Tuning reactivity of fischer–tropsch synthesis by regulating tiox overlayer over ru/tio2 nanocatalysts
Tuning reactivity of fischer–tropsch synthesis by regulating tiox overlayer over ru/tio2 nanocatalysts"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The activity of Fischer–Tropsch synthesis (FTS) on metal-based nanocatalysts can be greatly promoted by the support of reducible oxides, while the role of support remains elusive.
Herein, by varying the reduction condition to regulate the TiO_x_ overlayer on Ru nanocatalysts, the reactivity of Ru/TiO2 nanocatalysts can be differentially modulated. The activity in FTS
shows a volcano-like trend with increasing reduction temperature from 200 to 600 °C. Such a variation of activity is characterized to be related to the activation of CO on the TiO_x_
overlayer at Ru/TiO2 interfaces. Further theoretical calculations suggest that the formation of reduced TiO_x_ occurs facilely on the Ru surface, and it involves in the catalytic mechanism
of FTS to facilitate the CO bond cleavage kinetically. This study provides a deep insight on the mechanism of TiO_x_ overlayer in FTS, and offers an effective approach to tuning catalytic
reactivity of metal nanocatalysts on reducible oxides. SIMILAR CONTENT BEING VIEWED BY OTHERS INTERFACIAL COMPATIBILITY CRITICALLY CONTROLS RU/TIO2 METAL-SUPPORT INTERACTION MODES IN CO2
HYDROGENATION Article Open access 17 January 2022 GENERATION OF OXIDE SURFACE PATCHES PROMOTING H-SPILLOVER IN RU/(TIO_X_)MNO CATALYSTS ENABLES CO2 REDUCTION TO CO Article 09 October 2023
TACKLING ACTIVITY-STABILITY PARADOX OF RECONSTRUCTED NIIROX ELECTROCATALYSTS BY BRIDGED W-O MOIETY Article Open access 04 December 2024 INTRODUCTION Fischer–Tropsch synthesis (FTS) offers a
powerful way to convert syngas (a mixture of CO and H2) to long-chain hydrocarbons, by which the transformation of nonpetroleum resources (derived from coal, natural gas, or biomass) into
high value-added chemicals and fuels becomes economically efficient and environmentally friendly1,2,3. The most challenging step in this process is consented as the CO activation, with which
the further hydrogenation to CH_x_ (_x_ = 1, 2, 3) species for the carbon chain growth becomes facile on the surfaces of late transition metals like Fe, Co, Ru, and Rh4,5,6,7. Among these
metals, Ru is identified to be intrinsic of high activity and selectivity in FTS8. In particular, large particle sizes of Ru (~8 nm) are highly desirable9,10,11, on which the direct or
H-assisted CO dissociation is greatly facilitated12,13. As such, great efforts have been made on modulating the surface structure of Ru-based catalysts, including by tuning particle
size9,10,11, changing crystal phase14, and varying exposed plane15, to further promote the catalytic performance of Ru catalysts. Beyond that, there are strong metal–support interactions
(SMSI) on supported metal catalysts when reducible oxide such as TiO2 is used as a support16,17. More specifically, reducible oxide migrates to the metal surface by forming a thin overlayer
under the reduction condition18, which then results in a unique metal/support interface and variegates the behavior of catalyst in reactions19,20,21. The utilization of such SMSI has also
been demonstrated to be an alternative strategy to enhance the catalytic reactivity of metal catalysts in FTS22,23. This promoted effect is generally attributed to the interface between
metal and support, which serves as a new active site with an improved activity in the reactions18,23. However, the catalytic mechanism and the intrinsic roles of such newly generated
interfaces are remaining elusive. In this research, by regulating a TiO_x_ overlayer on the Ru nanoparticles (~2 nm), the catalytic activity of this Ru/TiO2 nanocatalyst in FTS can be
boosted. With various characterizations and theoretical modeling, the reduced TiO_x_ overlayer on Ru nanocatalysts is demonstrated to participate in and dramatically facilitate the bond
cleavage of CO. The results of this work are expected to play an important role in the mechanism understanding of SMSI in FTS, and also provide guidance with regard to tuning the catalytic
properties of metal nanocatalysts supported on reducible oxides. RESULTS STRUCTURAL CHARACTERIZATION With the aim to investigate the effect of TiO_x_ overlayer covered over the Ru
nanoparticles (NPs) on the reactivity in FTS, in the present work, we use wet impregnation method to fabricate Ru-based catalysts with small-sized metal nanoparticles by using rutile TiO2 as
a support24. In this text, catalysts reduced at specific temperatures were denoted as Ru/TiO2-_x_, where _x_ refers to reduction temperatures (_x_ = 200–600). The loading of Ru, after
calcination in air and the following thorough chlorides removal process, has been determined to be 2.2 wt% by ICP-OES. As indicated by Brunauer–Emmett–Teller (BET) testing (Supplementary
Table 1), different Ru/TiO2-_x_ samples are similar in physical textures, with almost the identical surface areas and pore volumes. Meanwhile, all Ru/TiO2 samples are found to possess
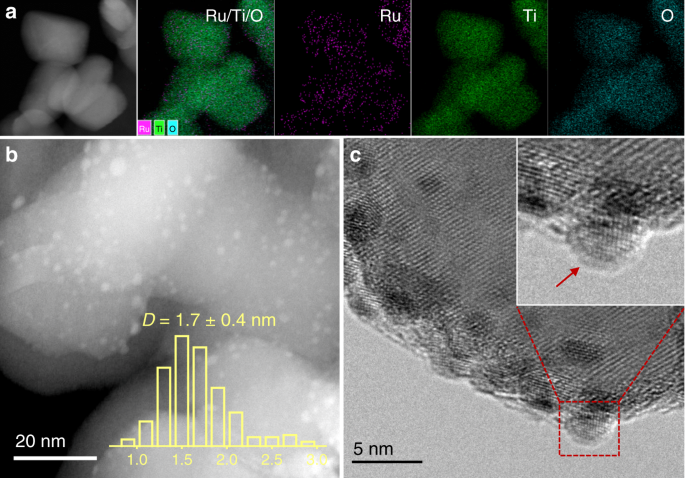
analogous morphologies of metal NPs, that is, the Ru NPs are all well dispersed on the support and have a uniform size distribution with an average diameter of ~2 nm (Fig. 1a, b and
Supplementary Figs. 1–3). It was attributed to the usage of rutile TiO2 as the support in our research, which possesses the same crystal phase and comparable lattice parameters with that of
RuO2 (Supplementary Table 2). As a result, the calcination step by formation of RuO2/TiO2 interphase helps to resist the aggregation of metal nanoparticles even after the high-temperature
