 16, 2512–2524 (2016). Article PubMed CAS Google Scholar *
Bosserman, R. E. et al. WhiB6 regulation of ESX-1 gene expression is controlled by a negative feedback loop in _Mycobacterium marinum_. _Proc. Natl. Acad. Sci. USA_ 114, E10772–e10781
(2017). Article ADS PubMed PubMed Central CAS Google Scholar * Kudhair, B. K. et al. Structure of a Wbl protein and implications for NO sensing by _M. tuberculosis_. _Nat. Commun._ 8,
2280 (2017). Article ADS PubMed PubMed Central Google Scholar * Khan, S. et al. Toxin-Antitoxin system of _Mycobacterium tuberculosis_: roles beyond stress sensor and growth regulator.
_Tuberculosis (Edinb.)_ 143, 102395 (2023). Article PubMed CAS Google Scholar * Levi, G. & Raiteri, M. Modulation of gamma-aminobutyric acid transport in nerve endings: role of
extracellular gamma-aminobutyric acid and of cationic fluxes. _Proc. Natl Acad. Sci. USA_ 75, 2981–2985 (1978). Article ADS PubMed PubMed Central CAS Google Scholar * Weidberg, H. et
al. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. _EMBO J._ 29, 1792–1802 (2010). Article PubMed PubMed Central CAS Google
Scholar * Wei, Q. et al. MAEA is an E3 ubiquitin ligase promoting autophagy and maintenance of haematopoietic stem cells. _Nat. Commun._ 12, 2522 (2021). Article ADS PubMed PubMed
Central CAS Google Scholar * Atabakhsh, E., Bryce, D. M., Lefebvre, K. J. & Schild-Poulter, C. RanBPM has proapoptotic activities that regulate cell death pathways in response to DNA
damage. _Mol. Cancer Res._ 7, 1962–1972 (2009). Article PubMed CAS Google Scholar * Liu, T., Roh, S. E., Woo, J. A., Ryu, H. & Kang, D. E. Cooperative role of RanBP9 and P73 in
mitochondria-mediated apoptosis. _Cell Death Dis._ 4, e476 (2013). Article PubMed PubMed Central CAS Google Scholar * Hawn, T. R., Matheson, A. I., Maley, S. N. & Vandal, O.
Host-directed therapeutics for tuberculosis: can we harness the host? _Microbiol. Mol. Biol. Rev._ 77, 608–627 (2013). Article PubMed PubMed Central Google Scholar * Young, C., Walzl, G.
& Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. _Mucosal Immunol._ 13, 190–204 (2020). Article PubMed CAS Google Scholar * Ye, Y., Tang,
X., Sun, Z. & Chen, S. Upregulated WDR26 serves as a scaffold to coordinate PI3K/ AKT pathway-driven breast cancer cell growth, migration, and invasion. _Oncotarget_ 7, 17854–17869
(2016). Article PubMed PubMed Central Google Scholar * Huffman, N., Palmieri, D. & Coppola, V. The CTLH complex in cancer cell plasticity. _J. Oncol._ 2019, 4216750 (2019). Article
PubMed PubMed Central Google Scholar * Jiang, G. et al. A novel biomarker ARMc8 promotes the malignant progression of ovarian cancer. _Hum. Pathol._ 46, 1471–1479 (2015). Article PubMed
CAS Google Scholar * Dartois, V. A. & Rubin, E. J. Anti-tuberculosis treatment strategies and drug development: challenges and priorities. _Nat. Rev. Microbiol._ 20, 685–701 (2022).
Article PubMed PubMed Central CAS Google Scholar * Sukumar, N., Tan, S., Aldridge, B. B. & Russell, D. G. Exploitation of _Mycobacterium tuberculosis_ reporter strains to probe the
impact of vaccination at sites of infection. _PLoS Pathog._ 10, e1004394 (2014). Article PubMed PubMed Central Google Scholar * Dhandayuthapani, S. et al. Green fluorescent protein as a
marker for gene expression and cell biology of Mycobacterial interactions with macrophages. _Mol. Microbiol._ 17, 901–912 (1995). Article PubMed CAS Google Scholar * Andreu, N. et al.
Optimisation of bioluminescent reporters for use with _Mycobacteria_. _PLoS One_ 5, e10777 (2010). Article ADS PubMed PubMed Central Google Scholar * Nazarova, E. V. & Russell, D.
