A conserved fungal morphogenetic kinase regulates pathogenic growth in response to carbon source diversity
A conserved fungal morphogenetic kinase regulates pathogenic growth in response to carbon source diversity"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Fungal pathogens must exhibit strong nutritional plasticity, effectively sensing and utilizing diverse nutrients to support virulence. How the signals generated by nutritional
sensing are efficiently translated to the morphogenetic machinery for optimal growth and support of virulence remains incompletely understood. Here, we show that the conserved
morphogenesis-related kinase, CotA, imparts isoform-specific control over _Aspergillus fumigatus_ invasive growth in host-mimicking environments and during infection. CotA-mediated invasive
growth is responsive to exogenous carbon source quality, with only preferred carbon sources supporting hyphal morphogenesis in a mutant lacking one of two identified protein isoforms.
Strikingly, we find that the CotA protein does not regulate, nor is _cotA_ gene expression regulated by, the carbon catabolite repression system. Instead, we show that CotA partially
mediates invasive growth in specific carbon sources and virulence through the conserved downstream effector and translational repressor, SsdA. Therefore, _A. fumigatus_ CotA accomplishes its
conserved morphogenetic functions to drive pathogenic growth by translating host-relevant carbon source quality signals into morphogenetic outputs for efficient tissue invasive growth.
SIMILAR CONTENT BEING VIEWED BY OTHERS A MULTILAYERED REGULATORY NETWORK MEDIATED BY PROTEIN PHOSPHATASE 4 CONTROLS CARBON CATABOLITE REPRESSION AND DE-REPRESSION IN _MAGNAPORTHE ORYZAE_
Article Open access 28 January 2025 LSR2 ACTS AS A CYCLIC DI-GMP RECEPTOR THAT PROMOTES KETO-MYCOLIC ACID SYNTHESIS AND BIOFILM FORMATION IN MYCOBACTERIA Article Open access 24 January 2024
CO-OPTION OF AN EXTRACELLULAR PROTEASE FOR TRANSCRIPTIONAL CONTROL OF NUTRIENT DEGRADATION IN THE FUNGUS _ASPERGILLUS NIDULANS_ Article Open access 17 December 2021 INTRODUCTION Invasive
aspergillosis, caused mainly by _Aspergillus fumigatus_, is the most threatening clinical manifestation of _Aspergillus_ spp. and is initiated by the inhalation of conidia that deposit in
the airways. In immunosuppressed hosts, these inhaled conidia are able to undergo germination, hyphal extension and tissue invasion which leads to fungal dissemination and critical
outcomes1. The human lung represents a hostile environment where germinating _Aspergillus_ conidia must rapidly adapt and respond to a series of challenges including, limited availability of
nutrients, fluctuations of oxygen saturation and temperature, and generation of reactive oxygen and nitrogen species by the host defense mechanisms2,3,4,5. Successful adaptation allows the
invading pathogen to restore cellular homeostasis and acquire nutrients that are not easily available6. The _Aspergilli_ display impressive nutritional and metabolic plasticity that allow
efficient growth and development, supporting virulence in a variety of host settings2,7. How the signals generated by nutrient sensing and utilization are transmitted to specific
morphogenetic machinery for support of fungal pathogenesis has been a focus of study for many years8. Unfortunately, this question remains poorly understood in pathogens like _Aspergillus_.
Here, through a screen to identify protein kinases required for invasive growth in a host-mimicking environment, we uncovered a previously unrecognized role for a conserved morphogenetic
protein kinase in the pathobiology of invasive aspergillosis. Through a series of in vitro and in vivo studies, we show that expression of two CotA kinase protein isoforms, long and short,
is required for tissue invasive hyphal morphogenesis. We show that loss of the long isoform imparts strict, carbon-source mediated growth requirements on _A. fumigatus_ that is independent
of the carbon catabolite repression (CCR) system. Finally, we show that the putative translational repressor, SsdA, partially regulates the carbon source-dependent phenotypes. Taken
together, our findings uncover new biological mechanisms underpinning pathogenic fungal growth in host-relevant carbon source environments. RESULTS A _COTA_ DISRUPTION MUTANT IS SEVERELY
GROWTH RESTRICTED IN HOST-MIMICKING CONDITIONS To identify protein kinases that are necessary for growth in the nutritional landscape of the host lung, we first generated a lung
environment-mimicking culture medium by combining a homogenized mouse lung tissue suspension, buffered to pH 7, with Noble agar (termed, Lung Agar). We then screened an _A. fumigatus_
protein kinase disruption mutant library, generated previously by our laboratory, for colony formation on this Lung Agar9 (Supplementary Fig. 1). As a growth control, we used glucose minimal
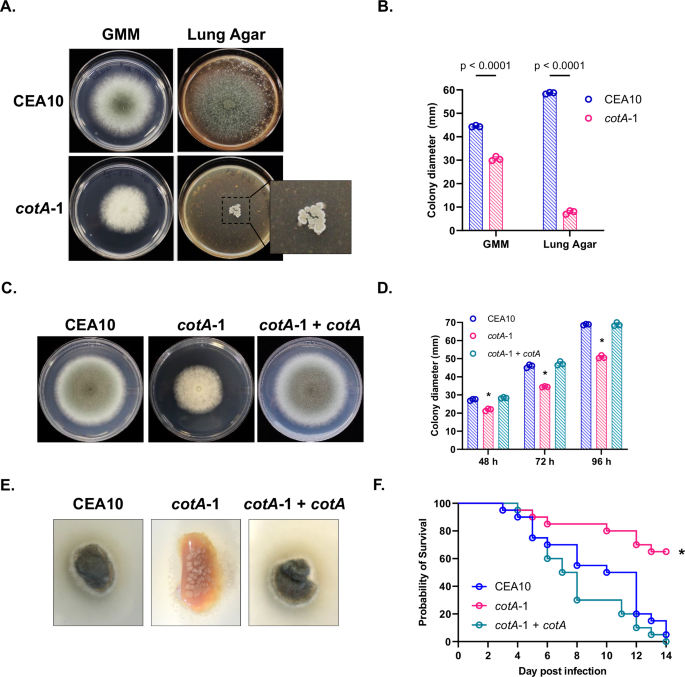
media (GMM) buffered to pH 710. Of the 118 mutants screened, only one mutant strain displayed a pronounced reduction in growth accompanied by an inability to support normal hyphal extension
on Lung Agar, in comparison to growth on GMM (Fig. 1A). This mutant (_cotA_-1) bears a disruption at the 5’ end of the gene encoding the conserved morphogenetic Nuclear Dbf2-Related (NDR)
protein kinase, CotA (AFUB_068890)9. As shown in Fig. 1A–D, the _cotA_-1 mutant develops a moderate growth defect in comparison to the parental strain, CEA10 (_p_ < 0.0001), accompanied
by a decrease in conidiation, when grown on GMM. However, this growth defect is greatly exacerbated when the strains are cultured on Lung Agar, as the _cotA-1_ mutant exhibits a striking
collapse of colony formation (_p_ < 0.0001) (Fig. 1A and B). Given this impressive phenotype, we attempted a complete deletion of the _cotA_ gene. Although successful, the resulting
Δ_cotA_ mutant was severely growth impaired and formed aconidial colonies on GMM agar making further characterization of this mutant unfeasible (Supplementary Fig. 2). Therefore, we utilized
the _cotA_-1 mutant for further study. Complementation of the _cotA_-1 mutant with the wild type allele restored growth to levels similar to the parental strain (Fig. 1C and D), confirming
that the disrupted _cotA_ allele is responsible for the phenotype observed. Culturing the isogenic set of strains in lung explants revealed an identical hyphal development impairment in the
_cotA_-1 mutant. Forty-eight hours after inoculation, the mutant generated only compact colonies over the lung explant, whereas the parental and complemented strains were able to cover the
lung tissue completely (Fig. 1E). These findings indicated that the Lung Agar-inducible poor growth phenotype of _cotA-1_ did not require tissue homogenization. Finally, we found _cotA_
disruption to cause significantly reduced virulence in a chemotherapeutic murine model of invasive aspergillosis (Fig. 1F and G). Sixty-five per cent of mice inoculated with the mutant were
able to survive after 14 days, in comparison to only 5% or 0% of mice infected with the parental (_p_ = 0.0002) or reverted (_p_ < 0.0001) strains, respectively (Fig. 1F). Although these
data revealed that growth impairment of the _cotA-1_ mutant was induced both ex vivo on lung explants and in vivo during infection, we found that the _cotA-1_ mutant did not display
differential susceptibility to a cadre of in vitro growth conditions known to mirror infection-related stress (Supplementary Fig. 3). Therefore, our findings together suggested that the CotA
kinase is likely required for the ability of _A. fumigatus_ to grow in the nutritional environment of the lung. THE _COTA-1_ POOR GROWTH PHENOTYPE IS INDUCED BY HOST-RELEVANT CARBON SOURCE
AVAILABILITY Lung Agar and the commonly used laboratory medium for _A. fumigatus_, GMM, differ significantly in their composition. Whereas GMM contains abundant and readily available
nutrients, Lung Agar is expected to reflect the nutritional landscape of the lung environment where free glucose concentrations are relatively low and microorganisms must obtain nutrients
from more complex sources of carbon and nitrogen11. To test if nutrient limitation was a triggering factor for the inability of the _cotA−1_ mutant to grow on Lung Agar, we next supplemented
Lung Agar with components present in GMM and assayed for improved radial growth of the mutant versus controls. Figure 2A and B show that addition of GMM salt solution (0.52 g KCl, 0. 52 g
MgSO4 x 7H20, 1.52 g KH2PO4, per liter of media; Lung + salt sol.) or nitrogen source (10 mM NaNO3; Lung + NaNO3) did not improve growth in the _cotA_-1 mutant, when compared to
non-supplemented Lung Agar (p = 0.9425 and p = 0.9987, for Lung Agar _vs_ Lung Agar + salt solution and Lung Agar + NaNO3, respectively). However, supplementation of Lung Agar with glucose
at concentrations present in GMM (1% or 55 mM) significantly increased the colony diameter of the _cotA-1_ mutant in comparison to growth on non-supplemented Lung Agar (p < 0.0001),
restoring colony growth to levels similar to those observed in GMM (Fig. 2A and B). The colony diameter of the mutant grown in GMM was 3.44 times larger than when grown on Lung Agar, but
only 1.18 times bigger than when grown on Lung Agar supplemented with 1% glucose (Fig. 2B), suggesting that carbon source availability may play an important role in the observed _cotA_-1
phenotype. On the contrary, in the case of the parental and complemented strains, the addition of glucose, NaNO3 or salt solution to Lung Agar did not alter the colony diameter when compared
to non-supplemented Lung Agar (p > 0.0879) (Fig. 2A and B). As Lung Agar is expected to contain a wide range of putative sources of carbohydrates for use by _A. fumigatus_, we next
employed a simple media supplementation assay to test the ability of the isogenic strains to form colonies in the presence of a diverse array of potential carbon sources. Upon supplementing
glucose-free GMM with various carbohydrates as the sole source of carbon, including monosaccharides (pentoses and hexoses), disaccharides and polysaccharides, we found that each were able to
support growth of the mutant, as well as the parental and complemented strains (Fig. 2C and Supplementary Fig. 4A). However, when employing non-carbohydrate compounds as the sole source of
carbon, including amino acids (Fig. 2C and Supplementary Fig. 4B), short and long chain fatty acids (Fig. 2C and Supplementary Fig. 4C) and the common fermentation product, ethanol (Fig.