pretreatment (Supplementary Fig. 4). On the other hand, however, there are significant discrepancies on the micro-structures of Ru NPs on the TiO2 support when pretreated at different
reduction temperatures (Fig. 1c and Supplementary Fig. 5). In detail, a distinct morphology of Ru NP can be resolved on the support when sample was reduced at a temperature below 300 °C,
while a visible coating on the Ru NPs can be distinguishable after higher temperature pre-reduction. In terms of the SMSI between Ru and rutile TiO2, it was ascribed to the TiO_x_ overlayer
over the Ru NPs under high-temperature reduction condition, and the migration of TiO_x_ over Ru NPs was initiated at a reduction temperature higher than 300 °C. To get a qualitative
comparison of the exposure of Ru species after coating by TiO_x_ overlayer, the chemisorption of CO and H2 were measured to estimate the Ru dispersion on these Ru/TiO2-_x_ samples. As seen
in Table 1, the values obtained by different probe molecules give the same tendency of the metal dispersions for different Ru/TiO2-_x_ samples, that is, the dispersion of Ru decreases with
increasing the reduction temperature from 300 to 600 °C. This can be explained by a gradual encapsulation of the Ru NPs by TiO_x_ overlayer as increasing the reduction temperature from 300
to 600 °C, which was in good agreement with the TEM observations. As compared, the dispersion derived from H2 chemisorption was lower than that from CO chemisorption. It might be caused by
the Ru_n_+ sites at the Ru–TiO2 interface, which are unavailable for H2 chemisorption due to the SMSI effects25, but it can be readily involved in CO chemisorption as indicated in our
further in situ DRIFT spectra experiments. Even so, the H2 uptakes on the Ru/TiO2-200 sample was found to be less than that of the Ru/TiO2-300. This can be explained by the results of H2
temperature-programmed reduction (H2-TPR), in which the predominant reduction of Ru/TiO2 to metallic Ru occurring at a temperature higher than 200 °C (Supplementary Fig. 6). In addition, the
decline of the surface metallic Ru exposure by a gradual encapsulation of the Ru NPs by TiO_x_ overlayer as increasing the reduction temperature was also confirmed by the underpotential
deposition of copper (Cu upd) experiments, with which the metallic surface area can be semi-quantified in terms of the integral area of current for the reduction deposition of copper on the
exposed metal surface26 (Supplementary Fig. 7 and Supplementary Table 3). The evolution of Ru/TiO2 catalysts at different temperature reductions was also investigated with X-ray absorption
spectroscopy (XAS). The extended X-ray absorption fine structure (EXAFS) of Ru _K_-edge and the fitting results (Fig. 2a and Supplementary Table 4) have demonstrated that the coordination
number (CN) associated with Ru–Ru pair (~2.67 Å) is increased gradually from 2.2 to 5.3 as the reduction temperature increased from 200 to 600 °C, while the CN of the Ru–O pair (~1.98 Å)
presents an inverse tendency by decreasing from 4.0 to 2.4. It suggests a gradual improvement of the reduction degree of ruthenium oxide to metallic phase. Correspondingly, a shift of edge
energy towards Ru foil was observed by the X-ray absorption near-edge structure (XANES) (Supplementary Fig. 8). Nevertheless, the inevitable Ru–O bonding for Ru/TiO2 samples indicates a
strong interfacial interaction between Ru and TiO2, further confirming the formation of TiO_x_ coating on the Ru NPs. On the other hand, the soft XANES spectra at the Ti _L_3,2-edge (Fig.
2b) exhibit a decline in peak intensities with the increase of pretreatment temperature, indicative of an increasing degree of the reduction of TiO2, owing to the formation of TiO_x_
overlayer on Ru NPs. The growth of the reduced TiO_x_ overlayer was also evidenced by the increased concentration of Ti3+ species accompanying by the decreased ratio of surface Ru/Ti
estimated from the Ti-2_p_ and Ru-3_p_ in XPS results (Supplementary Fig. 9 and Supplementary Table 5). On the basis of the above observations, the structure evolution of Ru/TiO2 at
different stages of reduction was then suggested in Fig. 2c. Benefiting from the lattice matching of RuO2/TiO2 interphase, a small size of Ru NPs with the improved sintering resistance can
be facilely acquired by the following reduction pretreatment. In the initial step of reduction, e.g., Ru/TiO2-300, a dominant metallic Ru will be exposed, and it serves as a typical Ru-based
nanocatalysts in FTS. With further increasing the reduction temperature, the SMSI between Ru and TiO2 governs the surface exposure of Ru NPs, and the TiO_x_ thin layer begins to migrate and
coating the Ru surface, resulting in a shrinkage of metallic Ru surface by the TiO_x_ overlayer (like that on Ru/TiO2-450). Finally, for the Ru/TiO2-600 sample, an excessive coverage of
TiO_x_ on Ru NPs causes a dominant TiO_x_ overlayer on Ru nanocatalysts. Accordingly, a tunable degree of TiO_x_ overlayer on Ru NPs can be easily achieved by varying the pretreatment
condition, and it thus provides us an opportunity to explore the effect of metal/support interface of Ru/TiO2 on the activity in FTS. CATALYTIC PERFORMANCE The catalytic performances of
various Ru/TiO2-_x_ catalysts in FTS were then evaluated at 160 °C with a 2 MPa reaction pressure according to our preliminary optimization of experiment conditions for achieving a high C5+
selectivity under a relatively mild condition (Supplementary Fig. 10). Notably, as shown in Fig. 3a and Supplementary Figs. 11, 12, all Ru/TiO2-_x_ catalysts possess an excellent C5+
