Cardiovascular and renal effects of apelin in chronic kidney disease: a randomised, double-blind, placebo-controlled, crossover study
Cardiovascular and renal effects of apelin in chronic kidney disease: a randomised, double-blind, placebo-controlled, crossover study"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Chronic kidney disease (CKD) affects ~10% of the population and cardiovascular disease is its commonest complication. Despite treatment, patient outcomes remain poor and newer
therapies are urgently needed. Here, we investigated the systemic and renal effects of apelin in CKD. In a randomized, double-blind, placebo-controlled, crossover study, 24 subjects (12
patients with CKD and 12 matched healthy subjects) received pyroglutamated apelin-13 ([Pyr1]apelin-13, 1 nmol/min and 30 nmol/min) or matched placebo on two separate visits. Systemic and
renal hemodynamics were monitored throughout. The co-primary endpoints were change in systemic vascular resistance index and renal blood flow. Secondary endpoints were change in blood
pressure, cardiac output, pulse wave velocity, glomerular filtration rate, natriuresis, free water clearance and urinary protein excretion. In both health and CKD, 30 nmol/min
[Pyr1]apelin-13 reduced mean arterial pressure by ~4%, systemic vascular resistance by ~12%, and increased cardiac index by ~10%, compared to placebo (p < 0.05 for all). Both doses of
[Pyr1]apelin-13 increased renal blood flow by ~15%, natriuresis by ~20% and free water clearance by ~10%, compared to placebo (p < 0.05 for all). In patients with chronic kidney disease
only, glomerular filtration rate fell by ~10%, effective filtration fraction by ~5% and proteinuria by ~25% (p < 0.01 for all). Apelin has short-term cardiovascular and renal benefits in
CKD. If maintained longer-term, these should improve patient outcomes. Clinical trials of long-acting oral apelin agonists are justified in CKD and other conditions with impaired salt and
water balance. Registration number at www.clinicalTrials.gov: NCT03956576. Funded by _Kidney Research UK. _ SIMILAR CONTENT BEING VIEWED BY OTHERS THE THERAPEUTIC POTENTIAL OF APELIN IN
KIDNEY DISEASE Article 13 August 2021 THE EFFECT OF ALDOSTERONE AND ALDOSTERONE BLOCKADE ON THE PROGRESSION OF CHRONIC KIDNEY DISEASE: A RANDOMIZED PLACEBO-CONTROLLED CLINICAL TRIAL Article
Open access 06 October 2020 EFFECTS OF RENAL DENERVATION ON KIDNEY FUNCTION IN PATIENTS WITH CHRONIC KIDNEY DISEASE: A SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 04 September
2023 INTRODUCTION Chronic kidney disease (CKD) is common and is predicted to be the fifth leading cause of life-years lost by 20401. Cardiovascular disease is independently associated with
CKD and is its commonest complication2. As the estimated glomerular filtration rate (eGFR) declines, the risks of major adverse cardiovascular events and cardiovascular and all-cause
mortality increase2. The current standard of care for CKD focuses on reducing blood pressure (BP) and proteinuria3,4, ideally using renin-angiotensin system antagonists alongside the recent
introduction of sodium-glucose co-transporter 2 (SGLT2) inhibitors. However, despite these, patient outcomes remain poor, and newer treatments are urgently needed that will not only slow CKD
progression but also reduce the associated risk of cardiovascular disease. The apelin system comprises the apelin receptor and its two endogenous ligands, apelin and elabela5. Apelin is an
endothelium-dependent vasodilator and a potent inotrope, and overall its actions oppose those of the renin-angiotensin system6. Clinical studies in healthy subjects and in patients with
heart failure have shown that apelin lowers BP and systemic vascular resistance, and increases cardiac output7,8. With respect to the kidney, pre-clinical studies demonstrate that apelin
regulates glomerular hemodynamics by opposing the actions of angiotensin II9. In addition, pre-clinical work shows that apelin opposes the actions of vasopressin10, relevant for CKD as
impaired salt and water balance is a feature and an important driver of hypertension. Thus, apelin offers major therapeutic promise in CKD. Here, we present the results of the first clinical
studies examining the systemic and renal actions of apelin in health and CKD. We find that the hemodynamic effects of apelin are equivalent in health and CKD: apelin lowers mean arterial
pressure and systemic vascular resistance and increases cardiac output and renal blood flow. Apelin also promotes urinary sodium excretion and free water clearance. In patients with CKD
alone, apelin reduces glomerular filtration rate, effective filtration fraction and proteinuria, and renoprotective effects analogous to an angiotensin-converting enzyme inhibitor. Overall,
acute apelin treatment has beneficial cardiovascular and renal effects in patients with CKD who are receiving standard-of-care. Our data support studies of chronic apelin receptor agonism in
this at-risk population. RESULTS In brief, we performed a randomised, double-blind, placebo-controlled, 2-way crossover study in 12 patients with stable CKD receiving recommended
renoprotective treatment and in 12 age- and sex-matched healthy subjects. All subjects received pyroglutamate apelin-13 ([Pyr1]apelin-13), 1 nmol/min and 30 nmol/min, or matched placebo on
two separate study visits. Impedance cardiography assessed systemic hemodynamics, and infusions of para-aminohippurate and iohexol assessed renal blood flow and glomerular filtration rate,
