Da-drd5 signaling controls colitis by regulating colonic m1/m2 macrophage polarization
Da-drd5 signaling controls colitis by regulating colonic m1/m2 macrophage polarization"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The decrease of neurotransmitter dopamine (DA) levels in the intestine is closely related to the development of inflammatory bowel disease (IBD). However, the functional relevance
and underlying mechanistic basis of the effects of DA signaling on IBD remains unclear. Here, we observed that the DRD5 receptor is highly expressed in colonic macrophages, and the
deficiency of DA-DRD5 signaling exacerbated experimental colitis. Moreover, DA-DRD5 signaling can inhibit M1 by negatively regulating NF-κB signaling but promote M2 macrophage polarization
through activation of the CREB pathway, respectively. The deficiency of DRD5 signaling increased colonic M1 macrophages but reduced M2 cells during colitis. Additionally, the administration
of a D1-like agonist that has a higher affinity to DRD5 can attenuate the colitogenic phenotype of mice. Collectively, these findings provide the first demonstration of DA-DRD5 signaling in
colonic macrophages controlling the development of colitis by regulating M1/M2 macrophage polarization. SIMILAR CONTENT BEING VIEWED BY OTHERS DOPAMINERGIC SIGNALLING LIMITS SUPPRESSIVE
ACTIVITY AND GUT HOMING OF REGULATORY T CELLS UPON INTESTINAL INFLAMMATION Article 12 November 2020 CHOLINERGIC ANTI-INFLAMMATORY PATHWAY AMELIORATES MURINE EXPERIMENTAL TH2-TYPE COLITIS BY
SUPPRESSING THE MIGRATION OF PLASMACYTOID DENDRITIC CELLS Article Open access 07 January 2022 COLITIS-ASSOCIATED INTESTINAL MICROBIOTA REGULATES BRAIN GLYCINE AND HOST BEHAVIOR IN MICE
Article Open access 29 September 2022 INTRODUCTION Inflammatory bowel disease (IBD) is a group of chronic relapsing inflammatory conditions of the colon and small intestine mainly comprising
Crohn’s disease (CD) and ulcerative colitis (UC)1. As the most sophisticated immune organ of the entire body, the extensive repertoire of intestinal immune cells is unique and plays an
important role in IBD development. In particular, large numbers of macrophages are present in the intestine and the shift on macrophage phenotype has been implicated in the establishment of
IBD2,3. M1 macrophages are classic inflammatory cells secreting proinflammatory cytokines and directly contribute to the defect of the barrier in IBD4. It was shown that in DSS-induced
colitis mice, the population of M1 macrophages increases5. Conversely, M2 macrophages express anti-inflammatory cytokines, including IL-10, and are involved in tissue repair and inflammation
resolution to relieve IBD6. Transfer of properly polarized M2 macrophages to _STAT6__−/−_ mice accelerated wound healing in the damaged mucosa7. Thus, the regulation of M1/M2 macrophages
balance might be a potential therapeutic strategy for IBD. Additionally, it is becoming increasingly clear that normal gastrointestinal (GI) function depends on not only immune-cell
populations but also the highly coordinated responses of the enteric nervous system (ENS)8. The neurons of the enteric nervous system control the functions of the GI system and communicate
through various neurotransmitters, including dopamine (DA), acetylcholine, and serotonin, etc9. In the inflamed mucosa of CD and UC patients, the DA levels were markedly lower than in
controls10. The study has shown that DA protects the homeostasis of the GI mucosal barrier through regulation of mucus secretion, intracellular pH, and submucosal blood flow11. In addition
to its effects on gastrointestinal secretory responses, DA also acts as an inhibitory modulator of gastrointestinal motility12. Although these studies have established a framework of the
relationship between DA and IBD, the functions and their underlying mechanisms of DA in the regulation of mucosal immunity and colitis remain not well understood. To date, DA has been
reported as an essential regulator of different immune cells by acting on its two primary subfamilies receptors: D1-like dopamine receptors that comprise dopamine receptor D1 (DRD1) and
dopamine receptor D5 (DRD5); D2-like dopamine receptors, including dopamine receptor D2 (DRD2), dopamine receptor D3 (DRD3) and dopamine receptor D4 (DRD4)13. There has been some progress in
understanding the role of DA receptors in regulating immune cell functions. Follicular helper T (TFH) cell-derived DA enhances T-B cell interactions through the DRD1 of germinal center B
cells14. DRD2 activation in astrocytes can suppress neuroinflammation and the development of Parkinson’s disease in the central nervous system via αB-crystallin protein15. Dopaminergic
agonists can reduce the ovalbumin antigen-induced activation of neutrophils via D1-like receptors in a mouse model of airway inflammation16. DA inhibits NLRP3 inflammasome activation and
system inflammation via DRD1 signaling in macrophages17. More recently, we described the detailed role and mechanisms of the macrophage DA-DRD5 signaling in controlling
inflammation-associated diseases such as meningitis and sepsis18. Thus, these studies highlight DA has an important influence on immune cell functions. However, the underlying functions of
DA in modulating intestinal immune cells are largely unknown. In this study, we found that DRD5 is highly expressed in colonic macrophages and DRD5 deficiency exacerbates DSS-induced
colitis. Moreover, DRD5 signaling rebalances the colonic M1/M2 macrophage ratio by negatively regulating NF-κB signaling and activating of the CREB pathway, and then the administration of
DRD5 agonist attenuates the colitogenic phenotype of mice. Thus, we proposed a novel neuroimmune regulatory pathway in which DA corrects the M1/M2 excessive polarization through DRD5 to
alleviate intestinal inflammation. RESULTS DRD5 RECEPTOR IS HIGHLY EXPRESSED IN COLONIC MACROPHAGES To systematically investigate the roles of DA-DRDs signaling in the gut mucosal immune
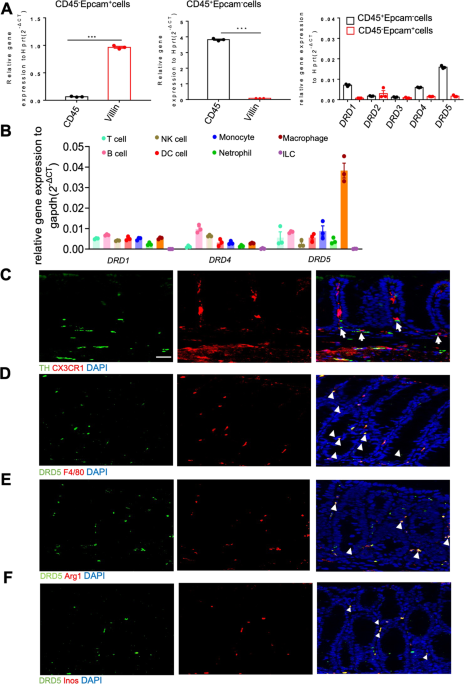
system and colitis, we firstly tested the expression of DA receptors in the isolated epithelial and CD45+ lamina propria (LP) hematopoietic cells (Fig. S1A) from the colon. We found that
DRD1, DRD4, and DRD5 were highly expressed in CD45+LP cells (Fig. 1A), whereas the expression of DRD2 and DRD3 were very low in both epithelial and LP immune cells. Since LP contains a range
of immune cells that maintain homeostasis and respond to a breakdown of epithelial protection19, we further measured the expression of DRD1, DRD4 and DRD5 in various immune cells of LP,
including Macrophages (CD45+CD11b+F4/80+), Monocytes (CD45+CD11b+Ly6c+), NK cells (CD45+ NK1.1+), Neutrophils (CD45+Ly6g+), Dendritic cells (CD45+ CD11c+), T cells (CD45+ TCR-β+), B cells
(CD45+ CD19+), ILCs (CD45+ Lin-) (Fig. S1B). Notably, a significantly high expression of DRD5 in macrophages was observed by RT-PCR analysis (Fig. 1B). Immunofluorescence analysis showed
that CX3CR1+ macrophages were close to tyrosine hydroxylase (TH) positive neurons in LP layer (Fig. 1C). Besides, we observed the wide distribution of DRD5 signaling in LP F4/80+ macrophages
(Fig. 1D), Arg1+M2 macrophages, and Inos+M1 macrophages (Fig. 1E, F). Thus, these data suggest that DA-DRD5 signaling could have an important regulatory effect on colonic macrophage
function. DRD5 DEFICIENCY IN IMMUNE CELLS EXACERBATES DSS-INDUCED COLITIS Notably, the public data sets (GSE47908 and GSE38713) showed that the gene expression of DRD5 was significantly
decreased in the colons of UC patients or active UC patients (Fig. S2A). To evaluate the role of DRD5 signaling in colitis, we used the dextran sodium sulfate (DSS) model of colitis in mice.