selectivity with a value up to 90%, indicating the promising application prospect of Ru/TiO2 in FTS for high-carbon products. While the intrinsic reaction rate (reflected as the TOF value)
greatly relies on the pretreatment temperature, and shows a volcano-like trend with the increase of reduction temperature (Fig. 3a, Supplementary Fig. 13 and Supplementary Table 6). As
compared, samples of Ru/TiO2-200 and Ru/TiO2-300 manifest a much lower intrinsic activity (0.003 s−1). A great promotion of activity was observed for the Ru/TiO2-400 catalyst. Among these
catalysts, the Ru/TiO2-450 exhibits the highest activity with an intrinsic TOF value of 0.039 s−1, which was also superior to other Ru-based catalysts reported previously (Supplementary
Table 7). The further increasing of pretreatment temperature, however, causes an activity decay of catalysts, with a TOF value of only 0.021 s−1 for Ru/TiO2-600. Correspondingly, a reverse
variation of apparent activation energy (_E_a) was obtained, i.e., the Ru/TiO2-450 presents the lowest _E_a with a calculated value of 62.0 kJ mol−1, which was much lower than the values of
other Ru/TiO2 catalysts (Fig. 3b). The particle size of metal was essential in determining the performance of Ru-based catalysts in FTS. The activity increases as the particle size of Ru
nanocatalyst increased, with the small-sized Ru NPs behaving a rather poor activity9,10,11. This explains well the low activity of our small-sized Ru/TiO2 catalysts reduced at low
temperatures (Ru/TiO2-200 and Ru/TiO2-300 samples) as well as Ru NPs supported on the irreducible support (Ru/Al2O3-450 in Supplementary Fig. 14 and Supplementary Table 8). While an enhanced
activity of Ru/TiO2-450 catalyst suggests that the TiO_x_ overlayer on Ru NPs has a positive contribution on the reactivity of Ru nanocatalysts. However, in terms of the decline of activity
for the Ru/TiO2-600 catalyst covered dominantly by TiO_x_ overlayer, the only TiO_x_ overlayer cannot achieve a high activity for the FTS reaction. In this regard, the interface between
metal and support of Ru/TiO2 plays a crucial role on the activity promotion, where both the metallic Ru and TiO_x_ overlayer are indispensable. The optimized composition of TiO_x_ overlayer
and Ru NPs on Ru/TiO2-450 catalyst makes it possess an enhanced activity. Furthermore, the Ru/TiO2-450 catalyst also owns an excellent stability in the steady running state of FTS
(Supplementary Figs. 10, 11). HAADF-STEM image of the spent Ru/TiO2-450 catalyst suggests that the size of Ru can keep constant after testing (Supplementary Fig. 15). This was also benefited
from the SMSI in the Ru/TiO2-450 catalyst, which greatly prohibits the size aggregation of Ru during FTS process. CATALYTIC MECHANISM The role of the TiO_x_ overlayer was then studied by
the steady-state isotopic transient kinetic analysis (SSITKA)27,28, by which the evolution of intermediates with the associated coverage and reactivity can be acquired (Supplementary Fig.
16). Limited by the atmospheric pressure condition in this analysis, as shown in Supplementary Fig. 17, CH4 selectivity has an increase because of the preference of hydrogenation over C–C
coupling for CH_x_ intermediates. Notably, a good correlation between the intrinsic activity (TOF) of CO consumption and methane generation can be set up. As such, the coverage of CH_x_
(represented as _θ_CH4 in SSITKA) was determined as a function of reduction temperature of Ru/TiO2 (Fig. 4a). From our results, the activity improvement of Ru/TiO2-450 can be attributed to
the increased coverage of CH_x_ intermediates on the catalyst surface29. By considering the comparable size of Ru for different Ru/TiO2 samples, a promoted effect toward CO activation to
generate CH_x_ intermediates with the aid of TiO_x_ overlayer can be expected on the Ru/TiO2-450 catalyst. To confirm our proposed mechanism of CO activation, the micro-calorimetry toward CO
was measured for Ru/TiO2 samples (Fig. 4b, c). The amount of CO chemisorption follows a trend of Ru/TiO2-300 > Ru/TiO2-450 > Ru/TiO2-600. This can be explained by the decrease
exposure of Ru for serving as the adsorbed sites toward CO as increasing reduction temperature. In particular, as compared with other catalysts, Ru/TiO2-450 owns a large portion of CO
chemisorption at a relative higher differential heat (>150 kJ mol−1). This was attributed to the CO chemisorption on the interface site, followed by a dissociation of CO with the aid of
TiO_x_ overlayer. For comparison, the Ru/TiO2-300 shows a predominant moderate chemisorption toward CO, with a differential heat of 120–150 kJ mol−1 for CO chemisorption on the Ru sites. In
contrast, the over-coverage of TiO_x_ on Ru NPs causes a shortage of both the Ru sites and the interfaces for CO chemisorption/dissociation on Ru/TiO2-600. In situ diffuse reflectance
infrared Fourier transform (DRIFT) spectra of CO chemisorption on Ru/TiO2-_x_ indicate that there are three distinct _υ_CO bands located at approximately 2136, 2075, and 2056 cm−1 in the
carbonyl region (Fig. 4d). Here, the bands at 2136 and 2075 cm−1 were often observed by performing CO adsorption on well-dispersed, partially oxidized Ru_n_+ with a low coordination
environment, which therefore were ascribed to multi-carbonyl (Ru_n_+(CO)_x_) and mono-carbonyl (Ru_n_+–CO) species adsorbed on partially oxidized Ru_n_+ sites on the interface,
respectively30,31,32. While the peak at 2056 cm−1 was assigned to linear CO adsorption on metallic Ru (Ru_x_–CO)33,34. After purging H2 into the CO-saturated Ru/TiO2, the gaseous CH4 product