2C), _cotA_-1 was unable to grow. The mutant, instead, displayed extensive impairment of hyphal elongation, characterized by constricted colony growth that mimicked Lung Agar culture. In
contrast, the CEA10 and _cotA_-1+_cotA_ strains were able to undergo hyphal extension and form colonies under all tested conditions. The lack of preferred carbon sources, such as simple
carbohydrates, might place strain on cellular demands for carbon source utilization in energy production versus gluconeogenesis for cell wall biogenesis. However, this carbon source-induced
growth defect was not worsened by increased culture temperature nor improved by the addition of a cell wall stabilizing agent, suggesting the diminished hyphal growth was not likely
associated with a weakened cell wall (Supplementary Fig. 5). To confirm that the phenotype observed was driven exclusively by carbon source, we also cultured the isogenic strains in minimal
media containing glucose or acetate as the sole carbon source, and nitrate or ammonium, as the sole nitrogen sources. We found that the _cotA_-1 mutant grows well in media containing
glucose, regardless of the nitrogen source present. However, the _cotA_-1 is unable to sustain hyphal growth on media containing acetate, regardless of the nitrogen source (Supplementary
Fig. 6). Therefore, the _cotA_-1 phenotype appears to be driven entirely by carbon source diversity. As no _A. fumigatus_ mutants have been reported to have such broadly restrictive carbon
source requirements, we next sought to confirm that this phenotype was consistent in different _A. fumigatus_ genetic backgrounds by generating the same _cotA_ disruption in Af293 and
Δ_akuB_-_pyrG_+12 (Supplementary Fig. 7). Regardless of the genetic background, disruption of the _cotA_ gene using the approach designed for the _A. fumigatus_ protein kinase mutant library
construction generated mutants unable to grow in de-repressing carbon sources (Supplementary Fig. 7). The inability of the _cotA_-1 mutant to grow in media containing non-sugars as the sole
carbon source suggests that the carbon catabolite repression (CCR) system might be constitutively activated upon _cotA_ mutation. To our knowledge, this is the first discovery of an _A.
fumigatus_ mutant with as striking a growth phenotype of constitutive CCR activation. THE IMPAIRED ABILITY OF THE _COTA_-1 MUTANT TO GROW IN DE-REPRESSING CONDITIONS IS NOT DUE TO
CONSTITUTIVE ACTIVATION OF CREA-MEDIATED CCR To examine the activation status of the CCR, the isogenic strains were cultured in GMM with the presence of 0.2 M allyl alcohol (AA), a compound
used to measure glucose-induced repression of alcohol dehydrogenases that are well-characterized targets of the CCR system in _A. nidulans_ and _A. fumigatus_13,14. Mutants with a defective
CCR typically display hypersusceptibility to AA, whereas constitutive CCR activation would be expected to result in AA resistance. In our study, when grown on 1% glucose supplemented with
AA, the parental and revertant strains displayed a 15.1% ± 0.017 and 13.4% ± 0.0091 growth reduction, respectively, when compared to AA-free conditions (Fig. 3A and B). These findings were
not significantly different from the results observed in the _cotA_−1 mutant (12.9% ± 0.0024) (Fig. 3A and B). In addition, supplementation of broth cultures with AA caused a slightly
greater reduction in growth for the _cotA_-1 mutant when compared to the parental strain (_p_ = 0.0409) (Fig. 3C). As AA resistance was not evident for the _cotA_-1 mutant, we hypothesized
that the mutant may not be in a state of constitutive CCR activation. To test this hypothesis, we next studied the effects of _cotA_ mutation on carbon source-induced nuclear localization of
the major CCR transcriptional regulator, CreA15. To localize CreA, we constructed chimeric proteins by tagging the C-terminus of CreA with eGFP in both CEA10 and _cotA_-1 genetic
backgrounds. When these new strains were grown under repressing conditions (glucose) for twenty hours, we observed that CreA-eGFP was primarily localized to nuclei in both genetic
backgrounds (Fig. 3D). These observations were supported by quantitation of GFP signal intensity that revealed fluorescence was significantly more abundant in the nuclei versus cytoplasm
under these culture conditions (Fig. 3E and F). However, we observed a significant redistribution of GFP signal from nuclei to cytoplasm when both strains were grown in the presence of
ethanol (Fig. 3D), and GFP intensity was similar in both nuclei and cytoplasm sections (Fig. 3E and F). As CreA-mediated constitutive CCR would require constitutive nuclear localization of
the CreA-eGFP protein, our results suggested that CCR may be normally regulated in the _cotA_-1 mutant. To further confirm that the _cotA_-1 phenotype was independent of CreA function, we
then generated CreA-deficient mutants in the CEA10 and _cotA_-1 genetic backgrounds. We reasoned that, if the _cotA_-1 phenotype of constitutive CCR was at all dependent on the presence of
CreA, then loss of CreA should relieve repression and generate growth recovery in de-repressing carbon sources. To do this, we replaced the promoter of _creA_ using the tetracycline
repressible promoter, TetOff, in both CEA10 and _cotA_-1 to allow for doxycycline-mediated control of _creA_ expression16,17,18. Culture of the wild type strain carrying the TetOff-_creA_
mutation in the presence of 1 µg/mL of doxycycline caused reduced growth in glucose (Fig. 3G, left panels), mimicking what has been previously observed for the _A. fumigatus creA_ deletion
mutant14. Similar levels of growth reduction were observed when _creA_ expression was repressed in the _cotA_-1 background (Fig. 3G). Expression analyzes by RT-qPCR confirmed down-regulation
of _creA_ when the strains were grown in the presence of doxycycline (Fig. 3H). Strikingly, we observed that down-regulation of _creA_ expression in the _cotA_ mutant was unable to rescue
the _cotA_-1 growth defect induced in de-repressing carbon sources (i.e., ethanol) (Fig. 3G, right panels). As it is possible that CreA-independent CCR mechanisms may be at play, we also
performed RNAseq analyzes of the wild type and _cotA_-1 mutant to broadly identify any differences in transcriptional induction of ethanol or acetate-related metabolism gene sets upon a
shift to these de-repressing conditions (Supplementary Data 1). To accomplish this, CEA10 and _cotA_-1 were inoculated into GMM broth and, after 18 h of culture at 37 °C, the spent media was
replaced with fresh minimal media containing glucose, acetate or ethanol as the sole carbon source and cultures were incubated for an additional 2 h. Although significant changes in gene
expression were noted between repressing and de-repressing culture conditions, gene expression correlation analysis revealed that disruption of _cotA_ did not significantly alter overall
transcriptional responses under either condition when compared to wild type (Supplementary Fig. 8). Specifically, of the 1,508 genes that were significantly upregulated (2-fold or more) in
ethanol versus glucose conditions, we found 974 (64.6%) in common between wild type and _cotA_-1 mutant (Fig. 4A). FunCat analysis revealed that around a third of these upregulated genes
were related to secondary metabolism (_n_ = 150), metabolism of C-compounds and carbohydrates (_n_ = 134) and lipid, fatty acids, and isoprenoid metabolism (_n_ = 87) (Fig. 4B). Other
significantly enriched FunCat terms included: cellular import (_n_ = 42), non-vesicular cellular import (_n_ = 40), C-compound and carbohydrate transport (_n_ = 39), among others (Fig. 4B).
