Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance
Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Proper storage of excessive dietary fat into subcutaneous adipose tissue (SAT) prevents ectopic lipid deposition-induced insulin resistance, yet the underlying mechanism remains
unclear. Here, we identify angiopoietin-2 (Angpt2)–integrin α5β1 signaling as an inducer of fat uptake specifically in SAT. Adipocyte-specific deletion of Angpt2 markedly reduced fatty acid
uptake and storage in SAT, leading to ectopic lipid accumulation in glucose-consuming organs including skeletal muscle and liver and to systemic insulin resistance. Mechanistically, Angpt2
activated integrin α5β1 signaling in the endothelium and triggered fatty acid transport via CD36 and FATP3 into SAT. Genetic or pharmacological inhibition of the endothelial integrin α5β1
recapitulated adipocyte-specific Angpt2 knockout phenotypes. Our findings demonstrate the critical roles of Angpt2–integrin α5β1 signaling in SAT endothelium in regulating whole-body fat
distribution for metabolic health and highlight adipocyte–endothelial crosstalk as a potential target for prevention of ectopic lipid deposition-induced lipotoxicity and insulin resistance.
SIMILAR CONTENT BEING VIEWED BY OTHERS PARACRINE ROLE OF ENDOTHELIAL IGF-1 RECEPTOR IN DEPOT-SPECIFIC ADIPOSE TISSUE ADAPTATION IN MALE MICE Article Open access 02 January 2025 LOSS OF BONE
MORPHOGENETIC PROTEIN-BINDING ENDOTHELIAL REGULATOR CAUSES INSULIN RESISTANCE Article Open access 26 March 2021 ANGIOCRINE POLYAMINE PRODUCTION REGULATES ADIPOSITY Article 14 March 2022
INTRODUCTION The adipose tissue plays a pivotal role in maintaining whole-body energy homeostasis by buffering lipids1,2,3. Impaired uptake of circulating lipids by subcutaneous adipose
tissue (SAT) can induce ectopic fat accumulation in major glucose-consuming organs such as the skeletal muscle and liver4,5, leading to lipotoxicity and insulin resistance6,7. Several
approaches have focused on preventing ectopic fat accumulation; one strategy inhibits trans-endothelial fatty acid (FA) transport8,9,10,11, because the vascular endothelial cell (EC) is the
anatomic and metabolic gatekeeper of lipid shuttling into tissues12,13. However, approaches targeting trans-endothelial FA transport have been only partly successful14,15, presumably because
of the systematic approach targeting the whole circulatory system rather than specific tissues. Therefore, it will be clinically important to develop new ways to control tissue-specific
endothelial FA transport, such as driving FA trafficking into SAT. Transducing roles for vascular ECs in FA trafficking into tissues have been uncovered through the recent characterization
of FA transport proteins (FATPs) and CD36 in ECs12,16. These proteins are regulated by autocrine or paracrine factors such as VEGF-B, apelin, or Notch ligands that act on receptors of
ECs8,10,11,17. Other than its corresponding receptors such as VEGFR1, NRP1, or APLNR, integrin receptors are also implicated in FA handling18,19, but their role in endothelial FA trafficking
is unknown. Among the well-known ligands of integrin receptors are ECM molecules such as fibronectin, Mfge8, and Angpt220,21,22. In particular, the effects of Angpt2 in regulating adipose
tissue metabolism through angiogenesis have been thoroughly investigated23,24. However, approaches targeting adipose tissue angiogenesis through systemic blockade or constant overexpression
have generated mixed results in metabolic outcome25,26,27,28. Thus, more thorough investigation is required to shed light on the fundamental mechanisms underlying adipocyte crosstalk with
ECs by other methods such as adipocyte-specific deletion of Angpt2. Here, using adipocyte-specific KO of Angpt2 and endothelial-specific KO of integrin receptors, we identify Angpt2–integrin
α5β1 signaling as a novel regulator of trans-endothelial FA transport, specifically in subcutaneous adipose depots. Angpt2–integrin α5β1 induced FA transport into SAT is critical for
clearance of circulating FAs and prevention of peripheral lipid accumulation and systemic glucose intolerance. Thus, stimulation of FA transport by targeting SAT endothelium through
Angpt2–integrin α5β1 signaling offers a new therapeutic avenue for combat against ectopic lipid-induced insulin resistance and related metabolic syndrome. RESULTS ADIPOCYTE-DERIVED ANGPT2
LEADS SUBCUTANEOUS FAT DISTRIBUTION To validate the expression of Angpt2 in adipose tissues23, we first examined the expressions and distributions of Angpt2 in various metabolic organs.
Angpt2 expression was highest in SAT compared with other adipose tissues or metabolically active organs in mice (Supplementary Fig. 1a–c). In SAT, Angpt2 expression was enriched in
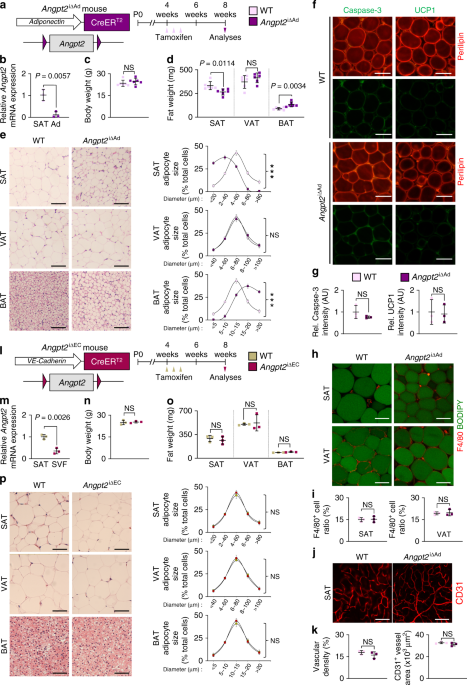
adipocytes compared with stromal vascular fraction (SVF) (Supplementary Fig. 1d). To investigate the role of adipocyte-derived Angpt2, we generated adipocyte-specific Angpt2 knockout (KO)
mice (_Angpt2_∆Ad) by crossing the adiponectin-Cre line with _Angpt2_fl/fl mice29 and analyzed them at 8 weeks after birth (Supplementary Fig. 2a). Cre-negative but flox/flox-positive mice
among the littermates were defined as wild-type (WT) mice for each experiment unless otherwise indicated. We found no differences in body weight (BW) and visceral adipose tissue (VAT)
weights between WT and _Angpt2_∆Ad mice (Supplementary Fig. 2b, c). However, we found altered fat distribution among different depots in _Angpt2_∆Ad mice, with significantly contracted SAT
and enlarged brown adipose tissue (BAT) (Supplementary Fig. 2c, d). Inducible adipocyte-specific Angpt2 KO (_Angpt2_i∆Ad) mice also showed almost identical phenotypes, indicating that this
alteration was not due to developmental defects (Fig. 1a–d). Interestingly, the fat mass change was largely due to the altered size in adipocytes: the size of SAT adipocytes was reduced
while the size of BAT adipocytes was increased (Fig. 1e). However, these smaller SAT adipocytes in the _Angpt2_i∆Ad mice did not show any signs of apoptosis, beiging, oxygen consumption,
immune cell infiltration, or defective vascularization (Fig. 1f–k and Supplementary Fig. 3a–g), suggesting that the reduced size is due to decreased fat contents. Compared with WT mice,
EC-specific Angpt2 KO mice (_Angpt2_i∆EC) showed no differences in BW or weights and adipocyte sizes of SAT, VAT, and BAT (Fig. 1l–p). Thus, Angpt2 derived from the adipocytes but not from
the ECs regulates the reciprocal distribution of fat into SAT and BAT. ANGPT2 STIMULATES ENDOTHELIAL FA UPTAKE We next sought to understand how fat contents were selectively reduced in SAT
by Angpt2 deletion. Thus, we examined if Angpt2 affects FA trafficking into adipocytes by measuring tissue uptake of orally administered radio-labeled FAs to _Angpt2_i∆Ad mice (Fig. 2a).