with a characterized frequency at 3015 cm−1 was detected35, accompanying by the consumption of CO (Fig. 4e and Supplementary Fig. 18). More importantly, by the complete consumption of CO of
Ru_n_+(CO)_x_ and Ru_n_+–CO, the further conversion of CO was restrained, with Ru_x_–CO as a predominant chemisorption species on Ru surface. In this case, the interface of partially
oxidized Ru_n_+ sites were supposed to be the active sites for the FTS reaction. As such, the intensity of CO related to FTS on the Ru/TiO2-450 was found to be more remarkable than that of
Ru/TiO2-300 and the Ru/TiO2-600 catalysts (Fig. 4d), which was responsible for its higher activity in FTS. According to our results, a catalytic mechanism for CO transformation on the
Ru/TiO2-_x_ catalysts was then proposed. Due to the SMSI over Ru/TiO2, the TiO_x_ overlayer on Ru NPs provides oxygen vacancies for anchoring the oxygen atoms from the dissociation of
carbonyl group; it thus greatly facilitates the dissociation of CO on the Ru/TiO_x_ interface of catalysts as also suggested by Bell and coworkers36, by which the hydrogenation and C–C
coupling can be realized on the Ru sites to produce carbon chain products. As for Ru/TiO2-450, the optimized TiO_x_ overlayer on Ru NP offers it a maximized activity in FTS, while the
shortage of interface on both the Ru/TiO2-300 and Ru/TiO2-600 samples leads to a lower activity in FTS. As such, the participation of the TiO_x_ overlayer in the C–O bond dissociation
process was responsible for the superior reactivity of the Ru/TiO2-450 catalyst. DFT CALCULATIONS Theoretically, we have performed a density functional theory (DFT) study on the CO
activation on the model catalyst on a TiO_x_ cluster decorated Ru(001) surface. In Fig. 5a and Supplementary Table 9, we have first studied the thermodynamic stability of different TiO_x_
clusters on the Ru(001) surface at different oxygen chemical potential. As compared with the TiO6 unit in bulk phase of rutile TiO2, the TiO_x_ was reduced facilely on the Ru(001) surface,
and the TiO4 cluster was found to be dominant under the oxygen-rich condition. By decreasing the oxygen chemical potential under reduction condition, the reduction of TiO4 occurred readily
on the Ru surface through a sequential reduction to TiO3/Ru(001) and TiO2/Ru(001), respectively. This was consistent with the experimental observation of a reduction of the TiO_x_ overlayer
under the reduction condition. On the TiO3/Ru(001) surface, the activation of CO by C–O bond cleavage was then estimated, with that occurring on parent Ru(001) surface as a comparison. As we
can see in Fig. 5b, the CO bond cleavage on the Ru (001) surface has a much high barrier (2.15 eV), and the laying down of the atop *CO adspecies adsorbed on the surface is the main
obstacle during its dissociation, in good agreement with the previous results37,38. In contrast, with a reduced TiO3 cluster decorating on the Ru(001) surface, the bond cleavage of CO
adsorbed on the Ru site of interface can be greatly promoted by experiencing a calculated barrier of 1.62 eV, with the aid of TiO3 as the O seizer of carbonyl group to transform to TiO4.
Taking into account that our experiments of FTS were conducted at a reaction temperature of 160–200 °C and a reaction pressure of 2 MPa, such a barrier is facile to overcome on the Ru/TiO2
catalysts at the reaction condition of FTS. The as-dissociated C* adspecies can then be conveniently diffused from the TiO_x_/Ru(001) interface to Ru(001) (0.73 eV) for the further
hydrogenation. Meanwhile, the reduction of TiO4/Ru(001) to TiO3/Ru(001) was even thermodynamically more favorable than the surface reduction of O adspecies on the parent Ru(001) surface
(Fig. 5a), which can then facilitate the catalytic cycle of CO activation on the interface. As indicated by the previous reports, such C* species is promising to be hydrogenated to CH_x_
species and realize the C–C coupling on the Ru surface to produce the C2+ products39. Accordingly, the Ru/TiO2-450 catalyst, owing to its optimized Ru/TiO_x_ interface for CO activation,
shows a superior activity to other Ru/TiO2-_x_ catalysts in FTS. In conclusion, we have successfully fabricated a highly active Ru/TiO2 catalyst for FTS by fine-tuning the catalyst
pre-reduction condition. With increasing the reduction temperature, the TiO_x_ overlayer is gradually enveloping the Ru NPs. The catalyst reduced at 450 °C exhibits a high intrinsic activity
under mild conditions with a low apparent activation energy. The participation of TiO_x_ overlayer in promoting CO dissociation plays a vital role in activity enhancement during FTS. An
optimized TiO_x_ overlayer on the Ru NPs formed during reduction at 450 °C is evidently able to capture oxygen from carbonyl group adsorbed on the interface of Ru/TiO_x_. This, in turn,
facilitates the cleavage of C–O bonds. This work not only provides an understanding of the mechanism of CO activation on Ru/TiO2 catalysts, but also suggests an effective approach to
tailoring the catalytic properties of metal nanocatalysts supported on reducible oxides. METHODS CATALYST PREPARATION The Ru/TiO2 catalysts were prepared by an impregnation method using
rutile TiO2 as the support. In a typical synthesis, 1.6 g of the aqueous RuCl3·3H2O solution (8.2 wt%, 0.0317 g Ru per gram of solution, AR) was diluted to 30 mL with deionized water. 2.0 g
of rutile TiO2 (40 nm, 99.8% metal basis) was then added to the solution and the resulting suspension was evaporated to dry in a 50 °C water bath with the vigorous stirring. The resulting
