Wnt/ss-catenin-mediated p53 suppression is indispensable for osteogenesis of mesenchymal progenitor cells
Wnt/ss-catenin-mediated p53 suppression is indispensable for osteogenesis of mesenchymal progenitor cells"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The developmental origins of mesenchymal progenitor cells (MPCs) and molecular machineries regulating their fate and differentiation are far from defined owing to their complexity.
Osteoblasts and adipocytes are descended from common MPCs. Their fates are collectively determined by an orchestra of pathways in response to physiological and external cues. The canonical
Wnt pathway signals MPCs to commit to osteogenic differentiation at the expense of adipogenic fate. In contrast to ß-catenin, p53’s anti-osteogenic function is much less understood. Both
activities are thought to be achieved through targeting _Runx2_ and/or Osterix (_Osx, Sp7_) transcription. Precisely, how Osx activity is dictated by ß-catenin or p53 is not clarified and
represents a knowledge gap that, until now, has largely been taken for granted. Using conditional lineage-tracing mice, we demonstrated that chondrocytes gave rise to a sizable fraction of
MPCs, which served as progenitors of chondrocyte-derived osteoblasts (Chon-ob). Wnt/ß-catenin activity was only required at the stage of chondrocyte-derived mesenchymal progenitor (C-MPC) to
Chon-ob differentiation. ß-catenin– C-MPCs lost osteogenic ability and favored adipogenesis. Mechanistically, we discovered that p53 activity was elevated in ß-catenin– MPCs including
ß-catenin– C-MPCs and deleting p53 from the ß-catenin– MPCs fully restored osteogenesis. While high levels of p53 were present in the nuclei of ß-catenin– MPCs, Osx was confined to the
cytoplasm, implying a mechanism that did not involve direct p53-Osx interaction. Furthermore, we found that p53’s anti-osteogenic activity was dependent on its DNA-binding ability. Our
findings identify chondrocytes as an additional source for MPCs and indicate that Wnt/ß-catenin discretely regulates chondrocyte to C-MPC and the subsequent C-MPC to osteoblast developments.
Most of all we unveil a previously unrecognized functional link between ß-catenin and p53, placing p53’s negative role in the context of Wnt/ß-catenin signaling-induced MPC osteogenic
differentiation. SIMILAR CONTENT BEING VIEWED BY OTHERS ATP6AP2, A REGULATOR OF LRP6/Β-CATENIN PROTEIN TRAFFICKING, PROMOTES WNT/Β-CATENIN SIGNALING AND BONE FORMATION IN A CELL TYPE
DEPENDENT MANNER Article Open access 29 May 2024 MAPK7 ENHANCES OSTEOGENESIS AND SUPPRESSES ADIPOGENESIS BY ACTIVATING LRP6/Β-CATENIN SIGNALING AXIS IN MESENCHYMAL STEM CELLS Article Open
access 26 February 2025 CUL4B ORCHESTRATES MESENCHYMAL STEM CELL COMMITMENT BY EPIGENETICALLY REPRESSING KLF4 AND C/EBPΔ Article Open access 02 June 2023 INTRODUCTION Endochondral bone
formation occurs through a cartilage to bone conversion process, during which cartilaginous tissue serves both as a template for ossification and as an innate source of
osteoblasts1,2,3,4,5,6. The cellular means by which a fully differentiated chondrocyte gains the plasticity to evolve into a mature osteoblast, as well as what signaling pathways govern this
event, remains elusive. Canonical Wnt signaling plays diverse roles at different stages of bone development and growth5,6,7,8,9,10. In _Osx_-expressing MPCS, Wnt/ß-catenin plays a switch
role between osteogenic and adipogenic fates. Despite the lack of convincing evidence7, it is currently accepted that ß-catenin promotes osteogenesis through activating _Runx2_ and/or _Osx_
transcription11,12,13. p53 is a well-established tumor suppressor. It is also a vital regulator of cell fate and differentiation14. The precise functions and regulatory mechanisms of p53’s
physiological roles remain much less understood and appreciated. In limited reports, crosstalk between p53 and Wnt/ß-catenin signaling has been shown to play various roles in a
context-dependent manner, such as in smooth muscle cells15 and in embryonic stem cells16. p53 exhibits osteo-inhibitory activity in various mouse models17,18 Nonetheless, p53 downstream
molecular events leading to osteogenic inhibition are not yet defined. p53 null marrow mesenchymal stem cells are more osteogenic and display no apparent difference in their adipogenic and
chondrogenic capacities19,20. One study shows that p53 inhibits osteoblastic differentiation through microRNA-34-mediated _Runx2_ suppression21,22. To date, the physiological context of this
inhibitory function remains entirely elusive. Here we used Collagen X (_Col10a1_) and Aggrecan (_Agc1_)-driven ß-catenin conditional lineage-tracing mice to delineate how Wnt/ß-catenin
regulates chondrocyte to osteoblast reprogramming. We showed that chondrocytes evolved into osteoblasts through at least two steps, which were differentially regulated by Wnt/ß-catenin.