G. Growing and handling of _Mycobacterium tuberculosis_ for macrophage infection assays. _Methods Mol. Biol._ 1519, 325–331 (2017). Article PubMed PubMed Central CAS Google Scholar *
Hart, T. et al. Evaluation and design of genome-wide CRISPR/SpCas9 knockout screens. _G3 (Bethesda)_ 7, 2719–2727 (2017). Article PubMed CAS Google Scholar * Chen, S. et al. Genome-wide
CRISPR screen in a mouse model of tumor growth and metastasis. _Cell_ 160, 1246–1260 (2015). Article PubMed PubMed Central CAS Google Scholar * Pisu, D., Huang, L., Rin Lee, B. N.,
Grenier, J. K. & Russell, D. G. Dual RNA-sequencing of _Mycobacterium tuberculosis_-infected cells from a murine infection model. _STAR Protoc._ 1, 100123 (2020). Article PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Jen K. Grenier and Ann E. Tate from the Cornell BRC Transcriptional Regulation and Expression Facility for
on-going support to improve the Dual RNA-sequencing protocols. Some graphics in Figs. 1C and S1B were obtained from Biorender.com. This work was supported by grants from the National
Institutes of Health (AI155319, AI162598, and OD032135), Bill and Melinda Gates Foundation, and the Mueller Health Foundation to D.G.R., and by a grant from the National Institutes of Health
(AI172433) to C.A. E.J. was supported by T32AI007349. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell
University, Ithaca, NY, USA Nelson V. Simwela, Luana Johnston & David G. Russell * Department of Population Medicine and Diagnostic Sciences, Cornell University, Ithaca, NY, USA Paulina
Pavinski Bitar & Craig Altier * Department of Microbiology and Physiological Systems, UMass Chan Medical School, Worcester, MA, USA Eleni Jaecklein & Christopher M. Sassetti Authors
* Nelson V. Simwela View author publications You can also search for this author inPubMed Google Scholar * Luana Johnston View author publications You can also search for this author
inPubMed Google Scholar * Paulina Pavinski Bitar View author publications You can also search for this author inPubMed Google Scholar * Eleni Jaecklein View author publications You can also
search for this author inPubMed Google Scholar * Craig Altier View author publications You can also search for this author inPubMed Google Scholar * Christopher M. Sassetti View author
publications You can also search for this author inPubMed Google Scholar * David G. Russell View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
N.V.S. and D.G.R. conceptualized the study. L.J., P.P.B., and E.J. contributed to the acquisition and analysis of data. C.A. and C.M.S. contributed to data interpretation. D.G.R. acquired
funding for the study. N.V.S. and D.G.R. wrote the original draft. N.V.S., D.G.R., L.J., P.P.B., E.J., C.A., and C.M.S. reviewed and edited the manuscript. CORRESPONDING AUTHOR
Correspondence to David G. Russell. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks
Vincenzo Coppola, Laura Jenniches and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY DATA 5 SUPPLEMENTARY DATA 6 REPORTING SUMMARY
SOURCE DATA TRANSPARENT PEER REVIEW FILE SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0
International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material
derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Simwela, N.V., Johnston, L., Bitar, P.P. _et al._ Genome-wide screen of _Mycobacterium tuberculosis-_infected macrophages revealed GID/CTLH
complex-mediated modulation of bacterial growth. _Nat Commun_ 15, 9322 (2024). https://doi.org/10.1038/s41467-024-53637-z Download citation * Received: 23 May 2024 * Accepted: 18 October
2024 * Published: 29 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53637-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Javascript support required...
636 mechanisms for sex differences in antacid-induced phosphate depletionABSTRACT Six wk. old male & female rats were gavaged daily with either Basaljel (lml/100gm body wt.) or distilled wa...
Making biofuel from algae - Los Angeles TimesBusiness Making biofuel from algae Chemist Matt Moranville watches another scientist’s experiment on algae at General At...
Something went wrong, sorry. :(Luc Rouban ne travaille pas, ne conseille pas, ne possède pas de parts, ne reçoit pas de fonds d'une organisation qui po...
Miranda kerr tucks into greasy burger and chips while naked in sexy inThe stunning 32-year-old looked like she was really enjoying a fast food feast in a picture she shared to her Instagram ...
Latests News
Genome-wide screen of mycobacterium tuberculosis-infected macrophages revealed gid/ctlh complex-mediated modulation of bacterial growthABSTRACT The eukaryotic Glucose Induced Degradation/C-Terminal to LisH (GID/CTLH) complex is a highly conserved E3 ubiqu...
Tribes hope to address key issues at upcoming conferenceNewsTribes hope to address key issues at upcoming conferenceByBrian BullNovember 22, 2010DownloadPresident Obama meets w...
Former Dgp House On Gm Land In Ranchiinextlive के साथ रहिए खबरों की दुनिया से जुड़े। यहां पढ़िए Former Dgp House On Gm Land In Ranchi से जुड़ी हिन्दी न्यूज़ ...
Bumpy ride for ba as fuel risesThe British flagcarrier and its new Spanish partner Iberia, together called the International Airlines Group (IAG), are ...
Piers morgan ripped apart by mesut ozil after arsenal transfer exit“He got all his likes and retweets and was doubtless a hero for the Arsenal faithful who want to stand by him. “We shoul...