solid was dried at 120 °C overnight, followed by the calcination in air at 300 °C for 3 h. Subsequently, to remove the residual chlorides, the sample was washed repeatedly with a dilute
ammonia solution (1 mol L−1), followed by filtrating and washing with deionized water until there was no more precipitate in the filtrate detected by AgNO3 solution (0.1 mol L−1). Finally,
the sample was dried at 60 °C overnight. The sample thus obtained was denoted as the fresh Ru/TiO2. Prior to catalytic performance tests, the fresh Ru/TiO2 sample were reduced in situ under
a H2 gas flow (20 mL min−1) at specific temperatures. The samples after reduction were denoted as Ru/TiO2-_x_, where _x_ indicates the reduction temperature (200, 300, 400, 450, 500, or 600
°C). The Ru loading in the Ru/TiO2 catalyst is 2.2 wt% as detected by ICP-OES. CATALYST CHARACTERIZATION High-angle annular dark field scanning transmission electron microscopy (HAADF-STEM)
together with the elemental mapping and high-resolution transmission electron microscopy (HRTEM) images were acquired using a JEOL JEM-2100F microscope operating at 200 kV. The Ru particle
size was determined from HAADF-STEM images, and at least 200 particles were counted for each sample. The Ru concentrations in specimens were determined by inductively coupled plasma optical
emission spectroscopy (ICP-OES) with an ICP-OES 7300DV instrument. CO AND H2 CHEMISORPTION EXPERIMENTS The exposure of Ru species after coating by TiO_x_ was determined by CO and H2 pulse
chemisorption on a Micromeritics AutoChem II 2920 instrument. For CO (or H2) chemisorption experiment, the sample (~100 mg) was pretreated with hydrogen at desired temperatures for 1 h,
followed by purging with high-purity helium (or argon) for 30 min. After the sample was cooled down to 50 °C, a 5% CO/He (or 10% H2/Ar) mixture was injected into the reactor repeatedly until
CO (or H2) adsorption was saturated. The dispersion of Ru was calculated from the amount of CO (or H2) adsorbed by assuming the CO/Ru (or H/Ru) adsorption stoichiometry to be 1/1. X-RAY
ABSORPTION SPECTROSCOPY Pseudo-in situ X-ray absorption spectroscopy (XAS), including acquiring X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure
(EXAFS) data at the Ru _K_-edge, was performed at the BL 14W1 of Shanghai Synchrotron Radiation Facility (SSRF), China. A double Si (311) crystal monochromator was used for energy selection.
The samples were pretreated in a H2 flow (20 mL min−1) at the desired temperatures for 2 h, followed by sealing with Capton film in a glove box without exposure to air. The spectra were
collected at room temperature in the transition mode. The Ti _L_3,2-edge XANES data was acquired at the XMCD beamline (BL12B) at the National Synchrotron Radiation Laboratory (NSRL), China.
The Athena software package was used to analyze the data. CO MICROCALORIMETRIC MEASUREMENTS The differential heat of CO adsorption was measured for each specimen at 40 °C using a BT 2.15
Calvet calorimeter connected to gas handling and volumetric systems equipped with MKS 698A Baratron capacitance manometers (±1.33 × 10−2 Pa). Previous to CO adsorption, the samples were
treated in a H2 flow at the desired temperatures for 1 h, followed by evacuation for 30 min at the same temperature. After cooling to room temperature in vacuum, the quartz tube was refilled
with He and tightly sealed, after which the samples were outgassed at 40 °C overnight in the calorimetric cell. CO adsorption was carried out during the stepwise introduction of pure CO up
to a pressure of ~10 Torr at 40 °C. IN SITU DRIFTS In situ diffuse reflectance infrared Fourier transform (DRIFT) spectra were acquired using a Bruker Equinox 55 spectrometer equipped with a
mercury cadmium telluride (MCT) detector, recorded with a resolution of 4 cm−1. Prior to CO adsorption, the samples were treated in situ in the DRIFT cell under a H2 flow (20 mL min−1) at
the desired temperatures for 1 h, followed by purging with a He flow at the same temperature for 30 min. After cooling to 160 °C, a background spectrum was collected, following which the He
flow was switched to a 5 vol% CO in He flow (20 mL min−1) that was maintained until saturated adsorption was achieved. The system was purged with He to remove non-adsorbed CO and IR spectra
were collected, such that CO adsorption data at 160 °C were obtained. After the treatment with He, the reactivity of adsorbed CO species (COad) was monitored by switching to a 10 vol% H2 in
He flow (20 mL min−1). Simultaneously, IR spectra were recorded every 30 s for 10 min. This experiment is referred to the reactivity of COad at 160 °C. FISCHER–TROPSCH TESTS FTS trials were
performed in a stainless-steel fixed-bed reactor with an inner diameter of 12 mm under high pressure. Typically, the Ru/TiO2 catalyst (20–40 mesh, 0.3 g) was diluted with quartz sand (20–40
mesh, 0.9 g) and loaded into the reactor. Prior to each reaction, the catalyst was reduced in a H2 gas flow (20 mL min−1) at the desired temperature (200–600 °C) for 2 h. After the reactor
was cooled down, a syngas with a H2/CO ratio of 2/1 (H2/CO/Ar = 64/32/4 (v/v/v)) was introduced into the reactor at a flow rate of 15 mL min−1 (space velocity = 3000 mL gcat−1 h−1). Ar was
used as an internal standard to calculate CO conversion and selectivity of CH4 and CO2. The reaction was carried out at 160 °C under 2.0 MPa. After passing through a hot trap (120 °C) and
then an ice-bath, the gaseous products were analyzed online using an Agilent 7890 gas chromatograph equipped with an HP-PLOT/Q capillary column connected to a flame ionization detector (FID)