Mechanistically, we discovered that the ß-catenin-deficient MPCS acquired elevated p53 activity and their defect in osteogenic capacity was fully reinstated by merely deleting p53 from them,
indicating that Wnt/ß-catenin promotes osteogenesis via a p53 suppression-dependent mechanism. RESULTS CHARACTERIZATIONS OF CHONDROCYTE CRE-MEDIATED Β-CATENIN MUTANT MICE REVEAL AN INVERSE
CORRELATION BETWEEN THE TRABECULAR VOLUMES AND THE NUMBERS OF CHONDROCYTE-DERIVED STROMAL CELLS To acquire a mechanistic understanding of the Wnt/β-catenin action initiated from
chondrocytes, we generated chondrocyte-lineage-tracing mouse models containing either deleted or stabilized ß-catenin alleles, and systematically quantified and compared the
reporter-expressing cells categorized by location and association with bone matrix. In the femurs of postnatal animals, an abundant number of Tomato-expressing (Tm+) cells was observed
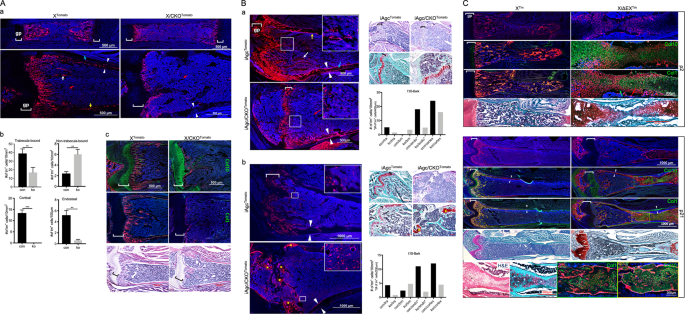
within the marrow cavity of both _Col10a1-Cre_;_ROSA26R-Tomato_ (XTomato) control and _Col10a1-Cre_;_Ctnnb1__fl/fl_;_ROSA26R-Tomato_ (X/CKOTomato) mutant animals, each of which presented a
distinct pattern of distribution. At postnatal day 2 (p2) of XTomato control animal, the majority of _Col10a1-Cre_-induced Tm+ (X/Tm+) cells were localized at the primary spongiosa
physically in contact with trabeculae (trabecula-bound) and showed mature osteoblast-like morphology (Fig. 1Aa). In contrast, most of the Tm+ (XCKO/Tm+) cells in the p2 X/CKOTomato mutant
animal were scattered rather evenly throughout the marrow cavity. Most of them were not connected with trabeculae (non-trabecula-bound) and were morphologically distinct from mature
osteoblasts (Fig. 1Aa). Similarly, at p16, there were more non-trabecula-bound Tm+ cells in the X/CKOTomato mutant than in the XTomato control mice (6/ko vs 2.4/con cells/10 mm2), whereas
fewer XCKO/Tm+ cells were found on the endostea (0.63/ko vs 6/con cells/100 µm), embedded within cortices (0/ko vs 13.4/con cells/10 mm2) or trabecular matrixes (16.6/ko vs 38.9/con cells/10
mm2) compared to the X/Tm+ cells (Fig. 1Ab). This phenotype persisted and became progressively pronounced with age. In the marrow of 4- and 8-month-old X/CKOTomato mice, there were visibly
more non-trabecula-bound XCKO/Tm+ cells than in that of p16, whose non-trabecula-bound XCKO/Tm+ cells remained scattered among the stromal cells and were not embedded within the cortexes
(Supplementary Fig. S1a, b). To validate, we generated _Agc1-CreERT2_;_ROSA26R-Tomato (_iAgcTomato) and _Agc1-CreERT2_;_Ctnnb1__fl/fl_;_ROSA26R-Tomato_ (iAgc/CKOTomato) mice. These mice were
analyzed after 6- and 8-week chases post tamoxifen injection at p10. The iAgc/CKOTomato mice developed a low trabecular bone volume phenotype resembling that of the X/CKOTomato mice (Fig.
1B). After the 6-week chase, numbers of non-trabecula-bound iAgcCKO/Tm+ cells were substantially higher than that of non-trabecula-bound iAgc/Tm+ cells (3.5/ko vs 0.3/con cells/10 mm2).
Conversely, numbers of the trabecula-bound, endosteum, and cortex-embedded iAgcCKO/Tm+ cells were all fewer than that of the corresponding iAgc/Tm+ cells (trabecula: 1.5/ko vs 5.2/con
cells/10 mm2; endosteum: 5/ko vs 8/con cells/400 µm; cortex: 16/ko vs 24/con cells/10 mm2) (Fig. 1Ba). The inverted correlation was preserved after the 8-week chase (non-trabecula-bound:
4.8/ko vs 2.4/con cells/10 mm2; trabecula-bound: 0.8/ko vs 4.8/con cells/10 mm2; endosteum: 2/ko vs 11/con cells/200 µm; cortex: 4.5/ko vs 12/con cells/10 mm2) (Fig. 1Bb). The phenotype
gradually intensified with prolonged chases (Supplementary Fig. S2a–c). In contrast, the _Col10a1-Cre_;_Ctnnb1EX3__fl/fl_;_ROSA26R-Tomato_ (X/ΔEXTomato) mice with stabilized β-catenin showed
delayed primary ossification and developed an osteopetrosis-like phenotype after birth, opposite of the X/CKOTomato mice (Fig. 1C and Supplementary Fig. S3a–c). In the p2 X/ΔEXTomato
animal, the humeral cavity was occupied by a rod-shaped structure in place of the primary ossification center as in XTm control (Fig. 1C). This aberrant structure was made of a mixture of
mineralized cartilage (Col10+) and bone (Col1+) matrixes and was filled with XΔEX/Tm+ cells. It appeared that many XEX/Tm+ cells, especially toward the middle, were producing both Col10 and
Col1 (Fig. 1C). Amounts of Col1 and Col10 by IF staining appeared in reverse correlation within the same cell (Fig. 1C). In addition, Col1 staining was lining the rod immediately adjacent to
XΔEX/Tm+ bone cells (Fig. 1C). In the p21 X/ΔEXTomato femur, Saf-O staining and anti-Col10 IF revealed an increased trabecular volume and a broadened hypertrophic zone of the distal growth
plate (Fig. 1C). Essentially all XΔEX/Tm+ cells at the metaphyseal region were in direct contact with either the trabeculae or the cortices, and very few were dispersed in the bone marrow
(Fig. 1C). The proximal marrow cavity was occupied by a rod-shaped mineralized tissue comprised of XΔEX/Tm+ cells similar to that of p2, suggesting that the anomalous structures might be