_Angpt2_i∆Ad mice showed 56% reduction in FA uptake by SAT, whereas they showed increased FA uptake by BAT (Fig. 2b and Supplementary Fig. 4a). Because BAT expresses much less Angpt2 than
SAT (Supplementary Fig. 1a), we speculate that the increased FA uptake by BAT reflects compensation mechanisms of reduced FA uptake by SAT. Importantly, FA production, uptake by liver, or
systemic levels of insulin or glucose, which are regulators of FA mobilization, were unchanged, indicating direct action of Angpt2 on FA uptake (Supplementary Fig. 4b–j). To elucidate the
mechanism of Angpt2 action on FA uptake, we measured FA intake in isolated SAT adipocytes in vitro (Fig. 2c). To our surprise, we found no difference in FA uptake between WT and
Angpt2-deficient (_Angpt2_∆Ad) adipocytes (Fig. 2d, e), indicating that Angpt2 induces fat uptake in a cell-nonautonomous manner. Given that blood vessels act as a gatekeeper of FA
trafficking into tissues12,30 and that ECs express Angpt2 receptors (e.g., Tie2 and integrins)21, we tested if Angpt2 regulates endothelial FA transport. We found that Angpt2 treatment
significantly increased FA uptake by ECs (Fig. 2f, g). These results indicate that Angpt2 acts as an adipokine to stimulate endothelial FA uptake in a cell-nonautonomous manner. ORGANOTYPIC
CHARACTERISTICS OF ENDOTHELIAL CELLS IN SAT To search for the receptor(s) that mediate the Angpt2 effect, we assessed which Angpt2 receptors are highly expressed in ECs of adipose tissues
using the RiboTag∆EC mouse31,32 to avoid disruption in cell surfaces. Upon tamoxifen treatment, this mouse tags hemagglutinin (HA) to the ribosome-associated actively transcribing mRNAs,
specifically in VE-Cadherin-expressing ECs (Fig. 3a–c). After successful validation of EC-specific marker enrichment [e.g., platelet endothelial cell adhesion molecule (PECAM)] in mRNAs
purified with an antibody against the HA tag (Fig. 3d), we compared a catalog of known Angpt2 receptors in ECs from various organs (Fig. 3e). Of note, the ECs in SAT expressed higher levels
of integrin α5 (ITGα5) and integrin β1 (ITGβ1) by ∼5.6-fold but ∼52% reduced levels of Tie2 compared with ECs in the other organs we examined (Fig. 3e). Immunohistochemical analyses
supported these reciprocal expression levels of integrin α5β1 and Tie2 in the ECs of SAT, which clearly differs with other depots (Fig. 3f, g and Supplementary Fig. 5a, b). Thus, integrins
are the strongest candidate receptors that are likely mediators of Angpt2 action on the endothelium in SAT. ANGPT2 DRIVES ENDOTHELIAL FA UPTAKE THROUGH INTEGRIN Α5Β1 To test this hypothesis,
we used uncoated plates to minimize integrin activation by ECM proteins such as gelatin or fibronectin33; nonetheless, the adherence of HUVECs was comparable to coated dishes (Supplementary
Fig. 6a). Next, we examined the effect of Mn2+, which enhances ligand-binding affinity of integrin21, on endothelial FA uptake (Fig. 4a). Treatment of Mn2+ alone showed no effect, but it
strongly augmented Angpt2-induced FA uptake by ECs by 3.0-fold (Fig. 4b, c). In the presence of Mn2+, Angpt2 stimulated FA uptake rapidly (Fig. 4d) and dose dependently (Fig. 4e). We found
no difference between vascular leakage by trans-well endothelial layer permeability assay, indicating that Angpt2 stimulates FA uptake independently of vascular permeabilizing actions (Fig.
4f). We next depleted various integrin subunits in ECs to further examine the importance of each integrin in mediating the Angpt2 effect. Depletion of integrin α5 or β1 completely blocked
Angpt2-induced FA uptake by ECs (Fig. 4g), whereas depletion of integrin αv or β5 did not (Supplementary Fig. 6b). Of interest, depletion of Tie2 or integrin β3 rather enhanced
Angpt2-induced FA uptake (Supplementary Fig. 6b), presumably because the absence of these receptors liberates Angpt2 and allows more Angpt2 to activate integrin α5β121,34. Consistent with
the depletion data, the α5β1-specific blocking peptide ATN-16135 completely suppressed Angpt2-induced FA uptake (Fig. 4h). Conversely, the conformational activator of integrin α5β1,
SNAKA-5122,36, enhanced Angpt2 activity (Fig. 4i). We observed similar effects of Angpt2 in a mouse EC cell line (MS1) but not in adipocytes isolated from SAT (Supplementary Fig. 6c–e). To
strengthen our finding that Angpt2 induces organotypic FA uptake in SAT ECs, we compared the effect of Angpt2 on primary ECs from SAT and VAT (Supplementary Fig. 7a). First, we employed a
previously published method for culturing primary ECs of murine organs37, and validated its 92.7% purity (Supplementary Fig. 7a–c). Next, we compared the effects of Angpt2 treatment with or
without Mn2+ in primary ECs from SAT and VAT (Supplementary Fig. 7d). Of note, Angpt2 treatment alone enhanced FA uptake in time- and dose-dependent manners only in SAT ECs (Supplementary
Fig. 7d–f). Importantly, this effect was inhibited by ATN-161 treatment (Supplementary Fig. 7g). These data demonstrate that the endothelial integrin α5β1 in SAT mediates Angpt2-induced FA
uptake. ANGPT2–INTEGRIN Α5Β1 DRIVES FA TRANSPORT THROUGH CD36/FATP3 Various FATPs mediate endothelial FA uptake12,30. Of note, Angpt2-induced FA uptake was specific for the long-chain FAs
(Fig. 5a). We thus depleted candidate FA transporters in ECs, including FA translocase (CD36) and FATPs (Fig. 5b). Also of interest, depletion of CD36 or FATP3, but not of FATP4, blocked
Angpt2-induced FA uptake and transport by ECs (Fig. 5c–f). However, we found no changes in gene expression levels of CD36 or FATP3 after Angpt2 treatment (Fig. 5g). Thus, Angpt2 activates
endothelial FA uptake, likely via redistribution or protein–protein interactions of CD36 or FATP39. Intracellular translocation of CD36 or FATPs, and consequently increased FA uptake, have
been reported in various cell types16,20. Therefore, we tracked protein expression of CD36 or FATP3 in ECs after Angpt2 treatment. Although we did not observe any changes in localization of
FATP3 in ECs (Fig. 5h), we detected rapid formation of punctate CD36 structures in perinuclear regions after only 5 min following Angpt2 addition (Fig. 5i). Intriguingly, these punctate CD36
signals were co-localized with ITGβ1 (Fig. 5i), suggesting that CD36 and ITGβ1 may physically interact upon activation by Angpt2. To test this possibility, we conducted an in situ proximity
ligation assay and observed strong signals indicative of complex formation between CD36 and ITGβ1 only after Angpt2 treatment (Fig. 5j, k). Moreover, ATN-161 markedly blunted CD36
co-localization with ITGβ1 and their interaction (Fig. 5l–n), indicating that activation of ITGβ1 by Angpt2 binding is required for CD36 translocation and interaction with ITGβ1.
Immunoprecipitation (IP) by anti-CD36 antibody also demonstrated that CD36 bound to ITGβ1 and that their interaction increased by ∼2.1-fold after Angpt2 treatment (Fig. 5o, p). Thus, Angpt2
facilitates CD36 translocation through integrin α5β1. Since ITGβ1 faces the basolateral side of ECs38, and CD36 is located on the luminal side of ECs39, we speculated that transmembrane
lipid rafts, which interacts with both molecules40, could mediate this binding complex. Indeed, we detected co-localization of this complex in Caveolin-1+ lipid rafts in plasma membrane of
ECs (Supplementary Fig. 8a, b). In line with this finding, we found that integrin α5β1 is mainly located on collagen IV+ CD34− basolateral membrane of ECs41,42 in SAT (Supplementary Fig. 8c,
d). ENDOTHELIAL ITGΒ1 STIMULATES FA TRANSPORT INTO SAT We next proceeded to recapitulate our findings in vivo. First, we generated EC-specific inducible ITGβ1 KO (_ITGβ1_∆EC) mice by
crossing VE-Cadherin-Cre-ERT2 mice and _Itgb1_flox/flox mice, and analyzed them 1 week after tamoxifen administration due to lethality43 (Fig. 6a). There were no changes in vascular
integrity or density in _ITGβ1_∆EC mice after 1 week of tamoxifen treatment (Supplementary Fig. 9a–d). Compared with WT mice, adipocyte sizes in SAT decreased by 63% and SAT weight by 23%,
but no differences were found in VAT in _ITGβ1_∆EC mice (Fig. 6b). We found no difference in BW, liver and muscle fat content, or BAT weight (Supplementary Fig. 9e–g). _ITGβ1_∆EC mice also
showed 42% reduced uptake of intravenously administered FAs toward SAT but not by VAT, BAT, or liver (Fig. 6c–e and Supplementary Fig. 9h, i). Thus, endothelial ITGβ1 is required for
vascular FA transport toward SAT. INHIBITION OF INTEGRIN Α5Β1 BLOCKS FA TRANSPORT INTO SAT We next administered the integrin α5β1-specific blocking peptide ATN-161 to WT mice (Supplementary
Fig. 10a) to evaluate whether the phenotypes of _ITGβ1_∆EC mice were caused by integrin α5β1 inhibition (Fig. 7a). Indeed, ATN-161 treatment recapitulated the _ITGβ1_∆EC mouse SAT
phenotypes, and the phenotype was even more enhanced after an additional week of treatment (Fig. 7b, c). This change was accompanied by a markedly diminished uptake of radioactive FAs in
SAT, by 44%, but not by other organs (Fig. 7d). Again, we found no changes in BW, liver, and BAT weights (Supplementary Fig. 10b–e). Therefore, we concluded that the endothelial integrin
α5β1 is essential for vascular FA transport, specifically in SAT. ANGPT2–INTEGRIN Α5Β1 IS ENRICHED IN NONDIABETIC OBESE SAT To investigate the clinical relevance of our findings, we compared
gene expression profiles of SAT and VAT between nondiabetic obese (NDO) and diabetic-obese (DO) individuals from the publicly available gene expression database, Gene Expression Omnibus
(GEO; GSE20950, GSE29226, GSE29231, GSE16415, GSE71416). Among the 34 genes that were specifically upregulated in SAT of NDO individuals, we identified Angpt2 as the sole secretory molecule
(Fig. 8a and Supplementary Tables 1, 2). To examine if dietary fat overload and obesity affect Angpt2 expression, we fed mice with high-fat diet (HFD). Surprisingly, with HFD, a gradual
increase in Angpt2 expression was detected in SAT but not in other organs or in the systemic circulation (Fig. 8b, c and Supplementary Fig. 11a, b). Intriguingly, the Angpt2 induction was
specific to the adipocytes (Fig. 8d and Supplementary Fig. 11c). This result was consistent with increased expression of Angpt2 in the isolated adipocytes from the SAT of NDO individuals
(Fig. 8e). In detail, saturated FA (palmitic acid) treatment alone for 24 h was sufficient to increase Angpt2 expression by 2.0- and 2.7-fold in SAT adipocytes in both mice and humans,
respectively (Fig. 8f). Thus, Angpt2 expression is rapidly induced by dietary fat intake in a SAT adipocyte-specific manner. By comparison analysis of human GEO data, we confirmed enhanced
expression of ITGα5 in SAT compared with VAT in NDO patients (Fig. 8g). Likewise, we confirmed enriched expression of integrin α5β1 in ECs of SAT of NDO patient (Supplementary Fig. 11d).
