Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes
Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Highly regio- and enantioselective intermolecular hydroamination of alkenes is a challenging process potentially leading to valuable chiral amines. Hydroamination of alkenes via
metal-catalyzed hydrogen atom transfer (HAT) with good regioselectivity and functional group tolerance has been reported, however, high enantioselectivity has not been achieved due to the
lack of suitable ligands. Here we report a ligand-promoted cobalt-catalyzed Markovnikov-type selective radical hydroamination of alkenes with diazo compounds. This operationally simple
protocol uses unsymmetric _NNN-_tridentate (UNT) ligand, readily available alkenes and hydrosilanes to construct hydrazones with good functional group tolerance. The hydrazones can undergo
nitrogen–nitrogen bond cleavage smoothly to deliver valuable amine derivatives. Additionally, asymmetric intermolecular hydroamination of unactivated aliphatic terminal alkenes using chiral
_N_-imidazolinylphenyl 8-aminoquinoline (IPAQ) ligands has also been achieved to afford chiral amine derivatives with good enantioselectivities. SIMILAR CONTENT BEING VIEWED BY OTHERS
NICKEL-CATALYSED ENANTIOSELECTIVE ALKENE DICARBOFUNCTIONALIZATION ENABLED BY PHOTOCHEMICAL ALIPHATIC C–H BOND ACTIVATION Article Open access 29 April 2024 ENANTIOSELECTIVE ALKENE
HYDROALKYLATION OVERCOMING HETEROATOM CONSTRAINTS VIA COBALT CATALYSIS Article 10 July 2024 REGIO‐ AND ENANTIOSELECTIVE NICKEL-ALKYL CATALYZED HYDROALKYLATION OF ALKYNES Article Open access
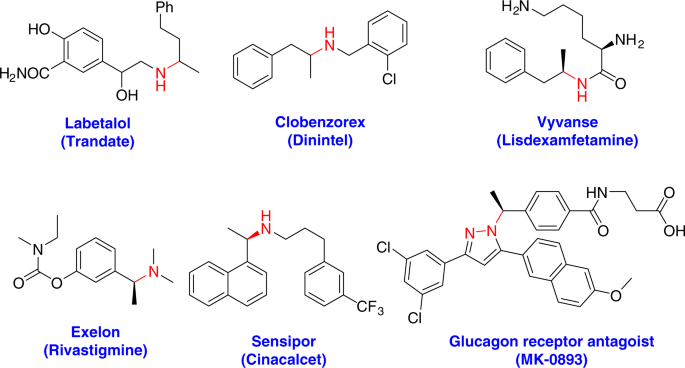
02 August 2024 INTRODUCTION Amine and its derivatives are significant in natural products and pharmaceutical chemistry (Fig. 1)1,2,3, (https://www.pharmacy-tech-test.com/top-200-drugs.html).
So development of novel methodologies for the synthesis of various amines and their derivatives is highly desirable, particularly from simple and readily available starting materials.
Metal-catalyzed hydrofunctionalization of readily available alkenes with nitrogen sources is one of the most efficient methods for the synthesis of nitrogen-containing molecules; however, to
achieve the high regio- and enantioselectivities of this transformation is still a challenge (Fig. 2a)4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19. Among several activation strategies for
alkene hydroamination, metal-catalyzed hydrogen atom transfer (HAT) reaction exhibits great Markovnikov selectivity and chemoselectivity (Fig. 2b)20. Okamoto and coworkers reported an early
example of cobalt-catalyzed hydronitrosation of styrenes with nitric oxide using a hydroborane salt as a hydrogen source to form oximes21. Since Mukaiyama and coworkers22 reported the
iron-catalyzed hydroamination of unactivated alkenes via HAT, using phenyl silane as a reductant and butyl nitrite as an aminating reagent, various aminating reagents, such as azo
compounds23,24,25,26, nitro compounds27,28, diazo compounds29, azides24,30, and amides31,32 have been explored by many research groups, which offers a great opportunity for retrosynthetic
possibility of new transformations. Although simple 1,3-dicarbonyl metal complexes could promote the reactions, stoichiometric amount or high loading of these complexes used to be
necessary20,33. Additionally, due to the generation of radical intermediates, asymmetric intermolecular hydroamination of alkenes via radical HAT process has not been explored. So the
development of new types of ligands for efficient radical hydroamination of alkenes via a HAT process is still highly desirable. It should be noted that the additional ligands used to
promote the formation of metal hydride species that could undergo classic alkene insertion34,35. Due to the inhibition of generation of the alkyl radical, the reactivity and selectivity of
transformation might decrease dramatically. Otherwise, the tetra- or more dentate ligand-based metal complexes could promote the generation of radical; however, no highly enantioselective
examples have been reported so far due to the weak coordination of alkyl radical with metal complexes. So the discovery of suitable ligand scaffolds for metal-catalyzed radical
hydroamination of alkenes is a challenge and also has great potential (Fig. 2c). Continuing our pursuit of efficient earth-abundant transition metal catalysis via ligand
design36,37,38,39,40,41,42,43,44,45; here, we report the use of unsymmetric _NNN_ tridentate ligands to promote the cobalt-catalyzed radical hydroamination of alkenes via HAT (Fig. 2d).