remnants from earlier stages (Fig. 1C). By 1 month of age, the distal phenotype became more pronounced (Supplementary Fig. S3c), whereas the proximal aberrant structure gradually ceased to
exist. Collectively, these data indicated an inverse correlation between the number of non-trabecula-bound Tm+ cells and trabecular volumes in chondrocyte-conditional β-catenin mutants. We
speculated that stromal non-trabecula-bound reporter+ cells may be precursors to trabecula-bound reporter+ cells. Β-CATENIN DISCRETELY REGULATES CHONDROCYTES TO MESENCHYMAL PROGENITOR CELLS
(C-MPCS) AND SUBSEQUENT C-MPCS TO MATURE OSTEOBLAST PROCESSES To establish the identity of non-trabecula-bound stromal reporter+ cells, the total marrow stromal cells were collected from
_Col10a1-Cre_;_ROSA26R-YFP_ (XYFP) mice and sorted for YFP-positive (YFP+: 0.08%/2.5-month, 0.16%/5.5-month) cells by fluorescence-activated cell sorting (FACS) (Supplementary Fig. S4a). The
sorted YFP+ cells were grown in culture and then harvested for analysis. Almost all YFP+ stromal cells showed positive signals for MSPC markers: Sca1+(99.81%), CD140a+(90.85%),
CD140b+(99.97%), CD105+(96.78%), and negative signals for hematopoietic cell marker CD45, endothelial cell marker CD31, and erythroid cell marker Ter119 (Fig. 2Aa). Alternatively, we
analyzed MSPC marker expression of fresh prepared stromal cells from XTomato mice and found that fractions of the Tm+ stromal cells showed positive signal for CD140a (11.9%) and CD105
(10.9%) (Supplementary Fig. S4b). Moreover, the YFP+ stromal cells were clonogenic and exhibited mesenchymal tri-lineage capacities in vitro (Fig. 2Aa). _Osx_ is expressed in bone marrow
mesenchymal progenitor cells (MPCs)23. To evaluate _Osx_ expression in chondrocyte-derived non-trabecula-bound stromal cells, we did experiments using _Col10a1-Cre_;_Osx__fl/+_ (X/Osxfl/+)
mice, in which _Osx_-expressing cells are identified by GFP upon Cre-mediated LoxP recombination24. Taking the approach shown in Supplementary Fig. S4, we found that approximately 35% of the
adherent stromal cells were GFP+ cells (Supplementary Fig. S4c). To confirm the histological quantification of non-trabecula-bound reporter+ cells shown in Fig. 1Ab, marrow stromal cells
were collected from 5- to 6-week-old XTomato (Con) and X/CKOTomato (CKO) mice for flow analysis. It revealed an elevated percentage of Tm+ stromal cells from CKO mice compared to that from
control mice (CKO/0.52% vs Con/0.15%, _n_ = 6, *_p_ < 0.05) (Fig 2Ab). Alternatively, marrow stromal cells of 3-week-old XTomato (Con), X/CKOTomato (CKO), and X/ΔEXTomato (ΔEX) mice were
collected and grown in culture. The attached cells were analyzed by FACS. As shown in the fresh isolated marrow cells (Fig. 2Ab), there was an increase in the percentage of β-catenin-Tm+
stromal cells (61.2%) and deep drop in percentage of Tm+ stromal cells (1.8%) compared to that of control Tm+ stromal cells (24.2%) (Fig. 2Ab) from X/ΔEXTomato mice. These results validated
histological quantification (Fig. 1Ab). We isolated the Tm+ stromal cells from the XTomato mice using the same approach as in Fig. 2Aa and found that they were negative for CD45 and CD31
proteins (Supplementary Fig. S4d). Unlike the chondrocyte-derived Tm+ stromal cells from control mice (Fig. 2Aa, Ac), the ß-catenin–Tm+ stromal cells completely failed to form any
mineralized nodules while intensely favoring adipogenic differentiation (Fig. 2Ac and Supplementary Fig. S6). In addition, both control Tm+ and ß-catenin–Tm+ cells were capable of forming
CFU-Fs. We observed a robust increase in the number of CFU-Fs from X/CKOTomato stromal cells compared to those from XTomato mice (Fig. 2Ac). Furthermore, after being transduced by
lenti-β-catenin, the non-trabecula-bound ß-catenin–Tm+ stromal cells reinstated osteoblastic differentiation (Fig. 2Ac), implying that the cell-autonomous ß-catenin deficiency was possibly
accountable for the altered differentiation potential. Histological analyses of tamoxifen chase experiments in our previous report1 show that the non-trabecula-bound Tm+ stromal cells first
appear in small numbers at the chondral-osteo junction of growth plate, and gradually increase in number and spread into marrow cavity. Here, we isolated stromal cells from tamoxifen-treated
iAgcTomato (iAgc) and _ROSA26R-Tomato_ (Tm/Con) mice after a 4- and a 10-day chase, and quantified Tm+ cell portions by FACS. Shown in Fig. 2Ad, the percentage of Tm+ stromal cells from the
mice chased for 10 days was significantly higher than that for 4 days (0.18% vs 0.11%, _n_ = 3, *_p_ < 0.05). In a separate experiment, we administered tamoxifen to p13
_Ctnnb1__fl/+_;_ROSA26R-Tomato_ (Con), _Agc1-CreERT2_;_Ctnnb1__fl/+_;_ROSA26R-Tomato_ (iAgc/Con), and _Agc1-CreERT2_;_Ctnnb1__fl/fl_;_ROSA26R-Tomato_ (iAgc/CKO) mice, and after a 10-week
chase, stromal cells were isolated and plated in culture. Flow analysis revealed a higher percentage of β-catenin– Tm+ cells from iAgc/CKO animals compared to that from iAgc/Con mice (Fig.