respectively. Twenty-four subjects (12 with CKD and 12 healthy subjects) were recruited and completed both study visits with no adverse events. Both doses of [Pyr1]apelin-13 were well
tolerated. Data from all 24 subjects are included in the analyses. Baseline subject demographics are shown in Table 1. Compared to healthy subjects (48 ± 4 years, 67% male), patients with
CKD (48 ± 4 years, 67% male) had a higher BP (systolic BP: 131 ± 3 _versus_ 121 ± 3 mmHg) and increased arterial stiffness (pulse wave velocity: 6.5 ± 0.3 _versus_ 5.7 ± 0.3 m/s). Patients
with CKD had a reduced estimated glomerular filtration rate (35 ± 4 _versus_ 100 ± 6 ml/min/1.73 m2) and proteinuria (~ 0.5 g/day). CARDIOVASCULAR EFFECTS OF [PYR1]APELIN-13 Baseline
cardiovascular parameters are shown in Table 2. As intended, only the infusion of 30 nmol/min [Pyr1]apelin-13 increased plasma apelin concentration (Supplementary Fig. 1). Accordingly,
infusion of [Pyr1]apelin-13 1 nmol/min did not have demonstrable hemodynamic effects in either healthy subjects or patients with CKD (Supplementary Figs. 2–4). APELIN REDUCES BP AND SYSTEMIC
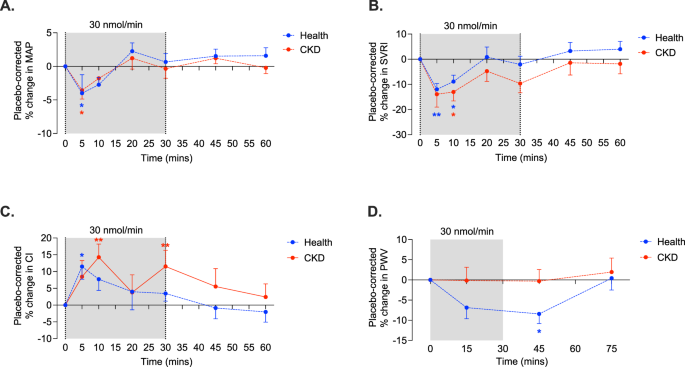
VASCULAR RESISTANCE IN HEALTH AND CKD [Pyr1]apelin-13 30 nmol/min reduced mean arterial pressure (MAP) in both healthy subjects and those with CKD (health: 92 ± 2 to 89 ± 2 mmHg; CKD: 97 ±
2 to 94 ± 3; _p_ < 0.05 for both), reductions of ~ 4% compared to placebo (Fig. 1A). Whereas systolic BP did not change in health, it fell from 131 ± 4 to 128 ± 3 mmHg in CKD (_p_ <
0.05 compared to placebo, Supplementary Fig. 5). In healthy subjects, diastolic BP fell from 78 ± 2 to 75 ± 2 mmHg and in CKD patients from 81 ± 2 to 77 ± 3 mmHg, (_p_ < 0.05 for both
compared to placebo, Supplementary Fig. 5), a reduction of ~ 5% in both groups. Systemic vascular resistance fell during infusion of [Pyr1]apelin-13 30 nmol/min (Fig. 1B). In healthy
subjects, this fell by a maximum of 309 dynes*s*cm−5m2 compared to placebo (_p_ < 0.01); in patients with CKD, this figure was 407 dynes*s*cm−5m2 (_p_ < 0.01), reductions of 12% and
13%, respectively. Overall, 8 of 12 patients with CKD showed a > 10% reduction in systemic vascular resistance. There was no demonstrable between-group difference in response to apelin.
APELIN INCREASES CARDIAC INDEX AND HEART RATE IN HEALTH AND CKD In keeping with its inotropic actions, [Pyr1]apelin-13 30 nmol/min increased cardiac index by ~ 10% in both healthy subjects
and patients with CKD patients (2.7 ± 0.1 to 3.0 ± 0.1 L/min/m2 and 2.5 ± 0.1 to 2.7 ± 0.2 L/min/m2 respectively, both _p_ < 0.05; Fig. 1C). Overall, patients with CKD showed a maximum
increase in cardiac index of 15 ± 3% and three patients had a ≥ 20% rise. Whilst the effect was again short-lived in healthy subjects, it persisted to the end of the infusion in patients
with CKD. Whereas apelin infusion was associated with an acute and short-lived 7% increase in heart rate in healthy subjects (56 ± 2 to 60 ± 2 bpm, _p_ < 0.01 compared to baseline), heart
rate did not change in patients with CKD (Supplementary Fig. 6). APELIN REDUCES ARTERIAL STIFFNESS IN HEALTH BUT NOT CKD In healthy subjects, pulse wave velocity fell by ~ 5% in response to
[Pyr1]apelin-13 30 nmol/min (6.3 ± 0.2 to 5.9 ± 0.2 m/s, _p_ < 0.01 compared to placebo; Fig. 1D). This reduction in arterial stiffness was still developing when the apelin infusion had
stopped and only returned to baseline after 45 min. Apelin did not affect arterial stiffness in patients with CKD. RENAL EFFECTS OF [PYR1]APELIN-13 APELIN INCREASES RENAL BLOOD FLOW IN
HEALTH AND CKD Baseline renal parameters are shown in Table 2. In healthy subjects and in patients with CKD, both low and high doses of [Pyr1]apelin-13 increased renal blood flow (Fig. 2A).
In health, and compared to placebo, renal blood flow increased by ~ 140 mL/min and ~ 110 mL/min in response to [Pyr1]apelin-13 1 nmol/min and 30 nmol/min, respectively (_p_ < 0.01 for
both), equivalent to increases of ~ 15%. In CKD, renal blood flow was still increasing at the end of both low and high-dose apelin infusions. Compared to placebo, and at maximum,
[Pyr1]apelin-13 1 nmol/min increased renal blood flow by ~ 40 mL/min and [Pyr1]apelin-13 30 nmol/min by ~ 55 mL/min, increases of 8% and 20% (_p_ < 0.05 and _p_ < 0.01), respectively.
APELIN REDUCES GLOMERULAR FILTRATION RATE IN CKD Whereas infusion of [Pyr1]apelin-13 did not affect glomerular filtration rate in healthy volunteers, both low and high dose [Pyr1]apelin-13
reduced glomerular filtration rate (1 nmol/min: 41 ± 6 to 36 ± 6 mL/min; 30 nmol/min: 38 ± 5 to 34 ± 5 mL/min, _p_ < 0.01 _versus_ placebo for both) in patients with CKD (Fig. 2B).
Notably, all patients with CKD experienced at least a 10% reduction in glomerular filtration rate in response to either dose of apelin. APELIN REDUCES FILTRATION FRACTION AND PROTEINURIA IN
CKD In healthy subjects, [Pyr1]apelin-13 1 nmol/min did not affect filtration fraction whereas [Pyr1]apelin-13 30 nmol/min reduced filtration fraction from 19.3 ± 0.8 to 17.8 ± 0.7% (_p_
< 0.05 _versus_ placebo). In patients with CKD, and compared to placebo, both low and high doses of [Pyr1]apelin-13 reduced filtration fraction (maximum response 1 nmol/min: 21.2 ± 1.2 to
19.4 ± 1.5%; 30 nmol/min: 22.0 ± 1.4 to 17.5 ± 0.8%; _p_ < 0.0001 for both, Fig. 2C). As seen with renal blood flow, these effects were still developing at the end of the study period.