Meanwhile, asymmetric reaction of unactivated aliphatic terminal alkenes using chiral _N_-imidazolinylphenyl 8-aminoquinoline (IPAQ) ligand has also been achieved to afford chiral amine
derivatives with good enantioselectivities. RESULTS REACTION OPTIMIZATION The reaction of styrene 1A with ethyl 2-diazo-2-phenylacetate 2A in the presence of phenyl silane as a hydrogen
donor in a solution of tetrahydrofura (THF) was selected as a model reaction. The reaction using Co(acac)2 afforded the hydroamination product 3A in a poor yield (entry 1, Table 1). The use
of Co(OAc)2 did not promote this transformation (entry 2). The known ligands for HAT, such as tpp, dmg, dppe, salen, and tridentate half salen L1 have been tested; however, no reactivity or
poor yields were observed (entry 3 and also see in Supplementary Table 1). _NN_-bidentate ligands such as 2,2′-bipyridine (bpy) and 1,10-phenanthroline (phen) could accelerate this reaction
to give 3A in 44 and 37% yields, respectively (entries 4 and 5). The reaction using _NNN_ tridentate _N_-oxazolinylphenyl picolinamide (OPPA) ligands42,46 L2 and L3 afforded 3A in 88 and 92%
yields, respectively (entries 6 and 7). Then, 8-aminoquinoline group was used as a directing group on the ligand instead of 2-picolinamide. The reaction using _N_-oxazolinylphenyl
8-aminoquinoline (OPAQ) L4 as a ligand delivered 3A in 96% yield (entry 8). So, the standard conditions were identified as 0.36 mmol of alkene 1, 0.3 mmol of _α_-diazo ester compound 2, 5
mol% of Co(OAc)2, 6 mol% of L4, and 1.2 equiv. of PhSiH3 in a solution of THF (0.25 M) at room temperature (r.t.) for 12 h. SUBSTRATE SCOPE With the optimized conditions in hand, the
substrate scope was shown in Table 2. A variety of styrenes bearing electron-donating or electron-withdrawing substituents at _para_-, _meta_-, or _ortho_-position on the phenyl ring
underwent the reactions to afford the corresponding benzylamine derivatives (3B–3T) in 50–95% yields. For more broad synthetic interests, various functional groups, such as halide, ether,
aniline, organoboronate, sulfide, nitrile, aldehyde, ester, and free alcohol were well tolerated. Meanwhile, 2-naphthyl (1U), pyridine derivated alkenes (1V and 1W) could be delivered to the
corresponding products in 71–92% yields. The reaction of alkyl-substituted terminal alkenes could undergo smoothly. Simple alkenes, such as 1-octene and 1-butene could be delivered to
aliphatic amines in 77 and 72% yields, respectively. Alkenes, bearing phenyl, ether, tosyl, free alcohol, amine, amide, as well as nitrogen containing heterocycles, could be converted to the
corresponding products in 49–84% yields. Notably, allylic alcohol and allylic amine derivatives could be reacted to deliver amino alcohol 3AE and diamine 3AF in 49 and 84% yields,
respectively. 1,1- and 1,2-disubstituted alkenes were suitable substrates and provided 3AJ–3AL in 79–86% yields. Natural products, such as amino acid, menthol, uracil, and estrone bearing a
terminal alkene moiety could be employed to deliver products (3AM–3AP) in 59–89% yields, which also demonstrated that this method could be suitable for late-stage functionalization of
complicated molecules. Terminal alkene bearing anti-inflammatory drug naproxen was converted to 3AQ in 61% yield. Configuration was confirmed by the X-ray diffraction of 3M, and the other
products were then assigned by analogy to 3M. FURTHER APPLICATIONS The gram-scale reaction could be smoothly performed to afford 3A in 94% yield (Fig. 3a). The reaction of 3M with
1,3-diketone afforded the pyrazole 4 in 86% yield (Fig. 3b). The three-step reactions containing alkene hydroamination, zinc-promoted reductive cleavage of _N_–_N_ bond, and acyl protection
of amine could be realized smoothly with once flash column chromatography purification process to deliver the corresponding amides 6 and 7 in 53–75% yields (Fig. 3c). The pharmaceutical