Among these commonly upregulated genes, we found multiple CreA-dependent genes known to regulate ethanol metabolism (_alcA_, _alcS_, _acuD_, _acuE_, _acuF_ and _fbp1_)14 (Fig. 4C). Similar
results were obtained when the strains were cultured in acetate. Of the 1749 genes that were significantly upregulated (2-fold or more) in acetate versus glucose conditions, we found that
1140 (65.2%) were commonly upregulated in both CEA10 and the _cotA-_1 mutant (Fig. 4D). The most enriched FunCat terms were the same as those observed in the presence of ethanol, which
included: secondary metabolism (_n_ = 190), C-compound and carbohydrate metabolism (_n_ = 184) and lipid, fatty acids, and isoprenoid metabolism (_n_ = 122) (Fig. 4E). Additional categories
included fatty acid metabolism (_n_ = 56), electron transport (_n_ = 55), C-compound and carbohydrate transport (_n_ = 50) and multiple categories related to amino acid and nitrogen
metabolism (Fig. 4E). Again, we observed upregulation of multiple CreA-regulated genes that are known or predicted to orchestrate acetate metabolism (_facB_, _facA_, a putative carnitine
acetyl transferase, a putative carnitine acylcarnitine carrier, and two putative acetyl-CoA transferases) (Fig. 4F). Significant overlap in down-regulated genes in derepressing conditions
for both CEA10 and _cotA_-1 was also noted (Supplementary Figs. 9 and 10). To verify the RNAseq findings, changes in mRNA expression levels of _alcA_, _aldA_, _acuD_ and _creA_ after
switching from repressing to de-repressing conditions were examined by RT-qPCR. Consistent with the RNAseq results, we confirmed that _alcA_, _aldA_, and _acuD_ are upregulated upon shifting
to ethanol culture for 2 h (Supplementary Fig. 11). Importantly, a broad analysis of additional gene sets predicted to be related to acetate, fatty acid and amino acid metabolism were
similarly regulated in both strains in acetate and ethanol (Supplementary Table 1). These similar signatures suggest that the mutant is able to transcriptionally activate the required
pathways to utilize these non-carbohydrate carbon sources even though hyphal growth is not supported by them. Acetyl-CoA is a key indicator of cellular metabolic state and is derived from
carbohydrate, fatty acid and amino acid metabolism19. Lack of the ability to functionally metabolize these nutrients, even though the mutant strain is appropriately transcriptionally
responsive, could be an underlying explanation for the _cotA_-1 phenotype. To determine if transcriptional activation of metabolic genes translates into functional metabolism in the _cotA_-1
mutant, we next determined cellular acetyl-CoA levels in both CEA10 and _cotA_-1 after culture in the presence of a preferred (glucose) or non-preferred (acetate) carbon source. Similar
concentrations of this key metabolite were detected in the _cotA_-1 mutant versus the parental strain, CEA10, in both glucose and acetate conditions (Supplementary Fig. 12). This finding
correlates with our transcriptional studies and supports the hypothesis that the disruption mutant expresses functional enzymes regulating the utilization of non-carbohydrate carbon sources.
Taken together, our results strongly support the hypothesis that CotA operates independently of CreA-mediated CCR to orchestrate growth in alternative carbon sources. THE _COTA_-1 PHENOTYPE
IS DRIVEN BY LOSS OF A LONG PROTEIN ISOFORM ESSENTIAL FOR GROWTH IN ALTERNATIVE CARBON SOURCES The fact that the _cotA_−1 disruption mutant displayed improved growth compared to the
deletion mutant (Supplementary Fig. 2) indicated that this mutant likely produced a gene product. To investigate the impacts of _cotA_ disruption at the protein level under varying carbon
source environments, we generated a fusion protein consisting of CotA tagged at the C-terminus with eGFP (Fig. 5A) in both the CEA10 and _cotA_−1 backgrounds. Attempts to analyze differences
in CotA-eGFP subcellular localization were unsuccessful, likely due to low gene expression levels driven by the endogenous _cotA_ promoter. Therefore, we utilized the eGFP tag as an epitope
to immunoprecipitate the CotA protein for further analyzes. Using a novel _A. fumigatus_-specific CotA antibody for western blot analysis of anti-GFP precipitated samples, we observed that
wild type _A. fumigatus_, grown in minimal medium containing glucose, produced two protein isoforms (long and short) with the short isoform being present in greater abundance (Fig. 5B, left
panel). As the predicted molecular weight of the CotA-eGFP fusion is ~101.1 kDa, the long isoform putatively represents the full-length protein (Supplementary Fig. 13). Interestingly, the
_cotA_-1 mutant only produced a short isoform under these growth conditions (Fig. 5B, left panel). When grown in the alternative carbon source, acetate, CEA10 was found to produce the same
two CotA protein isoforms but displayed a reduced abundance of the short isoform when compared to glucose culture. The reduction in CotA abundance was even more striking when CEA10 was grown
in the presence of ethanol, where only a small amount of the short isoform could be detected in the wild type (Fig. 5B, middle panels). Surprisingly, we observed that the CotA band was
completely absent when the mutant was grown in acetate or ethanol, suggesting a defect in the regulation of protein steady-state levels driven by the nature of the carbon source. Culture in
the presence of another sugar, sucrose, resulted in isoform abundance similar to what was seen in glucose (Fig. 5B, right panel). RT-qPCR analyzes of RNA extracted from the same growth
conditions revealed a decrease of _cotA_ expression when CEA10 was grown in acetate in comparison to both sugars, correlating with the reduction of the small protein isoform (Fig. 5C).
However, _cotA_ mRNA levels in the _cotA_-1 mutant were similar in all three conditions (Fig. 5C), suggesting that the loss of protein observed in the _cotA_-1 mutant when grown in acetate
is not due to a lack of gene expression, but rather to a possible defect in regulation at the protein level. In addition, further western blot analyzes revealed that abundance of the short
CotA isoform is not reduced at increased culture temperature suggesting the short isoform is likely not a destabilized truncated protein (Supplemental Fig. 14). Together, these results
suggest that _A. fumigatus_ CotA controls growth in alternative carbon sources in an isoform-dependent manner. _A. FUMIGATUS_ HYPHAL MORPHOGENESIS IN DIVERSE CARBON SOURCES IS NOT DEPENDENT
ON COTA ACTIVATION STATE As a conserved NDR kinase, CotA is expected to require three sequential steps for full activation: 1) binding of a Mob protein regulator; 2) autophosphorylation
within the kinase catalytic domain, and 3) phosphorylation of a C-terminal hydrophobic motif by an upstream STE20-like kinase (Fig. 6A). The conserved phosphorylation sites are denoted in
Fig. 6A and mutation of these sites to alanine ablates the function of CotA orthologs in other fungal species20,21,22. To test if the _cotA_-1 mutant phenotype is associated with altered
CotA activation, we mutated the conserved serine at position 464 (i.e., autophosphorylation site) and the threonine at position 635 (i.e., hydrophobic motif phosphorylation site), alone and
in combination, to alanine or glutamic acid (mimicking a constitutively activated state) in the wild type background. When grown in glucose, the S464A mutant displayed an almost 50% decrease
in colony diameter compared to the parental strain (CEA10), whereas the T635A mutant developed a more dramatic growth defect characterized by compact colonies that developed sectoring with
improved growth and conidiation after 48 h (Fig. 6B–D). Moreover, the S464A / T635A double mutant developed aconidial compact colonies with an 80% colony diameter reduction, compared to
CEA10 (Fig. 6B–D). Although these partially or completely inactive CotA mutants displayed defects when compared to the parental strain on glucose media, none of these
phosphorylation-deficient mutants showed inducible growth defects on alternative carbon sources in a manner that resembled the _cotA_-1 mutant (Fig. 6B and 6E–G). Further, we found that the
replacement of either S464 or T635 with glutamic acid did not significantly alter growth under any condition, whereas a strain carrying an S464E / T635E double mutation generated a slight
colony diameter reduction in all media tested (Supplementary Fig. 15). These findings suggested that a constitutively active CotA may be detrimental for _A. fumigatus_ growth and
morphogenesis. However, taken together, these data support the hypothesis that absence of the large protein isoform in the _cotA_-1 mutant, and not altered activation status of CotA, is the
cause of the carbon source-inducible phenotype uncovered here. THE PUTATIVE COTA ACTIVATOR, POD6, IS ESSENTIAL FOR HYPHAL MORPHOGENESIS BUT NOT FOR GROWTH IN NON-CARBOHYDRATE CONDITIONS NDR
protein kinases require a conserved phosphorylation event mediated by a member of the Ste20 family of kinases to become fully activated. In yeast, Cbk1 forms a heterodimer with its binding
partner, Mob2, and this complex is activated by the phosphorylation of Cbk1 by the Ste20-family kinase, Kic1, at a threonine residue in the C-terminus hydrophobic domain of the protein23.