Thus, integrin receptor expression is enriched in ECs of SAT in NDO individuals. Next, we observed changes in Angpt2 and its receptors during fast/fed cycle. Of note, Angpt2 expression was
reduced by 58.8% in fasted mice (Supplementary Fig. 11e). Consistently, expressions of both ITGα5β1 and CD36 were downregulated in ECs during fasting (Supplementary Fig. 11f, g). These
indicate that expressions of Angpt2 and its mediators in FA transport are reduced during energy output. ANGPT2 PREVENTS ECTOPIC LIPID-INDUCED INSULIN RESISTANCE Given that Angpt2 in
adipocytes is highly induced by dietary fat intake, we next challenged _Angpt2_i∆Ad mice with HFD (Fig. 9a and Supplementary Fig. 12a). After 8 weeks of HFD, SAT weighed 28% less, and BAT
weighed 71% more in _Angpt2_i∆Ad mice compared with WT animals (Fig. 9b). Obesity-associated inflammatory markers in VAT were upregulated, while thermogenic markers were downregulated in BAT
in _Angpt2_i∆Ad mice (Supplementary Fig. 12b, c). Likewise, electron microscope analysis revealed unpacked cristae and vacuole-filled mitochondria in BAT of _Angpt2_i∆Ad mice (Supplementary
Fig. 12d). Moreover, circulating triglyceride and leptin levels were each ∼1.5-fold and ∼2.0-fold higher, whereas plasma adiponectin level was 30% less in _Angpt2_i∆Ad mice (Fig. 9c and
Supplementary Fig. 12e, f). Of special note, histological analyses revealed profound lipid accumulation in the liver and skeletal muscle of _Angpt2_i∆Ad (Fig. 9d, e). _Angpt2_i∆Ad mice also
showed systemic glucose intolerance and insulin resistance (Fig. 9f, g), presumably because of ectopic fat deposition in these glucose-consuming organs6,8. Moreover, _Angpt2_i∆Ad mice showed
decreased metabolic rate without affecting food intake or activity (Fig. 9h, i and Supplementary Fig. 13a–e). Together, these data indicate that adipocyte-derived Angpt2 is required for
proper fat distribution toward SAT to prevent lipid overflow into glucose-metabolizing organs during high-fat intake. DISCUSSION Maintenance of metabolic health is of paramount importance in
a society where obesity is rapidly on the rise. Our various tissue-specific KO mouse models and mechanistic studies in primary cultured cells demonstrate that adipocyte-produced Angpt2
regulates endothelial FA uptake via CD36 and FATP3 through integrin α5β1 signaling. Intriguingly, this process is critical for FA uptake specifically in subcutaneous fat depots. Inhibition
of this process triggers fat accumulation in other fat depots and major glucose-consuming organs, leading to systemic insulin resistance with HFD, reminiscent of the pattern in
diabetic-obese patients (Fig. 10). Angpt2 is stored in repository granules of ECs called Weibel–Palade bodies, and rapidly released upon stimulation as an angiocrine factor44,45. Thus, it is
intriguing that only adipocyte-specific but not EC-specific Angpt2 KO mice exhibited phenotypes in SAT. How does Angpt2 in adipocytes exert different effects from Angpt2 in ECs? Adipocytes
do not possess Weibel–Palade bodies; thus, it is possible that the signals triggering Angpt2 release by adipocytes are distinct from those in ECs. For example, ECs release Angpt2 in
inflammatory conditions to increase vascular permeability for immune cell infiltration34,45. On the other hand, we found a gradual increase in Angpt2 expression in adipocytes after a
short-term high-fat regimen, which did not alter circulating Angpt2 levels. Thus, fat intake-induced Angpt2 released from adipocytes can stimulate FA uptake by neighboring ECs without
systemic impact. In eliciting this outcome, integrin receptors face the basolateral side to bind with the extracellular matrix46. Although ITGα5β1 is on the basolateral side and CD36 resides
on the luminal side, these seem to form a complex through transmembrane lipid rafts39,40. Thus, Angpt2 produced from the adipocytes may aggregate more easily than Angpt2 released from ECs
circulating inside the lumen. Another interesting feature of Angpt2 is its fat depot-specific effect. We found that adipocyte-specific deletion of Angpt2 affects SAT but not VAT. This
selectivity can be explained by the distribution of its receptors; ECs in SAT highly express ITGα5β1 but barely express Tie2, whereas ECs in VAT have the opposite expression. EC-specific
integrin KO mice phenocopied adipocyte-specific Angpt2 KO mice further confirming that the integrin but not Tie2 is a key determinant of Angpt2 action on endothelial FA uptake in SAT.
Therefore, endothelial heterogeneity among different fat depots mediates specific effects of Angpt2, which could be masked during systemic modulation of Angpt2 through pharmacological
blockade or constant overexpression affecting other organs23,24. The differences in adipose depots’ response to dietary fat intake could explain why VAT does not exhibit compensatory fat
uptake. While SAT expands through hypertrophy of existing adipocytes, VAT responds through hyperplasia of newly generated adipocytes upon dietary fat intake47,48. This means that SAT
expansion is mediated by fat intake itself whereas VAT expansion involves certain molecular cues to activate adipogenesis49,50. Meanwhile, it is well-known through clinical observation that
subcutaneous obesity is more frequent in females and is less morbid than visceral obesity that is more frequent in males2,51. Whether adipocyte-derived Angpt2 and its role in endothelial FA
transport is involved in the sexual dimorphism of body fat distribution needs to be studied. In order to do so, manipulating Angpt2 in a depot-specific manner could better define the role of
Angpt2 in various adipose depots. The important role of capillary ECs as a gatekeeper for fat trafficking has been demonstrated in various settings8,9,10,11,17,52. However, a study using
imaging mass spectrometry to visualize cardiac FA uptake showed that the vascular wall is not a substantial barrier to the lipid movement into cardiomyocytes53. In addition, these authors
reported that CD36 deficiency does not affect lipid entry into cardiomyocytes53. Yet another group recently reported that EC-specific deletion of CD36 leads to reduced lipid droplet
accumulation in cardiomyocytes16. In agreement, we found that CD36 is necessary for Angpt2-mediated endothelial FA uptake. Thus, it is possible that CD36 regulates FA uptake in a
context-dependent manner. On the other hand, genetic mouse models for FATPs and their phenotypes regarding endothelial fat metabolism have not yet been reported. Thus, studies using animal
models with genetic modifications in different FATPs in ECs will be useful to demonstrate their importance in regulating endothelial FA transport. Through comparative transcriptomics on
samples from NDO versus DO individuals, we identified Angpt2 as a potential adipokine that sustains metabolic health via regulation of body fat distribution. Accordingly, our
adipocyte-specific Angpt2 KO mice demonstrated significant ectopic fat accumulation in the BAT and in liver and skeletal muscle after HFD. This accumulation is accompanied by markedly
reduced FA uptake and SAT size, indicating that SAT adipocyte-released Angpt2 is critical for proper fat distribution to prevent fat spillover to other organs. The question then arises: Can
Angpt2–ITGα5β1 treatment be a therapeutic strategy to normalize fat distribution and treat obesity-induced metabolic disorders? The doses and administration route may be critical given that