Briefly, mice were given 2.5% DSS in the drinking water for six days and then switched to regular drinking water until day 9. First, we observed the colonic DA levels were markedly reduced
after DSS treatment from 1.5 × 10−7 M to 1 × 10−7 M (Fig. S2B), further suggesting the close relation of decrease of DA levels with colitis. Next, we treated age-matched DRD5 knockout
(_DRD5__−/−_) or wild-type (WT) mice with DSS to compare their colitis phenotype. Although no obvious differences in the weight, histopathology of colons between WT and _DRD5_−/− mice were
observed before DSS treatment (Fig. S2C, D), more severe colitis on day nine after DSS administration was observed in _DRD5__−/−_ mice than in WT controls, as characterized by significantly
greater body weight loss, higher disease activity index (DAI) score, and shorter colons in DSS-treated _DRD5__−/−_ mice (Fig. 2A–C). Histopathological analysis [hematoxylin and eosin
(H&E)] further showed that DRD5 deficiency increased inflammatory cell infiltration with more damage of mucosal epithelium (Fig. 2D). Also, the serum levels of TNF-α, IL-6, and CCL2 were
markedly increased in the _DRD5__−/−_ group compared with the WT group (Fig. 2E). Previous studies have suggested that the dynamic shifts in gut microbiota play a role during the
development of colitis20. To evaluate whether microbiota variation was associated with the increased severity in colitis observed in _DRD5__−/−_ mice, we cohoused littermate WT mice [WT
(_DRD5__−/−_)] and _DRD5__−/−_ mice [_DRD5__−/−_ (WT)] for 6 weeks to roughly equalize bacterial community before the administration of DSS. The 16s rRNA Sequencing showed that the microbial
community structure and composition of single-housed _DRD5__−/−_ mice were different from WT group, but they tend to be consistent after cohousing (Fig. S3A, B). Moreover, _DRD5__−/−_
single-housed mice and cohoused mice had slightly high abundance of colitis-associated microbiota, such as Prevotellaceae and Clostridia_UCG-014, and low abundance of protective bacteria,
including Bacteroidaceae and Tannerellaceae, which indicating _DRD5__−/−_ mice have a weak tendency to develop colitis (Fig. S3C). However, cohousing breeding did not change the development
of more severe DSS-induced colitis in _DRD5__−/−_ (WT) mice compared with the cohoused WT (_DRD5__−/−_) controls (Fig. S3D–G), suggesting microbiota changes did not involve in the protection
of DRD5 against colitis directly. To further determine whether DRD5 deficiency in immune cells or gut-resident cells contributes to the increased severity of colitis, we firstly generated
bone marrow chimeric mice by adoptively transferring WT or _DRD5__−/−_ bone marrow cells into lethally irradiated WT recipient mice. We found that WT mice reconstituted with _DRD5_−/− bone
marrow was easier to induce colitis with greater body weight loss, higher DAI score, and shorter colons than WT donors after DSS administration (Fig. 2F–H). H&E staining showed the more
infiltrating inflammatory cells and more severe disruption in the colon of _DRD5__−/−_ donor mice relative to WT donors during colitis (Fig. 2I). The levels of TNF-α, IL-6, and CCL2 were
also increased in _DRD5__−/−_ donor mice compared with the WT donor mice (Fig. 2J). However, a reverse bone marrow transfer experiment in which lethally irradiated WT or _DRD5__−/−_ mice
recipient mice were reconstituted with bone marrow cells isolated from WT, demonstrated the comparable body weight loss, DAI scores, colon length, colon pathology, and serum inflammatory
cytokines between these two recipients (Fig. 2F–J). Therefore, these results suggest that DRD5 signaling in immune cells is essential for its protective role in DSS-induced colitis. THE
DEFICIENCY OF DA-DRD5 SIGNALING INHIBITS M1 BUT ENHANCES M2 MACROPHAGE POLARIZATION IN VITRO Previous studies by us and other research groups have shown that DA can inhibit inflammation in
macrophages17,18. Given M1/M2 macrophages present in the intestine play essential roles in the initiation or resolution of inflammation in IBD21, we speculate DA may have an important role
in the polarization of macrophages. To test this, we first used Lps and Ifn-γ to treat BMDMs in vitro for M1 macrophage differentiation in the presence or absence of DA. We found that DA
treatment significantly inhibited Lps/Ifn-γ-induced expression of M1-associated genes, including _Inos_, _Tnf_, and _Il6_ at the mRNA level in a dose-dependent manner (Fig. 3A and Fig. S4A).
Meanwhile, ELISA analysis showed DA decreased the protein levels of TNF-α and IL-6 in a dose-dependent way (Fig. 3B and Fig. S4A). Consistent with previous reports showing that DA is
subject to degradation by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) to shorten its half-life22, we found that co-treatment with MAO and COMT inhibitors can greatly
improve the inhibitory effect of DA at the DA concentration of 0.5 μM (Fig. S4B, C). Moreover, fluorescence-activated cell sorting (FACS) revealed that DA markedly reduced the expression of
CD86 on macrophages under M1 conditions (Fig. 3C). The immunoblotting analysis also demonstrated DA significantly reduced the expression of Inos protein after Lps/Ifn-γ treatment (Fig. 3D).
Thus, these data suggest an inhibitory role of DA in M1 macrophage polarization. Given that DRD5 is highly expressed in colonic macrophages, we next address whether DA regulates M1
macrophage polarization through DRD5. Notably, the inhibitory effect of DA on the mRNA expression levels of M1-associated genes, including _Inos_, _Tnf_, and _Il6_ in response to Lps/Ifn-γ
was completely lost in _DRD5__−/−_ BMDMs (Fig. 3E and Fig. S4F). ELISA analysis showed that such inhibitory effects of DA on TNF-α and IL-6 production in response to Lps and Ifn-γ
stimulation were severely impaired in _DRD5__−/−_ cells (Fig. 3F and Fig. S4F). Also, FACS revealed _DRD5__−/−_ cells were resistant to the inhibitory effects of DA on CD86 expression under
M1 conditions (Fig. 3G). Moreover, Immunoblotting analysis demonstrated DRD5 deficiency precluded the inhibitory effect of DA on the expression of Inos protein in macrophages (Fig. 3H).