In keeping with the effects on filtration fraction, both [Pyr1]apelin-13 1 nmol/min and 30 nmol/min reduced proteinuria in patients with CKD (1 nmol/min: 349 ± 98 to 269 ± 91 μg/min; 30
nmol/min: 308 ± 86 to 252 ± 73 μg/min, _p_ < 0.01 for both, Fig. 2D), equivalent to reductions of ~ 25%. There was no relationship between baseline glomerular filtration rate and the
degree of proteinuria reduction. APELIN PROMOTES SALT AND WATER EXCRETION IN HEALTH AND CKD Both doses of [Pyr1]apelin-13 increased urinary sodium excretion in healthy subjects and patients
with CKD (Fig. 3A). In health, low dose [Pyr1]apelin-13 increased natriuresis by ~ 40 μmol/min in comparison to placebo (_p_ < 0.01), a rise of ~ 30%. Most healthy subjects had a ≥ 20%
increase in natriuresis at this dose. The effects of high-dose [Pyr1]apelin-13 were similar. In CKD, and compared to placebo, [Pyr1]apelin-13 1 nmol/min increased urinary sodium excretion by
~ 40 μmol/min (_p_ < 0.0001), with a similar response seen at [Pyr1]apelin-13 30 nmol/min. These increases in natriuresis were still developing at the study end. Overall, apelin
increased urinary sodium excretion in patients with CKD by ~ 30%. Finally, we examined the effects of apelin on the clearance of free water. In healthy subjects, [Pyr1]apelin-13 1 nmol/min
increased free water clearance by 1.2 ± 0.5 mL/min compared to placebo (_p_ < 0.05), an increase of ~ 25%. The higher dose of apelin had no effect. In patients with CKD, both low and high
doses of apelin increased free water clearance. [Pyr1]apelin-13 1 nmol/min increased free water clearance from 3.8 ± 0.4 mL/min to a maximum of 4.1 ± 0.4 mL/min (_p_ < 0.05). There was a
similar magnitude of response to [Pyr1]apelin-13 30 nmol/min. If maintained over a 24-hour period, this would equate to an increase in free water clearance of ~ 0.5 L. DISCUSSION Chronic
kidney disease (CKD) is an increasingly common public health concern. With a current global prevalence of ~ 10%, it now ranks as the twelfth leading cause of death worldwide11. Importantly,
patients with CKD are more likely to die from cardiovascular diseases than they are to reach kidney failure and the need for dialysis or kidney transplant2,12. Indeed, almost 8% of global
cardiovascular deaths in 2017 were attributable to CKD11. Unfortunately, these risks continue despite the current standard of care, and new add-on treatments are urgently needed. The ideal
treatment would provide direct renoprotection, whilst also offering broad cardiovascular protection. In this context, our study had four main findings. First, we demonstrated that apelin
lowers BP and causes peripheral vasodilation in patients with CKD. Second, apelin increases renal blood flow and reduces renal vascular resistance. Third, we observed important reductions in
filtration fraction and proteinuria in patients with CKD. Fourth, apelin promotes salt and water excretion. Our experimental design allowed us to show that these cardioprotective and
renoprotective effects are observed on top of renin-angiotensin system blockade and that apelin has direct renal effects. Overall, apelin was safe and well tolerated in CKD patients and our
data define the translational potential of apelin as a new therapy for CKD and its associated cardiovascular disease. These are the first clinical studies of apelin in kidney disease. In
keeping with the favourable systemic hemodynamic effects in heart failure7,8, we demonstrate that apelin reduces BP, causes peripheral vasodilation, and increases cardiac output in patients
with CKD. Hypertension is a frequent finding in patients with CKD13 and despite treatment with multiple antihypertensive agents, most patients with CKD fail to reach target BP14. Although
the BP-lowering effect of apelin was modest (~ 4 mmHg), it is similar in magnitude to that of the SGLT2 inhibitor, empagliflozin, which lowers systolic BP by ~ 3 mmHg and diastolic BP by ~ 1
mmHg in CKD, and reduces cardiovascular and all-cause mortality in those with and without CKD15,16. More broadly, a 5 mmHg reduction in systolic BP is recognised to reduce the risk of major
cardiovascular events by ~ 10%17. The effect of apelin on BP might have been more impressive had the subjects not had such good baseline BP control. Epidemiological data demonstrate that
arterial stiffness, measured by pulse wave velocity, is an independent risk factor for cardiovascular morbidity and mortality18,19. In the current study, apelin reduced pulse wave velocity
by ~ 5% in healthy subjects compared to placebo. The magnitude of this reduction is similar to that of a renin-angiotensin system inhibitor20,21, and the effect is likely due to the
reduction in BP seen with apelin. However, we did not observe vascular unstiffening in patients with CKD despite a comparable fall in BP. It is possible that the structural and functional
vascular changes characterising CKD (e.g., medial vascular calcification) are less likely to be overcome acutely and a longer duration of treatment is needed. Notably, in pre-clinical models
of pulmonary arterial hypertension, apelin agonism improves vascular hemodynamics and vascular remodelling22,23. If chronic apelin receptor agonism could reduce arterial stiffness in CKD,
it would be expected to improve patient outcomes more than BP reduction alone24. Changes in systemic hemodynamics will affect renal hemodynamics. Our protocol was designed to examine both
indirect and potential direct effects of apelin on the kidney. The higher apelin dose (30 nmol/min) was expected to affect systemic (and therefore renal) hemodynamics, and the lower
sub-systemic dose (1 nmol/min) was used to define direct renal effects. Whereas only the higher dose of apelin affected systemic hemodynamics, both low and high doses of apelin affected
renal hemodynamics supporting a direct effect of apelin on the kidney. Both low and high-dose apelin increased renal blood flow in healthy subjects and subjects with CKD. These findings have
two implications. First, that apelin causes glomerular afferent arteriolar vasodilation and second, that a downregulated apelin system contributes to the increased renovascular tone seen in
CKD. An increase in renal blood flow might be expected to increase the glomerular filtration rate. However, the glomerular filtration rate did not change in healthy subjects, suggesting