a constant or systemic increase in Angpt2 elicits other impacts, such as activation of angiogenesis and increased vascular permeability23,54,55. Alternatively, targeting the SAT endothelium
would be a more attractive approach. Further investigation is required to test these possibilities genetically or pharmaceutically to open new therapeutic paths for metabolically healthy
obesity. METHODS ANIMALS Specific pathogen-free C57BL/6J, _Adiponectin-_Cre_, Integrinβ1_ flox/flox, _Tie2_-GFP, and RiboTag mice were purchased from Jackson Laboratory (Jackson Labs, Bar
Harbor, ME). _Angpt2_-eGFP (Tg [Angpt2-EGFP] DJ90Gsat/Mmcd) were purchased from the Mutant Mouse Regional Resource Centers, _Angpt2_ flox/flox 29, _Angpt2-_lacZ56, _Adiponectin-_Cre-ERT2 57,
and _VE-Cadherin-_Cre-ERT2 58 mice were transferred and bred in our specific pathogen-free animal facilities in Korea Advanced Institute of Science and Technology (KAIST). Mice were housed
under 12 light/12 dark cycle, temperatures of 22 ± 2 °C with 50 ± 10% humidity. For all experiments, male mice aged 8-week-old under standard chow diet or 16-week-old under long term
high-fat diet (HFD) were used. In order to induce Cre activity in the Cre-ERT2 mice, 2 mg of tamoxifen was injected i.p. for 2 or 3 consecutive days from the indicated time points. For
integrin α5β1-specific inhibition, mice were treated with ATN-161 (30 mg/kg)35,59 for indicated time points and analyzed at indicated days. All mice were bred in our specific-pathogen-free
animal facility and were fed normal chow diet (PMI LabDiet, St. Louis, MO) or HFD (60 kCal% fat, Research Diets, New Brunswick, NJ) with ad libitum access to water. Animal care and
experimental procedures were performed under the approval from the Institutional Animal Care and Use Committee (IACUC; No. KA2013-39) of KAIST. HISTOLOGICAL ANALYSES Mice were anesthetized
with a combination of ketamine (80 mg/kg) and xylazine (12 mg/kg) by intramuscular injection. All adipose tissues were from male adult mice. For sampling of tissues, inguinal white adipose
tissue was used for SAT, epididymal white adipose tissue was used for VAT, interscapular BAT, and quadriceps (skeletal muscle) were used for this study. For hematoxylin and eosin (H&E)
staining, indicated organs were fixed overnight in 10% paraformaldehyde (PFA) at 4 °C. After tissue processing using standard procedures, samples were embedded in paraffin and cut into
sections followed by H&E staining. For immunofluorescence studies, harvested tissues were fixed overnight with 1% PFA in PBS at 4 °C, and whole mounted. After blocking with 5% goat or
donkey serum (Jackson ImmunoResearch) in PBST (0.3% Triton X-100 in PBS for whole-mount method, 0.03% Triton X-100 in PBS for paraffin-sections) for 1 h at RT, whole-mounted or sectioned
tissue was incubated overnight at 4 °C with the following primary antibodies (diluted at a ratio of 1:200 in blocking solution): anti-Perilipin (guinea pig polyclonal, 20R-PP004,
Fitzgerald), anti-mouse CD31 (hamster monoclonal, 2H8, Millipore), anti-GFP (rabit polycloncal, AB3080, Millipore), anti-cleaved caspase-3 (rabbit polyclonal, 9661, Cell Signaling),
anti-UCP1 (rabit polycloncal, ab23841, Abcam), anti-Integrin α5β1 (rat monoclonal, BMB5, Millipore), anti- active Integrin β1 (rat monoclonal, 9EG7, BD Biosciences), anti- Integrin β1 (mouse
monoclonal, 12G10, Abcam), anti-HA (rabbit polyclonal, H6908, Sigma-Aldrich), and anti-human CD31 (rabit polyclonal, ab28364, Abcam). After several washes with PBST, the samples were
incubated with the following secondary antibodies diluted at a ratio of 1:1000 in blocking solution (all from Jackson ImmunoResearch) for 2 h at RT: Cy3-conjugated anti-guinea pig antibody,
Cy5-conjugated anti-hamster antibody, FITC-conjugated anti-rabbit antibody, FITC-conjugated anti-rat antibody, and FITC-conjugated goat antibody. Hoechst 33342 (Sigma-Aldrich) was used to
detect nucleus, and boron-dipyrromethene (BODIPY, Invitrogen) was used to detect lipid accumulation. To evaluate β-galactosidase activity, the tissues were incubated with a staining solution
[5 mM potassium ferricyanide, 2 mM magnesium chloride, 5 mM potassium ferrocyanide, and 1 mg/ml 4-chloro-5-bromo-3-indolyl-β-D-galactopyranoside (X-gal) in PBS] at 37 °C for 16 h. For Oil
Red O staining, slides were washed with PBS and 60% isopropanol, and incubated with filtered Oil Red O working solution for 50 min at room temperature. After staining, several washes with
PBS and 60% isopropanol were performed to reduce nonspecific staining. MORPHOMETRIC ANALYSIS Confocal microscopes (LSM 800 and LSM 880, Carl Zeiss) and stereomicroscope (Axiozoom V16)
equipped with argon and helium–neon lasers were used to visualize fluorescence images. Morphometric analyses were performed with Image J software (NIH) or Zeiss image software (ZEN 2012).
Histological sections of adipose tissues were stained with H&E and studied under 40-fold magnification to compare adipocyte size. Four to six different mice of each genotype were
randomly selected to determine the adipocyte size by cross-sectioned area using Image J software. Cross-sectioned area were narrowed down into ranges of adipocyte size, and data were
presented as percentage of adipocytes of each range of adipocyte size. Vascular density was measured as CD31+ vessel area divided by total measured area and presented as percentage. RNA
EXTRACTION Total RNA was extracted from the samples using TRIzol® Reagent (Invitrogen) according to the manufacturer’s instructions. For adipocyte and SVF isolation, adipose tissues were
incubated in Hanks balanced salt solution (HBSS; Sigma-Aldrich) containing 0.2% collagenase type 2 (Worthington) for 30 min at 37 °C with constant shaking. After inactivating collagenase
activity with 10% fetal bovine serum (FBS) containing Dulbecco modified eagle medium (DMEM), the cell suspension was filtered through a 40 μm nylon mesh (BD Biosciences), followed by
centrifugation at 420 g for 5 min. Floating adipocytes were used as adipocyte fraction, remaining SVF pellet were isolated and further analyzed. 500–2000 ng of the RNA were reverse
transcribed into cDNA using GoScriptTM cDNA synthesis system (Promega, Madison, Wisconsin). QUANTITATIVE RT-PCR ANALYSES Quantitative real-time PCR was performed using FastStart SYBR Green
Master mix (Roche) and QuantStudio3 (Applied Biosystems) with the indicated primers. The real-time PCR data were analyzed with QuantStudio Software (Applied Biosystems). Results were
calculated using the delta delta CT method60, with _36b4_ used for normalization of in vivo samples and _Gapdh_ for normalization of in vitro samples. Primers for the quantitative real-time
PCR are shown in Supplementary Table 3. OXYGEN CONSUMPTION RATE The oxygen consumption rate was measured using the Seahorse XFe96 analyzer (Seahorse Bioscience) following the manufacturer’s
instructions. Briefly, ECAR and OCR were measured after primary cultured pre-adipocytes were stimulated with adipogenic cocktail on XFe96 microplates. Cells were maintained in non-buffered
assay medium in a non-CO2 incubator for 1 h before the assay. The Mito stress test kit (Seahorse Bioscience) was used to test the OCR under basal conditions in the presence of oligomycin
(1.5 μM), the mitochondrial uncoupler carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP; 1 μM), and the respiratory chain inhibitors rotenone and antimycin A (0.5 μM). FATTY ACID
UPTAKE IN VIVO For measuring FA absorption in vivo using radioisotopes, mice were given a bolus dose of 2 mCi of 14C-oleic acid (Perkin Elmer) dissolved in 200 µl olive oil by oral gavage.