Altogether, these data suggest that DA-DRD5 signaling inhibits M1 macrophage polarization. Next, we assessed whether DA regulates M2 macrophage differentiation in response to IL-4 and IL-13
stimulation. Interestingly, we observed that DA markedly augmented the expression of M2-associated genes, including _Arg1_, _Mrc1_, and _Ym1_, in response to the IL-4/IL-13 treatment (Fig.
3I and Fig. S4D). Also, MAO and COMT inhibitors can improve the promotion effects of DA on M2 macrophages at the lowest concentration of 0.5 μM (Fig. S4E). FACS revealed that DA could
increase the induction of CD206+ M2 macrophages in response to IL-4/IL-13 (Fig. 3J). Moreover, the significantly increased expression of Arg1 at the protein level was detected in M2
macrophages with DA treatment compared to the controls (Fig. 3K). In contrast to WT cells, DA failed to increase the expression of M2-associated genes, including _Arg1_, _Mrc1_, and _Ym1_ in
_DRD5__−/−_ cells (Fig. 3L and Fig. S4G). Additionally, FACS revealed the increased CD206 expression on M2 macrophages in the presence of DA was severely impaired in _DRD5__−/−_ cells (Fig.
3M). Moreover, the immunoblotting analysis demonstrated loss of DRD5 impaired the promoted effect of DA on the expression of Arg1 protein in macrophages (Fig. 3N). Thus, these results
suggested that DA can also promote M2 macrophage polarization through DRD5. DA-DRD5 SIGNALING CAN INHIBIT M1 MACROPHAGES POLARIZATION BY NEGATIVELY REGULATING NF-ΚB SIGNALING, AND PROMOTE M2
MACROPHAGES POLARIZATION THROUGH THE ACTIVATION OF THE CREB PATHWAY To further dissect the mechanistic of DA in regulating the balance of M1/M2 macrophage polarization, we performed RNA
sequencing to analyze the transcriptional profiles of Lps/Ifn-γ-stimulated M1 WT and _DRD5__−/−_ macrophage in the absence or presence of DA. Principal component (PC) analysis showed that
the profiles of DA-treated M1 WT BMDMs had a distinct gene clustered architecture compared with the communities of M1 WT, M1 _DRD5__−/−_, and DA-treated M1 _DRD5__−/−_ cells (Fig. 4A). Gene
set enrichment analysis (GSEA) revealed that the most prominent down-regulated pathways in DA-treated M1 WT BMDMs were the TNFA_SIGNALING_VIA_NFKB compared with untreated M1 WT cells.
Moreover, _DRD5__−/−_ BMDMs treated with DA were also enriched with the NFκB pathway relative to DA-treated WT cells (Fig. 4B). This finding is consistent with our previous report that
dopamine uses the DRD5 signaling to block TRAF6-mediated NF-κB activation and inflammation18. Accordingly, we found that DA remarkably reduced the capacity of Lps /Ifn-γ to induce
phosphorylation of IKKs or of their substrate IκBα, both of which are activation indices of the canonical NF-κB pathway. Moreover, IκBα degradation after stimulation with Lps/Ifn-γ was
significantly impaired in the presence of DA. However, such inhibitory effect of DA on the phosphorylation of IKKs and the degradation of IκBα under M1 conditions was completely lost in
BMDMs from _DRD5__−/−_ lines (Fig. 4C). Additionally, the heat map showed that DA treatment inhibited the expression of NF-κB-mediated M1 genes, including _Il6_, _Tnf_, _Nos2_, _Il12b_ in WT
but not _DRD5__−/−_ BMDMs after stimulation with Lps/Ifn-γ (Fig. 4D). Besides, consistent with the previous findings that DA/DRD5 plays anti-NF-κB effects by ARRB/PP2A signaling18, PP2A
inhibitor okadaic acid (OA) rescued the inhibitory effect of DA on NF-κB and M1 differentiation (Fig. S5A–D). Taken together, these data suggest that DA-DRD5 signaling specifically inhibits
Lps/Ifn-γ-mediated NF-κB signaling and thereby inhibits M1 macrophage polarization. Next, we use RNA-seq to analyze the transcriptional profiles of IL-4/IL-13-stimulated WT and _DRD5__−/−_
M2 macrophage in the absence or presence of DA. PCA revealed that an entirely different clustered pattern in DA-treated M2 WT BMDMs compared with those of M2 WT, M2 _DRD5__−/−_, and
DA-stimulated M2 _DRD5__−/−_ cells (Fig. 4E). Moreover, GSEA showed that CREB_PATHWAY was the top upregulated pathways in M2 WT BMDMs after DA treatment, and CREB_PATHWAY was also the
top-ranking gene set in M2 WT BMDMs relative to _DRD5__−/−_ cells in the presence of DA (Fig. 4F). Consistently, the immunoblotting analysis showed that DA significantly increased the
phosphorylation of CREB in WT BMDMs after IL-4/IL-13 stimulation, but such effects of DA were severely impaired in _DRD5__−/−_ BMDMs. Protein kinase A (PKA) is a well-known critical upstream
protein kinase of CREB and can be activated by DA-DRD5 signaling18. Therefore, we next detected the phosphorylation level of PKA catalytic (PKAc) subunits, which phosphorylate the
downstream targets such as CREB and ATF123,24. The upregulation of phosphorylation of PKAc by DA was observed in WT but not _DRD5__−/−_ BMDMs after stimulation with IL-4 and IL-13 (Fig. 4G).