The activity of Kic1 is enhanced by Hym1 and Sog2 and the two kinase complexes are stabilized by the scaffold protein Tao3, facilitating Cbk1 activation24 (Fig. 7A). In _N. crassa_, the
Ste20-like kinase, Pod6, has been shown to phosphorylate CotA at threonine 589, not only regulating its activity, but also its localization25. In addition, a null _A. nidulans pod6_ mutant
displays the same phenotype as the _A. nidulans cotA_ deletion mutant26, suggesting that CotA and Pod6 function in the same network in both model filamentous fungi. To investigate a possible
role for the _A. fumigatus pod6_ ortholog in the _cotA_-1 phenotype, we first attempted to delete the complete _pod6_ gene multiple times. However, no correct transformants were recovered,
suggesting gene essentiality. We then generated a hypomorphic _pod6_ allele through the replacement of the native promoter with the tetracycline regulatable promoter, TetOff, allowing
regulatable gene repression by the addition of doxycycline to the culture medium. Figure 7B shows that, in the absence of doxycycline, the TetOff-_pod6_ mutant displayed wild-type growth and
colony morphology characteristics in glucose conditions but increasing concentrations of doxycycline resulted in restricted colony diameter. In contrast, the wild type strain, CEA10, is
unaffected by the addition of doxycycline morphology (Fig. 7B). Although culture in the presence of 0.25 µg/ml doxycycline resulted in a ~ 4-fold (log2) decrease in _pod6_ expression in the
TetOff-_pod6_ mutant (Fig. 7C), doxycycline addition did not generate an exaggerated growth defect under acetate, ethanol or casamino acids conditions (Fig. 7D). These data suggest that Pod6
is important for growth and morphogenesis, but it is not essential for _A. fumigatus_ ability to grow in non-carbohydrate carbon sources. Taken further, these findings provide additional
support for the hypothesis that the _cotA_-1 phenotype is likely not dependent on CotA activation status but rather by the loss of the large protein isoform. DELETION OF _SSDA_ PARTIALLY
RESTORES GROWTH OF THE _COTA_-1 MUTANT IN NON-CARBOHYDRATE CARBON SOURCES CotA orthologs (Cbk1 in _S. cerevisiae_, COT-1 in _N. crassa_, and CotA in _A. nidulans_) function in the Regulation
of Ace2 and Morphogenesis (RAM) network, a conserved signaling pathway that controls numerous cellular processes through multiple effectors. In _S. cerevisiae_, the RAM network controls
cell separation through phosphorylation of the transcription factor Ace224,27, maintenance of polarity24,28 and cell wall integrity by modulating the function and localization of Ssd129,
secretion and Golgi function during polarized growth by binding and phosphorylating Sec230, cell cycle progression31, mating efficiency through Mpt5 and Brr132,33 and stress signaling
through Bck234. In _C. albicans_, Cbk1 is essential for hyphal development and regulates biofilm formation through the transcription factor Bcr135. In the filamentous fungi, _A. nidulans_
and _N. crassa_, COT-1 / CotA controls morphogenesis through largely unknown mechanisms26,36 (Fig. 8A). As our data indicates that CotA orchestrates growth in host niche-relevant carbon
source environments in a manner that is independent of the known CCR system, we hypothesized that the CotA kinase may impart this control instead through conserved downstream effectors. Ssd1
is a translational repressor and is a previously characterized substrate of Cbk1 in _S. cerevisiae_ and _C. albicans_37. In addition, GUL-1, an Ssd1 ortholog, is a known effector of COT-1
activity in _N. crassa_38. In all three organisms, Ssd1 orthologs have been implicated in cell wall integrity and polarized growth. Cbk1 is a negative regulator of Ssd1 in yeast, as
Cbk1-mediated phosphorylation of Ssd1 blocks mRNA binding37 (Fig. 8B). Although interactions with CotA are as of yet undescribed, the Ssd1 ortholog of _A. fumigatus_, SsdA, has been shown to
regulate trehalose and chitin biosynthesis and _ssdA_ overexpression reduces virulence39. To define a possible role for SsdA in the CotA network responsible for the carbon source inducible
phenotype, we deleted _ssdA_ in both CEA10 and the _cotA_-1 disruption mutant. Loss of _ssdA_ in the wild type strain caused a significant growth defect that was consistent in all carbon
sources tested (_p_ < 0.0001) (Fig. 8C and D). These results correlated well with previously published phenotypes of an _ssdA_ deletion mutant39. However, in submerged culture using media
with various carbon sources, significant growth alterations were not evident in this strain (Fig. 8E). Deletion of _ssdA_ in the _cotA_-1 mutant caused only a slight colony diameter
reduction when glucose was used as the sole carbon source (_p_ < 0.0001), but this mutation resulted in significant growth recovery in ethanol and casamino acids (_p_ = 0.0075, and _p_ =
0.0002, respectively) compared to the _cotA_-1 disruption mutant (Fig. 8C and F). These results correlated with biomass accumulation observations, where the Δ_ssdA_/_cotA_-1 double mutant
displayed a significant growth increase in acetate (_p_ = 0.0013) and casamino acids (_p_ = 0.0006) (Fig. 8G). These findings support the hypothesis that SsdA operates downstream of CotA to,
at least partially, regulate the carbon source-dependent roles of CotA signaling. To determine if the improved growth of the double mutant correlated with an increase in virulence in our
mouse model of invasive aspergillosis, we inoculated CD-1 mice with CEA10, _cotA_-1 or their respective _ssdA_ deletion mutants. Figure 9A shows that 100% of mice infected with CEA10
succumbed to the infection after 9 days, and deletion of _ssdA_ in this background did not affect virulence. The _cotA_-1 mutant generated 60% mortality (Fig. 9A). This is greater than that
observed in earlier experiments (Fig. 1) and is likely associated with the larger inoculum used in this experiment (1 × 105 _vs_. 5 × 105 conidia). However, mortality in the _cotA_−1
experimental arm remained significantly reduced compared to CEA10 (_p_ = 0.0002). Interestingly, deletion of _ssdA_ in the _cotA_-1 background significantly increased mortality to levels
similar to CEA10 and ∆_ssdA_/CEA10 (Fig. 9A). Correlating with the mortality results, histological analyzes revealed that all lung sections from mice infected with CEA10, ∆_ssdA_/CEA10 and
∆_ssdA/cotA_-1 displayed multiple foci of infection spread throughout the tissue, characterized by large areas of necrosis and fungal hyphal growth (Fig. 9B). In contrast, only three of five
lung specimens analyzed from mice infected with the _cotA_-1 mutant displayed areas of fungal growth (Fig. 9B). These lesions were restricted to areas proximal to the airways and were
surrounded by healthy tissue (Fig. 9B). A possible hypothesis to explain the improved growth of the disruption mutant in non-preferred carbon sources and the increased virulence is that loss
of _ssdA_ causes recovery of the short CotA protein isoform. To test this, CotA protein abundance in CEA10, _cotA_-1 and the respective _ssdA_ deletion mutants was detected by western blot
after culture in the presence of glucose or acetate. Figure 9C and Supplementary Fig. 16 show that both CEA10 and Δ_ssdA_/CEA10 produce two CotA isoforms (long and short). Similar to CEA10,
we observed that the abundance of the short isoform in Δ_ssdA_/CEA10 is also reduced when grown in acetate (Fig. 9C). The _cotA_-1 mutant again produced only the short isoform when grown in
glucose and protein levels were indetectable after growth in alternative carbon sources (Fig. 9C). The Δ_ssdA_/_cotA_-1 mutant displayed similar CotA isoform profiles when compared to
_cotA_-1, suggesting that its improved growth in alternative carbon sources is independent of CotA isoform presence. DISCUSSION Although the yeast NDR serine-threonine kinase, Cbk1, is
well-characterized, the orthologous CotA proteins of filamentous fungi have remained relatively understudied. Cbk1 is a major regulator of the Regulation of Ace2 and Morphogenesis (RAM)
network that is essential for yeast cell separation during24,37,40. This protein operates through a multitude of downstream effectors to orchestrate growth and cytokinesis, including the
translational repressor Ssd137,41,42. In filamentous fungi, Cbk1/CotA orthologs appear to mediate multiple cellular processes important for filamentation including, growth, hyphal tip
extension, and branching, through completely uncharacterized mechanisms43,44. Although regulation of morphogenesis is a conserved function, no previous studies have described CotA orthologs
as mediators of pathogenic fungal growth in response to carbon source diversity. Here, we obtained a mutant with an N-terminal disruption of CotA that exhibits a striking restriction of
carbon source preference wherein only carbohydrate-based sources support morphogenesis. Thus, the CotA disruption mutant displays a strong phenotype of constitutive carbon catabolite
repression (CCR). To the best of our knowledge, this is the first _A. fumigatus_ mutant strain with such profoundly restricted carbon source preference. It is a logical presumption that this
phenotype might be caused by constitutive activation of CCR, a process that is positively regulated by the C2H2 type transcription factor, CreA, in multiple fungal organisms11,15. In _A.
fumigatus_, a _creA_ deletion strain is unable to grow in the presence of allyl alcohol14. However, our _cotA_-1 mutant did not present this defect. A study in _A. nidulans_ revealed that
CreA shuttles between the nucleus and cytoplasm under repressing and de-repressing conditions, respectively, to exert its’ functions in gene repression for optimal growth45. As we find that
_A. fumigatus_ CreA undergoes normal nucleo-cytoplasmic shuttling when the _cotA_-1 mutant is cultured in repressing and derepressing conditions, our results support the hypothesis that
CreA-mediated CCR is not orchestrating the _cotA_-1 phenotype. In further support of this hypothesis, transcriptional profiles did not reveal a fingerprint of characteristic CCR activation
in the _cotA_-1 mutant. For example, the transcription factor governing acetate metabolism, FacB, was upregulated in both CEA10 and _cotA_-1 in acetate versus glucose. FacB activates acetate
utilization genes, like _facA_ (acetyl-coenzyme A synthetase), the carnitine acetyltransferases _facC_, _acuH_, and _acuJ_, the succinate/fumarate antiporter _acuL_, and the glyoxylate
cycle malate synthase _acuE_46,47 and is also essential for growth in the presence of ethanol as the sole carbon source. We observed upregulation of each of these genes in both strains grown
in acetate as well. In fact, no significant differences in gene expression between wild type and _cotA_-1 were detected for a host of genes known or predicted to regulate carbon source
sensing, uptake and metabolism (Supplementary Table 1). Therefore, our findings strongly support the hypothesis that CotA controls host-relevant carbon source-responsive hyphal morphogenesis
through uncharacterized CCR-independent mechanisms. In attempts to decipher these mechanisms, we found that our _cotA_-1 disruption mutant produced only one protein isoform (short), whereas
the wild type parental strain produced two isoforms (long and short). Precedence for multiple isoforms of CotA kinase orthologs can be found in previously published work with _N. crassa_.
In this organism, the CotA ortholog, COT-1, is known to exist as two isoforms (long and short) mediated by an alternative transcription start site that produces an N-terminally truncated
COT-1 short isoform48. Regulated abundance of the _N. crassa_ COT-1 isoforms is responsive to environmental stimuli, including light and temperature, with the long COT-1 isoform often being
in greater abundance than the short isoform48,49,50. Although we found the abundance of the _A. fumigatus_ short CotA protein isoform to be higher in carbohydrate carbon sources and reduced
in acetate, the long isoform was detectable at a stable and lower level regardless of carbon source. Whereas the short isoform in _cotA_-1 was present at levels similar to the wild type in
glucose and sucrose, we noted that loss of growth in _cotA_-1 under non-preferred carbon sources (acetate and ethanol) was associated with an inability to detect even this short isoform.