After 2 h, indicated organs were dissected. The organs were incubated overnight at 50° in 1 ml tissue solubilizer (Solvable, Perkin Elmer), decolorized with 0.3 ml 30% hydrogen peroxide for
1 h in room temperature. We added scintillation solution (Ultima Gold, Perkin Elmer) to each vial. Total radioactivity was measured by liquid scintillation using a Tri-Carb 2910 TR Liquid
Scintillator (Perkin Elmer). 14C-oleic acid content was normalized to g of tissue. For measuring FA absorption in vivo using fluorescent dyes, we revised a previously described method20. In
brief, the appropriate amount of BODIPY fluorescent-conjugated FAs (BODIPY FL C16 and BODIPY 558/568 C12, Invitrogen) was calculated (0.5 mg/kg) for each mice and dissolved in control
solution (HBSS supplemented with 20 mM HEPES and 0.2% FFA free BSA) in a total volume of 200 µl. Injection was performed intravenously, and tissues were harvested and snap freezed at
indicated time points. Tissues were then homogenized in RIPA buffer, centrifuged and supernatant was used. Fluorescence intensity was measured (_λ_ex = 485/_λ_em = 520 nm) in black, 96-well
flat-bottom plates using a fluorescence microplate reader (Tecan). We subtracted the fluorescence signal of each tissue from mice treated with control solution, and normalized by the weight
of the extracted tissue. For both methods, those with insufficient FA intake in plasma were ruled out for data analysis. CELL CULTURE Human Umbilical Vein Endothelial Cells (HUVECs) were
cultured according to the manufacturer’s protocols (Lonza, Walkersville, Maryland). In brief, the cells were cultured in a humidified atmosphere with 5% CO2 at 37 °C in endothelial growth
medium (EGM, Lonza) on cell culture plates without gelatin or fibronectin coating to minimize effects of integrin activation from other growth factors or matrix. The cells used were between
passages 3–6. For SVF induction, dissected SAT were chopped with scissors and incubated in digestion medium containing 0.2% collagenase type 2 (Worthington) for 30 min at 37 °C with constant
shaking. The resulting suspension were lysed with ACK Lysing Buffer (Life Technologies) for 5 min at 37 °C, and centrifuged at 470 × _g_ for 5 min. After several washes, SVF pellet was
resuspended in culture medium. Two days after confluent state, SVF cells were cultured with adipogenic differentiation medium. After 3 days, adipogenic differentiation medium was changed to
maintenance medium. Human primary subcutaneous pre-adipocytes (PCS-210-010; ATCC) were cultured in fibroblast basal medium (PCS-201-030; ATCC) added with fibroblast growth kit-low serum
(PCS-201-041; ATCC) and differentiated using adipocyte differentiation toolkit (PCS-500-050; ATCC). MS1 mouse pancreas ECs (CRL-2279; ATCC) were cultured in DMEM medium supplemented with 5%
fetal calf serum (FCS) at 37 °C and 5% CO2. For primary culture of ECs from SAT and VAT, we employed and modified a previously published method for culturing primary ECs of murine adipose
tissues37. Dissected SAT and VAT were chopped with scissors and incubated in digestion medium containing 0.2% collagenase type 2 (Worthington) for 30 min at 37 °C on a shaker. The resulting
suspension was lysed with ACK Lysing Buffer (Life Technologies) for 5 min at 37 °C and centrifuged at 470 × _g_ for 5 min. After several washes, cell suspension was filtered through 100 μm
nylon cell strainer and washed. To enrich the EC fraction, cells were incubated for 20 min with anti-CD31 Microbeads (Miltenyi) and selected using AutoMACS (Miltenyi) according to the
manufacturer’s instructions. In order to maximize EC survival and culture, cells were incubated with EGM2 containing 10% FBS and 400 ng/ml of EC growth supplement (ECGS; Sigma).
Culture-expanded monolayer of ECs under single passage that were validated to be CD31+ were used for all experiments. For SVF induction, dissected SAT were chopped with scissors and
incubated in digestion medium containing 0.2% collagenase type 2 (Worthington) for 30 min at 37 °C with constant shaking. The resulting suspension were lysed with ACK Lysing Buffer (Life
Technologies) for 5 min at 37 °C, and centrifuged at 470 × _g_ for 5 min. After several washes, SVF pellet was resuspended in culture medium. Two days after confluent state, SVF cells were
cultured with adipogenic differentiation medium. After 3 days, adipogenic differentiation medium was changed to maintenance medium. Human primary subcutaneous pre-adipocytes (PCS-210-010;
ATCC) were cultured in fibroblast basal medium (PCS-201-030; ATCC) added with fibroblast growth kit-low serum (PCS-201-041; ATCC) and differentiated using adipocyte differentiation toolkit
(PCS-500-050; ATCC). MS1 mouse pancreas ECs (CRL-2279; ATCC) were cultured in DMEM medium supplemented with 5% FCS at 37 °C and 5% CO2. CELL ADHESION ASSAY For cell adhesion assays, 48-well
tissue culture plates were either uncoated or coated with 0.1% gelatin or 10 μg/mL fibronectin in PBS at 4 °C for 1 h, air dried, and rinsed once with PBS. After being serum deprived at 37
°C for 8 h, HUVECs were detached and washed twice, and plated onto the wells in serum-free medium containing 0.1% BSA at 5 × 104 per well. Cells in three wells of the quadruplicate were
allowed to adhere to the coated/uncoated surface for 30 min, followed by four intensive washes to remove nonadherent cells, and incubated with 44 µM resazurin (#R7017, Sigma-Aldrich) in
complete medium for 2 h. Resazurin fluorescence was then measured with a microplate reader (excitation 530 nm, emission 590 nm, cutoff 550 nm). Values were normalized to control. SIRNA
TRANSFECTION For siRNA experiments, HUVECs were transfected with siRNAs targeting human CD36 (5′-AAGAGGAACTATATTG-TGCCTCCTGTCTC-3′), FATP3 (Santa Cruz Biotechnology, SASI_Hs01_00100092),
FATP4 (Santa Cruz Biotechnology, SASI_Hs01_00047-530), ITGβ1 (5′-TGATAGATCCAATGGCTTA-3′), ITGα5 (Bioneer, 1075709), ITGαV (Bioneer, 1075799), ITGβ3 (Bioneer, 1075875), ITGβ5 (Bioneer,
1075906), TIE2 (5′-GGCUAGUAAGAUCAAUGGUdTdT-3′), NFATc1 (5′-CCCGUUCACGUCAGUUUCUACGUCU-3′)or a scrambled control (5′-UAGCGACUAAACACAUCAA-3′) were used. Transfections of siRNA into the HUVECs
were performed using Lipofectamine® RNAiMAX (Invitrogen, Waltham, Massachusetts) according to the manufacturer’s protocol. Briefly, 20 nM of siRNA was transfected with RNAiMAX and knockdown
was assessed by RT-PCR. Experiments were conducted 48 h after siRNA transfection. FATTY ACID UPTAKE AND TRANSPORT IN VITRO For measuring FA absorption in vitro, we revised a previously
described method9. In brief, confluent HUVECs were transferred from a 10 cm dish to a non-coated, 96-well, black, clear-bottom plate (Corning, 3603), with empty corner wells for no-cell
controls. After overnight incubation, the cells were serum-starved for at least 6 h. The cells were then treated with Mn2+ to activate integrin motifs21 with vehicle or human recombinant
Angpt2 (2.5 µg/ml). Then, BODIPY-FA (Invitrogen, D3823), preincubated with fatty acid-free bovine serum albumin (BSA) (2:1 molar ratio) in PBS for 30 min in a 37 °C water bath, was added to
the cells for 5–30 min at 37 °C. The BODIPY solution was then completely aspirated, and the cells were washed with 0.5% BSA in PBS for 1.5 min twice (100 µl per well). One percent PFA was
added (100 µl per well) to minimize degradation, and intracellular fluorescence was measured (excitation 488 nm, emission 515 nm) immediately with a microplate reader (Tecan, BioTek).
Readings from wells without BODIPY addition were used to subtract background signals. The cells were then incubated with HOECHST (100 µl per well) and was measured with a microplate reader
(excitation 350 nm, emission 461 nm) to normalize BODIPY signals to cell number. BODIPY FL C16 (D3821) and BODIPY FL C5 (D3834) were purchased from Invitrogen. To block integrin α5β1, 10 μM
ATN-16135 were treated 15 min prior to Angpt2 addition. To activate integrin α5β1-specific motif, 10 μg/ml SNAKA-5122 were treated 15 min prior to Angpt2 addition. For all experiments, a
fresh batch of recombinant Angpt261 were treated in freshly isolated HUVECs (less than four passages). For measuring FA transport in vitro, we revised a previously described method8. HUVECs
were grown on a 24-well 0.4 µm trans-well inserts (SPL, 35024), and they were then grown for 2–3 days until the cells formed compact layers. Phenol red-free ECBM (Promocell, C-22215) were
used to minimize overlapping fluorescence of medium samples that were collected from the bottom chamber at the indicated time points. Fifty microliters of medium sample was measured using a
fluorescence plate reader (excitation 488 nm, emission 515 nm). IN VITRO VASCULAR PERMEABILITY ASSAY For assessment of endothelial layer permeability, we modified the manufacturer’s
instructions of in vitro vascular permeability assay (Millipore). Briefly, HUVECs were grown until confluence for 2–3 days on trans-well inserts. FITC-dextran solution (Millipore) was added
to the upper chamber and transferred solution was measured. IN VIVO VASCULAR PERMEABILITY ASSAY Vascular leakage was analyzed after i.v. injection of 100 μl of FITC-conjugated dextran (4
mg/ml, 70 kDa, Sigma-Aldrich) 5 min before sacrifice. Mice were anesthetized and perfused by intracardiac injection of 1% PFA to remove circulating dextran. MRNA ISOLATION USING RIBOTAG
METHOD RiboTag mouse was used to isolate polysome-bound mRNAs of EC with minor modification from previously described method32. Briefly, tissues were harvested and immediately snap freezed.