2Ad), which was similar to that in X/CKO mice (Fig. 2Ad). These results validated findings shown in Fig. 1Ba, Bb. FACS analysis found the presence of iAgc/Tm+Sca1+ and iAgc/Tm+CD140a+
stromal cells in the marrow of iAgcTomato mice (Supplementary Fig. S5). Our data demonstrated that chondrocyte-derived non-trabecular-bound stromal cells were _Osx_-expressing cells and
processed MSPC capacities. In addition, β-catenin– Tm+ cells heavily favored adipogenic differentiation at the expense of osteogenic differentiation in a similar fashion to the
_Osx_-Cre-mediated β-catenin– MPCs7. Together these results supported the idea that chondrocytes were able to give rise to a population of MPCs—C-MPCs, which is a subpopulation in the total
_Osx_-expressing MSPC pool. The finding of C-MPCs along with our previous observation of a sequential emergence of iAgc/Tm+ stromal cells followed by iAgc/Tm+GFP+ osteoblasts1 leads us to
hypothesize that chondrocyte to osteoblast reprogramming may take place in at least two steps: chondrocytes to C-MPCs and subsequently C-MPCs to osteoblasts. To delineate how precisely
Wnt/β-catenin signaling governs chondrocyte to osteoblast transformation, we did two separate pairs of expression profiling comparisons: (1) between growth plate chondrocytes_;_ and (2)
between C-MPCs of X/CKOTomato mutant and control animals (Fig. 2B). Total chondrocyte RNAs were extracted from the growth plate chondrocytes dissected from 7-day-old X/CKOTomato and control
littermates, and total C-MPC RNAs were extracted from the non-trabecula-bound Tm+ stromal cells of 5-week-old X/CKOTomato and _Col10a1-Cre_;_Ctnnb1__fl/+_;_ROSA26R-Tomato_ (X/CHetTomato)
control mice. The RNA-seq expression profiling of growth plate chondrocytes showed only 23 genes with equal to or more than two-fold changes in expression levels, out of over ten thousand
genes detected in the experiment (Table 1 and Supplementary Table S8a), consistent with anti-Col10 staining shown in Fig. 1Ac. In sharp contrast, the expression profile of ß-catenin– C-MPCs
was extensively different from that of control C-MPCs. A total of 1633 genes showed equal to or more than two-fold difference in expression levels, with 790 genes up and 843 genes
downregulated (Table 1 and Supplementary Table S8b). This result is in line with the severely altered bone and marrow phenotype. The profiling results offered additional validity to our
interpretation that ß-catenin activity in hypertrophic chondrocytes is not needed for their C-MPCs-forming activity. The ß-catenin– C-MPCs expressed lower levels of osteoblast marker genes
such as _Col1a1_, _Dmp1_, and _Bglap_, as one would expect (Table 2). However, expression levels of osteogenic transcription factors _Runx2_, _Osx_, and _Dlx5_ were not found to be
significantly changed (Table 2). The result was validated by qPCR (data not shown). Collectively, the data indicate that loss of ß-catenin activity in hypertrophic chondrocytes did not
prevent formation of C-MPCs, which nonetheless were dependent on ß-catenin function to differentiate into mature osteoblasts. Β-CATENIN NEGATIVELY REGULATES P53 IN MPCS INCLUDING
CHONDROCYTE-DERIVED PROGENITOR CELLS (C-MPC) Ingenuity pathway analysis (IPA) projected p53 as the top upstream regulator (Table 3) contributing to the cellular outcomes due to loss of
ß-catenin. To validate, we did culture-based recombination experiments to achieve ß-catenin (_Ctnnb1_) and/or p53 deletions in MPCs. The qPCR confirmed that adeno-Cre (Ad-Cre) efficiently
deleted _Ctnnb1_ and/or _p53_ conditional alleles (Fig. 3Aa). The Ad-Cre infected _Ctnnb1__fl/fl__tm_ MPCs (ß-catenin–Tm+ MPCs) showed a 2.85-fold increase in _p53_ expression compared to
the mock treated _Ctnnb1__fl/fl__tm_ MPCs (Fig. 3Aa). A slight increase of _p53_ expression was detected in _Ctnnb1__fl/fl__tm_ C-MPCs from X/CKOTomato mice relative to control C-MPCs
(Supplementary Fig. S7). The p53 protein was slightly higher in the ß-catenin–Tm+ MPCs, in spite of incomplete ß-catenin deletion (Fig. 3Ab). Immunocytochemistry (ICC) validated that
ß-catenin was efficiently removed from both ß-catenin–Tm+ and ß-catenin–p53–Tm+ MPCs, in reference to control MPCs (Fig. 3Ac). The nuclei of ß-catenin–Tm+ MPCs were intensely stained by
anti-p53 antibody (green), while no signal was detected in control MPCs (Fig. 3Ac). The ß-catenin–Tm+ C-MPCs exhibited characteristic senescent cell morphology, being cube-shaped, much
larger, and flatter with little or no dendritic extensions distinct from spindle-shaped control MPCs including C-MPCs. Acidic β-galactosidase (β-gal) assay revealed a higher number of β-gal+