substances and biologically active amines 8, such as appetite-suppressant drug clobenzorex, antianginal drug fendiline, and anti-hyperparathyroidism active NPS _R_568, could be efficiently
synthesized in 32–45% yields from simple alkenes using a three-step synthetic protocol (Fig. 3c). SUBSTRATE SCOPE OF ASYMMETRIC HYDROAMINATION It should be noted that metal-catalyzed highly
enantioselective hydroamination of unactivated aliphatic terminal alkenes is still an unsolved problem47. A primary study on asymmetric reaction using chiral unsymmetric _NNN_-tridentate
ligand was explored. A chiral _N_-imidazolinylphenyl 8-aminoquinoline (IPAQ) ligand was designed and synthesized for asymmetric hydroamination of unactivated aliphatic terminal alkenes
followed by cleavage of _N_–_N_ bond and benzyl protection to afford chiral amine derivatives with up to 92.7 : 7.3 _er_ (Table 3)48. The scope of substrate was quite broad. Various
functional groups, such as halide, ether, and indole, could be tolerated. Alkene-bearing free alcohol moiety could underdo this transformation to afford the corresponding amide with a
decreased yield. Chiral products with up to 98.6 : 1.4 _er_ could be obtained after recrystallization. Particularly, the reaction of 1-butene afforded corresponding amide in 67% yield and
89.0 : 11.0 _er_ (97.3 : 2.7 _er_ after recrystallization). The chiral antihypertensive drug labetalol could be obtained from 14 via a known procedure49. MECHANISTIC STUDIES Control
experiments were conducted to illustrate the possible mechanism. The reaction in the presence of TEMPO did not occur, which indicated a possible radical pathway (Fig. 4a). The reaction of
vinyl cyclopropane 28 afforded the ring-opening product 29 in 62% yield (Fig. 4b) via cleavage of the more substituted carbon–carbon bond50, which supported the radical promoted ring-opening
pathway51. A deuterium-labeled experimental reaction of indene using PhSiD3 was conducted to afford 30 in 89% yield with 94% D and 1/1 _dr_ (Fig. 4c) which demonstrated that the hydrogen
came from hydrosilane and the carbon-centered radical formed as an intermediate. Based on the experimental studies and previously reported literatures24,27,52,53,54,55,56, a possible
mechanism was shown in Fig. 5. The cobalt hydride species A obtained from the reaction of Co(OAc)2 with ligand and silane could undergo a metal hydride HAT process to deliver the carbon
radical intermediate and cobalt species B. Due to the possible redox non-innocent property of the ligand, the chemical valence of cobalt was not consistent. The cobalt species B might
coordinate with diazo compound and undergo one electron oxidation with the carbon radical intermediate to afford the cobalt–carbon species C, which could undergo alkyl group migration from
cobalt to nitrogen atom to generate cobalt oxide species D. The possibility that carbon radical directly attacked the cobalt coordinated diazo compound could not be ruled out. The cobalt
species D could react with hydrosilane to regenerate the cobalt hydride species A and afford the vinyl silyl ether intermediate, which could undergo hydrolysis and isomerization to give the
product. Further studies are undergoing in our laboratory to gain an accurate understanding of the mechanism. DISCUSSION In summary, we reported the use of unsymmetric _NNN_-tridentate OPAQ
ligand to promote the cobalt-catalyzed radical hydroamination of alkenes via HAT. The protocol uses simple and commercially available alkenes to deliver the amination products with good
functional group tolerance and high Markovnikov selectivity. The hydrazone compounds could undergo nitrogen–nitrogen bond cleavage smoothly to afford a series of biologically active
molecules. In particularly, asymmetric reaction of unactivated aliphatic terminal alkenes using newly developed chiral IPAQ ligand has also been achieved to afford chiral hydroamination