Then, polysome buffer (50 mM Tris, pH 7.5, 100 mM KCl, 12 mM MgCl2, 1% Nonidet P-40, 1 mM DTT, 200 U/ml RNasin, 1 mg/ml heparin, 100 μg/ml cyclohexamide, and 1× protease inhibitor mixture)
were added to each sample and homogenized using Precellys lysis kit (Bertin). For IP against HA, anti-HA antibody-conjugated magnetic beads (MBL, M180-11) were added to the supernatant after
centrifugation for 10 min at 13500 × _g_ 4 °C, and incubated on a rotating shaker at 4 °C overnight. Beads were washed for four times with high-salt buffer (50 mM Tris, pH 7.5, 300 mM KCl,
12 mM MgCl2, 1% Nonidet P-40, 1 mM DTT, and 100 μg/ml cyclohexamide) and resuspended in 350 μl of RLT plus buffer with β-mercaptoethanol. Total RNAs were extracted using the RNA isolation
mentioned in methods. The quality and quantity of the RNA samples were analyzed using Agilent 2100 Bioanalyzer with RNA 6000 pico kit (Agilent), and further performed to RNA-seq. RNA-SEQ
ANALYSIS OF RIBOTAG-CAPTURED MRNA For RNA-seq data analysis, four to five biological replicates of mRNA isolated by RiboTag method were used for analysis. The normalized count values were
processed based on Quantile normalization method using the Genowiz™ version 4.0.5.6 (Ocimum Biosolutions) and used for heatmaps and bioinformatics analysis. RNA-Seq gene expression heatmap
was generated with Multiple Experiment Viewer from The Institute of Genomic Research. The indicated expression ratio in a heatmap reflects the normalized count of each replicate apart from
the mean gene expression value over all condition. BIOINFORMATICS For RNA-seq experiment, SENSE 3′ mRNA-Seq Library Prep Kit (Lexogen, Inc.) were used according to the manufacturer’s
instructions. In brief, each 500 ng total RNA were prepared and an oligo-dT primer containing an Illumina-compatible sequence at its 5′ end was hybridized to the RNA and reverse
transcription was performed. After degradation of the RNA template, second strand synthesis was initiated by a random primer containing an Illumina-compatible linker sequence at its 5′ end.
The double-stranded library was purified by using magnetic beads to remove all reaction components. The library was amplified to add the complete adapter sequences required for cluster
generation. The finished library is purified from PCR components. High-throughput sequencing was performed as single-end 75 sequencing using NextSeq 500 (Illumina, Inc.). For RNA-Seq, SENSE
3′ mRNA-Seq reads were aligned using Bowtie2 version 2.1.0. Bowtie2 indices were either generated from genome assembly sequence or the representative transcript sequences for aligning to the
genome and transcriptome. The alignment file was used for assembling transcripts, estimating their abundances and detecting differential expression of genes. Differentially expressed gene
were determined based on counts from unique and multiple alignments using EdgeR within R version 3.2.2 (R development Core Team) using BIOCONDUCTOR version 3.0. The RT (Read Count) data were
processed based on Quantile normalization method using the Genowiz™ version 4.0.5.6 (Ocimum Biosolutions). IMMUNOBLOTTING For immunoblot analysis, cells were lysed on ice in RIPA lysis
buffer supplemented with protease and phosphatase inhibitors (Roche). Cell lysates were centrifuged for 10 min at 4 °C, 16,000 × _g_. Protein concentrations of the supernatants were
quantitated using the detergent-insensitive Pierce BCA protein assay kit (Thermo Scientific, 23227). Aliquots of each protein lysate (10–20 μg) were subjected to SDS polyacrylamide gel
electrophoresis. After electrophoresis, proteins were transferred to nitrocellulose membranes and blocked for 30 min with 5% skim milk in TBST (0.1% Tween 20 in TBS). For phosphorylated
protein detection, membranes were blocked with 2% BSA in TBS. Primary antibodies were incubated overnight at 4 °C. After washes, membranes were incubated with anti-rabbit (CST, 7074) or
anti-mouse (CST, 7076) secondary peroxidase coupled antibody for 1 h at RT. Target proteins were detected using ECL western blot detection solution (Millipore, WBKLS0500). The uncropped and
unprocessed scans with marker positions of all blots were included in the Source data file. IMMUNOPRECIPITATION Cells were lysed in NETN lysis buffer (20 mM Tris-HCl pH 7.4, 100 mM NaCl, 1
mM EDTA, 0.5% Nonidet P-40) with protease inhibitors (Roche). Antibody was added to the cleared lysate and incubated overnight. Then, 30 μl of protein A/G agarose (Pierce) was added to the
lysate, incubated for 2 h, washed with NETN buffer three times, and boiled in Laemmli’s sample buffer. The samples were then subjected to SDS-PAGE gels for western blot analysis. IN SITU
PROXIMITY LIGATION ASSAY Cells cultured on confocal dishes were fixed with 4% PFA for 20 min at room temperature, permeabilized and incubated with primary antibodies at 4 °C. All primary
antibodies were used at a 1:200 dilution. For in situ proximity ligation assay, protein–protein interactions between CD36 (Thermo) and Integrin β1 (abcam) were detected with secondary
proximity probes (Anti-Rabbit Plus and Anti-Mouse Minus) according to the Duolink In Situ Fluorescence protocol (Sigma-Aldrich). STABLE EXPRESSION OF FATP3 IN HUVECS The cDNA sequence of
human FATP3 was cloned into FUtdTW vector (addgene) containing td-Tomato sequence. Sequencing confirmed that no errors were introduced. Lentivirus production was performed with Lenti-X cells
co-transfected with FUtdTW-FATP3, Delta 8.2 and Ampho plasmids using Lipofectamine LTX (Invitrogen). At day 3 after transfection, culture supernatants were collected and centrifuged at 450
× _g_ for 10 min to remove cell debris. Supernatants were filtered with 0.22 μm syringe filter and concentrated by using Centricon filters (30 kDa cutoff, Amicon). Lentiviral particles were
transduced into HUVECs with hexadimethrine bromide for 20 h. Two days after lentiviral transduction, HUVECs were stably expressed, giving rise to a cell population with FATP3 expression.
Cells were used immediately for experiments. Live cell imaging was performed using the incubator chamber equipped with optimal environment settings of 5% CO2 and 37 °C, live cell imaging was
performed and recorded for 30 min by microscope (Cell Observer, Carl Zeiss). SAMPLING OF HUMAN ADIPOSE TISSUES Human SATs were collected from female patients (ages 39–56) undergoing breast
reconstruction after mastectomy for breast cancer. The Institutional Review Board of Seoul National University Hospital (1708-043-876) approved experimental procedures with human adipose
tissue specimens. All human samples were collected in an unbiased manner by the tissue bank of Seoul National University Hospital, Seoul, Korea, with the informed consents from the donors
following the bioethics and safety regulations. COMPARISON OF NONDIABETIC OBESE AND DIABETIC-OBESE PATIENTS For the analysis of differentially expressed genes in NDO versus DO, each gene
expression data were collected from NCBI-GEO (GSE20950, GSE29226, GSE29231, GSE16415, GSE71416), a publically available database repository of high-throughput gene expression data. Collected
datasets were annotated with official gene symbols, and normalized (log2). Differential expressed genes were analyzed separately in each gene sets. Among each datasets, common genes that
were specifically upregulated in SAT (fold change > 1.5, _p_ < 0.05) were narrowed down with genes that were not upregulated in VAT (<1.5). Datasets including GEO reference number,
sample number, organ, normalized Angpt2 expression, and status of patient (diabetic, obese) are indicated in Supplementary Table 2. Gene classification analysis was performed with Ingenuity
Pathway Analysis (IPA, Qiagen) and indicated in Supplementary Table 1. For analysis of Angpt2 mRNA expression in human SAT, indicated gene sets was annotated and each value for Angpt2
expression was normalized (log2). Relative expression of Angpt2 in human SVF was compared between human adipocytes (GSE80654), and adipocytes from NDO individuals (BMI 55 ± 8) were compared
with lean individuals (GSE2508). Relative expression of ITGα5 in NDO SAT was compared with lean individuals (GSE55200), and SAT from NDO individuals (obese, nondiabetic) were compared with
VAT (GSE20950). BIOCHEMICAL ANALYSIS OF SERUM A 0.5 ml blood sample harvested in Vacutainer tubes (BD) were centrifuged for 20 min at 2000 × _g_ t 4 °C twice. The plasma activity
triglyceride (TG) was measured using an automated analyzer (VetScan, Abaxis, CA, USA). To measure circulating Angpt2, mouse/rat Ang2 ELISA kit was used (R&D Systems) according to the
manufacturer’s instruction and measured using a Spectra MAX340 plate reader (Molecular Devices). To measure plasma level of FFA, mouse/rat/human FFA kit was used (abcam) according to the
manufacturer’s instruction using a Spectra MAX340 plate reader (Molecular Devices). INTRAPERITONEAL GLUCOSE/INSULIN TOLERANCE TEST For intraperitoneal glucose tolerance test, mice fasted for
16 h were injected with D-glucose (2 g/kg) (Sigma-Aldrich) intraperitoneally. For intraperitoneal insulin tolerance test, mice fasted for 4 h were injected with insulin (1 U/kg)
(Sigma-Aldrich) intraperitoneally. For analysis of blood glucose concentrations, blood was collected from the tail vein at 0, 15, 30, 60, 90, and 120 min after insulin administration, and