Therefore, it is likely that the striking growth reduction of the _cotA_-1 mutant on non-preferred carbon sources results from loss of nearly all cellular CotA abundance. This hypothesis is
further supported by multiple findings, including: 1) an inactive CotA mutant bearing mutations in two highly conserved CotA phosphorylation sites could not form invasive hyphae under any
condition, 2) deletion of _cotA_ resulted in significantly growth-restricted colonies regardless of carbon source, and 3) previously published data from _N. crassa_ showing that hyphal cell
elongation and branching are independently and strongly regulated by the activation state of COT-120. Taken together, we predict that the presence of the long CotA isoform in _A. fumigatus_
may be essential for regulation of CotA small isoform abundance, which acts as the major source of cellular CotA activity to drive efficient hyphal morphogenesis in diverse carbon source
environments. Similar to mechanisms in _N. crassa_, we hypothesize that the difference between long and short isoforms of _A. fumigatus_ CotA may be an N-terminus that is shortened during
transcription or translation to produce the short isoform. Our _cotA_-1 disruption event, which results in only the short isoform being produced, was designed to occur at the extreme 5’ end
of the putative _cotA_ coding sequence, making this a possibility. Indeed, analysis of the _cotA_-1 mutant transcript read coverage from our RNAseq studies suggests the mutant generates a 5’
truncated transcript compared to the parental control (Supplementary Fig. 17). Alignment of the N-terminal protein regions of multiple Cbk1/CotA orthologs from various fungi revealed a
species-specific N-terminal domain that is composed of variably sized glutamine-rich regions in many organisms (Supplemental Fig. 18). Though not well understood, incorporation of glutamine
rich regions into proteins are suggested to be one mechanism to promote protein phase separation, a phenomenon that has important implications for regulation of signal transduction and RNA
processing51,52,53,54. It is enticing to speculate that a major difference between the two isoforms is the ability of the N-terminally glutamine-rich long isoform to undergo phase separation
in response to host-relevant carbon source availability. This process could, in turn, regulate morphogenetic CotA signaling or mediate CotA short isoform abundance via translational control
or RNA processing mechanisms. As we also find that the conserved translational repressor, SsdA, plays a partial role in the _cotA_-1 phenotype and SsdA orthologs are important drivers of
virulence in plant and animal pathogens36,39,55,56, it will be of interest to test if SsdA partially contributes to this process. Although further studies are needed to decipher the specific
mechanisms at play, our findings suggest that CotA functions in an isoform-specific and CreA-independent manner to support hyphal morphogenesis and virulence in host-relevant carbon source
environments. METHODS ETHICS STATEMENT All research was performed in compliance with all ethical regulations under protocol 22-0373.0, approved by the Institutional Biosafety Committee at
the University of Tennessee Health Science Center. GROWTH CONDITIONS The common laboratory strain, CEA10, was used as the wild type, with mutant strains generated in this genetic background,
unless stated otherwise (see complete list of strains in Supplementary Table 2). Glucose Minimal Medium (GMM) (6 g NaNO3, 0.52 g KCl, 0.52 g MgSO4 x 7H2O, 1.52 g KH2PO4, 1 mL trace elements
[2.2 g ZnSO4 x 7H2O, 1.1 g H3BO3, 0.5 g MnCl2 x 4H2O, 0.5 g FeSO4 x 7H2O, 0.16 g CoCl2 x 5H2O, 0.16 g CuSO4 x 5H2O, (NH4)6Mo7O24 x 4H2O, 5 g Na4EDTA in 100 mLl distilled H2O], 10 g glucose,
15 g agar, pH 6.5, in 1 liter distilled H2O) (Shimizu & Keller, 2001) was used for general growth conditions and conidia were harvested from 4-day-old cultures by flooding the plates
with sterile water and recovering the conidial suspension through a filter to remove hyphal fragments. For growth rate analyzes, five microliters of sterile distilled water containing 104
conidia were inoculated in the center of agar plates. The colony diameters were measured every 24 h, starting 48 h post-inoculation and for a period of 4 days. For biomass quantification,
fifty milliliters of minimal media containing 1% glucose, 1% potassium acetate, 1% EtOH or 1% casamino acids, as the sole carbon sources, were inoculated with 2 × 107 conidia and incubated
at 37 °C for 24 h in static conditions. After this time, the mycelia were recovered, washed and lyophilized, and weights were recorded. To analyze the germination rates of the isogenic set
of strains, 104 conidia were inoculated in liquid GMM. Starting 5 h after inoculation, and every hour for the course of 12 h, the samples were observed and the number of conidia forming a
germ tube were recorded. At least 300 conidia were counted for each sample and the experiment was performed in biological triplicates. Doxycycline (0.05 – 200 µg/mL) was added to the culture
medium, when needed, to downregulate the expression of those genes that were expressed under the tetracycline repressible promoter, pTetOff18. Lung Agar preparation and protein kinase
mutant library screening was as follows. After euthanasia by anoxia with CO2, the lungs of 6-week-old CD-1 female mice were aseptically extracted, and the blood excess was removed using
sterile water and gauze. The lung tissue from each mouse was then homogenized in 15 mL of 0.165 M MOPS adjusted to pH 7, containing 100 µg/mL of gentamycin and 100 µg/mL of chloramphenicol,
in a gentleMACS tissue dissociator (Milteny Biotec). Afterwards, the homogenates were mixed with equal volumes of 3% Noble agar and poured over 100 mm petri dishes. The medium was allowed to
dry before spot inoculation with 5 µL of sterile water containing 500 conidia on the surface of the agar. GMM agar, unbuffered and buffered with 0.165 M MOPS and adjusted to pH 7, was used
as control. Each assay plate contained 20 different mutant strains and the parental strain CEA10 was inoculated in each plate as control for growth (Supplementary Fig. 1). Colony growth was
monitored every 24 h over the course of 72 h at 37 °C. Those strains showing a significant growth defect on Lung Agar during library screening were confirmed in single strain assays. To
determine the ability to grow on intact lung tissue ex vivo, lung explants of healthy female CD-1 mice were placed onto plates lacking nutrients (water with agarose) and inoculated with 103
conidia. The plates were incubated at 37 °C for 48 h. For evaluation of growth in diverse carbon source and stress environments, equal amounts of conidia (104 in 5 µL of sterile distilled
water) from the isogenic strains were cultured on minimal medium agar supplemented with amino acids (1% arginine, 0.5% aspartic acid, 1% glutamine, 1% histidine, 1% leucine, 1% methionine,
1% phenylalanine, 1% proline and 1% serine), short and long chain fatty acids or their corresponding salts (1% potassium acetate, 1% sodium butyrate, 1% sodium propionate and 1% oleic acid),
simple and complex carbohydrates (1% glucose, 1% sucrose, 1% ribose, 1% maltose, or 1% glycogen from bovine liver), 1.2 M sorbitol, 1% ethanol or 0.2 M allyl alcohol. All the compounds were
purchased from Sigma Aldrich or Fisher Scientific. GENETIC MANIPULATIONS The _cotA_ disruption mutant (_cotA-1_) was obtained from our _A. fumigatus_ protein kinase disruption mutant
library9. A complemented strain was generated by replacing the disrupted _cotA_ allele in the _cotA_-1 genetic background with a wild type allele. Therefore, the re-introduced _cotA_ gene is
expressed by the endogenous promoter. We employed a CRISPR-Cas9 gene editing approach, previously established in our laboratory57, that included targeting two Protospacer Adjacent Motif
(PAM) sites located upstream and downstream of the predicted _cotA_ START and STOP codons, respectively. As we have previously described, the crRNA sequences were designed to include 20
nucleotides directly upstream (5’) of each PAM site and BLAST analyzes were performed to reduce risk of off-target Cas9 activity. Purified Cas9 enzyme and tracrRNAs were obtained from IDT,
as previously described57. Ribonucleoprotein (RNP) complexes were assembled in vitro and used for the transformation of _cotA_-1 protoplasts. For a repair template, the complete _cotA_ ORF
was amplified from CEA10 gDNA. In parallel, a phleomycin resistance cassette was amplified from plasmid pAGRP58. Both amplicons contained an overlapping region of 40 bp, as well as
microhomology arms complementary to areas upstream and downstream of the chosen PAM sites. A _cotA_ deletion strain was generated through the replacement of the entire predicted ORF with a
hygromycin resistance cassette in the CEA10 genetic background. The resistance cassette was amplified from plasmid pUCGH59 and contained 40 nucleotides complementary to upstream and
downstream target sequences. Selected PAM sites custom crRNA sequences are listed in Supplementary Table 2. To express CotA- or CreA-Green Fluorescent Protein (GFP) chimeric proteins, repair
templates consisting of the _egfp_ coding sequence upstream of a phleomycin resistance cassette were amplified from pAGRP58 and contained 40 bp regions of microhomology targeting a PAM site
downstream of the predicted _cotA_ or _creA_ ORFs. To generate regulatable _pod6_ and _creA_ alleles, the doxycycline responsive promoter system was PCR amplified from plasmid
pSK606-phleo18 to contain 40 bp microhomology regions targeting the PAM site selected just upstream of each gene. Deletion of _ssdA_ was accomplished by replacing the predicted _ssdA_ ORF
with a phleomycin resistance cassette in both CEA10 and _cotA_-1 backgrounds. G-blocks containing _cotA_ mutant alleles encoding point mutations in predicted phosphorylation sites (Ser464
and Thr635 mutated to Alanine or Glutamic acid, single and in combination) were synthesized by Genewiz and cloned into pUCGH59. Repair templates containing the respective _cotA_ mutant
allele and a hygromycin resistance cassette were PCR amplified for transformation into CEA10. All the transformations were carried out with 60% PEG, as described previously (Yelton et al.,
1984). Transformation mixtures were spread in SMM agar plates and incubated at RT overnight to allow the protoplasts to recover. The following day, transformation plates were overlayed with
top agar (SMM containing 7.5 g/L of agar) containing 150 µg/mL of hygromycin or 125 µg/mL of phleomycin, as needed. Hygromycin plates were placed at 37 °C, while the phleomycin-containing
plates were incubated at RT overnight, before they were placed at 30 °C until colonies were observed. Putative transformants were sub-cultured in GMM containing the adequate selection
antibiotic, and subsequently genotyped by multiple PCRs to confirm a correct integration. All primers, custom crRNA sequences, and PAM site used for genetic manipulations can be found in