products with good enantioselectivity. Further studies on asymmetric hydrofunctionalization of simple alkenes are undergoing in our laboratory. METHODS MATERIALS For the optimization of
reaction conditions and control experiments of alkene 1a (Supplementary Table 1), and for the experimental procedures and analytic data of compounds synthesized (Supplementary Methods). For
nuclear magnetic resonance (NMR) spectra of compounds in this manuscript (Supplementary Fig. 1–166). For high-performance liquid chromatography (HPLC) spectra of compounds in this manuscript
(Supplementary Fig. 167–185). GENERAL PROCEDURE A FOR HYDROAMINATION OF ALKENES A 25 mL Schlenk flask equipped with a magnetic stirrer and a flanging rubber plug was dried with flame under
vacuum. When cooled to ambient temperature, it was vacuumed and flushed with N2 and repeated for three times. To the flask, Co(OAc)2 (0.015 mmol), L3 or L4 (0.018 mol), and THF (1.2 mL) were
added. The flask was degassed and stirred for 30 min at r.t. Then, PhSiH3 (0.36 mol), diazo compound (0.3 mmol), and alkene (0.36 mmol) were added in sequence. After 12 h, the reaction was
quenched with 10 ml of petroleum ether (PE) and the mixture was filtered through a pad of silica gel and washed with PE/EtOAc (5/1, 50 mL). The combined filtrates were concentrated and
purified by flash column chromatography using PE/EtOAc as the eluent to afford the corresponding product. GENERAL PROCEDURE B FOR ASYMMETRIC HYDROAMINATION OF ALKENES A 25 mL Schlenk flask
equipped with a magnetic stirrer and a flanging rubber plug was dried with flame under vacuum. When cooled to ambient temperature, it was vacuumed and flushed with N2 and repeated for three
times. To the flask, Co(OAc)2 (0.015 mmol), IPAQ (0.018 mol), ethyl acetate (1.2 mL), and 2-ethylethanol (100 μL, 0.93 g/mL, 0.9 mmol) were added. The flask was degassed and cooled down to
−10 °C and stirred for 30 min. Then, PhSiH3 (0.36 mol), diazo compound (0.3 mmol), and alkene (0.36 mmol) were added in sequence. After 24 h, the reaction was warmed up to r.t. and quenched
with 10 ml of PE. The mixture was filtered through a pad of silica gel and washed with PE/EtOAc (5/1, 50 mL). The combined filtrates were concentrated to afford a yellow oil that was used
for the next step without further purification. CLEAVAGE OF _N_–_N_ BOND To the above suspension, AcOH–THF–H2O (3:1:1 v/v/v, 3 mL) was added, followed by addition of activated Zn powder (0.5
g, 7.5 mmol) in several portions at r.t. After that, the mixed solution was warmed up to 60 °C and stirred until completion monitored by thin-layer chromatography (usually 3 h). Then, the
reaction mixture was cooled down to r.t. and quenched with water (20 mL). The reaction mixture was basified with a solution of NaOH (6 N) until the solution turned clear (pH > 10) and
then extracted with Et2O (20 mL × 4). The combined organic layers were dried over anhydrous Na2SO4, filtered, concentrated to give a yellow oil that was used for the next step without
further purification. PROTECTION OF FREE AMINES WITH BZ GROUP To the above oil, 3 mL (0.1 M) of THF, 55 μL (1.211 g/mL, 0.45 mmol) of BzCl, and 84 μL (0.728 g/mL, 0.6 mmol) of Et3N were
added, followed by 2 h stirring. The mixture was quenched with water (20 mL) and then extracted with Et2O (20 mL × 4). The combined organic layers were dried over anhydrous Na2SO4, filtered,
concentrated, and purified by flash column chromatography using PE/EtOAc as the eluent to give the corresponding amide. DATA AVAILABILITY The authors declare that the data supporting the
findings of this study are available within the paper and its Supplementary Information file. The X-ray crystallographic coordinates for structures of 3M has been deposited at the Cambridge
Crystallographic Data Centre (CCDC) under deposition numbers CCDC 194446. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via
http://www.ccdc.cam.ac.uk/data_request/cif. The experimental procedures and characterization of all new compounds are provided in Supplementary Information. REFERENCES * Funayama, S. &