glucose was measured with a glucometer (Gluco Dr. Plus, All Medicus). STATISTICS AND REPRODUCIBILITY Sample sizes were chosen on the basis of standard power calculations (with _α_ = 0.05 and
power of 0.8) performed for similar experiments and statistical methods were not used to predetermine sample size. No samples were excluded from the analysis. Unless otherwise indicated,
experiments were replicated at least once for all analyses and number of reproductions of each experimental finding is described in each figure legend. All attempts at experimental
replication were successful. Animals from different cages, but within the same experimental group, were selected to assure randomization. Experiments involving in vitro and in vitro study
was assured randomization through double-blind experiments. The investigators were not blinded during experiments involving long term HFD challenge due to clear appearance of body mass
changes among the groups. However, two independent investigators have performed most of experiments in parallel and administration of chemicals was carried out as blinded experiments. Data
are presented as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). Statistical differences between the means were compared by the two-tailed, unpaired _t_ test for
two groups, or determined using one-way ANOVA followed by Tukey’s multiple comparison test for multiple groups, unless otherwise noted. Statistical analysis was performed with PASW
Statistics 18 (SPSS) or Prism 7 (GraphPad). Statistical significance was set to _P_ value < 0.05. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY The RNA-seq data are available in the European Bioinformatics Institute (EMBL-EMI’s) ArrayExpress under the accession
number E-MTAB-6161. Other transcriptomic datasets analyzed in this study can be retrieved from the GEO repository under the accessions GSE20950, GSE29226, GSE29231, GSE16415, GSE71416 for
the comparison of NDO vs. DO, GSE80654 for human SVF and adipocytes, GSE2508 for NDO vs. lean human adipocytes, GSE55200 for NDO vs. lean human SAT, and GSE20950 for NDO human SAT vs. VAT
datasets. The source data underlying all Figs. and Supplementary Figs. are provided as a Source Data file. A reporting summary for this article is available as a Supplementary Information
file. All other data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Stefan, N., Häring, H.-U., Hu, F. B. &
Schulze, M. B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. _Lancet Diabetes Endocrinol._ 1, 152–162 (2013). Article PubMed Google Scholar * Karpe,
F. & Pinnick, K. E. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. _Nat. Rev. Endocrinol._ 11, 90–100 (2015). Article CAS PubMed Google Scholar *
Stefan, N., Schick, F. & Haring, H. U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. _Cell Metab._ 26, 292–300 (2017). Article CAS
PubMed Google Scholar * Despres, J. P. Body fat distribution and risk of cardiovascular disease: an update. _Circulation_ 126, 1301–1313 (2012). Article PubMed Google Scholar *
Spalding, K. L. et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. _Nat. Commun._ 8, 15253 (2017). Article ADS CAS PubMed PubMed Central Google
Scholar * Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. _N. Engl. J. Med_ 371, 1131–1141 (2014). Article PubMed CAS Google Scholar *
Schweiger, M. et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. _Nat. Commun._ 8, 14859 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar * Hwangbo, C. et al. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects.
_Sci. Transl. Med_. 9, https://doi.org/10.1126/scitranslmed.aad4000 (2017). * Jang, C. et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin
resistance. _Nat. Med._ 22, 421–426 (2016). Article CAS PubMed PubMed Central Google Scholar * Hagberg, C. E. et al. Vascular endothelial growth factor B controls endothelial fatty acid
uptake. _Nature_ 464, 917–921 (2010). Article ADS CAS PubMed Google Scholar * Hagberg, C. E. et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes.
_Nature_ 490, 426–430 (2012). Article ADS CAS PubMed Google Scholar * Mehrotra, D., Wu, J., Papangeli, I. & Chun, H. J. Endothelium as a gatekeeper of fatty acid transport. _Trends
Endocrinol. Metab._ 25, 99–106 (2014). Article CAS PubMed Google Scholar * Augustin, H. G. & Koh, G. Y. Organotypic vasculature: From descriptive heterogeneity to functional
pathophysiology. _Science_ 357, https://doi.org/10.1126/science.aal2379 (2017). * Kivela, R. et al. VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in
the heart. _EMBO Mol. Med_ 6, 307–321 (2014). Article PubMed PubMed Central CAS Google Scholar * Robciuc, M. R. et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts
obesity and related metabolic complications. _Cell Metab._ 23, 712–724 (2016). Article CAS PubMed PubMed Central Google Scholar * Son, N. H. et al. Endothelial cell CD36 optimizes
tissue fatty acid uptake. _J. Clin. Investig._ https://doi.org/10.1172/JCI99315 (2018). * Jabs, M. et al. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to
metabolic and vascular remodeling of the adult heart. _Circulation_ 137, 2592–2608 (2018). Article CAS PubMed Google Scholar * Beeson, C. C. et al. Integrin-dependent Akt1 activation
regulates PGC-1 expression and fatty acid oxidation. _J. Vasc. Res._ 49, 89–100 (2012). Article CAS PubMed PubMed Central Google Scholar * Ata, R. & Antonescu, C. N. Integrins and
cell metabolism: an intimate relationship impacting cancer. _Int. J. Mol. Sci._ 18, https://doi.org/10.3390/ijms18010189 (2017). * Khalifeh-Soltani, A. et al. Mfge8 promotes obesity by
mediating the uptake of dietary fats and serum fatty acids. _Nat. Med._ 20, 175–183 (2014). Article CAS PubMed PubMed Central Google Scholar * Felcht, M. et al. Angiopoietin-2
differentially regulates angiogenesis through TIE2 and integrin signaling. _J. Clin. Investig._ 122, 1991–2005 (2012). Article CAS PubMed PubMed Central Google Scholar * Clark, K. et
al. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. _J. Cell Sci._ 118, 291–300 (2005). Article CAS PubMed
Google Scholar * An, Y. A. et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. _Elife_ 6, https://doi.org/10.7554/eLife.24071 (2017).
* Xue, Y. et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. _Proc. Natl Acad. Sci. USA_ 105, 10167–10172
(2008). Article ADS CAS PubMed PubMed Central Google Scholar * Rupnick, M. A. et al. Adipose tissue mass can be regulated through the vasculature. _Proc. Natl Acad. Sci. USA_ 99,
10730–10735 (2002). Article ADS CAS PubMed PubMed Central Google Scholar * Brakenhielm, E. et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice.
_Circ. Res._ 94, 1579–1588 (2004). Article CAS PubMed Google Scholar * Sung, H. K. et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis.
_Cell Metab._ 17, 61–72 (2013). Article CAS PubMed Google Scholar * Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. _Proc. Natl Acad. Sci. USA_ 109,
5874–5879 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Shen, B. et al. Genetic dissection of tie pathway in mouse lymphatic maturation and valve development.
_Arterioscler. Thromb. Vasc. Biol._ 34, 1221–1230 (2014). Article CAS PubMed Google Scholar * Hagberg, C., Mehlem, A., Falkevall, A., Muhl, L. & Eriksson, U. Endothelial fatty acid
transport: role of vascular endothelial growth factor B. _Physiology_ 28, 125–134 (2013). Article CAS PubMed PubMed Central Google Scholar * Sanz, E. et al. Cell-type-specific isolation
of ribosome-associated mRNA from complex tissues. _Proc. Natl Acad. Sci. USA_ 106, 13939–13944 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Jeong, H. W. et al.
Transcriptional regulation of endothelial cell behavior during sprouting angiogenesis. _Nat. Commun._ 8, 726 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Davidenko,
N. et al. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. _J. Mater. Sci. Mater. Med_ 27, 148 (2016). Article
PubMed PubMed Central CAS Google Scholar * Hakanpaa, L. et al. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. _Nat. Commun._ 6, 5962 (2015). Article ADS
CAS PubMed Google Scholar * Donate, F. et al. Pharmacology of the novel antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2): observation of a U-shaped dose-response curve in several preclinical
models of angiogenesis and tumor growth. _Clin. Cancer Res_. 14, 2137–2144 (2008). Article CAS PubMed Google Scholar * Albarran-Juarez, J. et al. Piezo1 and Gq/G11 promote endothelial
inflammation depending on flow pattern and integrin activation. _J. Exp. Med_. 215, 2655–2672 (2018). Article CAS PubMed PubMed Central Google Scholar * Kajimoto, K. et al. Isolation
and culture of microvascular endothelial cells from murine inguinal and epididymal adipose tissues. _J. Immunol. Methods_ 357, 43–50 (2010). Article CAS PubMed Google Scholar * Ojakian,
G. K. & Schwimmer, R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. _J. Cell Sci._ 107, 561–576 (1994). (Pt 3). CAS PubMed Google Scholar * Tran, T. T.
et al. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. _J. Biol. Chem._ 286, 25201–25210 (2011). Article CAS PubMed PubMed Central Google
Scholar * Bodin, S. et al. Integrin-dependent interaction of lipid rafts with the actin cytoskeleton in activated human platelets. _J. Cell Sci._ 118, 759–769 (2005). Article CAS PubMed
Google Scholar * Pitter, B., Werner, A. C. & Montanez, E. Parvins are required for endothelial cell-cell junctions and cell polarity during embryonic blood vessel formation.