Supplementary Table 3. RNA EXTRACTION AND RT-QPCR RNA extraction and downstream processing was as previously described with slight modification60. In brief, a one hundred milliliter volume
culture of liquid GMM was inoculated with 5 × 107 conidia of CEA10 or _cotA_-1 and allowed to grow for 18 h at 37 °C / 250 rpm. Then, the cultures were washed three times with sterile
distilled water and the medium was replaced by minimal medium containing 1% glucose, 1% potassium acetate or 1% ethanol. The cultures were incubated for 2 additional hours in the same
conditions and after this time, the hyphal mats were filtered, washed with sterile distilled water, dried, and crushed under liquid N2. Immediately, the resulting powder was resuspended in 1
mL of Trizol (Invitrogen) and the RNA extraction was performed using the Qiagen RNeasy kit, as previously described61. On-column DNAse I (Qiagen) treatment was performed to remove genomic
DNA (gDNA) contamination, according to the manufacturers’ instructions. The quality of the RNAs, and lack of degradation or gDNA contamination, were ensured by running RNA aliquots in a 1%
agarose gel supplemented with 1% bleach (Aranda et al., 2012). Concentrations and quality were also determined using Nanodrop (ThermoFisher Scientific) and Qubit (Invitrogen). cDNA was
synthesized from 1 µg of total RNA using the ProtoScript II First strand cDNA synthesis kit (New England Biolabs). Quantitative reverse transcription PCR was performed using SYBR® Green
Master Mix (Bio‐Rad) in a CFX Connect Real‐Time System (Bio‐Rad). Transcript levels were calculated and normalized to those of the housekeeping _tubA_ and the relative expression was
determined using the 2ΔΔCt method62. Each analysis was performed in technical and biological triplicates. RNASEQ AND DIFFERENTIAL GENE EXPRESSION ANALYZES DNAse-treated RNA samples in
biological triplicates were submitted to NovoGene for RNA sequencing analyzes. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent
Technologies). mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in First Strand
Synthesis Reaction Buffer (5X). First strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-). Second strand cDNA synthesis was subsequently
performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, an
adapter with a hairpin loop structure was ligated to prepare for hybridization. To select cDNA fragments of preferentially 370 ~ 420 bp in length, the library fragments were purified with
AMPure XP system (Beckman Coulter, Beverly, USA). PCR was then performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, PCR products were
purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation
System using TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina
Novaseq platform and 150 bp paired-end reads were generated. Raw reads of fastq format were first processed through in-house perl scripts. The reference genome of _A. fumigatus_ A1163 and
gene model annotation files were downloaded from FungiDB63. Index of the reference genome was built using Hisat2 v2.0.5 and paired-end clean reads were aligned to the _A. fumigatus_ A1163
reference genome using Hisat2 v2.0.5. The mapped reads of each sample were assembled by StringTie (v1.3.3b)64 in a reference-based approach. featureCounts v1.5.0-p3 was used to count the
reads numbers mapped to each gene and then FPKM of each gene was calculated based on the length of the gene and reads count mapped to this gene. Differential expression analysis was
performed using the DESeq2 R package (1.20.0). The resulting _P_-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an
adjusted _P_-value ≤ 0.05 found by DESeq2 were assigned as differentially expressed. ACETYL COA ASSAY CEA10 and _cotA_-1 (5 × 107 conidia / 100 ml) were grown in minimal media containing
glucose or acetate as the sole carbon source for 20 h at 37 °C and 230 rpm. After this time, the mycelia were filtered through miracloth and washed three times with dH2O. Two hundred
milligrams of mycelia of each sample were crushed with liquid N2 and the resulting powder was resuspended in 1 ml of ice-cold PBS and centrifuged for 10 min at 1900 x _g_ at 4 °C. The
supernatants were transferred to new tubes and dilutions were performed to determine acetyl CoA concentration using a colorimetric Universal Acetyl-CoA ELISA kit (Novus Biologicals, LLC). A
standard curve was constructed, and samples were processed according to the manufacturer’s recommendations. PROTEIN EXTRACTION, IMMUNOPRECIPITATION, AND WESTERN BLOT ANALYZES Three hundred
milliliter cultures of minimal medium containing either 1% glucose, 1% sucrose, 1% ethanol, 1% casamino acids or 1% potassium acetate were inoculated with 2 × 108 conidia and incubated for
22 h at 37 °C and 250 rpm. After incubation, cultures were filtered, and the hyphal mats were washed with sterile distilled water and dried. The hyphal mats were immediately submerged in
liquid N2 and crushed with mortar and pestle. The resulting powder was resuspended in ice-cold lysis buffer (0.5% NP-40; 10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5 mM EDTA; 10 mM MgCl2x6H2O)
containing protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO) and 1 mM Pefabloc (Roche, Indianapolis, IN). After ten minutes of centrifugation at 1900 x g and 4 °C, the supernatants
were collected, and the protein concentration was determined using a Bradford assay kit (Thermo Scientific, Waltham, MA). For immunoprecipitation and subsequent western blot analyzes, four
milligrams of total protein from _cotA_-GFP/CEA10 and _cotA_-GFP/_cotA_-1 strains were diluted in 1.5 volumes of wash buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5 mM EDTA; 10 mM
MgCl2x6H2O) supplemented with 1 mM Pefabloc (Roche) and protease inhibitor cocktail (Sigma Aldrich), and subsequently mixed with 70 µL of GFP-trap magnetic agarose beads (Chromotek). The
samples were incubated for one hour at 4 °C with agitation and then the beads were recovered using a magnetic stand and washed 3 times with wash buffer. Finally, the beads were resuspended
in 30 µL of wash buffer containing reducing SDS Laemmli sample buffer (Alfa Aesar, Tewksbury, MA) and 5% β-mercaptoethanol (Sigma Aldrich, St. Louis, MO), and incubated at 95 °C for 10 min
to release the protein complexes from the beads. The supernatants were run on a 12% polyacrylamide gel (BioRad) for 1 hour at 200 V. Separated proteins were then transferred to a PVDF
membrane using a semi-dry transfer system (BioRad) and membranes were blocked for one hour at RT in TTBS (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1% Tween 20) + 2% skim milk. After this time,
the membrane was incubated overnight at 4 °C with a novel _A. fumigatus_ CotA-specific polyclonal antibody (GenScript) in TTBS + 2% skim milk (1:5000 dilution). This novel antibody was
generated against an antigenic peptide (DKMIRDPSISKEKKC) selected from within the core CotA kinase domain such that both wild type and N-terminally truncated CotA proteins could be detected.
After three washes with TTBS, the membrane was incubated for one hour at room temperature with the secondary antibody (1:5000 dilution) anti-rabbit conjugated with horseradish peroxidase
(A00098, Genscript), stabilized with TBS (50 mM Tris-HCl, pH 7.4; 150 mM NaCl) for 10 min and incubated for 5 min with the working detection solution (SuperSignal West Pico Plus,
ThermoFisher Scientific). Finally, blots were imaged using a Bio‐Rad ChemiDoc XRS HQ System. As loading control, fifty micrograms of total protein were run on a separate gel and stained with
Coomassie Blue (BioRad) for 2 h, followed by incubation overnight in de-staining solution (15% methanol and 10% acetic acid). IN VIVO STUDIES Infection experiments were as previously
described16. Groups of 6-week-old CD-1 female mice or 4-week-old CD-1 male mice (_n_ = 20 for survival, _n_ = 3 to 5 for histopathology) were treated with 40 mg/kg of triamcinolone acetonide
(Kenalog) the day before the infection, plus 150 mg/kg of cyclophosphamide intraperitoneally on days -3, +1, +4, +7 and +10. On day 0, mice were transiently anesthetized with isoflurane and
inoculated by nasal instillation with 105 or 5×105 (for survival studies) or 106 conidia (for histopathology) of CEA10, _cotA_-1, _cotA_-1 + _cotA_, Δ_ssdA/_CEA10 or Δ_ssdA/cotA_-1. Three
days after the infection, mice from histopathology groups were euthanized and lungs were inflated with 10% buffered neutral formalin and aseptically removed. Then, 5 µm tissue sections were
stained with hematoxylin-eosin or with Grocott Methenamine Silver stains. For survival studies, mice were monitored at least twice a day for 14 days after inoculation, and those animals
showing severe signs of disease were humanely euthanized. Drinking water was supplemented with sulfamethoxazole and trimethoprim (Bactrim, Ani Pharmaceuticals, Inc.) to avoid bacterial
infections. The animals were housed under standard conditions in sterile microisolator cages on a 12 h dark/light cycle at ambient temperature and humidity and were supplied with water and
food _ad libitum_. STATISTICS AND REPRODUCIBILITY With the exception of the survival studies involving animals, no statistical tests were utilized to pre-determine sample size. For survival
studies, sample size power analyzes were conducted at 80% power and an a of 0.05 to detect an anticipated incidence of mortality of 80% and 20% for the control and treated experimental arms,
respectively. This analysis indicated the need for 20 animals per experimental group. As it is the minimum number of replicates required for inferential analysis, at least three biological
replicates were utilized for all other experiments. No data were excluded from analyzes, the experiments were not randomized, and investigators were not blinded to allocation during
experiments of outcomes assessment. Statistical analyzes were performed using GraphPad Prism 10.0.0 for Windows (GraphPad Software, San Diego, CA, USA). Specific tests used to determine
statistical analyzes are noted in each figure legend. p values are depicted, with a value of _p_ < 0.05 considered significant. REPORTING SUMMARY Further information on research design is
available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Transcriptome sequencing data files have been deposited in NCBI SRA under Bioproject accession
number PRJNA1147328. Source data are provided with this paper. REFERENCES * Latgé, J.-P. & Chamilos, G. _Aspergillus fumigatus_ and Aspergillosis in 2019. _Clin. Microbiol. Rev._ 33,
e00140–00118 (2019). Article PubMed PubMed Central Google Scholar * Tekaia, F. & Latgé, J. P. _Aspergillus fumigatus_: saprophyte or pathogen? _Curr. Opin. Microbiol_ 8, 385–392
(2005). Article PubMed CAS Google Scholar * Shibuya, K. et al. Catalases of _Aspergillus fumigatus_ and Inflammation in Aspergillosis. _Nippon Ishinkin Gakkai Zasshi_ 47, 249–255 (2006).
Article CAS Google Scholar * Lambou, K., Lamarre, C., Beau, R., Dufour, N. & Latge, J. P. Functional analysis of the superoxide dismutase family in _Aspergillus fumigatus_. _Mol.