and a TDX-01 column connected to a thermal conductivity detector (TCD). The data of the catalytic performances of Ru/TiO2 catalysts were collected at the stable stage after at least 6 h of
running. The calculation method for FTS catalytic performance was described in detail in the supplementary information. SSITKA EXPERIMENTS Steady-state isotopic transient kinetic analysis
(SSITKA) is a combination of steady-state and transient techniques that can provide a reliable kinetic model to gain insights into the reaction mechanism. In this study, we used SSITKA to
explore the activity of the Ru/TiO2-_x_ catalysts with the aim of providing insights into the intrinsic promotional effects. During each SSITKA experiment, 50 mg of the sieved catalyst mixed
with 200 mg of SiC was pretreated in situ in a H2/Ar flow (20/20 mL min−1) at the desired temperature (300, 450, or 600 °C). After cooling to 100 °C, the feed was switched to a 12CO/Ar
mixture (35 mL min−1, 0.85 bar CO, 1.77 bar inert). CO adsorption was determined by switching from 12CO/Ar to 13CO/Kr without changing the other reaction conditions. Subsequently, the feed
was switched to 12CO/H2/Ar (1.5/15/33.5 mL min−1, 1.85 bar) for CO hydrogenation at 200 °C. After 6 h on stream, a switch from 12CO/H2/Ar to 13CO/H2/Kr was employed to study isotopic
transients, while maintaining the CO conversion at ~10%. The isotopic transient response was determined by mass spectrometer. The TOF values were calculated as
$${\mathrm{TOF}}_{{\mathrm{CO}}} = \frac{{R_{{\mathrm{CO}}} \cdot M_{{\mathrm{Ru}}}}}{{D \cdot x_{{\mathrm{Ru}}}}}$$ (1) and $${\mathrm{TOF}}_{{\mathrm{CH}}_4} = \frac{{R_{{\mathrm{CO}}}
\cdot S_{{\mathrm{CH}}_4} \cdot M_{{\mathrm{Ru}}}}}{{D \cdot x_{{\mathrm{Ru}}}}}$$ (2) where _R_CO is the CO consumption in moles per gram of catalyst, _S_CH4 is the methane selectivity,
_M_Ru is the atomic mass of Ru, _D_ is the Ru dispersion and _x__Ru_ is the Ru loading of the sample. The surface coverage of intermediates leading to CH4 was calculated as $$\theta
_{{\mathrm{CH}}_4} = \frac{{N_{{\mathrm{CH}}_4}}}{{N_{{\mathrm{Total}}}}}$$ (3) where _N_CH4 is the number of intermediates leading to methane and _N_Total is the total number of active
sites, as determined by the reversible adsorption of CO in the SSITKA experiments. COMPUTATIONAL DETAILS Density functional theory (DFT) calculations were performed using the Vienna
Ab-initio Simulation Package (VASP, a version of 5.4.4)40,41. The Perdew–Burke–Ernzerhof (PBE) exchange-correlation functional was used to initial calculation42. The core and valence
electrons were represented by the projector augmented wave (PAW) potential, and the plane wave basis set with a cut-off energy of 500 eV was used. A TiO_x_ cluster covered on a four-layered
slab of the close-packed (001) surface derived from the hcp phase of Ru, was used as a model to the TiO_x_ overlayer covered Ru nanocatalyst. A vacuum gap of 15 Å was used to separate
periodic images of the slab in the direction perpendicular to the surface. The atoms in the top two layers of Ru(001) were allowed to relax during optimization. The Brillouin zone of the 4 ×
4 surface unit cell of Ru(001) was sampled with a 2 × 2 × 1 Monkhorst-Pack grid. Optimized geometries were obtained by minimizing the forces on the atoms below 0.02 eV Å−1. The transition
state was first isolated using the climbing image nudged elastic band (CI-NEB) method and then refined using the dimer method to until force is below 0.02 eV Å−143. After that, the newly
developed GW potential was adopted for the further optimization of adsorption geometries and transition states. The resulting transition state was finally confirmed by the normal mode
frequency analysis, showing only one imaginary mode. We have first compared the relative stability of different TiO_x_ (_x_ = 1–4) clusters on the Ru(001) surface under different reduction
degree condition which can be represented as the variation of chemical potential of oxygen, and was calculated according to the procedure of previous research44. In our calculation, the data
of the formation energy of rutile TiO2 was acquired from the reference45. Other computational details are shown in the Supplementary Information. DATA AVAILABILITY The data that support the
findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Zhong, L. et al. Cobalt carbide nanoprisms for direct production of lower olefins
from syngas. _Nature_ 538, 84–87 (2016). Article ADS CAS PubMed Google Scholar * Li, J. et al. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. _Nat.
Catal._ 1, 787–793 (2018). Article CAS Google Scholar * Torres Galvis, H. M. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. _Science_ 335,
835–838 (2012). Article ADS CAS PubMed Google Scholar * Torres Galvis, H. M. et al. Iron particle size effects for direct production of lower olefins from synthesis gas. _J. Am. Chem.
Soc._ 134, 16207–16215 (2012). Article CAS PubMed Google Scholar * Fischer, N., Clapham, B., Feltes, T. & Claeys, M. Cobalt-based Fischer–Tropsch activity and selectivity as a
function of crystallite size and water partial pressure. _ACS Catal._ 5, 113–121 (2015). Article CAS Google Scholar * Sun, J. et al. Highly-dispersed metallic Ru nanoparticles sputtered
on H-beta zeolite for directly converting syngas to middle isoparaffins. _ACS Catal._ 4, 1–8 (2014). Article CAS Google Scholar * Ligthart, D. A. J. M. et al. EJM. Identification of
step-edge sites on Rh nanoparticles for facile CO dissociation. _Catal. Commun._ 77, 5–8 (2016). Article CAS Google Scholar * Simonetti, D. A., Rass-Hansen, J., Kunkes, E. L., Soares, R.