_Arterioscler.Thromb. Vasc. Biol._ 38, 1147–1158 (2018). Article CAS PubMed Google Scholar * Strilic, B. et al. The molecular basis of vascular lumen formation in the developing mouse
aorta. _Dev. Cell_ 17, 505–515 (2009). Article CAS PubMed Google Scholar * Yamamoto, H. et al. Integrin beta1 controls VE-cadherin localization and blood vessel stability. _Nat. Commun._
6, 6429 (2015). Article ADS CAS PubMed Google Scholar * Jang, C. et al. Angiopoietin-2 exocytosis is stimulated by sphingosine-1-phosphate in human blood and lymphatic endothelial
cells. _Arterioscler Thromb. Vasc. Biol._ 29, 401–407 (2009). Article CAS PubMed Google Scholar * Fiedler, U. et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released
upon stimulation from endothelial cell Weibel–Palade bodies. _Blood_ 103, 4150–4156 (2004). Article CAS PubMed Google Scholar * Lee, J. L. & Streuli, C. H. Integrins and epithelial
cell polarity. _J. Cell Sci._ 127, 3217–3225 (2014). Article CAS PubMed PubMed Central Google Scholar * Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis
during white adipose tissue development, expansion and regeneration. _Nat. Med._ 19, 1338–1344 (2013). Article PubMed PubMed Central CAS Google Scholar * Jeffery, E., Church, C. D.,
Holtrup, B., Colman, L. & Rodeheffer, M. S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. _Nat. Cell Biol._ 17, 376–385 (2015). Article CAS
PubMed PubMed Central Google Scholar * Chau, Y. Y. et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. _Nat. Cell Biol._ 16, 367–375
(2014). Article CAS PubMed PubMed Central Google Scholar * Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. _Nat. Rev. Mol. Cell Biol._ 20, 242–258 (2019). Article
CAS PubMed Google Scholar * Jeffery, E. et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. _Cell Metab._ 24, 142–150 (2016). Article CAS
PubMed PubMed Central Google Scholar * Kuo, A., Lee, M. Y. & Sessa, W. C. Lipid droplet biogenesis and function in the endothelium. _Circ. Res._ 120, 1289–1297 (2017). Article CAS
PubMed PubMed Central Google Scholar * He, C. et al. NanoSIMS analysis of intravascular lipolysis and lipid movement across capillaries and into cardiomyocytes. _Cell Metab._ 27,
1055–1066.e3 (2018). Article CAS PubMed PubMed Central Google Scholar * Kim, M. et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. _J. Clin. Investig._
126, 3511–3525 (2016). Article PubMed PubMed Central Google Scholar * Korhonen, E. A. et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. _J. Clin.
Investig._ 126, 3495–3510 (2016). Article PubMed PubMed Central Google Scholar * Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and
only the latter role is rescued by Angiopoietin-1. _Dev. Cell_ 3, 411–423 (2002). Article CAS PubMed Google Scholar * Sassmann, A., Offermanns, S. & Wettschureck, N.
Tamoxifen-inducible Cre-mediated recombination in adipocytes. _Genesis_ 48, 618–625 (2010). Article CAS PubMed Google Scholar * Okabe, K. et al. Neurons limit angiogenesis by titrating
VEGF in retina. _Cell_ 159, 584–596 (2014). Article CAS PubMed Google Scholar * Lee, S. J. et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction.
_J. Clin. Investig._ 128, 5018–5033 (2018). Article PubMed PubMed Central Google Scholar * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time
quantitative PCR and the 2(-Delta Delta C(T)) Method. _Methods_ 25, 402–408 (2001). Article CAS PubMed Google Scholar * Noh, S. M., Shin, S. & Lee, G. M. Comprehensive
characterization of glutamine synthetase-mediated selection for the establishment of recombinant CHO cells producing monoclonal antibodies. _Sci. Rep._ 8, 5361 (2018). Article ADS PubMed
PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Intae Park for proof reading of paper, Sujin Seo, Hyun Tae Kim, Jeomil Bae, and Taechang Yang for their
technical assistances, Professor Yoshiaki Kubota (Keio University) for providing _VE-Cadherin-_Cre-ERT2 mice, and Dr. Nicholas Gale (Regeneron Pharmaceuticals) for providing _Angpt2_-_LacZ_
reporter knock-in mice. H.B. is supported by NRF (National Research Foundation of Korea) Grant funded by the Korean Government (NRF-2014-Fostering Core Leaders of the Future Basic Science
Program/Global Ph.D. Fellowship Program). This study was supported by the Institute for Basic Science funded by the Ministry of Science, ICT and Future Planning, Korea (IBS-R025-D1, G.Y.K.).
AUTHOR INFORMATION Author notes * Cholsoon Jang Present address: Department of Biological Chemistry, University of California Irvine, 92697, Irvine, CA, US AUTHORS AND AFFILIATIONS * Center
for Vascular Research, Institute for Basic Science, Daejeon, 34141, Republic of Korea Hosung Bae, Choong-kun Lee, Seung-Jun Lee, Hyuek Jong Lee & Gou Young Koh * Department of Plastic
and Reconstructive Surgery, Dongguk University Ilsan Hospital, Goyang, 10326, Republic of Korea Ki Yong Hong * Graduate School of Medical Science and Engineering, Korea Advanced Institute of
Science and Technology (KAIST), Daejeon, 34141, Republic of Korea Choong-kun Lee & Gou Young Koh * Lewis Sigler Institute for Integrative Genomics and Department of Chemistry, Princeton
University, Washington Rd, Princeton, NJ, 08544, USA Cholsoon Jang * Graduate School of Nanoscience and Technology, Korea Advanced Institute of Science and Technology (KAIST), Daejeon,
34141, Republic of Korea Kibaek Choe * Department of Pharmacology, Max Planck Institute for Heart and Lung Research, 61231, Bad Nauheim, Germany Stefan Offermanns * Cyrus Tang Hematology
Center, Collaborative Innovation Center of Hematology, Soochow University, 215123, Suzhou, China Yulong He Authors * Hosung Bae View author publications You can also search for this author
inPubMed Google Scholar * Ki Yong Hong View author publications You can also search for this author inPubMed Google Scholar * Choong-kun Lee View author publications You can also search for
this author inPubMed Google Scholar * Cholsoon Jang View author publications You can also search for this author inPubMed Google Scholar * Seung-Jun Lee View author publications You can also
search for this author inPubMed Google Scholar * Kibaek Choe View author publications You can also search for this author inPubMed Google Scholar * Stefan Offermanns View author
publications You can also search for this author inPubMed Google Scholar * Yulong He View author publications You can also search for this author inPubMed Google Scholar * Hyuek Jong Lee
View author publications You can also search for this author inPubMed Google Scholar * Gou Young Koh View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS H.B. designed, organized, and performed the experiments, generated the figures, and wrote the paper. K.Y.H. provided human samples and critical comments on this study. C.L.,
S.L., and K.C. provided important idea and technical supports. C.J. and H.J.L. supervised and participated in paper preparation. S.O. provided the mice and critical comments on this study.
Y.H. and G.Y.K. designed, organized, supervised the project, and wrote the paper. CORRESPONDING AUTHORS Correspondence to Yulong He or Gou Young Koh. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Antonio Giordano, Tara Haas and the other, anonymous, reviewer(s)
for their contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bae, H., Hong, K.Y., Lee, Ck. _et
al._ Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance. _Nat Commun_ 11, 2980 (2020).
https://doi.org/10.1038/s41467-020-16795-4 Download citation * Received: 28 May 2019 * Accepted: 22 May 2020 * Published: 12 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16795-4 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Hse launches ‘falls from height’ inspection campaign - farmers weekly© FLPA/REX/Shutterstock Health and safety inspectors will be visiting farms across the country over the next month to en...
Reality tv producer discovers the truth about mair smyth(MUSIC SEGUE) [00:00:01] Bob: This week on The Perfect Scam. [00:00:04] The tragedy is, at the time I confronted her, I ...
New york wedding guide - the album - barnyard bacchanal -- new york magazine - nymagCharlotte Adams & Max Bertz _Grasmere Farm in Rhinebeck, N.Y._ October 1, 2011 Marketing manager Charlotte Adams and...
Cll flow cytometry test: what it's used for and how it worksA flow cytometry test is an important part of the diagnostic process for CLL. It can determine that you have CLL and not...
Vaccinia virus on the move | Nature Reviews Molecular Cell BiologyAccess through your institution Buy or subscribe The new reports show that actin-based motility only occurs close to the...
Latests News
Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistanceABSTRACT Proper storage of excessive dietary fat into subcutaneous adipose tissue (SAT) prevents ectopic lipid depositio...
Water of india~ii - the statesmanWater is essential for healthy and sustainable livelihood. Scarcity can disrupt social stability, economic prosperity an...
How do environments talk to genes?A report elucidates the widely recognized, but poorly understood, concept of gene-environment interaction, finding a mol...
Claim for demotion of tenancy and suspension of right to buy: form n6Form CLAIM FOR DEMOTION OF TENANCY AND SUSPENSION OF RIGHT TO BUY: FORM N6 Use this form to claim for demotion of tenanc...
Connecting sympathetic and renin–angiotensin system overdrive in neurogenic hypertension through mirna-181aHypertension is a primary risk factor for cardiovascular and renal impairments that increases the likelihood of heart di...