that apelin has a similar vasodilatory effect at the efferent arteriole. In patients with CKD, we observed a fall in glomerular filtration rate which suggests that apelin preferentially
vasodilates the efferent arteriole, although not excluding an effect on mesangial cells and filtration coefficient. Our data are consistent with pre-clinical studies9, and these hemodynamic
effects of apelin are similar to those of an endothelin blocker25, agents that have recently been licensed for CKD26. In line with the effects of apelin on efferent arteriolar tone and
glomerular perfusion pressure, we observed a reduction in filtration fraction and proteinuria in those with CKD. These effects are analogous to but occur in addition to, those seen with
renin-angiotensin system blockade27. Proteinuria reduction is important both for reducing the risk of CKD progression28 and its associated cardiovascular disease29,30. However, despite the
standard of care, many patients with proteinuric CKD have residual proteinuria31. In the current study, all subjects were established on maximally tolerated treatment with an
angiotensin-converting enzyme inhibitor or angiotensin receptor blockers with good BP control. Despite this, mean baseline proteinuria remained at ~ 0.5 g/d. Interestingly, the magnitude of
proteinuria reduction we see (~ 25%) is similar to that observed with an endothelin receptor antagonist and an SGLT2 inhibitor25,32. In both healthy subjects and those with CKD, apelin
promoted natriuresis. If the natriuretic response observed was maintained over 24 h, then patients would excrete an extra ~ 60 mmol of sodium, which is equivalent to the effect of 25 mg of
spironolactone33. Natriuresis was seen with both doses of [Pyr1]apelin-13 but was more impressive with the lower dose. This might be expected given the higher dose of apelin-affected
systemic hemodynamics in a manner that should promote salt (and water) retention by the kidney. We have previously shown that apelin and its receptor are expressed throughout the human
kidney34, including nephron segments intimately involved in regulating sodium balance, so direct tubular action is not unexpected. In addition, pre-clinical studies have shown that apelin
decreases epithelial sodium channel expression and activity35, which would lead to reduced sodium reabsorption, although we recognise that the effects seen here are probably too rapid to be
explained by this. Finally, we have previously shown co-localisation of the apelin receptor and the SGLT2 in the proximal tubule34. SGLT2 inhibitors are also recognised to promote
natriuresis36, and a synergistic crosstalk between apelin and SGLT2 to promote salt excretion is an intriguing possibility that should be explored in future work. In keeping with
pre-clinical studies9,37, apelin increased the clearance of free water – the volume of solute-free water excreted by the kidneys. The mechanism for this may relate to an inhibition of
vasopressin signalling. Apelin and vasopressin show reciprocal regulation in humans in response to changes in osmolality, and in vitro and in vivo studies find apelin directly inhibits the
actions of vasopressin in the collecting duct, thus promoting free water loss37,38,39. Notably, vasopressin also activates the epithelial sodium channel, promoting distal sodium
reabsorption, and so its downregulation by apelin may be another mechanism whereby apelin promotes natriuresis40. Overall, our findings are relevant to conditions beyond CKD where salt and
water excretion is impaired, such as heart failure, liver disease and syndrome of inappropriate antidiuresis. The effects of apelin on renal parameters appeared delayed in patients with CKD
compared to healthy subjects, with a suggestion of prolonged and perhaps increased systemic hemodynamic effects in this group. There are several potential explanations for these differences.
The apelin system is expressed throughout the human kidney34, and whilst there is no published human data, the system is downregulated in animal models of CKD41. Thus, we expect the system
to be modified in patients with CKD. The relationship between the apelin system and the renin-angiotensin system is also implicated. There is a crosstalk between the systems. For example, in
a model of heart failure, angiotensin II-induced downregulation of the apelin receptor is restored by angiotensin receptor blockade42. In our study, most patients with CKD were established
on renin-angiotensin system blockers, and these drugs may alter apelin signalling. Inhibition of angiotensin II may also affect the speed at which compensatory mechanisms are able to respond
to acute changes in systemic hemodynamics. We acknowledge the limitations of our data. This is an acute study, and it will be important to confirm that these effects are maintained
longer-term. In addition, we studied a relatively homogeneous CKD population, and further work is needed in a broader population of patients with CKD, including those with diabetes, renal
vascular disease, and active multi-system inflammatory disease. However, our patients were at elevated cardiovascular risk as reflected by their increased arterial stiffness and proteinuria.
Our study was also only powered to examine the effects of apelin on systemic vascular resistance and renal blood flow. Finally, although optimised on antagonists of the renin-angiotensin
system, only a minority of subjects were receiving SGLT2 inhibitors as these were not recommended for use in CKD until the final months of our study. Future work in this area should examine
the role of apelin agonism in patients with CKD receiving both renin-angiotensin system and SGLT2 inhibitors. In conclusion, we have shown in a randomised, double-blind, placebo-controlled
crossover study that apelin has beneficial systemic and renal effects in healthy subjects, and these effects are preserved and accentuated in patients with CKD already optimised on current
treatment. If these effects were maintained longer-term, they would be expected to reduce cardiovascular and renal risk and improve patient outcomes. Our data, therefore, provide
justification for future studies in kidney disease and beyond. Encouragingly, this will be facilitated by long-acting, orally available apelin receptor agonists, which are in development43.