Microbiol._ 75, 910–923 (2010). Article PubMed CAS Google Scholar * Kowalski, C. H. et al. Heterogeneity among isolates reveals that fitness in low oxygen correlates with _Aspergillus
fumigatus_ virulence. _mBio_ 7, e01515–e01516 (2016). Article PubMed PubMed Central CAS Google Scholar * Brown, A. J. P., Cowen, L. E., Pietro, A. D. & Quinn, J. Stress adaptation.
_Microbiol. Spectr._ 5, 5.4.04 (2017). Article Google Scholar * Davis, D. A. How human pathogenic fungi sense and adapt to pH: the link to virulence. _Curr. Opin. Microbiol_ 12, 365–370
(2009). Article PubMed CAS Google Scholar * Johns, L. E., Goldman, G. H., Ries, L. N. A. & Brown, N. A. Nutrient sensing and acquisition in fungi: mechanisms promoting pathogenesis
in plant and human hosts. _Fungal Biol. Rev._ 36, 1–14 (2021). Article CAS Google Scholar * Souza, A. C. O. et al. Loss of Septation Initiation Network (SIN) kinases blocks tissue
invasion and unlocks echinocandin cidal activity against _Aspergillus fumigatus_. _PLOS Pathog._ 17, e1009806 (2021). Article PubMed PubMed Central CAS Google Scholar * Shimizu, K.
& Keller, N. P. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in _Aspergillus nidulans_.
_Genetics_ 157, 591–600 (2001). Article PubMed PubMed Central CAS Google Scholar * Ries, L. N. A., Beattie, S., Cramer, R. A. & Goldman, G. H. Overview of carbon and nitrogen
catabolite metabolism in the virulence of human pathogenic fungi. _Mol. Microbiol._ 107, 277–297 (2018). Article PubMed CAS Google Scholar * Al Abdallah, Q., Martin-Vicente, A., Souza,
A. C. O., Ge, W. & Fortwendel, J. R. C-terminus proteolysis and palmitoylation cooperate for optimal plasma membrane localization of rasA in _Aspergillus fumigatus_. _Front Microbiol_ 9,
562 (2018). Article PubMed PubMed Central Google Scholar * Kulmburg, P., Mathieu, M., Dowzer, C., Kelly, J. & Felenbok, B. Specific binding sites in the alcR and alcA promoters of
the ethanol regulon for the CREA repressor mediating carbon catabolite repression in _Aspergillus nidulans_. _Mol. Microbiol_ 7, 847–857 (1993). Article PubMed CAS Google Scholar *
Beattie, S. R. et al. Filamentous fungal carbon catabolite repression supports metabolic plasticity and stress responses essential for disease progression. _PLOS Pathog._ 13, e1006340
(2017). Article PubMed PubMed Central Google Scholar * Adnan, M. et al. Carbon Catabolite Repression in Filamentous Fungi. _Int. J. Mol. Sci._ 19, 48 (2017). Article PubMed PubMed
Central Google Scholar * Martin-Vicente, A., Souza, A. C. O., Al Abdallah, Q., Ge, W. & Fortwendel, J. R. SH3-class Ras guanine nucleotide exchange factors are essential for
_Aspergillus fumigatus_ invasive growth. _Cell Microbiol_ 21, e13013 (2019). Article PubMed PubMed Central Google Scholar * Wanka, F. et al. Tet-on, or Tet-off, that is the question:
advanced conditional gene expression in _Aspergillus_. _Fungal Genet Biol._ 89, 72–83 (2016). Article PubMed CAS Google Scholar * Xie, J. et al. The sterol C-24 methyltransferase
encoding gene, _erg6_, is essential for viability _of Aspergillus_ species. _Nat. Commun._ 15, 4261 (2024). Article ADS PubMed PubMed Central Google Scholar * Shi, L. & Tu, B. P.
Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. _Curr. Opin. Cell Biol._ 33, 125–131 (2015). Article PubMed PubMed Central CAS Google Scholar * Ziv, C. et al.
Cell elongation and branching are regulated by differential phosphorylation states of the nuclear Dbf2-related kinase COT1 in _Neurospora crassa_. _Mol. Microbiol_ 74, 974–989 (2009).
Article PubMed CAS Google Scholar * Liu, N. et al. The NDR kinase-MOB complex FgCot1-Mob2 regulates polarity and lipid metabolism in _Fusarium graminearum_. _Environ. Microbiol_ 23,
5505–5524 (2021). Article PubMed CAS Google Scholar * Jansen, J. M., Barry, M. F., Yoo, C. K. & Weiss, E. L. Phosphoregulation of Cbk1 is critical for RAM network control of
transcription and morphogenesis. _J. Cell Biol._ 175, 755–766 (2006). Article PubMed PubMed Central CAS Google Scholar * Hsu, J. & Weiss, E. L. Cell cycle regulated interaction of a
yeast hippo kinase and its activator MO25/Hym1. _PLoS ONE_ 8, e78334 (2013). Article ADS PubMed PubMed Central CAS Google Scholar * Nelson, B. et al. RAM: a conserved signaling
network that regulates ace2p transcriptional activity and polarized morphogenesis. _Mol. Biol. Cell_ 14, 3782–3803 (2003). Article ADS PubMed PubMed Central CAS Google Scholar * Maerz,
S., Dettmann, A. & Seiler, S. Hydrophobic motif phosphorylation coordinates activity and polar localization of the _Neurospora crassa_ Nuclear Dbf2-Related Kinase COT1. _Mol. Cell.
Biol._ 32, 2083–2098 (2012). Article PubMed PubMed Central CAS Google Scholar * De Souza, C. P. et al. Functional analysis of the _Aspergillus nidulans_ Kinome. _PLoS One_ 8, e58008
(2013). Article ADS PubMed PubMed Central Google Scholar * Bidlingmaier, S., Weiss, E. L., Seidel, C., Drubin, D. G. & Snyder, M. The Cbk1p pathway is important for polarized cell
growth and cell separation in _Saccharomyces cerevisiae_. _Mol. Cell. Biol._ 21, 2449–2462 (2001). Article PubMed PubMed Central CAS Google Scholar * Racki, W. J. Cbk1p, a protein
similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in _Saccharomyces cerevisiae_. _EMBO J._ 19, 4524–4532 (2000). Article PubMed PubMed Central CAS
Google Scholar * Kurischko, C., Kuravi, V. K., Herbert, C. J. & Luca, F. C. Nucleocytoplasmic shuttling of Ssd1 defines the destiny of its bound mRNAs. _Mol. Microbiol._ 81, 831–849
(2011). Article PubMed PubMed Central CAS Google Scholar * Kurischko, C. et al. The yeast LATS/Ndr kinase Cbk1 regulates growth via golgi-dependent glycosylation and secretion. _Mol.
Biol. Cell_ 19, 5559–5578 (2008). Article PubMed PubMed Central CAS Google Scholar * Bogomolnaya, L. M., Pathak, R., Guo, J. & Polymenis, M. Roles of the RAM signaling network in
cell cycle progression in Saccharomyces cerevisiae. _Curr. Genet._ 49, 384–392 (2006). Article PubMed CAS Google Scholar * Bourens, M. et al. Mutations in the _Saccharomyces cerevisiae_
kinase Cbk1p lead to a fertility defect that can be suppressed by the absence of Brr1p or Mpt5p (Puf5p), proteins involved in RNA metabolism. _Genetics_ 183, 161–173 (2009). Article PubMed
PubMed Central CAS Google Scholar * Weiss, E. L. et al. The _Saccharomyces cerevisiae_ Mob2p–Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to
facilitate daughter cell–specific localization of Ace2p transcription factor. _J. Cell Biol._ 158, 885–900 (2002). Article PubMed PubMed Central CAS Google Scholar * Kuravi, V. K.,
Kurischko, C., Puri, M. & Luca, F. C. Cbk1 kinase and Bck2 control MAP kinase activation and inactivation during heat shock. _Mol. Biol. Cell_ 22, 4892–4907 (2011). Article PubMed
PubMed Central CAS Google Scholar * Gutiérrez-Escribano, P. et al. The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator bcr1 during biofilm formation in
_Candida albicans_. _PLoS Pathog._ 8, e1002683 (2012). Article PubMed PubMed Central Google Scholar * Herold, I. & Yarden, O. Regulation of _Neurospora crassa_ cell wall remodeling
via the cot-1 pathway is mediated by gul-1. _Curr. Genet._ 63, 145–159 (2017). Article PubMed CAS Google Scholar * Saputo, S., Chabrier-Rosello, Y., Luca, F. C., Kumar, A. & Krysan,
D. J. The RAM network in pathogenic fungi. _Eukaryot. Cell_ 11, 708–717 (2012). Article PubMed PubMed Central CAS Google Scholar * Herold, I. et al. Transcriptional profiling and
localization of GUL-1, a COT-1 pathway component, in _Neurospora crassa_. _Fungal Genet. Biol._ 126, 1–11 (2019). Article PubMed CAS Google Scholar * Thammahong, A., Dhingra, S.,
Bultman, K. M., Kerkaert, J. D. & Cramer, R. A. An Ssd1 Homolog Impacts Trehalose and Chitin Biosynthesis and Contributes to Virulence in _Aspergillus fumigatus_. _mSphere_ 4,
https://doi.org/10.1128/msphere.00244−19 (2019). * Song, Y. et al. Role of the RAM network in cell polarity and hyphal morphogenesis in _Candida albicans_. _Mol. Biol. Cell_ 19, 5456–5477
(2008). Article PubMed PubMed Central CAS Google Scholar * Kurischko, C. A Role for the _Saccharomyces cerevisiae_ regulation of Ace2 and polarized morphogenesis signaling network in
cell integrity. 171, 443-455 https://doi.org/10.1534/genetics.105.042101 (2005) * Chadwick, B. J. et al. The RAM signaling pathway links morphology, thermotolerance, and CO2 tolerance in the
global fungal pathogen _Cryptococcus neoformans_. _eLife_ 11, https://doi.org/10.7554/elife.82563 (2022). * Yarden, O., Plamann, M., Ebbole, D. J. & Yanofsky, C. cot-1, a gene required
for hyphal elongation in _Neurospora crassa_, encodes a protein kinase. _EMBO J._ 11, 2159–2166 (1992). Article PubMed PubMed Central CAS Google Scholar * Johns, S. A., Leeder, A. C.,
Safaie, M. & Turner, G. Depletion of _Aspergillus nidulans cotA_ causes a severe polarity defect which is not suppressed by the nuclear migration mutation nudA2. _Mol. Genet Genomics_
275, 593–604 (2006). Article PubMed CAS Google Scholar * De Assis, L. J. et al. Carbon catabolite repression in filamentous fungi is regulated by phosphorylation of the transcription
factor CreA. _mBio_ 12, https://doi.org/10.1128/mbio.03146-20 (2021). * Hynes, M. J., Murray, S. L., Duncan, A., Khew, G. S. & Davis, M. A. Regulatory genes controlling fatty acid
catabolism and peroxisomal functions in the filamentous fungus _Aspergillus nidulans_. _Eukaryot. Cell_ 5, 794–805 (2006). Article PubMed PubMed Central CAS Google Scholar * Ries, L. N.