Moreover, the heatmap analysis also displayed a significant increase in the expression of a variety of genes associated with M2 macrophage and CREB signaling, such as _Arg1, Chil3, Mrc1,
Socs1, Il10_, in WT but not _DRD5__−/−_ BMDMs under M2 conditions in the presence of DA (Fig. 4H). Additionally, the inhibitor of CREB KG501 blocked the enhanced effects of DA/DRD5 on M2
differentiation (Fig. S5E–G). Collectively, these data clearly demonstrate that DA-DRD5 signaling can promote IL-4/IL-13-triggered M2 macrophage polarization through its activation of the
CREB pathway. THE DEFICIENCY OF DRD5 SIGNALING INCREASED M1 MACROPHAGES BUT REDUCED M2 CELLS IN THE COLON OF DSS COLITIS MICE To further investigated whether DA-DRD5 signaling could regulate
colonic M1/M2 polarization in vivo to protect against colitis, we next characterized macrophages in the colon from untreated and DSS-induced colitis mice. An increase in the number of
F4/80+ macrophages was observed in the colons of _DRD5__−/−_ colitis mice relative to those of WT mice by immunofluorescence staining. Moreover, immunofluorescence showed that _DRD5__−/−_
mice had significantly increased numbers of Inos+ M1 cells but markedly reduced numbers of Arg1+ M2 cells in the colon compared with WT mice (Fig. 5A). Consistently, FACS revealed that
compared with WT mice, the expression of CD86, Inos, and TNF-α in colonic macrophages sorted from _DRD5__−/−_ mice were modestly increased, but significantly increased on day 6 after DSS
treatment. Moreover, the expression of Arg1 in colonic macrophages was markedly reduced in _DRD5__−/−_ mice during colitis (Fig. 5B and Fig. S6). Additionally, we further validated the
requirements for DRD5 signaling in macrophage polarization by constructing mixed bone marrow chimeras. The sublethally irradiated mice were reconstituted with _DRD5__−/−_ CD45.2/WT CD45.1
bone marrow (1:1 ratio), and a control WT CD45.2/WT CD45.1 group (1:1 ratio). We found that mice that received DRD5 deficient bone marrow cells had higher M1 polarization and lower M2
polarization than WT donors after colitis (Fig. 5C). Collectively, these data suggest DRD5 signaling is indispensable for regulating the balance of colonic macrophage polarization and
thereby its protective role in DSS-induced colitis. THE ADMINISTRATION OF DRD5 AGONIST ATTENUATES THE COLITOGENIC PHENOTYPE OF MICE Given that DRD5 signaling could protect against colitis,
we were keen to assess if pharmacological activation of DRD5 could prevent colitis in mice. To test this, we treated WT and _DRD5__−/−_ mice with intraperitoneal administration of the
D1-like agonist SKF-38393 (10 mg/kg of body weight), which has a higher affinity to DRD525. Such treatment significantly attenuated the clinical signs of colitis in WT mice as indicated by
less weight loss, lower DAI score, less shortening in colon length, less histopathological findings, and lower serum cytokines of TNF-α, IL-6, and CCL2, whereas such effects of SKF-38393
were severely impaired in _DRD5__−/−_ mice (Fig. 6A–E). Thus, these data further indicate a vital role of DRD5 signaling in the pathogenesis of colitis and suggest the development of
potential therapeutic strategies to target DA-DRD5 signaling that might be useful for protection against colitis. DISCUSSION The most striking finding from this study is that DA-DRD5
signaling can inhibit the development of colitis by regulating the balance of colonic M1/M2 macrophages. Actually, DA or its agonists have been reported to act as protective agents in
various rat ulcer models26. It is known that the gastrointestinal tract (GI) is one of the main sources of peripheral DA27. GI DA is mainly produced by the dopaminergic neuron in ENS and
epithelial cells in the gut lumen28,29. There have been some studies that reported the reduction of dopamine content altered the status of immune cells in the intestine30. In this study, we
further showed the role of DA via DRD5 in regulating the balance of colonic M1/M2 macrophage polarization and explained the underlying mechanism of DA in ameliorating colitis. Macrophages in
the GI tract represent the largest population of mononuclear macrophages in the body31. There is an increasing awareness of the role of macrophages in the regulation and development of
gastrointestinal disease. The proinflammatory cytokines such as TNF-α released from M1 macrophages contribute to the development of IBD32. Besides, TNF-α neutralization induces
CD206-positive M2 macrophages33 and contributes to mucosal healing34. IL-10, a potent cytokine derived from M2-like macrophages, is necessary for recovery from postoperative ileus and
colitis35,36. DA receptors are widely distributed in almost all immune cell subsets including macrophages and serve to regulate the release of immune factors to affect immune cell function
and inflammatory response37. In our study, we observed a high expression of DRD5 receptor in colonic LP macrophages, including F4/80+, Inos+M1, and Arg1+M2 cells. Consistent with the
previous report that DA-DRD5 signaling suppresses macrophage-mediated inflammation18, we revealed that DA-DRD5 signaling is likely to play an important role in controlling the balance of
colonic macrophage polarization, which is required for colonic homeostasis and prevent colitis. Notably, DA has recently been reported to inhibit LPS-induced NO production and inflammation
in microglia, a type of central nervous system (CNS) macrophage, through DRD5-mediated signaling38, further suggesting an inhibitory role of DRD5 signaling in macrophage inflammation.
RNA-seq analysis revealed that DA-DRD5 signaling regulated macrophage polarization by inhibiting the NF-κB signaling pathway for the suppression of M1 macrophages while activating the CREB
signal pathway to promote M2 macrophages. This finding is consistent with our previous report that dopamine uses DRD5 signaling to block TRAF6-mediated NF-κB inflammation18. Given that NF-κB
signaling is critical for the induction of a large number of inflammatory M1 genes39,40, our study highlighted a vital role of DA-DRD5 signaling in suppressing NF-κB activation to control
colonic M1 polarization. Additionally, PKA is a well-known critical upstream protein kinase of CREB and can be activated by DRD5 signaling23,24. Moreover, the PKA-CREB cascade has been
reported to induce M2 macrophage-specific gene expression and M2 polarization41,42,43. Thus, our study also elucidates the mechanism map of DA-DRD5 signaling in facilitating M2 colonic
macrophage polarization to control inflammation. Overall, in our model, colonic DA released by ENS dopaminergic neuron or colonic epithelial cells, acting to DRD5 receptor, inhibits M1 but
promotes M2 macrophages polarization through the suppression of the NF-κB pathway and activation of the CREB pathway respectively, thereby driving anti-inflammatory protective effects in
colitis (Fig. S7). In this study, we found that the administration of D1-like agonist (prefer to target DRD5) attenuates the colitogenic phenotype of mice, suggesting targeting of DRD5
signaling could be a potential therapeutic manipulation to treat IBD. Moreover, the effects of DA on macrophages polarization might be not limited in IBD, because the dopaminergic signaling
is widely distributed in the peripheral tissues of the body and the balance of M1/2 macrophage polarization has a close relationship with the development of various other diseases, such as
infections44, lung injury45, and tumors46. Therefore, targeting the DA-DRD5 signaling pathway to regulate macrophage polarization has great potential to achieve a therapeutic effect to treat
not only IBD but other macrophage-mediated inflammatory diseases. MATERIALS AND METHODS MICE We used C57BL/6 background male mice in this study. _DRD5__−/−_ mice were generated by Cyagen
Biosciences Inc (Guangzhou, China) using CRISPR-Cas9 technology, as described previously18. To mark lamina propria macrophages, Rosa26-tdTomato mice were crossed with Cx3cr1-Cre mice.