METHODS STUDY DESIGN AND OVERSIGHT We performed a randomised, double-blind, placebo-controlled crossover study in patients with chronic kidney disease and age- and sex-matched healthy
subjects. Recruitment and all studies were completed at the University of Edinburgh between May 2021 and December 2022 in accordance with the principles of the Declaration of Helsinki. The
study ended when the recruitment target was met. Ethical approval was obtained from the South-East Scotland Research Ethics Committee (Reference18/SS/0145). All subjects provided written
informed consent prior to participation, and the study was registered at www.clinicaltrials.gov (NCT 03956576). The study protocol is provided. SUBJECTS Adult patients with stable CKD stages
1–4 were recruited from the renal outpatient clinic within NHS Lothian. To be included, subjects had to be over the age of 18 years and able to give informed consent. Stable CKD was defined
as < 10% variability in eGFR over at least two measurements taken three months apart. Where the primary renal disease was due to a multi-system inflammatory condition (e.g.,
ANCA-associated vasculitis), this was in clinical remission. Exclusion criteria were: overt cardiovascular disease or a diagnosis of diabetes mellitus as these are recognised to affect the
apelin system44,45; kidney failure (estimated glomerular filtration rate < 15 mL/min/1.73 m2) or receiving kidney replacement therapy (including kidney transplant recipients); serum
albumin < 30 g/L; a diagnosis of polycystic kidney disease or tolvaptan therapy due to the relationship between apelin and vasopressin10; multiple or severe drug or food allergies;
pregnancy or breastfeeding; uncontrolled hypertension (BP > 160/100 mmHg). Sex was self-reported. Age- and sex-matched healthy subjects were recruited from local ethically approved
databases. ‘Health’ was defined as no current or previous medical issues and no regularly prescribed medication. RANDOMISATION A randomised and numbered study visit schedule was generated
prior to subject recruitment. This specified the order in which subjects received apelin or placebo. Upon recruitment by the investigator, the study nurse randomly allocated each subject to
a numbered visit schedule to which both the investigator and subject were blinded. The investigators remained blinded to the visit schedule until all study visits were complete. DRUGS
Pyroglutamate apelin-13 ([Pyr1]apelin-13, Severn Biotech Ltd., Kidderminster, UK) was dissolved in 5 ml 0.9% saline and administered at 1 and 30 nmol/min for 30 min as two separate infusions
with a 90-min washout between doses (Supplementary Fig. 7). These doses were based on dose-ranging studies and our previous work7,8. In these studies, 1 nmol/min [Pyr1]apelin-13 caused
local vasodilation but had no discernible effects on systemic hemodynamics. Therefore, we hypothesised that it might allow us to identify isolated renal hemodynamic effects of apelin. The
higher 30 nmol/min dose had significant systemic hemodynamic effects in both local and systemic vascular studies. Iohexol (OmnipaqueTM 140 mg Iodine/mL, 200 ml, GE Healthcare, Chicago, USA)
and para-aminohippurate (PAH, Sodium 4-aminohippurate 2 g in 10 ml, Clinalfa AG and Basic Pharma, Geleen, The Netherlands) were diluted in 100 ml 5% dextrose and a weight-based loading dose
was given over 15 min, followed by a maintenance infusion. For subjects with an estimated glomerular filtration rate < 60 mL/min/1.73 m2, doses of PAH and iohexol were reduced as in our
previous studies25. ASSAYS At prespecified time points (Supplementary Fig. 7), venous blood was collected into EDTA tubes (Monovette® Sarstedt, Numbrecht, Germany) for the measurement of
plasma apelin, haematocrit, iohexol, and PAH, and serum gel tubes (Monovette® Sarstedt, Numbrecht, Germany) for measurement of serum sodium, potassium, and osmolality. In addition, 20 ml
aliquots of urine from each void were collected into plain universal containers for the measurement of PAH, iohexol, sodium, potassium, and protein. Haematocrit was measured immediately on
whole blood using a Coulter counter in the hospital laboratory. All other blood samples were centrifuged within 90 min of sampling (2500 × _g_ for 20 min for EDTA, 3000 × _g_ for 15 min for
gel) with plasma, serum and urine samples, then stored at − 80 °C until required. [Pyr1]apelin-13 concentration was measured using an enzyme-linked immunosorbent assay (EK-057-23, Phoenix
Pharmaceuticals, Burlingame, USA). Plasma and urine iohexol and PAH concentrations were measured using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection46,47.
Plasma and urine sodium and potassium concentrations were measured using an ion-selective electrode (Roche Diagnostics AVL 9180 series electrolyte analysers, Fisher Scientific,
Leicestershire, UK)48 and osmolality using an osmometer (Loser micro-digital osmometer, Camlab, Cambridge, UK). Urine protein was measured using a Bradford assay49 with volumes adapted for
use on a Cobas Fara or Mira analyser (Roche Diagnostics Ltd, Welwyn Garden City, UK). CARDIOVASCULAR ASSESSMENTS BP was measured using the Omron HEM-705CP, a validated oscillometric
sphygmomanometer50. BP was recorded as an average of two readings from the right arm with ≤ 10 mmHg difference. Mean arterial pressure was calculated (diastolic BP + 1/3 pulse pressure).
Arterial stiffness was measured by gold standard pulse wave velocity using the SphygmoCor® system (SphygmoCor® Mx, AtCor Medical, Sydney, Australia, version 6.31)51. A high-fidelity
micromanometer (SPC-301, Millar Instruments, Texas, USA) was used to determine carotid-femoral pulse wave velocity. Values were recorded as the average of two measurements within 0.5 m/s.