A. et al. _Aspergillus fumigatus_ acetate utilization impacts virulence traits and pathogenicity. _mBio_ 12, https://doi.org/10.1128/mbio.01682-21 (2021). * Lauter, F. R. et al.
Photoregulation of cot-1, a kinase-encoding gene involved in hyphal growth in _Neurospora crassa_. _Fungal Genet Biol._ 23, 300–310 (1998). Article PubMed CAS Google Scholar * Gorovits,
R., Propheta, O., Kolot, M., Dombradi, V. & Yarden, O. A mutation within the catalytic domain of COT1 kinase confers changes in the presence of two COT1 isoforms and in Ser/Thr protein
kinase and phosphatase activities in _Neurospora crassa_. _Fungal Genet Biol._ 27, 264–274 (1999). Article PubMed CAS Google Scholar * Gorovits, R. & Yarden, O. Environmental
Suppression of _Neurospora crassa_ cot-1 Hyperbranching: a Link between COT1 Kinase and Stress Sensing. _Eukaryot. Cell_ 2, 699–707 (2003). Article PubMed PubMed Central CAS Google
Scholar * Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. _Science_ 324, 1729–1732 (2009). Article ADS PubMed CAS
Google Scholar * Mitrea, D. M. & Kriwacki, R. W. Phase separation in biology; functional organization of a higher order. _Cell Commun. Signal._ 14.
https://doi.org/10.1186/s12964-015-0125-7 (2016) * Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. _Science_ 352, 595–599 (2016). Article
ADS PubMed PubMed Central CAS Google Scholar * Sfakianos, A. P., Whitmarsh, A. J. & Ashe, M. P. Ribonucleoprotein bodies are phased in. _Biochem Soc. Trans._ 44, 1411–1416 (2016).
Article PubMed CAS Google Scholar * Seiler, S., Vogt, N., Ziv, C., Gorovits, R. & Yarden, O. The STE20/Germinal Center Kinase POD6 Interacts with the NDR Kinase COT1 and Is Involved
in Polar Tip Extension in _Neurospora crassa_. _Mol. Biol. Cell_ 17, 4080–4092 (2006). Article PubMed PubMed Central CAS Google Scholar * Tanaka, S. et al. Saccharomyces cerevisiae
SSD1 orthologues are essential for host infection by the ascomycete plant pathogens _Colletotrichum lagenarium_ and _Magnaporthe grisea_. _Mol. Microbiol._ 64, 1332–1349 (2007). Article
PubMed CAS Google Scholar * Al Abdallah, Q., Ge, W. & Fortwendel, J. R. A Simple and Universal System for Gene Manipulation in _Aspergillus fumigatus In Vitro_-Assembled Cas9-Guide
RNA Ribonucleoproteins Coupled with Microhomology Repair Templates. _mSphere_ 2, https://doi.org/10.1128/mSphere.00446-17 (2017). * Fortwendel, J. R. et al. Plasma membrane localization is
required for RasA-mediated polarized morphogenesis and virulence of _Aspergillus fumigatus_. _Eukaryot. Cell_ 11, 966–977 (2012). Article PubMed PubMed Central CAS Google Scholar * Al
Abdallah, Q., Norton, T. S., Hill, A. M., LeClaire, L. L. & Fortwendel, J. R. A Fungus-specific protein domain is essential for rasa-mediated morphogenetic signaling in _Aspergillus
fumigatus_. _mSphere_ 1, https://doi.org/10.1128/mSphere.00234-16 (2016) * Martin-Vicente, A., Souza, A. C. O., Nywening, A. V., Ge, W. & Fortwendel, J. R. Overexpression of the
_Aspergillus fumigatus_ small GTPase, RsrA, promotes polarity establishment during germination. _J. Fungi_ 6, 285 (2020). Article CAS Google Scholar * Martin-Vicente, A., Souza, A. C. O.,
Al Abdallah, Q., Ge, W. & Fortwendel, J. R. SH3-class Ras guanine nucleotide exchange factors are essential for _Aspergillus fumigatus_ invasive growth. _Cell. Microbiol._ 0, e13013
(2019). * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. _Methods_ 25, 402–408 (2001). Article PubMed
CAS Google Scholar * Basenko, E. et al. FungiDB: an integrated bioinformatic resource for fungi and oomycetes. _J. Fungi_ 4, 39 (2018). Article Google Scholar * Pertea, M. et al.
StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. _Nat. Biotechnol._ 33, 290–295 (2015). Article PubMed PubMed Central CAS Google Scholar Download
references ACKNOWLEDGEMENTS This work was funded in part by the National Institutes of Health (NIH) / National Institute of Allergy and Infectious Disease (NIAID) awards R01AI143197,
R01AI158442, and R21AI178048 to JRF. Portions of figures were created with BioRender.com. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Clinical Pharmacy and Translational
Science, College of Pharmacy, University of Tennessee Health Science Center, Memphis, TN, 38163, USA Adela Martin-Vicente, Xabier Guruceaga, Harrison I. Thorn, Jinhong Xie & Jarrod R.
Fortwendel * Department of Pharmacy and Pharmaceutical Sciences, Division of Pharmaceutical Sciences, St Jude Children’s Research Hospital, Memphis, TN, 38105, USA Ana Camila Oliveira Souza
& Wenbo Ge * Graduate Program in Pharmaceutical Sciences, College of Graduate Health Sciences, University of Tennessee Health Science Center, Memphis, TN, 38103, USA Harrison I. Thorn
& Jinhong Xie * Integrated Program in Biomedical Sciences, College of Graduate Health Sciences, University of Tennessee Health Science Center, Memphis, TN, 38103, USA Ashley V. Nywening
* Department of Microbiology, Immunology, and Biochemistry, College of Medicine, University of Tennessee Health Science Center, Memphis, TN, 38103, USA Jarrod R. Fortwendel Authors * Adela
Martin-Vicente View author publications You can also search for this author inPubMed Google Scholar * Ana Camila Oliveira Souza View author publications You can also search for this author
inPubMed Google Scholar * Xabier Guruceaga View author publications You can also search for this author inPubMed Google Scholar * Harrison I. Thorn View author publications You can also
search for this author inPubMed Google Scholar * Jinhong Xie View author publications You can also search for this author inPubMed Google Scholar * Ashley V. Nywening View author
publications You can also search for this author inPubMed Google Scholar * Wenbo Ge View author publications You can also search for this author inPubMed Google Scholar * Jarrod R.
Fortwendel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization and planning: J.R.F. and A.M.-V.; Experimentation: A.M.-V.,
A.C.O.S.; W.G., J.X., X.G., A.V.N., and H.I.T.; Data analysis: J.R.F., A.M-V., and A.C.O.S.; Manuscript and figures preparation: A.M.-V., J.R.F., and A.C.O.S. CORRESPONDING AUTHOR
Correspondence to Jarrod R. Fortwendel. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks
the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES
SUPPLEMENTARY DATA 1 REPORTING SUMMARY TRANSPARENT PEER REVIEW FILE SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Martin-Vicente, A., Souza, A.C.O., Guruceaga, X. _et al._ A conserved fungal
morphogenetic kinase regulates pathogenic growth in response to carbon source diversity. _Nat Commun_ 15, 8945 (2024). https://doi.org/10.1038/s41467-024-53358-3 Download citation *
Received: 13 November 2023 * Accepted: 09 October 2024 * Published: 17 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53358-3 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Carnegie Endowment for International Peace | Carnegie Endowment for International PeaceGlobal LocationsresearchemissaryaboutexpertsmoresupportprogramseventsblogspodcastsvideosNewslettersAnnual Reportscareers...
Soft ground conditions a concern in east lothian - farmers weeklySoft ground conditions are concerning James Grant-Suttie at Balgone Farms, North Berwick, East Lothian, but he hopes to ...
Putin dismisses us hacking allegations as campaign rhetoricRussian President Vladimir Putin Sergei Karpukhin | Reuters Russian President Vladimir Putin on Sunday shrugged off new ...
Roddick fined for quick getawayThe twice Wimbledon finalist left in such a hurry that he was fined by the ATP, the players’ union. Roddick, 24, who mad...
Attention Required! | CloudflarePlease enable cookies. Sorry, you have been blocked You are unable to access defatoonline.com.br Why have I been blocked...
Latests News
A conserved fungal morphogenetic kinase regulates pathogenic growth in response to carbon source diversityABSTRACT Fungal pathogens must exhibit strong nutritional plasticity, effectively sensing and utilizing diverse nutrient...
Cindy lamp by ferruccio laviani for kartellCINDY LAMP BY FERRUCCIO LAVIANI FOR KARTELL ‘cindy’ by ferruccio laviani for kartell is influenced by lamp designs of th...
Page not found - Just SecurityYou can use the search tool to locate content, authors or media.DON'T MISS A THING. Stay up to date with Just Security c...
Jobs support scheme ‘will not halt rising unemployment’Fears are growing of a surge in unemployment before Christmas as the recovery in the economy falters under pressure from...
Profile: The field medic | NatureWhen emergencies happen in remote settings, field researchers can be left with little recourse. Erik Vance meets a man t...