Cordell, G. A. (eds) _Alkaloids: A Treasury of Poisons and Medicines_ (Waltham, MA, 2014). * Brown, B. R. _The Organic Chemistry of Aliphatic Nitrogen Compounds_. (Oxford University, New
York, 1994). Google Scholar * Nugent, T. C. _Chiral Amine Synthesis: Methods, Developments and Applications_ (Wiley-VCH, 2010). * Reznichenko, A. L. & Hultzsch, K. C. in _Organic
Reactions_, Vol. 88 (eds Denmark, S. E.) Ch 1 (John Wiley & Sons Inc., 2006). * Huang, L.-B., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed
hydroamination and hydroamidation. _Chem. Rev._ 115, 2596–2697 (2015). Article CAS PubMed Google Scholar * Leporl, C. & Hannedouche, J. First-row late transition metals for catalytic
(formal) hydroamination of unactivated alkenes. _Synthesis_ 49, 1158–1167 (2017). Google Scholar * Hannedouche, J. & Schulz, E. Hydroamination and hydroaminoalkylation of alkenes by
group 3−5 elements: recent developments and comparison with late transition metals. _Organometallics_ 37, 4313–4326 (2018). Article CAS Google Scholar * Pirnot, M. T., Wang, Y.-M. &
Buchwald, S. L. Copper hydride catalyzed hydroamination of alkenes and alkynes. _Angew. Chem. Int. Ed._ 55, 48–57 (2016). Article CAS Google Scholar * Zhang, Z.-B., Lee, S. D. &
Widenhoefer, R. A. Intermolecular hydroamination of ethylene and 1-alkenes with cyclic ureas catalyzed by achiral and chiral gold(i) complexes. _J. Am. Chem. Soc_. 131, 5372–5373 (2009). *
Zhu, S.-L., Niljianskul, N. & Buchwald, S. L. Enantio- and regioselective cuh-catalyzed hydroamination of alkenes. _J. Am. Chem. Soc._ 135, 15746–15749 (2013). Article CAS PubMed
Google Scholar * Sevov, C. S., Zhou, J.-R. & Hartwig, J. F. Iridium-catalyzed, intermolecular hydroamination of unactivated alkenes with indoles. _J. Am. Chem. Soc._ 136, 3200–3207
(2014). Article CAS PubMed Google Scholar * Yang, Y., Shi, S.-L., Niu, D.-W., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to
aliphatic amines. _Science_ 349, 62–66 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Nishikawa, D., Hirano, K. & Miura, M. Asymmetric synthesis of α-aminoboronic
acid derivatives by copper-catalyzed enantioselective hydroamination. _J. Am. Chem. Soc._ 137, 15620–15623 (2015). Article CAS PubMed Google Scholar * Yu., F., Chen, P. & Liu, G.-S.
Pd(II)-catalyzed intermolecular enantioselective hydroamination of styrenes. _Org. Chem. Front._ 2, 819–822 (2015). Article CAS Google Scholar * Gurak, J. A. Jr, Yang, K. S., Liu, Z.
& Engle, K. M. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. _J. Am. Chem. Soc._ 138, 5805–5808 (2016). Article CAS PubMed Google Scholar *
Nakafuku, K. M., Fosu, S. C. & Nagib, D. A. Catalytic alkene difunctionalization via imidate radicals. _J. Am. Chem. Soc._ 140, 11202–11205 (2018). Article CAS PubMed PubMed Central
Google Scholar * Vanable, E. P. et al. Rhodium-catalyzed asymmetric hydroamination of allyl amines. _J. Am. Chem. Soc._ 141, 739–742 (2019). Article CAS PubMed PubMed Central Google
Scholar * Bathamonde, A., Rifaie, B. A., Martín-Heras, V., Allen, J. R. & Sigman, M. S. Enantioselective Markovnikov addition of carbamates to allylic alcohols for the construction of
α-secondary and α-tertiary amines. _J. Am. Chem. Soc._ 141, 8708–8711 (2019). Article CAS Google Scholar * Li, H.-Z., Shen, S.-J., Zhu, C.-L. & Xu, H. Direct intermolecular
anti-Markovnikov hydroazidation of unactivated olefins. _J. Am. Chem. Soc._ 141, 9415–9421 (2019). Article PubMed CAS PubMed Central Google Scholar * Crossley, S. W. M., Obradors, C.,
Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and co-catalyzed radical hydrofunctionalizations of olefins. _Chem. Rev._ 116, 8912–9000 (2016). Article CAS PubMed PubMed Central Google
Scholar * Okamoto, T., Kobayashi, K., Oka, S. & Tanimoto, S. Cobalt-catalyzed reaction of nitric oxide with aryl-substituted olefins in the presence of tetrahydroborate ion. _J. Org.