R. & Dumesic, J. A. Coupling of glycerol processing with Fischer–Tropsch synthesis for production of liquid fuels. _Green. Chem._ 9, 1073–1083 (2007). Article CAS Google Scholar *
Carballo, J. M. G. et al. Catalytic effects of ruthenium particle size on the Fischer–Tropsch synthesis. _J. Catal._ 284, 102–108 (2011). Article CAS Google Scholar * Kang, J., Deng, W.,
Zhang, Q. & Wang, Y. Ru particle size effect in Ru/CNT-catalyzed Fischer-Tropsch synthesis. _J. Energ. Chem._ 22, 321–328 (2013). Article CAS Google Scholar * Kang, J., Zhang, S.,
Zhang, Q. & Wang, Y. Ruthenium nanoparticles supported on carbon nanotubes as efficient catalysts for selective conversion of synthesis gas to diesel fuel. _Angew. Chem. Int Ed._ 48,
2565–2568 (2009). Article CAS Google Scholar * Ojeda, M. et al. CO activation pathways and the mechanism of Fischer–Tropsch synthesis. _J. Catal._ 272, 287–297 (2010). Article CAS
Google Scholar * Loveless, B. T., Buda, C., Neurock, M. & Iglesia, E. CO chemisorption and dissociation at high coverages during CO hydrogenation on Ru catalysts. _J. Am. Chem. Soc._
135, 6107–6121 (2013). Article CAS PubMed Google Scholar * González Carballo, J. M. et al. Support effects on the structure and performance of ruthenium catalysts for the Fischer–Tropsch
synthesis. _Catal. Sci. Technol._ 1, 1013–1023 (2011). Article CAS Google Scholar * Li, W. Z. et al. Chemical insights into the design and development of face-centered cubic ruthenium
catalysts for Fischer-Tropsch synthesis. _J. Am. Chem. Soc._ 139, 2267–2276 (2017). Article CAS PubMed Google Scholar * Tauster, S. J., Fung, S. C., Baker, R. T. K. & Horsley, J. A.
Strong interactions in supported-metal catalysts. _Science_ 211, 1121–1125 (1981). Article ADS CAS PubMed Google Scholar * Tauster, S. J., Fung, S. C. & Garten, R. L. Strong
metal-support interactions. Group 8 noble metals supported on titanium dioxide. _J. Am. Chem. Soc._ 100, 170–175 (1978). Article CAS Google Scholar * Komaya, T. et al. Effects of
dispersion and metal-metal oxide interactions on Fischer-Tropsch synthesis over Ru/TiO2 and TiO2-promoted Ru/SiO2. _J. Catal._ 150, 400–406 (1994). Article CAS Google Scholar * Xu, J. et
al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. _J. Catal._ 333, 227–237 (2016). Article CAS Google
Scholar * Abdel-Mageed, A. M. et al. Selective CO methanation on Ru/TiO2 catalysts: role and influence of metal–support interactions. _ACS Catal._ 5, 6753–6763 (2015). Article CAS Google
Scholar * van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. _Nat. Catal._ 2,
955–970 (2019). Article CAS Google Scholar * Kikuchi, E., Matsumoto, M., Takahashi, T., Machino, A. & Morita, Y. Fischer-Tropsch synthesis over titania-supported ruthenium catalysts.
_Appl Catal._ 10, 251–260 (1984). Article CAS Google Scholar * Hernandez Mejia, C., van Deelen, T. W. & de Jong, K. P. Activity enhancement of cobalt catalysts by tuning metal-support
interactions. _Nat. Commun._ 9, 4459–4466 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Lin, Q. et al. Crystal phase effects on the structure and performance of
ruthenium nanoparticles for CO2 hydrogenation. _Catal. Sci. Technol._ 4, 2058–2063 (2014). Article CAS Google Scholar * Badyal, J. P. S. & Lambert, R. M. Surface oxide films and H2/CO
chemisorption at the Ru/TiO2 interface: studies with a model planar catalyst. _J. Catal._ 130, 173–180 (1991). Article CAS Google Scholar * Green, C. L. & Kucernak, A. Determination
of the platinum and ruthenium surface areas in platinum-ruthenium alloy electrocatalysts by underpotential deposition of copper. I. Unsupported catalysts. _J. Phys. Chem. B_ 106, 1036–1047
(2002). Article CAS Google Scholar * Yang, J. et al. Reaction mechanism of CO activation and methane formation on Co Fischer–Tropsch catalyst: a combined DFT, transient, and steady-state
kinetic modeling. _J. Catal._ 308, 37–49 (2013). Article CAS Google Scholar * Ledesma, C., Yang, J., Chen, D. & Holmen, A. Recent approaches in mechanistic and kinetic studies of
catalytic reactions using SSITKA technique. _ACS Catal._ 4, 4527–4547 (2014). Article CAS Google Scholar * Lohitharn, N. & Goodwinjr, J. Impact of Cr, Mn and Zr addition on Fe
Fischer–Tropsch synthesis catalysis: investigation at the active site level using SSITKA. _J. Catal._ 257, 142–151 (2008). Article CAS Google Scholar * Hadjiivanov, K. et al. FTIR study
of CO interaction with Ru/TiO2 catalysts. _J. Catal._ 176, 415–425 (1998). Article CAS Google Scholar * González-Carballo, J. M. et al. In-situ study of the promotional effect of chlorine
on the Fischer–Tropsch synthesis with Ru/Al2O3. _J. Catal._ 332, 177–186 (2015). Article CAS Google Scholar * Elmasides, C., Kondarides, D. I., Grünert, W. & Verykios, X. E. X. P. S.
and FTIR study of Ru/Al2O3 and Ru/TiO2 catalysts: reduction characteristics and interaction with a methane-oxygen mixture. _J. Phys. Chem. B_ 103, 5227–5239 (1999). Article CAS Google
Scholar * Robbins, J. L. Chemistry of supported Ru: CO-induced oxidation of Ru at 310 K. _J. Catal._ 115, 120–131 (1989). Article CAS Google Scholar * Solymosi, F. & Raskó, J. An
infrared study of the influence of CO adsorption on the topology of supported ruthenium. _J. Catal._ 115, 107–119 (1989). Article CAS Google Scholar * Gupta, N. M. et al. FTIR
spectroscopic study of the interaction of CO2 and CO2 + H2 over partially oxidized Ru/TiO2 catalyst. _J. Catal._ 146, 173–184 (1994). Article CAS Google Scholar * Johnson, G. R., Werner,
S. & Bell, A. T. An investigation into the effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch synthesis: evidence for enhanced CO adsorption and
dissociation. _ACS Catal._ 5, 5888–5903 (2015). Article CAS Google Scholar * Shetty, S. & van Santen, R. A. CO dissociation on Ru and Co surfaces: the initial step in the
Fischer–Tropsch synthesis. _Catal. Today_ 171, 168–173 (2011). Article CAS Google Scholar * Ciobica, I. M. & van Santen, R. A. Carbon monoxide dissociation on planar and stepped
Ru(0001) surfaces. _J. Phys. Chem. B_ 107, 3808–3812 (2003). Article CAS Google Scholar * Filot, I. A., van Santen, R. A. & Hensen, E. J. The optimally performing Fischer-Tropsch
catalyst. _Angew. Chem. Int Ed._ 53, 12746–12750 (2014). Article CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations
using a plane-wave basis set. _Phys. Rev. B_ 54, 11169–11186 (1996). Article ADS CAS Google Scholar * Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector
augmented-wave method. _Phys. Rev. B_ 59, 1758–1775 (1999). Article ADS CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple.
