Stability of the adeno-associated virus 8 reference standard material
Stability of the adeno-associated virus 8 reference standard material"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Adeno-associated virus (AAV) vectors are extensively used for gene therapy clinical trials. Accurate and standardized titration methods are essential for characterizing and dosing
AAV-based drugs and thus to assess their safety and efficacy. To this end, the Reference Standard Materials (RSM) working group generated standards for AAV serotype 2 and serotype 8. The
AAV8RSM (ATCC® VR-1816™) was deposited to the American Type Culture Collection in 2014 and is available to the scientific community. Here, three independent laboratories of the RSM working
group provide stability data of the AAV8RSM 2 years after the initial characterization and after container relabeling performed at the ATCC. The AAV8RSM showed constant titers across
experimental conditions: 1.48 ± 0.62 × 1012 vector genome (vg)/ml, 9.38 ± 11.4 × 108 infectious units (IU)/ml and 5.76 ± 2.39 × 1011 total particles (p)/ml as determined by qPCR, TCID50 and
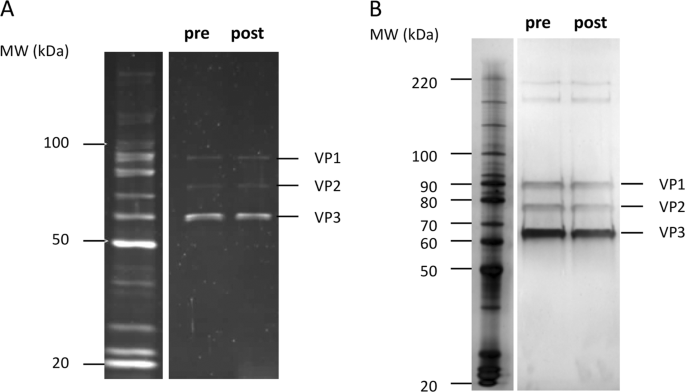
ELISA, respectively. Additionally, the AAV8RSM capsid protein integrity assessed by SDS-PAGE was equivalent to the original analyses. In conclusion, the AAV8RSM titers remained stable for
two years under appropriate storage conditions ( <−70° C). The use of RSM is strongly recommended and endorsed by regulatory agencies to normalize laboratory internal controls and to
provide accurate titration of AAV vectors lots. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 6 print issues and online access $259.00 per year only $43.17 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support REFERENCES * Aucoin MG, Perrier M, Kamen AA. Critical assessment of current adeno-associated viral vector production and
quantification methods. Biotechnol Adv. 2008;26:73–88. Article CAS Google Scholar * Clement N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials.
Mol Ther Methods & Clin Dev. 2016;3:16002. Article Google Scholar * Schnodt M, Buning H. Improving the quality of adeno-associated viral vector preparations: the challenge of
product-related impurities. Human gene Ther Methods. 2017;28:101–8. Article Google Scholar * Wilson JM. A call to arms for improved vector analytics! Human gene Ther Methods. 2015;26:1–2.
Article CAS Google Scholar * Lock M, McGorray S, Auricchio A, Ayuso E, Beecham EJ, Blouin-Tavel V, et al. Characterization of a recombinant adeno-associated virus type 2 Reference
Standard Material. Hum Gene Ther. 2010;21:1273–85. Article CAS Google Scholar * Ayuso E, Blouin V, Lock M, McGorray S, Leon X, Alvira MR, et al. Manufacturing and characterization of a
recombinant adeno-associated virus type 8 reference standard material. Hum Gene Ther. 2014;25:977–87. Article CAS Google Scholar * Moullier P, Snyder RO. Recombinant adeno-associated
viral vector reference standards. Methods Enzymol. 2012;507:297–311. Article CAS Google Scholar * Moullier P, Snyder RO. International efforts for recombinant adeno-associated viral
vector reference standards. Mol Ther. 2008;16:1185–8. Article CAS Google Scholar * Gavin DK. FDA statement regarding the use of adeno-associated virus reference standard materials. Human
gene Ther Methods. 2015;26:3. Article CAS Google Scholar * Lock M, Alvira MR, Chen SJ, Wilson JM. Absolute determination of single-stranded and self-complementary adeno-associated viral
vector genome titers by droplet digital PCR. Human gene Ther Methods. 2014;25:115–25. Article CAS Google Scholar * D’Costa S, Blouin V, Broucque F, Penaud-Budloo M, Francois A, Perez IC,
et al. Practical utilization of recombinant AAV vector reference standards: focus on vector genomes titration by free ITR qPCR. Mol Ther Methods Clin Dev. 2016;5:16019. Article Google
Scholar * Allay JA, Sleep S, Long S, Tillman DM, Clark R, Carney G, et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a
hemophilia B clinical trial. Hum Gene Ther. 2011;22:595–604. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank ATCC for their contribution labeling the vials and
sending materials to the testing laboratories involved in this study. We thank Sven Kuhlendahl and Progen team for providing data, helpful discussions and the ELISA kits used in this study
along with Diego Matayoshi, Kristy Guo, Jessica Stone and Kathy Lastra at Brammer Bio. Financial support to E.A.’s laboratory was provided by INSERM, University of Nantes, CHU of Nantes,