Chem._ 52, 5089–5092 (1987). Article CAS Google Scholar * Kato, K. & Mukaiyama, T. Iron(III) complex catalyzed nitrosation of terminal and 1,2-disubstituted olefins with butyl nitrite
and phenylsilane. _Chem. Lett._ 21, 1137–1140 (1992). Article Google Scholar * Waser, J. & Carreira, E. M. Convenient synthesis of alkylhydrazides by the cobalt-catalyzed
hydrohydrazination reaction of olefins and azodicarboxylates. _J. Am. Chem. Soc._ 126, 5676–5677 (2004). Article CAS PubMed Google Scholar * Waser, J., Gaspar, B., Nambu, H. &
Carreira, E. M. Hydrazines and azides via the metal-catalyzed hydrohydrazination and hydroazidation of olefins. _J. Am. Chem. Soc._ 128, 11693–11712 (2006). Article CAS PubMed Google
Scholar * Waser, J. & Carreira, E. M. Catalytic hydrohydrazination of a wide range of alkenes with a simple Mn complex. _Angew. Chem. Int. Ed._ 43, 4099–4102 (2004). Article CAS
Google Scholar * Zhang, Y. et al. Modular synthesis of alkylarylazo compounds via iron(III)-catalyzed olefin hydroamination. _Org. Lett._ 21, 2261–2264 (2019). Article CAS PubMed Google
Scholar * Gui, J.-H. et al. Practical olefin hydroamination with nitroarenes. _Science_ 348, 886–891 (2015). Article ADS CAS PubMed Google Scholar * Zhu, K.-L., Shaver, M. P. &
Thomas, S. P. Amine-bis(phenolate) iron(III)-catalyzed formal hydroamination of olefins. _Chem. Asian J._ 11, 977–980 (2016). Article CAS PubMed Google Scholar * Zheng, J., Qi, J.-F.
& Cui, S.-L. Fe-catalyzed olefin hydroamination with diazo compounds for hydrazone synthesis. _Org. Lett._ 18, 128–131 (2016). Article CAS PubMed Google Scholar * Waser, J., Nambu,
H. & Carreira, E. M. Cobalt-catalyzed hydroazidation of olefins: convenient access to alkyl azides. _J. Am. Chem. Soc._ 127, 8294–8295 (2005). Article CAS PubMed Google Scholar *
Shigehisa, H. et al. Catalytic hydroamination of unactivated olefins using a co catalyst for complex molecule synthesis. _J. Am. Chem. Soc._ 136, 13534–13537 (2014). Article CAS PubMed
Google Scholar * Zhou, X.-L. et al. Cobalt-catalyzed intermolecular hydrofunctionalization of alkenes: evidence for a bimetallic pathway. _J. Am. Chem. Soc._ 141, 7250–7255 (2019). Article
CAS PubMed Google Scholar * Ishikawa, H. et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. _J. Am. Chem. Soc._ 131, 4904–4916
(2009). Article CAS PubMed PubMed Central Google Scholar * Chen, J.-H., Guo, J. & Lu, Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition
metal catalysis. _Chin. J. Chem._ 36, 1075–1109 (2018). Article CAS Google Scholar * Ai, W.-Y., Zhong, R., Liu, X.-F. & Liu, Q. Hydride transfer reactions catalyzed by cobalt
complexes. _Chem. Rev._ 119, 2876–2953 (2019). Article CAS PubMed Google Scholar * Chen, J.-H., Cheng, B., Cao, M.-Y. & Lu, Z. Iron-catalyzed asymmetric hydrosilylation of
1,1-disubstituted alkenes. _Angew. Chem. Int. Ed._ 54, 4661–4664 (2015). Article CAS Google Scholar * Guo, J. & Lu, Z. Highly chemo-, regio-, and stereoselective cobalt-catalyzed
Markovnikov hydrosilylation of alkynes. _Angew. Chem. Int. Ed._ 55, 10835–10838 (2016). Article CAS Google Scholar * Guo, J., Shen, X.-Z. & Lu, Z. Regio- and enantioselective