cells in ß-catenin– MSPC population. This phenotype was attenuated by removal of p53 (Fig. 3Ba). In addition, there was a significant decline in percentage of EdU+ ß-catenin–Tm MPSCs
compared to that of control MPCs, and this reduction was entirely reversed by depleting p53 (Fig. 3Bb). Reintroducing β-catenin to β-catenin–Tm+ C-MPCs reversed their senescent cell-like
morphology (Fig. 3Bc). A similar morphological transformation took place in β-catenin–Tm+ C-MPCs infected by lenti-shp53 (Fig. 3Bc). Furthermore, lenti-β-catenin-infected ß-catenin–Tm C-MPCs
lowered expressions of p53 targets, including _p21_, _Mdm4_, _Puma_, _Bax_, and _Noxa_ (Fig. 3Bd). Collectively, these data substantiated that p53 activity is indeed elevated in ß-catenin–
MPCs including C-MPC subpopulation, indicating that Wnt/ß-catenin negatively regulates p53 in these cells. DELETING P53 FROM Β-CATENIN– MPCS FULLY RESTORED OSTEOGENIC DIFFERENTIATION The
ß-catenin–p53–Tm+ MPCs showed higher ALP activity than the ß-catenin–Tm+ MPCs (Fig. 4A). Consistently, the ß-catenin–p53–Tm+ MPCs fully mineralized in vitro shown by Von Kossa staining and
by Col1 and Ocn productions revealed by ICC (Fig. 4B), demonstrating that p53 depletion from β-catenin– MPCs sufficiently released osteogenic inhibition. To verify, we used an alternative
method to attain β-catenin inactivation. Shown in Fig. 4C, β-catenin inhibitor XAV939-treated p53–Tm+ MPCs maintained osteogenic capacity as the vehicle-treated p53–Tm+ MPCs. The uCT imaging
revealed that trabecular bone loss in the X/CKO mice was mostly recovered in the Col_10a1-Cre_;_Ctnnb1__fl/fl_;_p53__fl/fl_ (X/DKO) mice (Fig. 4D), presenting in vivo proof for the
essential negative role of p53 in ß-catenin– MSPC osteogenic defect. To evaluate if p53 DNA-binding activity is involved in its anti-osteogenic function, we crossed _p53__R245W_ allele to
_Ctnnb1__fl/fl__tm_ mice to generate _p53__R245W/+__Ctnnb1__fl/fl__tm_ and _p53__R245W/R245W__Ctnnb1__fl/fl__tm_ mice. The p_53__R245W_ allele contains a hot spot mutation in DNA-binding
domain that completely abolishes its DNA-binding ability25. It took as few as 4 days for ß-catenin–p53R245WR245WTm+ MPCs to mineralize in in vitro osteogenic differentiation assay (Fig. 4E),
implying that p53’s anti-osteogenic ability is dependent on its DNA-binding activity. ICC revealed that in control and β-catenin–Tm+ MPCs, Runx2 was barely detectable in the absence of
osteogenic stimuli except for sporadic relatively brighter Runx2+ granules in the cytoplasm of control MPCs (Fig. 5A). In comparison, the nuclei of ß-catenin–p53–Tm+ MPCs showed relatively
more apparent Runx2 signal (Fig. 5A). Meanwhile, Osx was detected in both cytoplasmic and nuclear compartments of control MPCs, with a slightly brighter nuclear peripheral staining and a few
in granule-form (Fig. 5A), whereas in the β-catenin–Tm+ MPCs, Osx was devoid from the nuclei and was only observed in granule-from in cytoplasm (Fig. 5A). Remarkably, the nuclei of
β-catenin–p53– Tm+ MPCs were intensely stained by anti-Osx antibody and the signal became even stronger after 7 days of osteogenic induction, while in the β-catenin–Tm+ MPCs, osteogenic
induction led to an increase in the number of Osx+ granules in cytoplasm, verified by apparent nuclear staining on the neighboring Tm– control cells (Fig. 5A). To validate, we did ICC on
DMSO and XAX939-treated MPCs (Figs. 4C and 5B). The anti-β-catenin and anti-p53 ICC confirmed sufficient inhibition of β-catenin and upregulation of p53 in the XAX939-treated MPCs (Fig. 5B).
Similar to β-catenin–Tm+ MPCs shown in Fig. 5A, Osx was mostly localized outside the nuclei of XAX939-treated MPCs, in contrast to vehicle-treated MPCs (Fig. 5B). In addition, the
β-catenin–Tm+ C-MPCs from X/CKOTomato mice exhibited Osx cellular localization pattern (Fig. 5C) similar to the β-catenin–Tm+ (Fig. 5A) and the XAX939-treated MPCs (Fig. 5B). β-Catenin–
C-MPCs were more adipogenic than their control counterparts (Fig. 2Ac and Supplementary Fig. S6), consistent with β-catenin’s pro-osteo/anti-adipogenic property. We queried whether p53 had
any role in β-catenin’s anti-adipogenic function. We found that the β-catenin–p53–Tm+ MPCs maintained as much enhanced adipogenic capacity as the β-catenin–Tm+ MPCs (Fig. 5D), meaning that
removal of p53 from β-catenin–Tm+ MPCs had no impact on β-catenin deficiency-mediated adipogenic acceleration. In accordance, ICC with anti-C/EBPa and anti-Pparr antibodies showed no
discernable differences between the β-catenin–p53–Tm+ and β-catenin–Tm+ MPCs (Fig. 5D). We noticed that an ample number of β-catenin–p53– MPCs were adipocytes in their default state (Fig.