Rosa26-tdTomato and Cx3cr1-Cre mice were kindly provided by Dr. Jiawei Zhou (Institute of neuroscience, Chinese academy of sciences). For cohousing experiments, age-matched heterozygous male
wild-type and _DRD5__−/−_ mice from the same littermates were cohoused at a 1:1 ratio for 6 weeks. All mice were kept in a barrier facility, and all animal experiments were conducted
following the procedure approved by the Ethical Review Committee for Laboratory Animal Welfare of Nanjing Medical University. ANTIBODIES AND REAGENTS Antibody to Inos (ab15323) was from
Abcam. Anti-β-actin (A1978) antibody was from Sigma. Anti-Arg1 (PA585267), anti-F4/80 (14-4801-82), anti-PE IgG (35-4914-81), anti-PEcy7 IgG 25-4914-82 antibodies were from ebioscience. The
anti-phosphorylated PKA C (5661) antibody was from SCT. Anti-PKA C-α (55388-1-AP) and anti-Drd5 (20310-1-AP/ADR-005) antibodies were from Proteintech/Alomone. Anti-phosphorylated IKKα/β
(2697), anti-IKKβ (2370), anti-phosphorylated IκBα (9246) and anti-phosphorylated CREB (9198s) were from Cell Signaling Technology. Anti-CREB (48601-2) antibody was from SAB. IRDye
680RDanti-mouse (926-68070) and IRDye 800CW anti-rabbit (926-32211) were from LI-COR Biosciences. Anti-mouse-HRP and anti-rabbit-HRP were from Jackson ImmunoResearch. Flow antibodies
including Anti-TCR-β-eFlour450 (48-5961-80), anti-CD45-AF700 (30-F11, 85-11-0112-81), anti-APC-CD45.1 (17-0453-82), anti-APC-eflour-780-CD45.2(47-0454-82), anti-CD11b-FITC
(M1/70,85-12-0114-81), anti-F4/80-APC (BM8, 17-4801-82), anti-Ly6c-PE-Cy7 (HK1.4, 25-5932-82), anti-Ly6g-percpcy5.5 (48-9668-82), FVD-eFlour®506 (65-0866), anti-APC-TNF-α (17-7321-82) were
from eBioscience. anti-CD11c (12-0114-82), anti-CD19 (17-0193-80) and anti-NK1.1-PE-cy7 (25-5941-81) were from Thermo. LPS (ALX-581-013-L002) was from EnzoLife Sciences. IL-4 (214-14), IL-13
(210-13-10), IFN-γ (500-M90) was from peprotech. SKF-38393 hydrobromide (0922) was from TOCRIS. KG501 (CSN22252) was from CSNpharm. OA (O7885-25UG) was from Sigma-Aldrich. DSS (DB001-38)
was from TdB Consultancy. DSS-INDUCED COLITIS For acute experimental colitis induction, 6-8w age- and sex-matched WT or DRD5_−/−_ mice were distributed randomly according to their genotypes,
which received 2.5% DSS from water for 6 days, followed by normal drinking water until the end of the experiment on days 9. During the experiment, body weights, stool, and body posture were
monitored daily to assess the disease activity index (DAI) in a blinded fashion. The DAI is the combined score of weight loss compared to initial weight, stool consistency, and body
posture. The scores are evaluated as follow: 0 (No weight loss or weight gain), 1 (5–10% weight loss), 2 (10–15% weight loss), 3 (>15% weight loss); stool consistency: 0 (normal and
well-formed), 1 (very soft and formed), 2 (loose stool), 3 (bloody stools); body posture: 0 (Smooth fur without a hunchback), 1 (mild fur and hunchback), 2 (moderate fur and hunchback), 3
(Severe fur and heavy hunchback). Mice were sacrificed at the indicated time points, and colons were collected immediately for colon length measure, colonic macrophage analysis, and
histology analysis. BONE MARROW CHIMERAS The recipient mice were subjected to lethal-dose irradiation (10 Gy), and 1 d later, bone marrow cells (10 × 106) derived from the tibiae and femurs
of _DRD5__−/−_ CD45.2/WT CD45.1 bone marrow (1:1 ratio), and a control WT CD45.2/WT CD45.1 group (1:1 ratio) were i.v. injected into lethally irradiated mice. After 8 weeks, the chimeric
mice were then subjected to DSS induction. D1-LIKE AGONIST SKF-38393 TREATMENT IN DSS-INDUCED COLITIS MICE The D1-like agonist SKF-38393 was dissolved in water. The agonist was i.p. injected
into WT and _DRD5__−/−_ mice at a dose of 10 mg/kg daily, starting 1 d before the DSS challenge. HISTOLOGICAL ANALYSIS For histology, tissue sections were stained with hematoxylin &
eosin (H&E). Histology was scored in a blinded fashion as a combination of inflammatory cell infiltration (score 0–4) and intestinal architecture damage (score 0–4). The presence of
occasional inflammatory cells in the lamina propria was scored as 0; increased numbers of inflammatory cells in the lamina propria was scored as 1; inflammatory cells extending into the
mucosa and submucosa was scored as 2; inflammatory cells extending into the mucosa, submucosa, and sometimes transmural infiltration was scored as 3; and severe transmural extension of the
infiltrate was scored as 4. For architecture damage, no mucosal damage was scored as 0; focal erosions were scored as 1; slight crypt loss and focal ulcerations was scored as 2; extended
ulcerations and moderate crypt loss was scored as 3; and extensive crypt loss, mucosal damage, and extension into deeper structures of the bowel wall was scored as 4. The total histologic
score was derived by summing each individual score. Images were acquired with a Nikon 50i inverted microscope. M1/M2 POLARIZATION For isolation of BMDMs, tibias and femurs were removed from
WT or _DRD5__−/−_ mice by sterile techniques, and the bone marrow was flushed with fresh medium. BMDMs were plated in DMEM supplemented with 10% FBS in the presence of 10% L929 conditioned
medium for 4–6 d at 37 °C in a humidified atmosphere of 5% CO2. Primary BMDMs were seeded (1.5 × 106 cells per well) in 12-well plates and were grown for 24 h. Cells were then stimulated
with LPS (100 ng/ml), IFN-γ (50 ng/ml) or IL-4 (10 ng/ml), IL-13 (10 ng/ml) for 12 h to induce M1 or M2 macrophages differentiation with or without DA at different concentrations of 1, 10,
20, 50 μM. In addition, 10 μM MAO inhibitor of PLZ and COMT inhibitor of DNC were added to test the lowest effective concentration of DA. RT-QPCR Total RNA was extracted by using TRIzol
reagent (Life) and subjected to cDNA synthesis. Quantitative RT-PCR was performed using SYBR Green Supermix (Vazyme) according to the manufacturer’s instructions. The following primers were
used: _Drd1_ S AS 5-CCGCTGTCATCAGGTTTCG-3 5-GATGTCAAAGGCTACCCAAATG-3 _Drd2_ S AS 5-CATCTCTTGCCCACTGCTCTT-3 5-CGATGGAGGAGTAGACCACGA-3 _Drd3_ S AS 5-CATCATCTTTGGCAACGGTCT-3
5-CGGCTGAAATTCCAGACTCC-3 _Drd4_ S AS 5-GGTGTGTTGGACGCCTTTCT-3 5-TTGAGGGCACTGTTGACATAGC-3 _Drd5_ S AS 5-CTATTTCCAGACCCTTCCGCT-3 5-CTGCCTTGTCTCTGTGCCAAT-3 _Inos_ S AS
5-AGGGAATCTTGGAGCGAGTTG-3 5-TGAGGGCTTGGCTGAGTGAG-3 _Il-6_ S AS 5-CTTGGGACTGATGCTGGTGAC-3 5-GCCATTGCACAACTCTTTTCTC-3 _Tnf_ S AS 5-TACTGAACTTCGGGGTGATCG-3 5-TCCTCCACTTGGTGGTTTGC-3 _Ym1_ S
AS 5- CATTGGAGGATGGAAGTTTGG -3 5-GGTACTGCCAGTCCAGGTTGA-3 _Mrc1_ S AS 5-CTCAACCCAAGGGCTCTTCTAA -3 5-AAGGTGGCCTCTTGAGGTATGT-3 _Arg1_ S AS 5-GTCTGGCAGTTGGAAGCATCT -3
5-GCTGGTTGTCAGGGGAGTGT-3 _Hprt_ S AS 5-GTCCCAGCGTCGTGATTAGC-3 5-TGGCCTCCCATCTCCTTCA-3 _Gapdh_ S AS 5-TGGATTTGGACGCATTGGTC-3 5-TTTGCACTGGTACGTGTTGAT-3 ELISA Primary BMDMs were seeded (1.5
× 106 cells per well) in 12-well plates and were grown for 24 h. Cells were then untreated or treated DA and stimulated with LPS (100 ng/ml), IFN-γ (50 ng/ml) or IL-4 (10 ng /ml), IL-13 (10
ng/ml) for 12 h. Conditioned media from these groups above and the serum from DSS or SKF38393 treated mice were collected and measured for levels of IL-6 (DY406), TNF-α (DY410) and CCL2
(DY479) according to manufacturer’s instructions (R&D Systems). IMMUNOBLOTTING Primary BMDMs were seeded (1.5 × 106 cells per well) in 12-well plates and were grown for 24 h. Cells were
then untreated or treated DA and stimulated with LPS (100 ng/ml), IFN-γ (50 ng/ml) or IL-4 (10 ng /ml), IL-13 (10 ng/ml). BMDMs were lysed in SDS buffer and boiled for 10 min. Samples were
resolved by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by immunoblot with the appropriate antibodies. IMMUNOFLUORESCENCE STAINING Tissue sections were incubated with
primary antibody to Drd5, F4/80, Inos, and Arg1 sections at 4 °C overnight, and then incubated with secondary antibody as indicated. The nuclei were counterstained with
4,6-diamidino-2-phenylindole (DAPI) (sigma). Slides were dried and mounted using ProLong Antifade mounting medium (Beyotime Biotechnology). At last, slides were visualized using a Nikon 50i
fluorescent microscope. The number of macrophages from three images that were randomly selected from each tissue section were quantified using Image pro plus. The cell number shown in Fig.