cobalt-catalyzed sequential hydrosilylation/hydrogenation of terminal alkynes. _Angew. Chem. Int. Ed._ 56, 615–618 (2017). Article CAS Google Scholar * Cheng, B., Lu, P., Zhang, H.-Y.,
Cheng, X.-P. & Lu, Z. Highly enantioselective cobalt-catalyzed hydrosilylation of alkenes. _J. Am. Chem. Soc._ 139, 9439–9442 (2017). Article CAS PubMed Google Scholar * Guo, J.,
Cheng, B., Shen, X.-Z. & Lu, Z. Cobalt-catalyzed asymmetric sequential hydroboration/hydrogenation of internal alkynes. _J. Am. Chem. Soc._ 139, 15316–15319 (2017). Article CAS PubMed
Google Scholar * Cheng, B., Liu, W.-B. & Lu, Z. Iron-catalyzed highly enantioselective hydrosilylation of unactivated terminal alkenes. _J. Am. Chem. Soc._ 140, 5014–5017 (2018).
Article CAS PubMed Google Scholar * Chen, X., Cheng, Z.-Y., Guo, J. & Lu, Z. Asymmetric remote C-H borylation of internal alkenes via alkene isomerization. _Nat. Commun._ 9, 3939
(2018). Article ADS PubMed PubMed Central CAS Google Scholar * Cheng, Z.-Y. et al. Highly regioselective sequential 1,1-dihydrosilylation of terminal aliphatic alkynes with primary
silanes. _Chin. J. Chem._ 37, 457–461 (2019). Article CAS Google Scholar * Guo, J., Wang, H.-L., Xing, S.-P., Hong, X. & Lu, Z. Cobalt-catalyzed asymmetric synthesis of
gem-bis(silyl)alkanes by double hydrosilylation of aliphatic terminal alkynes. _Chem_ 5, 881–895 (2019). Article CAS Google Scholar * Cheng, X.-K., Lu, H.-Z. & Lu, Z. Enantioselective
benzylic C–H arylation via photoredox and nickel dual catalysis. _Nat. Commun._ 10, 3549 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Chen, X., Cheng, Z.-Y. &
Lu, Z. Iron-catalyzed, Markovnikov-selective hydroboration of styrenes. _Org. Lett._ 19, 969–971 (2017). Article CAS PubMed Google Scholar * Coombs, J. R. & Morken, J. P. Catalytic
enantioselective functionalization of unactivated terminal alkenes. _Angew. Chem. Int. Ed._ 55, 2636–2649 (2016). Article CAS Google Scholar * Das, S. et al. Asymmetric catalysis of the
carbonyl-amine condensation: kinetic resolution of primary amines. _J. Am. Chem. Soc._ 139, 1357–1359 (2017). Article CAS PubMed Google Scholar * Sanfilippo, C., Paternò, A. A. &
Patti, A. Resolution of racemic ̀amines via lipase-catalyzed benzoylation: chemoenzymatic synthesis of the pharmacologically active isomers of labetalol. _Mol. Catal._ 449, 79–84 (2018).
Article CAS Google Scholar * O’Reilly, M. E., Dutta, S. & Veige, A. S. β-Alkyl elimination: fundamental principles and some applications. _Chem. Rev._ 116, 8105–8145 (2016). Article
PubMed CAS Google Scholar * Chen, C.-H., Shen, X.-Z., Chen, J.-H., Hong, X. & Lu, Z. Iron-catalyzed hydroboration of vinylcyclopropanes. _Org. Lett._ 19, 5422–5425 (2017). Article
CAS PubMed Google Scholar * Green, S. A. et al. The high chemofidelity of metal-catalyzed hydrogen atom transfer. _Acc. Chem. Res._ 51, 2628–2640 (2018). Article CAS PubMed PubMed
Central Google Scholar * Li, W. et al. New electrophilic addition of α-diazoesters with ketones for enantioselective C–N bond formation. _J. Am. Chem. Soc._ 133, 15268–15271 (2011).