5D). DISCUSSION Despite emerging recognition of p53’s roles in non-transformed cells, the level of understanding of its anti-osteogenic function has barely progressed since over a decade
ago. Given that p53 is the most frequently mutated gene in osteosarcoma (OS)26, which arises from osteoblastic lineage cells, defining its role in osteoblast differentiation holds special
value to the hunt for etiological and pathogenetic mechanisms underlying OS tumorigenesis and an ultimate cure for OS. The revelation of chondrocytes as an innate source of osteoblasts has
received a divided response, largely due to the lack of a plausible explanation. Little has been accomplished since its discovery more than 6 years ago. Two independent studies found that
there are reduced numbers of Chon-obs in mice with Wnt/ß-catenin deletion in chondrocytes1,2. Both groups tested a variety of hypothetical causes for the declines including potential
alteration in proliferation and/or apoptosis in chondrocytes and/or Chon-obs. Their findings are conflicting and are inadequate to justify the Chon-obs phenotype. In our study we did not
find any evidence for the chondrocyte-derived “Osx” cells1 or for the severe defects in proliferation in both ß-catenin– chondrocytes and Chon-obs2 (Supplementary Fig. S8a, b). The
sequential emergence phenomenon of chondrocyte-derived non-trabecula-bound stromal cells and Chon-obs3 along with the “de-differentiation” feature shared by various organisms undergoing
trans-differentiation27 suggests that chondrocytes to osteoblasts reprogramming may also follow a similar “de-differentiation” mechanism. Here we gathered several lines of evidence in favor
of our hypothesis: (1) the C-MPCs’ progenitor cell-like properties (Fig. 2Aa, Ac and Supplementary Fig. S4). Although we do not yet fully understand their precise identity, our data are
sufficient to distinguish them from differentiated cells such as mature chondrocytes and osteoblasts. The marker profiles of Tm+ C-MPCs in fresh prepared samples represented a snapshot of
these cells in various progenitor states and should be expected to differ from that of culture-synchronized C-MPCs (Fig. 2Aa). (2) Sequential temporal relationship of Cre-induced reporter+
cells in the order of: chondrocytes (Tm+) – C-MPCs (Tm+GFP+ cells) – Chon-obs (Tm+GFP+) shown by tamoxifen chase experiments. (3) The finding of discrete regulations by Wnt/ß-catenin
substantiated by RNA-seq profiling and histological and differentiation analyses favors the stepwise idea (Fig. 2B and Supplementary Fig. S8). The full rescue by solely deleting p53 from
ß-catenin– MPCs suggested that ß-catenin likely does not directly regulate _Runx2_ and _Osx_ promoter activity. The partial rescue of trabecular volume in X/DKO mice solidified the key
negative role of p53 in the context of Wnt/ß-catenin pro-osteogenic function. Evaluation of whether there is any change in osteoclast differentiation compared to the X/CKO mice would prove
to be an interesting follow-up. Since p53 and Osx were not concurrently localized in the same cellular compartment of ß-catenin– MPCs, it is implausible that a p53-Osx physical interaction
could be the reason for osteogenic inhibition28. Of great ongoing interest is further understanding of the granule-form of Osx and its transport regulation. Likewise, p53 upstream events
triggered by ß-catenin signaling are equally important and yet to be elucidated. Our study advanced understanding in two fundamental subjects: (1) identification of p53 as a key node
negatively involved in Wnt/ß-catenin-mediated osteogenesis; (2) revealing of a stepwise chondrocyte to osteoblast process independently regulated by Wnt/ß-catenin signaling (Fig. 6). Given
the broad roles of both p53 and canonical Wnt signaling, we hope that our basic findings will translate to benefit clinical research beyond the scope of bone disease. MATERIALS AND METHODS
EXPERIMENTAL ANIMALS _Col10a1-Cre_29, _Agc1-CreERT2_30, _Osx__fl/fl_31, _Ctnnb1__fl/fl_, _Ctnnb1EX3__fl/f_, _p53__R245W_25, _p53__fl/fl_, and _2.3Col1a1-GFP_ mice have been described.