6A (_y_) is derived from this formula. y = N/S (_y_, number of cells per mm2; N, the total number of cells per tissue section; S, area). FACS ANALYSIS AND SORTING OF COLONIC EPITHELIAL AND
IMMUNE CELLS Colons were excised and washed thoroughly by flushing with PBS several times. They were opened longitudinally and transferred into PBS contained 1 mM DTT and 5 mM EDTA and
shaken for 20 min at 37 °C, repeated twice. All supernatants were collected and passed through a 70 μm cell strainer for staining, then the epithelial cells (CD45-CD326+) were sorted out by
flow cytometry. The remaining tissues were digested with DMEM media contained 1 mg/ml collagenase IV(Sigma) and 10 U/ml DNAse I (Roche) for 25 min. After repeating twice, all supernatants
were collected and passed through a 70 μm cell strainer for staining. The immune cells were collected from the middle layer of the liquid surface after density gradient centrifugation with
40/80% Percoll (GE Healthcare). After intensive washing, single suspensions were stained with FVD eFlour® 506, anti-CD45, anti-CD45.1, anti-CD45.2, anti-CD11b, anti-F4/80, anti-CD86,
anti-CD206, anti-Inos, anti-Arg1, anti-TNF-α for FACS analysis. All flow cytometry was performed on an Attune NxT flow cytometer (Thermo Fisher) and data were analyzed by FlowJo 10 software.
For colonic immune cells sorting, we use flow cytometry to sort out CD45+ immune cells (CD45+CD326-), T cells (CD45+TCR-β+), B cells (CD45+CD19+), NK cells (CD45+NK1.1+), macrophages
(CD45+F4/80+CD11b+), monocytes (CD45+Ly6c+CD11b+), DC cells (CD45+CD11c+), neutrophils (CD45+Ly6G+), intrinsic lymphoid cells (CD45+Lin-). RNA-SEQ ANALYSIS BMDMs from WT or _DRD5__−/−_ mice
were untreated or treated DA and stimulated with LPS (100 ng/ml), IFN-γ (50 ng/ml) or IL-4 (10 ng /ml), IL-13 (10 ng/ml) and then collected for RNA extraction. RNA samples were constructed
and sequenced on a BGISEQ-500 (Beijing Genomic Institution, BGI). The filtered data were mapped to the mouse genome (GRCm38.p5) through HISAT2. For gene expression analysis, the matched
reads were calculated and then normalized to FPKM. Fold changes were calculated for all possible comparisons and a 1.2-fold and FDR < 0.001 cutoff was used to select genes with expression
changes for the Heat map. The whole-genome were analyzed for the Principle component (PC). GSEA analysis was performed using the R package, using whole-genome as target genes. The Raw data
files and processed files have been uploaded to Gene Expression Omnibus public database (Accession: GSE159206). 16S RIBOSOMAL RNA GENE SEQUENCING Microbial DNA was extracted from fecal
samples of the indicated mice using the TIANamp Stool DNA Kit (TIANGEN) according to the manufacturer’s protocols. The final DNA concentration and purification were determined by Onedrop,
and the quality of DNA was determined by agarose gel electrophoresis. The purified DNA amplicons were then added with Illumina adapters by ligation (TruSeq DNA LT Sample Prep Kit), the
adapter-ligated DNA fragments were further pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform for sequencing according to the standard protocols by Majorbio
Bio-Pharm Technology Co. Ltd. (Shanghai, China). Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) with a
novel ‘greedy’ algorithm that performs chimera filtering and OTU clustering simultaneously. The taxonomy of each 16S rRNA gene sequence was analyzed by the RDP Classifier algorithm
(http://rdp.cme.msu.edu/) against the Silva (SSU123) 16S rRNA database using a confidence threshold of 70%. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database
(SRA accession: PRJNA664271). HPLC ANALYSIS HPLC analysis was employed to measure dopamine from the distal colon. This analysis used a DIONEX HPLC system with a Coulochem III Electrochemical
Detector together with a Uniget C-18 reverse phase microbore column as the stationary phase. The mobile phase consisted of buffer [1.7 mM OSA, 0.05 mM Na-EDTA, 90 mM NaH2PO4·2H2O, and 50 mM
C6H8O7·H2O] and acetonitrile. The flow rate was 0.2 ml/min, and the working electrode was set at 350 mV versus Ag/Ag/Cl reference electrode. Detection gain was 100 nA and filter was 5 s.10
mL of the sample supernatant was directly injected into the HPLC for analysis. Standard dopamine (Sigma) was used to quantify and identify the peaks on the chromatographs. The detection
limits for dopamine was determined by analyzing the known concentrations of dopamine in the HPLC system under the set condition. For this purpose, standard solutions of 1 mg per ml were made
with pure dopamine and diluted accordingly to the desired concentrations of the stock solutions for running on HPLC. Concentration of dopamine was determined using the following formula:
y(nmol/L) = 0.0217x–0.0132 (r2 = 0.9998) (y, peak area; x, analyte concentration in μM). To more effectively compare dopamine concentrations between studies, all values of tissues were
converted to final molar concentrations by dividing original tissue weights and multiplying the density of tissues which we averaged to be around 1 kg/L. STATISTICAL ANALYSES The data were
analyzed by GraphPadPrism 7.0 and GraphPadPrism 8.0 software and are presented as the mean ± standard error of the mean (SEM). The statistics were analyzed by using a two-tailed unpaired
_t_-test for two groups, one-way ANOVA for multiple groups. _P_ values were provided as ∗_p_ < 0.05, ∗∗_p_ < 0.01 and ∗∗∗_p_ < 0.001. DATA AVAILABILITY Sequencing data are deposited
into the Gene Expression Omnibus (accession no. SRP574780 and GSE159206). REFERENCES * Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. _Lancet_
369, 1627–1640 (2007). Article CAS PubMed Google Scholar * You, Y. et al. Sorting nexin 10 acting as a novel regulator of macrophage polarization mediates inflammatory response in
experimental mouse colitis. _Sci. Rep._ 6, 20630 (2016). Article CAS PubMed PubMed Central Google Scholar * Kuhl, A. A., Erben, U., Kredel, L. I. & Siegmund, B. Diversity of