Fondation d’Entreprises pour la Thérapie Génique en Pays de la Loire, and Région Pays de la Loire and Pre-industrial gene therapy consortium (Agence Nationale de la Recherche -
Investissements d’Avenir, grant number 10-DPBS-0001). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * INSERM UMR1089, University of Nantes, Centre Hospitalier Universitaire, Nantes, France
Magalie Penaud-Budloo, Frédéric Broucque, Katell Harrouet, Mohammed Bouzelha, Sylvie Saleun, Sandy Douthe, Oumeya Adjali, Véronique Blouin & Eduard Ayuso * Brammer Bio, Alachua, FL,
32615, USA Susan D’Costa, Sushma Ogram & Richard O. Snyder * Gene Therapy Program, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, 19104,
USA Martin Lock Authors * Magalie Penaud-Budloo View author publications You can also search for this author inPubMed Google Scholar * Frédéric Broucque View author publications You can also
search for this author inPubMed Google Scholar * Katell Harrouet View author publications You can also search for this author inPubMed Google Scholar * Mohammed Bouzelha View author
publications You can also search for this author inPubMed Google Scholar * Sylvie Saleun View author publications You can also search for this author inPubMed Google Scholar * Sandy Douthe
View author publications You can also search for this author inPubMed Google Scholar * Susan D’Costa View author publications You can also search for this author inPubMed Google Scholar *
Sushma Ogram View author publications You can also search for this author inPubMed Google Scholar * Oumeya Adjali View author publications You can also search for this author inPubMed Google
Scholar * Véronique Blouin View author publications You can also search for this author inPubMed Google Scholar * Martin Lock View author publications You can also search for this author
inPubMed Google Scholar * Richard O. Snyder View author publications You can also search for this author inPubMed Google Scholar * Eduard Ayuso View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Eduard Ayuso. ETHICS DECLARATIONS CONFLICT OF INTEREST ROS is an inventor on patents related to recombinant AAV
technology. ROS owns equity in a gene therapy company that is commercializing AAV for gene therapy applications. To the extent that the work in this manuscript increases the value of these
commercial holdings, ROS has a conflict of interest. ML is an inventor on patents related to recombinant AAV technology. The other authors declare that they have no conflict of interest.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Penaud-Budloo, M., Broucque, F., Harrouet, K. _et al._ Stability of the adeno-associated virus 8 reference standard material.
_Gene Ther_ 26, 211–215 (2019). https://doi.org/10.1038/s41434-019-0072-9 Download citation * Received: 18 December 2018 * Revised: 05 March 2019 * Accepted: 08 March 2019 * Published: 29
March 2019 * Issue Date: May 2019 * DOI: https://doi.org/10.1038/s41434-019-0072-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
11 quick questions for lisa ling | members only accessMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Why we cried #injustiss at the guwahati campusA hunger strike called by students of the Tata Institute of Social Sciences (TISS), Guwahati, against withdrawal of fee ...
Disney streaming boss leaves to become the new ceo of tiktokThe head of streaming for Disney is leaving the Magic Kingdom and headed to TikTok. Kevin Mayer, chair of Walt Disney di...
'Emma'DÍAS DE CINE 'EMMA' 'Emma' Disponible hasta 21-02-2222 30/10/2020 00:03:27 Recomendado para mayores...
Kodi crackdown - police make arrest, seize stash of fully-loaded boxesPolice have arrested a 53 year-old man and seized a stash of some 40 Android-powered set-top boxes preloaded with the Ko...
Latests News
Stability of the adeno-associated virus 8 reference standard materialABSTRACT Adeno-associated virus (AAV) vectors are extensively used for gene therapy clinical trials. Accurate and standa...
Oops! That page can't be foundOops! That page can't be found It seems we can't find what you're looking for.Latest from HITC More latest from HITC...
No amnesty for death squads | thearticleIn the late 1990s I spent two years hunting war criminals in Bosnia as commander of a small Nato search team. Locating t...
Covid-19: india cases near 1. 6 lakh-mark; death toll 4,531 | livePANDEMIC MAY PUSH 86 MILLION MORE CHILDREN INTO POVERTY BY YEAR ENS: REPORT 3 JUDGE BENCH BEGINS HEARING ON MIGRANTS’ IS...
Ceo won't divulge details of negotiations : texaco-icahn talks a try to ease sale of assetsTexaco’s latest talks with shareholder Carl C. Icahn were motivated in part by the fact that uncertainty over Icahn’s in...