Thoracic electrical impedance was performed using a validated impedance cardiograph, the BioZ® Dx (CardioDynamics®, Sonosite, Bedford, UK), with the non-invasive assessment of heart rate
(beats per min), cardiac output (L/min) and systemic vascular resistance (dynes*sec*cm−5). Data were corrected for body surface area to give cardiac index (L/min/m2) and systemic vascular
resistance index (dynes*sec*cm−5m−2), respectively, to allow direct comparison between subjects. Measurements were recorded as the average of two consecutive readings with < 10%
difference between the two. If the required criteria for measurements were not met, readings were repeated until they were achieved, or the data point was omitted at the discretion of the
investigator in the overall interests of the study. STUDY PROTOCOL Studies were performed in the University of Edinburgh’s Clinical Research Centre, Western General Hospital. All studies
were carried out in a quiet, temperature-controlled room (22–24 °C). Subjects were asked to adhere to a low-salt diet for 3 days prior to each study day and to abstain from smoking, alcohol,
and caffeine for 24 hours prior to each study. Subjects completed a 24-hour urine collection the day prior to each study. All subjects attended for two study days separated by at least 2
weeks. They received systemic infusions of [Pyr1]apelin-13 on one visit and matched placebo on the other. Subjects received a light breakfast on arrival and thereafter remained supine
throughout the study except when voiding. Routine medications were taken other than diuretics and SGLT2 inhibitors (omitted for 24 h and 7 days, respectively, prior to each study). Three
cannulae (two 18 G and one 20 G) were inserted into the forearms. Diuresis was induced by a bolus of 500 ml 5% dextrose given over 30 min, with a loading dose of iohexol and PAH commenced 15
min later. Thereafter, maintenance infusions of iohexol and PAH and 5% dextrose to a total volume of 400 mL/h continued throughout the study. A 2.5-h equilibration period was followed by
either infusion of [Pyr1]apelin-13 or placebo. Cardiovascular assessments and blood sampling were performed at pre-specified time points. Urine was collected by spontaneous voiding every 30
min (Supplementary Fig. 7, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license). STUDY ENDPOINTS AND DATA ANALYSIS
Pre-specified coprimary endpoints were changes in systemic vascular resistant index and renal blood flow. Secondary endpoints were changes in cardiac output, blood pressure, pulse wave
velocity, glomerular filtration rate, natriuresis, free water clearance and urinary protein excretion. Glomerular filtration rate and effective renal plasma flow were calculated from iohexol
and PAH clearances, respectively, as follows: Glomerular filtration rate = UrineIohexol / PlasmaIohexol x urine flow rate (mL/min) Effective renal plasma flow = UrinePAH / PlasmaPAH x urine
flow rate (mL/min) Effective renal blood flow was calculated by dividing the effective renal plasma flow by (1-haematocrit) and effective renal vascular resistance by dividing mean arterial
pressure by effective renal blood flow. Urinary protein and sodium excretion were calculated as their respective urinary concentrations multiplied by urinary flow rate. Effective filtration
fraction was calculated as the ratio of glomerular filtration rate to effective renal plasma flow and expressed as a percentage. SAMPLE SIZES & STATISTICS As apelin has not been given
to patients with CKD before, sample sizes were based on our previous studies in healthy subjects and patients with heart failure. For the coprimary endpoints of systemic vascular resistance
index and renal blood flow, a sample size of 12 subjects per group would have 85% power to detect a 10% reduction in systemic vascular resistance index and a 15% increase in renal blood flow
in response to apelin compared to placebo with a two-sided 5% significance level7,8. Previous studies examining apelin in heart failure have included similar sample sizes7,8. All data were
tested for normality with log transformation as appropriate. For the purpose of analyses, sex/gender were grouped together due to the small sample size. Data are presented as mean ± standard
error of the mean (SEM) or median (interquartile interval) as appropriate. Data were analysed using paired and unpaired _t_ tests or non-parametric equivalents as appropriate, analysis of
variance (ANOVA) with repeated measures and multiple comparison corrections or mixed effect analysis. For ANOVA and mixed effect models, where the model identified a significant result, the
change in the variable of interest was then compared at prespecified time points to baseline (within-group comparison, using Dunnett’s multiple comparison correction) and between groups
(using Šidák’s multiple comparison correction). Simple linear regression was used to explore relationships between variables, with Pearson correlation coefficients. Results were considered
statistically significant if a two-sided _p_-value was < 0.05. All statistical analyses were carried out on Prism® (version 9.4.1, GraphPad Software Inc, San Diego, USA). REPORTING
SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Source data are provided in this paper.
De-identified individual participant data are available on request from the corresponding author ([email protected]) for up to 6 months from the online publication date without
restrictions. A full study protocol is available on request. Source data are provided in this paper. REFERENCES * Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and
all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. _Lancet_ 392, 2052–2090 (2018). Article
PubMed PubMed Central Google Scholar * Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. _Lancet_ 382, 339–352 (2013).
Article PubMed Google Scholar * UK Kidney Association Clinical Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease. UKKA.
https://ukkidney.org/sites/renal.org/files/UKKA%20guideline_SGLT2i%20in%20adults%20with%20kidney%20disease%20v1%2018.10.21.pdf (2021). * Stevens, P. E. Levin A. Evaluation and management of
chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. _Ann. Intern. Med._ 158, 825–830 (2013). Article ADS PubMed Google
Scholar * Chapman, F. A. et al. The therapeutic potential of apelin in kidney disease. _Nat. Rev. Nephrol._ 17, 840–853 (2021). Article CAS PubMed PubMed Central Google Scholar * Read,
C. et al. International union of basic and clinical pharmacology. CVII. structure and pharmacology of the apelin receptor with a recommendation that Elabela/Toddler is a second endogenous
peptide ligand. _Pharm. Rev._ 71, 467–502 (2019). Article CAS PubMed PubMed Central Google Scholar * Japp, A. G. et al. Acute cardiovascular effects of apelin in humans: potential role
in patients with chronic heart failure. _Circulation_ 121, 1818–1827 (2010). Article CAS PubMed Google Scholar * Barnes, G. D. et al. Sustained cardiovascular actions of APJ agonism
during renin-angiotensin system activation and in patients with heart failure. _Circ. Heart Fail._ 6, 482–491 (2013). Article CAS PubMed Google Scholar * Hus-Citharel, A. et al. Effect
of apelin on glomerular hemodynamic function in the rat kidney. _Kidney Int._ 74, 486–494 (2008). Article CAS PubMed Google Scholar * Girault-Sotias, P. E., Gerbier, R., Flahault, A., de
Mota, N. & Llorens-Cortes, C. Apelin and vasopressin: The Yin and Yang of water balance. _Front. Endocrinol._ 12, 735515 (2021). Article Google Scholar * Bikbov, B. et al. Global,
regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. _Lancet_ 395, 709–733 (2020). Article Google Scholar
* Keith, D. S., Nichols, G. A., Gullion, C. M., Brown, J. B. & Smith, D. H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care
organisation. _Arch. Intern. Med._ 164, 659–663 (2004). Article PubMed Google Scholar * Coresh, J. et al. Prevalence of high blood pressure and elevated serum creatinine level in the
United States: findings from the third National Health and Nutrition Examination Survey (1988-1994). _Arch. Intern. Med._ 161, 1207–1216 (2001). Article CAS PubMed Google Scholar *
Peralta, C. A. et al. Control of hypertension in adults with chronic kidney disease in the United States. _Hypertension_ 45, 1119–1124 (2005). Article CAS PubMed Google Scholar *
Herrington W. G. et al. Empagliflozin in Patients with Chronic Kidney Disease. _N. Engl. J. Med_. 388, 117–127 (2022). * Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and
mortality in type 2 diabetes. _N. Engl. J. Med._ 373, 2117–2128 (2015). Article CAS PubMed Google Scholar * Canoy, D. et al. How much lowering of blood pressure is required to prevent
cardiovascular disease in patients with and without previous cardiovascular disease? _Curr. Cardiol. Rep._ 24, 851–860 (2022). Article PubMed PubMed Central Google Scholar * Blacher, J.