intestinal macrophages in inflammatory bowel diseases. _Front Immunol._ 6, 613 (2015). Article PubMed PubMed Central CAS Google Scholar * Lissner, D. et al. Monocyte and M1
macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. _Inflamm. Bowel Dis._ 21, 1297–1305 (2015). PubMed Google Scholar * Lin, Y. et al. Chemerin
aggravates DSS-induced colitis by suppressing M2 macrophage polarization. _Cell. Mol. Immunol._ 11, 355–366 (2014). Article CAS PubMed PubMed Central Google Scholar * Shouval, D. S. et
al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. _Immunity_ 40, 706–719 (2014). Article CAS PubMed
PubMed Central Google Scholar * Cosin-Roger, J. et al. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. _Mucosal Immunol._
9, 986–998 (2016). Article CAS PubMed Google Scholar * Liu, M.-T., Kuan, Y.-H., Wang, J., Hen, R. & Gershon, M. D. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the
enteric nervous system of adult mice. _J. Neurosci._ 29, 9683–9699 (2009). Article CAS PubMed PubMed Central Google Scholar * Veiga-Fernandes, H. & Pachnis, V. Neuroimmune
regulation during intestinal development and homeostasis. _Nat. Immunol._ 18, 116–122 (2017). Article CAS PubMed Google Scholar * Magro, F. et al. Impaired synthesis or cellular storage
of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. _Digestive Dis. Sci._ 47, 216–224 (2002). Article CAS Google Scholar * Hiltebrand, L. B., Krejci,
V. & Sigurdsson, G. H. Effects of dopamine, dobutamine, and dopexamine on microcirculatory blood flow in the gastrointestinal tract during sepsis and anesthesia. _Anesthesiology_ 100,
1188–1197 (2004). Article CAS PubMed Google Scholar * Dive, A., Foret, F., Jamart, J., Bulpa, P. & Installé, E. Effect of dopamine on gastrointestinal motility during critical
illness. _Intensive Care Med._ 26, 901–907 (2000). Article CAS PubMed Google Scholar * Beaulieu, J.-M. et al. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic
neurotransmission and behavior. _Cell_ 122, 261–273 (2005). Article CAS PubMed Google Scholar * Papa, I. et al. T FH-derived dopamine accelerates productive synapses in germinal centres.
_Nature_ 547, 318–323 (2017). Article CAS PubMed PubMed Central Google Scholar * Shao, W. et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin.
_Nature_ 494, 90–94 (2013). Article CAS PubMed Google Scholar * Nakagome, K. et al. Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin
antigen-induced neutrophilic airway inflammation. _J. Immunol._ 186, 5975–5982 (2011). Article CAS PubMed Google Scholar * Yan, Y. et al. Dopamine controls systemic inflammation through
inhibition of NLRP3 inflammasome. _Cell_ 160, 62–73 (2015). Article CAS PubMed Google Scholar * Wu Y. et al. Dopamine Uses the DRD5-ARRB2-PP2A signaling axis to block the TRAF6-Mediated
NF-κB pathway and suppress systemic inflammation. _Mol. Cell_ 78, 42–56.e6 (2020). * Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal
microbiota. _Nat. Rev. Immunol._ 10, 159–169 (2010). Article CAS PubMed Google Scholar * Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during
health and disease. _Nat. Rev. Immunol._ 9, 313–323 (2009). Article CAS PubMed PubMed Central Google Scholar * Na Y. R., Stakenborg M., Seok S. H. & Matteoli G. Macrophages in
intestinal inflammation and resolution: a potential therapeutic target in IBD. _Nat. Rev. Gastro. Hepato._ 16, 531–543 (2019). * Youdim, M. B., Edmondson, D. & Tipton, K. F. The
therapeutic potential of monoamine oxidase inhibitors. _Nat. Rev. Neurosci._ 7, 295–309 (2006). Article CAS PubMed Google Scholar * Thomson, D. M. et al. AMP-activated protein kinase
phosphorylates transcription factors of the CREB family. _J. Appl. Physiol._ 104, 429–438 (2008). Article CAS PubMed Google Scholar * Masson, N., John, J. & Lee, K. A. In vitro
phosphorylation studies of a conserved region of the transcription factor ATF1. _Nucleic acids Res._ 21, 4166–4173 (1993). Article CAS PubMed PubMed Central Google Scholar * Habuchi, Y.
et al. Dopamine stimulation of cardiac β–adrenoceptors: the involvement of sympathetic amine transporters and the effect of SKF38393. _Br. J. Pharmacol._ 122, 1669–1678 (1997). Article CAS
PubMed PubMed Central Google Scholar * Sikirić, P. et al. The role of dopamine in the formation of gastric ulcers in rats. _Eur. J. Pharmacol._ 112, 127–128 (1985). Article PubMed
Google Scholar * Eisenhofer, G. et al. Substantial production of dopamine in the human gastrointestinal tract. _J. Clin. Endocrinol. Metab._ 82, 3864–3871 (1997). Article CAS PubMed
Google Scholar * Vieira-Coelho, M. & Soares-da-Silva, P. Dopamine formation, from its immediate precursor 3, 4-dihydroxyphenylalanine, along the rat digestive tract. _Fundamental Clin.
Pharmacol._ 7, 235–243 (1993). Article CAS Google Scholar * Pacheco, R., Contreras, F. & Zouali, M. The dopaminergic system in autoimmune diseases. _Front. Immunol._ 5, 117 (2014).
Article PubMed PubMed Central CAS Google Scholar * Powell, N., Walker, M. M. & Talley, N. J. The mucosal immune system: master regulator of bidirectional gut–brain communications.
_Nat. Rev. Gastroenterol. Hepatol._ 14, 143 (2017). Article CAS PubMed Google Scholar * Smith, P. et al. Intestinal macrophages and response to microbial encroachment. _Mucosal Immunol._
4, 31–42 (2011). Article PubMed CAS Google Scholar * Lynch, J., Metz, D., Rutgeerts, P., Vermeire, S. & Assche, G. V. Biological Therapies for 1108 Inflammatory Bowel Diseases.
_Gastroenterology_ 136, 1182–1197 (2009). Article CAS Google Scholar * Vos, A. C. W. et al. Anti–tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent
manner. _Gastroenterology_ 140, 221–230. e3 (2011). Article CAS PubMed Google Scholar * Vos, A. C. W. et al. Regulatory macrophages induced by infliximab are involved in healing in vivo
and in vitro. _Inflamm. Bowel Dis._ 18, 401–408 (2012). Article PubMed Google Scholar * Stoffels, B. et al. Role of interleukin 10 in murine postoperative ileus. _Gut_ 58, 648–660
(2009). Article CAS PubMed Google Scholar * Zhu, W. et al. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases.