_Phys. Rev. Lett._ 77, 3865–3868 (1996). Article ADS CAS PubMed Google Scholar * Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding
saddle points and minimum energy paths. _J. Chem. Phys._ 113, 9901–9904 (2000). Article ADS CAS Google Scholar * Reuter, K. & Scheffler, M. Composition, structure, and stability of
RuO2(110) as a function of oxygen pressure. _Phys. Rev. B_ 65, 035406 (2001). Article ADS CAS Google Scholar * Lide, D. R. _CRC Handbook of Chemistry and Physics_, 88th edn (Taylor &
Francis Group, Boca Raton, 2007). Download references ACKNOWLEDGEMENTS This work was supported by the National Key R&D Program of China (2016YFA0202804), the Strategic Priority Research
Program of the Chinese Academy of Sciences (XDB36030200), the National Natural Science Foundation of China (21978286, 21925803, 21776269), the Youth Innovation Promotion Association CAS.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China Yaru Zhang, Xiaoli
Yang, Xiaofeng Yang, Hongmin Duan, Haifeng Qi, Yang Su, Binglian Liang, Xiong Su, Yanqiang Huang & Tao Zhang * University of Chinese Academy of Sciences, Beijing, 100049, China Yaru
Zhang, Xiaoli Yang & Haifeng Qi * Department of Chemical Engineering, Norwegian University of Science and Technology, Trondheim, 7494, Norway Xiaoli Yang & De Chen * School of
Chemical and Biomedical Engineering, Nanyang Technological University Singapore, 637459, Singapore, Singapore Huabing Tao & Bin Liu Authors * Yaru Zhang View author publications You can
also search for this author inPubMed Google Scholar * Xiaoli Yang View author publications You can also search for this author inPubMed Google Scholar * Xiaofeng Yang View author
publications You can also search for this author inPubMed Google Scholar * Hongmin Duan View author publications You can also search for this author inPubMed Google Scholar * Haifeng Qi View
author publications You can also search for this author inPubMed Google Scholar * Yang Su View author publications You can also search for this author inPubMed Google Scholar * Binglian
Liang View author publications You can also search for this author inPubMed Google Scholar * Huabing Tao View author publications You can also search for this author inPubMed Google Scholar
* Bin Liu View author publications You can also search for this author inPubMed Google Scholar * De Chen View author publications You can also search for this author inPubMed Google Scholar
* Xiong Su View author publications You can also search for this author inPubMed Google Scholar * Yanqiang Huang View author publications You can also search for this author inPubMed Google
Scholar * Tao Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Z., X.F.Y., X.S., and Y.H. conceived and designed the project. Y.Z.
performed the experiments. X.F.Y. carried out the theoretical calculations. X.L.Y. and D.C. finished the SSITKA experiments. H.Q. performed the X-ray absorption experiments. Y.S. conducted
the TEM observations. H.D., B.L.L., H.T., and B.L. contributed to the structure characterizations. Y.Z., X.F.Y., X.S., Y.H., and T.Z. analyzed the experimental data and prepared the paper.
All authors reviewed and contributed to the paper. CORRESPONDING AUTHORS Correspondence to Xiaofeng Yang, Xiong Su or Yanqiang Huang. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Doan Pham Minh and the other, anonymous, reviewer(s) for their contribution to
the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Y., Yang, X., Yang, X. _et al._ Tuning reactivity of Fischer–Tropsch
synthesis by regulating TiO_x_ overlayer over Ru/TiO2 nanocatalysts. _Nat Commun_ 11, 3185 (2020). https://doi.org/10.1038/s41467-020-17044-4 Download citation * Received: 09 December 2019 *
Accepted: 02 June 2020 * Published: 24 June 2020 * DOI: https://doi.org/10.1038/s41467-020-17044-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
We've got some trouble | 404 - Resource not foundResource not found Error 404 The requested resource could not be found but may be available again in the future....
We've got some trouble | 404 - Resource not foundResource not found Error 404 The requested resource could not be found but may be available again in the future....
We've got some trouble | 404 - Resource not foundResource not found Error 404 The requested resource could not be found but may be available again in the future....
PM Modi offers prayers at Padmanabhaswamy templeModi, who entered the sprawling shrine through the eastern entrance, spent about 20 minutes there Prime Minister Narendr...
Competing couple : jody and harry lockmann don't let marriage get in the way of racingEL CAJON — Jody and Harry Lockmann made a deal one Saturday night at Cajon Speedway. If Jody won the four-car trophy das...
Latests News
Tuning reactivity of fischer–tropsch synthesis by regulating tiox overlayer over ru/tio2 nanocatalystsABSTRACT The activity of Fischer–Tropsch synthesis (FTS) on metal-based nanocatalysts can be greatly promoted by the sup...
Exercise Proteinuria and Proteinuria induced by KallikreinEXERCISE proteinuria was first reported1 in 1878, and since then increased excretion of protein in the urine during and ...
Family-Oriented, Classic Amusement Parks to Visit for Any AgeBy Stacey Freed, AARP En español Published July 26, 2024Kathy Kenez, 65, and Ralph Meranto, 60, love the adrenaline ru...
Community Care | VA Northern California Health Care | Veterans AffairsThe MISSION Act gives Veterans greater access to health care in VA facilities and the community, expands benefits for ca...
Abramovich flies into moscow as more oligarch assets are seized in spainAbramovich flies into Moscow as more oligarch assets are seized in Spain | WTVB | 1590 AM · 95.5 FM | The Voice of Branc...