et al. Impact of aortic stiffness on survival in end-stage renal disease. _Circulation_ 99, 2434–2439 (1999). Article CAS PubMed Google Scholar * Laurent, S. et al. Aortic stiffness is
an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. _Hypertension_ 37, 1236–1241 (2001). Article CAS PubMed Google Scholar * Asmar, R., Gosse,
P., Topouchian, J., N’Tela, G., Dudley, A. & Shepherd, G. L. Effects of telmisartan on arterial stiffness in Type 2 diabetes patients with essential hypertension. _J. Renin Angiotensin
Aldosterone Syst._ 3, 176–180 (2002). Article CAS PubMed Google Scholar * Hirata, K., Vlachopoulos, C. & Adji, A. O’Rourke MF. Benefits from angiotensin-converting enzyme inhibitor
‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? _J. Hypertens._ 23, 551–556 (2005). Article CAS PubMed Google Scholar * Brame, A. L. et al. Design,
characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. _Hypertension_ 65, 834–840 (2015). Article CAS PubMed Google Scholar *
Yang, P. et al. A novel cyclic biased agonist of the apelin receptor, MM07, is disease modifying in the rat monocrotaline model of pulmonary arterial hypertension. _Br. J. Pharm._ 176,
1206–1221 (2019). Article CAS Google Scholar * Guerin, A. P. et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. _Circulation_ 103, 987–992
(2001). Article CAS PubMed Google Scholar * Dhaun, N. et al. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in
chronic kidney disease. _Hypertension_ 54, 113–119 (2009). Article CAS PubMed Google Scholar * Rovin, B. H. et al. Efficacy and safety of sparsentan versus irbesartan in patients with
IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. _Lancet_ 402, 2077–2090 (2023). Article CAS PubMed Google Scholar * Lant, A. F. Evolution
of diuretics and ACE inhibitors, their renal and antihypertensive actions–parallels and contrasts. _Br. J. Clin. Pharm._ 23, 27s–41s (1987). Article CAS Google Scholar * Jafar, T. H. et
al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. _Ann. Intern. Med._
139, 244–252 (2003). Article CAS PubMed Google Scholar * de Zeeuw, D. et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy.
_Circulation_ 110, 921–927 (2004). Article PubMed Google Scholar * de Zeeuw, D. et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from
RENAAL. _Kidney Int._ 65, 2309–2320 (2004). Article PubMed Google Scholar * Ruggenenti, P. et al. Role of remission clinics in the longitudinal treatment of CKD. _J. Am. Soc. Nephrol._
19, 1213–1224 (2008). Article PubMed PubMed Central Google Scholar * Jardine, M. J. et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: A
secondary analysis of the CREDENCE randomized trial. _J. Am. Soc. Nephrol._ 31, 1128–1139 (2020). Article CAS PubMed PubMed Central Google Scholar * McInnes, G. T., Perkins, R. M.,
Shelton, J. R. & Harrison, I. R. Spironolactone dose-response relationships in healthy subjects. _Br. J. Clin. Pharm._ 13, 513–518 (1982). Article CAS Google Scholar * Nyimanu, D. et
al. Apelin is expressed throughout the human kidney, is elevated in chronic kidney disease & associates independently with decline in kidney function. _Br. J. Clin. Pharm._
https://doi.org/10.1111/bcp.15446 (2022). Article Google Scholar * Ayari, H. & Chraibi, A. Apelin-13 decreases epithelial sodium channel (ENaC) expression and activity in kidney
collecting duct cells. _Cell Physiol. Biochem._ 56, 1–12 (2022). Article CAS PubMed Google Scholar * Mordi, N. A. et al. Renal and cardiovascular effects of SGLT2 inhibition in
combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The RECEDE-CHF trial. _Circulation_ 142, 1713–1724 (2020). Article CAS PubMed PubMed Central
Google Scholar * Hus-Citharel, A. et al. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat
collecting duct. _Endocrinology_ 155, 4483–4493 (2014). Article PubMed Google Scholar * Boulkeroua et al. Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in
kidney collecting duct cells. _Cell Physiol. Biochem._ 53, 687–700 (2019). Article CAS PubMed Google Scholar * Azizi, M. et al. Reciprocal regulation of plasma apelin and vasopressin by
osmotic stimuli. _J. Am. Soc. Nephrol._ 19, 1015–1024 (2008). Article CAS PubMed PubMed Central Google Scholar * Stockand, J. D. Vasopressin regulation of renal sodium excretion.
_Kidney Int._ 78, 849–856 (2010). Article CAS PubMed Google Scholar * Najafipour, H., Soltani Hekmat, A., Nekooian, A. A. & Esmaeili-Mahani, S. Apelin receptor expression in ischemic
and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. _Regul. Pept._ 178, 43–50 (2012). Article CAS PubMed Google Scholar
* Iwanaga, Y., Kihara, Y., Takenaka, H. & Kita, T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1
receptor system. _J. Mol. Cell Cardiol._ 41, 798–806 (2006). Article CAS PubMed Google Scholar * APIE Therapeutics, Therapeutic Pipeline. https://apie-therapeutics.com/pipeline/ (2024).
* Japp, A. G. & Newby, D. E. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. _Biochem. Pharm._ 75, 1882–1892 (2008). Article CAS PubMed
Google Scholar * Noori-Zadeh, A., Bakhtiyari, S., Khanjari, S., Haghani, K. & Darabi, S. Elevated blood apelin levels in type 2 diabetes mellitus: A systematic review and meta-analysis.