_Immunological Investig._ 43, 638–652 (2014). Article CAS Google Scholar * Matt S. M, & Gaskill P. J. Where is dopamine and how do immune cells see it?: dopamine-mediated immune cell
function in health and disease. _J. Neuroimmune Pharmacol._ 15, 114–164 (2019). * Wang, B. et al. Dopamine alters lipopolysaccharide-induced nitric oxide production in microglial cells via
activation of D1-like receptors. _Neurochem. Res._ 44, 947–958 (2019). Article PubMed CAS Google Scholar * Saccani, A. et al. p50 nuclear factor-κB overexpression in tumor-associated
macrophages inhibits M1 inflammatory responses and antitumor resistance. _Cancer Res._ 66, 11432–11440 (2006). Article CAS PubMed Google Scholar * Liu, T., Zhang, L., Joo, D. & Sun,
S.-C. NF-κB signaling in inflammation. _Signal Transduct. Target. Ther._ 2, 1–9 (2017). CAS Google Scholar * Ruffell, D. et al. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene
expression and promotes muscle injury repair. _Proc. Natl Acad. Sci. USA_ 106, 17475–17480 (2009). Article CAS PubMed PubMed Central Google Scholar * Luan, B. et al. CREB pathway links
PGE2 signaling with macrophage polarization. _Proc. Natl Acad. Sci. USA_ 112, 15642–15647 (2015). Article CAS PubMed PubMed Central Google Scholar * Ma, L., Dong, F., Zaid, M., Kumar,
A. & Zha, X. ABCA1 protein enhances Toll-like receptor 4 (TLR4)-stimulated interleukin-10 (IL-10) secretion through protein kinase A (PKA) activation. _J. Biol. Chem._ 287, 40502–40512
(2012). Article CAS PubMed PubMed Central Google Scholar * Benoit, M., Desnues, B. & Mege, J.-L. Macrophage polarization in bacterial infections. _J. Immunol._ 181, 3733–3739
(2008). Article CAS PubMed Google Scholar * Johnston, L. K., Rims, C. R., Gill, S. E., McGuire, J. K. & Manicone, A. M. Pulmonary macrophage subpopulations in the induction and
resolution of acute lung injury. _Am. J. Respir. Cell Mol. Biol._ 47, 417–426 (2012). Article CAS PubMed PubMed Central Google Scholar * Jandaghi, P. et al. Expression of DRD2 is
increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. _Gastroenterology_ 151, 1218–1231 (2016). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS Not applicable. FUNDING This work was supported by the National Natural Science Foundation of China (82070567/ 81771773/91742116/81570499 to S. Yang; 81802393 to
B.W. Wang), the Start Fund for Specially-Appointed Professor of Jiangsu Province (S. Yang), the Major Project of Nanjing Medical University Science and Technology Development Fund
(NMUD2018003 to S. Yang and L. Lin.), the cultivation project of “high level young scientific and technological talents” of Nanjing Medical University (NMUR2019003 to S. Yang) and the Youth
Project Funding of Nanjing University of Chinese medicine (NZY81802393 to B. W. Wang). AUTHOR INFORMATION Author notes * These authors contributed equally: Lu Liu, Yuqing Wu, Bingwei Wang
AUTHORS AND AFFILIATIONS * Department of Immunology, Key Laboratory of Immunological Environment and Disease, State Key Laboratory of Reproductive Medicine, Center for Global Health, Nanjing
Medical University, Nanjing, 211166, China Lu Liu, Yuqing Wu, Yuying Jiang, Xiaoxi Li & Shuo Yang * Department of Pharmacology, Nanjing University of Chinese Medicine, Nanjing, China
Bingwei Wang * Department of Gastroenterology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China Lin Lin Authors * Lu Liu View author publications You can also
search for this author inPubMed Google Scholar * Yuqing Wu View author publications You can also search for this author inPubMed Google Scholar * Bingwei Wang View author publications You
can also search for this author inPubMed Google Scholar * Yuying Jiang View author publications You can also search for this author inPubMed Google Scholar * Lin Lin View author publications
You can also search for this author inPubMed Google Scholar * Xiaoxi Li View author publications You can also search for this author inPubMed Google Scholar * Shuo Yang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.L., Y.W., Y.J., and X.L. designed and performed the experiments, analyzed the data, and prepared the
figures; L. Lin. provided the key research source; B.W. provided the key technique mentoring, research reagents, and mice; S.Y. supervised the project; L.L., Y.W., X.L., and S.Y. wrote the
manuscript. CORRESPONDING AUTHORS Correspondence to Bingwei Wang, Xiaoxi Li or Shuo Yang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests. ETHICS
STATEMENT All research protocols involving animal experiments were approved by Institutional Animal Care and Use Committee of Nanjing Medical University (Protocol Number: IACUC-1806012-1).
No patient study was involved and the consent to participate is not applicable ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. Edited by H.-U. Simon SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, L., Wu, Y., Wang, B. _et al._ DA-DRD5 signaling controls
colitis by regulating colonic M1/M2 macrophage polarization. _Cell Death Dis_ 12, 500 (2021). https://doi.org/10.1038/s41419-021-03778-6 Download citation * Received: 18 January 2021 *
Revised: 23 April 2021 * Accepted: 26 April 2021 * Published: 17 May 2021 * DOI: https://doi.org/10.1038/s41419-021-03778-6 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Future large hydropower dams impact global freshwater megafaunaABSTRACT Dam construction comes with severe social, economic and ecological impacts. From an ecological point of view, h...
Infrastructure UK - GOV.UKInfrastructure UK Part of HM Treasury Infrastructure UK is now part of Infrastructure and Projects Authority On 1 Januar...
Javascript support required...
Meet The Cops On The Financial BeatBy Laura Petrecca, AARP En español Published June 15, 2022 If your home’s been burglarized, you know exactly whom to cal...
Pac-10 swimming championships : evans keys stanford to big lead over calThe Stanford women’s swim team continued to dominate the Pacific 10 women’s swimming and diving championships, winning f...
Latests News
Da-drd5 signaling controls colitis by regulating colonic m1/m2 macrophage polarizationABSTRACT The decrease of neurotransmitter dopamine (DA) levels in the intestine is closely related to the development of...
Credit card rates, balances soar3. THINK TWICE BEFORE SIGNING UP FOR A NEW CARD AT CHECKOUT. It’s not uncommon for store check-out clerks to try to enti...
Kobbie mainoo made roy keane break his own golden rule at man unitedTHE ICONIC FORMER MAN UNITED CAPTAIN WAXED LYRICAL ABOUT KOBBIE MAINOO AFTER HIS PREMIER LEAGUE DEBUT. 05:30, 21 Dec 202...
France’s marmots are back as they emerge from a winter of hibernatingTHE MOUNTAIN-DWELLING ‘GROUND SQUIRRELS’ HAVE BEEN SPOTTED IN THE FRENCH ALPS Marmots - France’s answer to the American ...
Javascript support required...