Article CAS PubMed Google Scholar * Discolo, C. A., Touney, E. E. & Pronin, S. V. Catalytic asymmetric radical−polar crossover hydroalkoxylation. _J. Am. Chem. Soc._ 141, 17527–17532
(2019). Article CAS PubMed Google Scholar * Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. _Nat. Chem._ 11, 1158–1166 (2019). Article CAS
PubMed Google Scholar * Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. _Acc. Chem. Res_. https://doi.org/10.1021/acs.accounts.9b00381
(2019). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS Financial support was provided by NSFC (21922107 and 21772171), Zhejiang Provincial Natural Science
Foundation of China (LR19B020001), Center of Chemistry for Frontier Technologies, and the Fundamental Research Funds for the Central Universities (2019QNA3008). AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Department of Chemistry, Zhejiang University, Hangzhou, 310058, China Xuzhong Shen, Xu Chen, Jieping Chen, Yufeng Sun, Zhaoyang Cheng & Zhan Lu Authors * Xuzhong Shen
View author publications You can also search for this author inPubMed Google Scholar * Xu Chen View author publications You can also search for this author inPubMed Google Scholar * Jieping
Chen View author publications You can also search for this author inPubMed Google Scholar * Yufeng Sun View author publications You can also search for this author inPubMed Google Scholar *
Zhaoyang Cheng View author publications You can also search for this author inPubMed Google Scholar * Zhan Lu View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Z. L. designed the experiments. X. S., J. C. and Y. S. performed the experiments. Z. L. and X. C. designed the racemic ligands. Z. L. and Z. C. designed the chiral
ligands. Z. L. and X. S. prepared this manuscript. X. S., J. C., Y. S. and Z. L. prepared the supplementary information. CORRESPONDING AUTHOR Correspondence to Zhan Lu. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s) for their contribution
to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shen, X., Chen, X., Chen, J. _et al._ Ligand-promoted cobalt-catalyzed radical
hydroamination of alkenes. _Nat Commun_ 11, 783 (2020). https://doi.org/10.1038/s41467-020-14459-x Download citation * Received: 28 October 2019 * Accepted: 10 January 2020 * Published: 07
February 2020 * DOI: https://doi.org/10.1038/s41467-020-14459-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Farms can claim £20,000 each for flood repairs, defra says - farmers weekly© FLPA/REX Shutterstock Flood-hit farmers in north-west England will be able to claim up to £20,000 to help restore dama...
Caregiver tips: organize for doctor appointmentsTo help get everything organized for a doctor’s appointment, color code important documents and then place them in an en...
Channelnews : microsoft takes on apple, google with mobile games app storeMicrosoft is planning to launch a new app store for games on both iPhones and Android smartphones – as long as its A$100...
Treatment privacy laws protect me and 43 million other americans living with addiction or in recoveryFive years ago, I was in the darkest grip of substance use disorder. Constant use of opioids and other drugs had led to ...
Va puget sound health care system annual report | va puget sound health care | veterans affairsPRESS RELEASE May 27, 2021 Seattle , WA — The Annual Report is a look back at the accomplishments of the previous year a...
Latests News
Ligand-promoted cobalt-catalyzed radical hydroamination of alkenesABSTRACT Highly regio- and enantioselective intermolecular hydroamination of alkenes is a challenging process potentiall...
Elon Musk's team now has access to US Treasury's payments systemNewsletters ePaper Sign in HomeIndiaKarnatakaOpinionWorldBusinessSportsVideoEntertainmentDH SpecialsOperation SindoorNew...
Is a rift brewing between producers of ‘stree’ over pending dues?One half of the producers of _Stree_, Raj Nidimoru and Krishna DK—aka Raj & DK—have been sidelined by Dinesh Vijan, ...
Un seeks record $4. 4 billion in aid for afghanistan’s futureUN seeks record $4.4 billion in aid for Afghanistan’s future | WTVB | 1590 AM · 95.5 FM | The Voice of Branch County Clo...
Javascript support required...