_Diab. Res. Clin. Pr._ 148, 43–53 (2019). Article CAS Google Scholar * Soman, R. S., Zahir, H. & Akhlaghi, F. Development and validation of an HPLC-UV method for determination of
iohexol in human plasma. _J. Chromatogr. B Anal. Technol. Biomed. Life Sci._ 816, 339–343 (2005). Article CAS Google Scholar * Chan, K., Miners, J. O. & Birkett, D. J. Direct and
simultaneous high-performance liquid chromatographic assay for the determination of p-aminobenzoic acid and its conjugates in human urine. _J. Chromatogr. B Biomed. Sci. Appl._ 426, 103–109
(1988). Article CAS Google Scholar * Pelleg, A. & Levy, G. B. Determination of Na+ and K+ in urine with ion-selective electrodes in an automated analyzer. _Clin. Chem._ 21, 1572–1574
(1975). Article CAS PubMed Google Scholar * Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye
binding. _Anal. Biochem._ 72, 248–254 (1976). Article CAS PubMed Google Scholar * O’Brien, E. M. F., Atkins, N. & Thomas, M. Evaluation of three devices for self-measurement of b
lood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. _Blood Press Monit._ 1, 55–61 (1996). PubMed Google
Scholar * Oliver, J. J. & Webb, D. J. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. _Arterioscler. Thromb. Vasc. Biol._ 23, 554–566 (2003). Article
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS F.A.C. was funded through a Clinical Research Training Fellowship from Kidney Research UK (TF_006_20171124) with additional
grants from the Scottish Society of Physicians and Mason Medical Research Trust. A.P.D. and J.J.M. received funding from the Wellcome Trust (203151/Z/16/Z). D.E.N. is funded by the British
Heart Foundation (CH/09/002/26360, RE/18/5/34216, RG/F/22/110093). N.D. is supported by a Senior Clinical Research Fellowship from the Chief Scientist Office (SCAF/19/02). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * University/BHF Centre for Cardiovascular Science, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK Fiona A. Chapman, Vanessa
Melville, Emily Godden, Beth Morrison, Lorraine Bruce, David E. Newby & Neeraj Dhaun * Department of Renal Medicine, Royal Infirmary of Edinburgh, Edinburgh, UK Fiona A. Chapman &
Neeraj Dhaun * Division of Experimental Medicine and Immunotherapeutics, Addenbrooke’s Centre for Clinical Investigation, University of Cambridge, Cambridge, UK Janet J. Maguire &
Anthony P. Davenport Authors * Fiona A. Chapman View author publications You can also search for this author inPubMed Google Scholar * Vanessa Melville View author publications You can also
search for this author inPubMed Google Scholar * Emily Godden View author publications You can also search for this author inPubMed Google Scholar * Beth Morrison View author publications
You can also search for this author inPubMed Google Scholar * Lorraine Bruce View author publications You can also search for this author inPubMed Google Scholar * Janet J. Maguire View
author publications You can also search for this author inPubMed Google Scholar * Anthony P. Davenport View author publications You can also search for this author inPubMed Google Scholar *
David E. Newby View author publications You can also search for this author inPubMed Google Scholar * Neeraj Dhaun View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS F.A.C., J.J.M., A.P.D., D.E.N., and N.D. designed the study; F.A.C. recruited all participants; F.A.C., V.M., E.G. and B.M. completed all study visits; L.B.
performed laboratory analyses; F.A.C. performed statistical analyses; F.A.C. and N.D. wrote the manuscript; all authors critically appraised and approved the final manuscript. CORRESPONDING
AUTHOR Correspondence to Neeraj Dhaun. ETHICS DECLARATIONS COMPETING INTERESTS D.E.N. has historically received consultancy fees from Bristol-Myers Squibb and Amgen regarding the development
of apelin receptor agonists for heart failure and was a sub-investigator in a clinical trial for Bristol-Myers Squibb (NCT03281122). All other authors have no competing interests. PEER
REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Mirjam Christ-Crain, Andreas Kronbichler and the other anonymous reviewer(s) for their contribution to the peer review of this
work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chapman, F.A., Melville, V., Godden, E. _et al._ Cardiovascular and
renal effects of apelin in chronic kidney disease: a randomised, double-blind, placebo-controlled, crossover study. _Nat Commun_ 15, 8387 (2024). https://doi.org/10.1038/s41467-024-52447-7
Download citation * Received: 21 March 2024 * Accepted: 09 September 2024 * Published: 14 October 2024 * DOI: https://doi.org/10.1038/s41467-024-52447-7 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
Trending News
Real madrid will call tottenham boss mauricio pochettino - balagueZidane resigned as head coach of Real Madrid just days after leading the Spanish club to a third straight Champions Leag...
Were team gb’s skeleton suits responsible for fantastic three medal haul?Team GB skeleton rider Lizzie Yarnold won a stunning Winter Olympic gold on February 17, backed up by bronzes for Laura ...
Ski stations in pyrénées close early due to lack of snowMAJOR RESORT SHUT DOORS TWO WEEKS EARLY AS RAIN AND STORMS MADE IT TOO DANGEROUS TO SKI A ski station in the Pyrénées ha...
Certificates programs can boost your career and skillsDerrick Lewis has new appreciation for something a coworker told him 20 years ago: “You have to keep learning.” That adv...
Comment: weeds can make a garden cheaper and more manageable in franceCOLUMNIST SAMANTHA DAVID TELLS HOW EMBRACING NATURE HAS HIDDEN BENEFITS The day after I moved into my French farmhouse, ...
Latests News
Cardiovascular and renal effects of apelin in chronic kidney disease: a randomised, double-blind, placebo-controlled, crossover studyABSTRACT Chronic kidney disease (CKD) affects ~10% of the population and cardiovascular disease is its commonest complic...
Clc-5 cl--channel disruption impairs endocytosis in a mouse model for dent's diseaseABSTRACT Dent's disease is an X-linked disorder associated with the urinary loss of low-molecular-weight proteins, ...
Fugitiva season 2 netflix release date: will there be another series?Fugitiva dropped on Netflix on Friday, November 23. The show has already aired in Spain on TV channel RTVE which means a...
Generation of targeted mouse mutants by embryo microinjection of talen mrnaABSTRACT Genetically engineered mice are instrumental for the analysis of mammalian gene function in health and disease....
Neuroscience: Halving it all | NatureDouwe Draaisma enjoys the autobiography of Michael Gazzaniga, who has studied split brains for half a century. Access th...