_Ctnnb1EX3__fl/fl_ mice were provided by Dr Makoto Taketo of Kyoto University. _2.3Col1a1-GFP_ mice were provided by Dr David Rowe of University of Connecticut. _Ctnnb1__fl/fl_
(_B6.129-Ctnnb1__tm2Kem__/KnwJ_, Stock No: 004152), _ROSA26R-tdTomato_ (also as Ai9) (Gt (ROSA)26Sortm9(CAG-tdTomato) Hze, Stock No: 007909) and _ROSA26R-YFP_ (B6.129 × 1-_Gt
(ROSA)26Sor__tm1(EYFP)Cos_/J, Stock No: 006148) mice were purchased from the Jackson Laboratory. Tamoxifen (Sigma-Aldrich T-5648) was injected intraperitoneally at 1.5–3.0 mg/10 g body. All
animals were housed in pathogen-free conditions, procedures followed the rules and regulations of AAALAC and were approved by Institutional Animal Care and Use Committee of University of
Texas MD Anderson Cancer Center. ISOLATION OF BONE MARROW STROMAL CELLS Bone marrow nonhematopoietic stromal cells were isolated as described32. Total bone marrow cells were cultured in
alpha-MEM media containing 20% FBS under 5% O2 up to around 10 days. Attached cells were passaged for expansion. CELL SORTING AND FLOW CYTOMETRY Cell sorting experiments were performed on
Arial II Cell Sorter (BD Bioscience). Flow cytometry analyses were carried out using Gallios 561 (Beckman Coulter). Services were provided by MDACC NORTH Campus Flow Cytometry and Cellular
Imaging Core Facility. Data were analyzed using FlowJo or Kaluza. CFU-F AND IN VITRO DIFFERENTIATION ASSAYS Bone marrow plugs flushed out of femurs, tibias, and humeri were treated with
Collagenase I (3 mg/ml, Worthington) and Dispase II (4 mg/ml, Roche) as previously described32. Collected cells were plated 0.5–1 × 106/well in 6-well plates and cultured in alpha-MEM
media/20% FBS under 5% O2 for 10 days without changing media and were stained with crystal violet with methanol. R&D Mouse Mesenchymal Stem Cell Functional Identification Kit (R&D)
was used for in vitro osteogenic, adipogenic, and chondrogenic assays. IMMUNOFLUORESCENCE STAINING Long bones were fixed in freshly made 4% paraformaldehyde/PBS (pH 7.2) at 4 °C overnight
and changed to 14% EDTA for 2–7 days at 4 °C. Decalcified bones were immersed in 30% sucrose/PBS for 1 h before embedded in OCT compound. Then, 8–12 µm sections were prepared using CryoStar
NX70 Cryostat. Hyaluronidase treatment (2 mg/ml in PBS [pH 5.0]) was used for antigen retrieval, 20’ for embryonic or 30’ for postnatal tissue at 37 °C. Primary antibodies used were
anti-mouse collagen type I (Millipore AB765P, 1:50) and anti-Collagen X antibody (ab58632, 1:200). Secondary antibodies were Alexa fluor 488 goat anti-rabbit IgG and Alexa fluor 488 goat
anti-mouse IgG (Molecular probes). IF sections were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen P36931). CONFOCAL MICROSCOPY IMAGING Fluorescence images were captured
using A1 Laser scanning confocal microscope by Nikon Instruments at Microscopy Laboratory in the Department of Genetics at MDACC. RNA-SEQ AND ANALYSIS Tm+ C-MPCs were sorted by FACS from
fresh marrow of 5-week-old X/CKOTomato and X/CHetTomato mice, two of each genotype. Total RNAs were isolated using Quick-RNA Micro-prep kit (Zymo research), followed by additional DNase
treatment and purification (RNA clean and concentrator-5 kit, Zymo research). Around 100 ng total RNAs of each sample was sent to Sequencing and Microarray Facility at MDACC for
strand-specific RNA-Seq analysis. Libraries were made with Illumina’s TruSeq Stranded Total RNA Library Prep Kit and were sequenced in 76 paired-end format on Illumina Next Generation
Sequencing-HiSeq4000. X-GAL STAINING X-gal staining procedure was as described33. LENTIVIRUS AND ADENOVIRUS TRANSDUCTION Ad5-cmv-GFP and Ad5-cmv-Cre were purchased from Baylor College of
Medicine’s Vector Development Lab. Primary stromal cells were transduced with Ad5-cmv-Cre or Ad5-cmv-GFP at a concentration of 5000 pv/cell (8 µg/ml polybrene). After around 24 h, fresh
media was added to replace media containing adenoviruses. Lenti-ß-catenin was generously provided by Andrew Gladden of the Genetics department. Lentivirus plasmids pGIPZ2 (empty vector),
pGIPZ3 (non-specific shRNA), and pGIPZ-shp53 were purchased from MDACC Functional Genomics Core. REAL-TIME QPCR Quick-RNA Micro-prep kit (Zymo Research) was used to extract total RNAs. cDNAs
were synthesized using amfiRivert cDNA Synthesis Platinum Master Mix (Gendepot). qPCR reactions were made using amfiSure qGreen Q-PCR Master Mix(2X), Low Rox (Gendepot), and QuantStudio 6
(Applied Biosystems). Primer sequences were designed using Integrated DNA Technologies’s PrimerQuest tool. STATISTICS Statistical analysis was calculated by two-tailed, unpaired Student’s
_t_-test in GraphPad Prism 7.0. The mean values were presented. The error bars indicated SEM. DATA AVAILABILITY The data reported in the current study are available from the corresponding
authors upon request. REFERENCES * Zhou, X. et al. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice.
_PLoS Genet_ 10, e1004820 (2014). Article Google Scholar * Yang, L., Tsang, K. Y., Tang, H. C., Chan, D. & Cheah, K. S. Hypertrophic chondrocytes can become osteoblasts and osteocytes
in endochondral bone formation. _Proc. Natl Acad. Sci. USA_ 111, 12097–12102 (2014). Article CAS Google Scholar * Enishi, T. et al. Hypertrophic chondrocytes in the rabbit growth plate
can proliferate and differentiate into osteogenic cells when capillary invasion is interposed by a membrane filter. _PLoS ONE_ 9, e104638 (2014). Article Google Scholar * Park, J. et al.
Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. _Biol. Open_ 4, 608–621 (2015). Article Google Scholar *
Hill, T. P., Spater, D., Taketo, M. M., Birchmeier, W. & Hartmann, C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. _Dev. Cell_ 8,
727–738 (2005). Article CAS Google Scholar * Day, T. F., Guo, X., Garrett-Beal, L. & Yang, Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte
differentiation during vertebrate skeletogenesis. _Dev. Cell_ 8, 739–750 (2005). Article CAS Google Scholar * Song, L. et al. Loss of wnt/beta-catenin signaling causes cell fate shift of
preosteoblasts from osteoblasts to adipocytes. _J. Bone Min. Res._ 27, 2344–2358 (2012). Article CAS Google Scholar * Bao, Q. et al. Constitutive beta-catenin activation in osteoblasts
impairs terminal osteoblast differentiation and bone quality. _Exp. Cell Res._ 350, 123–131 (2017). Article CAS Google Scholar * Kramer, I. et al. Osteocyte Wnt/beta-catenin signaling is
required for normal bone homeostasis. _Mol. Cell Biol._ 30, 3071–3085 (2010). Article CAS Google Scholar * Houben, A. et al. beta-catenin activity in late hypertrophic chondrocytes
locally orchestrates osteoblastogenesis and osteoclastogenesis. _Development_ 143, 3826–3838 (2016). CAS PubMed PubMed Central Google Scholar * Nakashima, K. et al. The novel zinc
finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. _Cell_ 108, 17–29 (2002). Article CAS Google Scholar * Otto, F. et al. Cbfa1,
a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. _Cell_ 89, 765–767 (1997). Article CAS Google Scholar * Ducy, P.,
Zhang, R., Geoffroy, V., Ridall, A. L. & Karsenty, G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. _Cell_ 89, 747–754 (1997). Article CAS Google Scholar *
Jain A. K., Barton M. C. p53: emerging roles in stem cells, development and beyond. _Development_ 145, dev158360 (2018). * Riascos-Bernal, D. F. et al. beta-Catenin C-terminal signals
suppress p53 and are essential for artery formation. _Nat. Commun._ 7, 12389 (2016). Article CAS Google Scholar * Wang, Q. et al. The p53 family coordinates Wnt and nodal inputs in
mesendodermal differentiation of embryonic stem cells. _Cell Stem Cell_ 20, 70–86 (2017). Article CAS Google Scholar * Wang, X. et al. p53 functions as a negative regulator of
osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. _J. Cell Biol._ 172, 115–125 (2006). Article CAS Google Scholar * Lengner, C. J. et al. Osteoblast
differentiation and skeletal development are regulated by Mdm2-p53 signaling. _J. Cell Biol._ 172, 909–921 (2006). Article CAS Google Scholar * He, Y. et al. p53 loss increases the
osteogenic differentiation of bone marrow stromal cells. _Stem Cells_ 33, 1304–1319 (2015). Article CAS Google Scholar * Boregowda, S. V. et al. Basal p53 expression is indispensable for
mesenchymal stem cell integrity. _Cell Death Differ._ 25, 679–692 (2018). Article CAS Google Scholar * Kwak, B., Kim, D. U., Kim, T. O., Kim, H. S. & Kim, S. W. MicroRNA-552 links Wnt
signaling to p53 tumor suppressor in colorectal cancer. _Int J. Oncol._ 53, 1800–1808 (2018). CAS PubMed Google Scholar * Kim, N. H. et al. p53 and microRNA-34 are suppressors of
canonical Wnt signaling. _Sci. Signal_ 4, ra71 (2011). PubMed PubMed Central Google Scholar * Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal
progenitors during bone marrow development. _Dev. Cell_ 29, 340–349 (2014). Article CAS Google Scholar * Akiyama, H. et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing
precursors. _Proc. Natl Acad. Sci. USA_ 102, 14665–14670 (2005). Article CAS Google Scholar * Xiong, S. et al. Pla2g16 phospholipase mediates gain-of-function activities of mutant p53.
_Proc. Natl Acad. Sci. USA_ 111, 11145–11150 (2014). Article CAS Google Scholar * Roberts, R. D. et al. Provocative questions in osteosarcoma basic and translational biology: a report
from the Children’s Oncology Group. _Cancer_ 125, 3514 (2019). Article Google Scholar * Merrell, A. J. & Stanger, B. Z. Adult cell plasticity in vivo: de-differentiation and trans
differentiation are back in style. _Nat. Rev. Mol. Cell Biol._ 17, 413–425 (2016). Article CAS Google Scholar * Artigas, N. et al. p53 inhibits SP7/Osterix activity in the transcriptional
program of osteoblast differentiation. _Cell Death Differ._ 24, 2022–2031 (2017). Article CAS Google Scholar * Gebhard, C., Hattori, T., Bauer, E., Schlund, B., Bösl, M. R., de
Crombrugghe, B., von der Mark, K. Specific expression of Cre recombinase in hypertrophic cartilage under the control of a BAC-Col10a1 promoter. Matrix Biology 27(8), 693-699 (2008). * Henry,
S. P., Jang, C.-W., Deng, J. M., Zhang, Z., Behringer, R. R., de Crombrugghe, B. Generation of aggrecan-CreERT2knockin mice for inducible Cre activity in adult cartilage. genesis: NA-NA
(2009). * Akiyama, H., Kim, J.-E., Nakashima, K., Balmes, G., Iwai, N., Deng, J. M., Zhang, Z., Martin, J. F., Behringer, R. R., Nakamura, T., de Crombrugghe, B. Osteo-chondroprogenitor
cells are derived from Sox9 expressing precursors. Proceedings of the National Academy of Sciences 102(41), 14665–14670 (2005). * Suire, C., Brouard, N., Hirschi, K. & Simmons, P. J.
Isolation of the stromal-vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. _Blood_ 119, e86–e95 (2012). Article CAS Google Scholar *
Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells
in culture and in vivo. _Nat. Protoc._ 4, 1798–1806 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank K. von der Mark, D. Rowe, and M. Taketo for providing
_Col10a1-cre_, _2.3Col1a1-GFP_, and _Ctnnb1EX3__fl/fl_ mice, and A. Gladden for providing Lenti-ß-catenin. We would like to acknowledge Ailing Huang for technical assistant. We are grateful
for the excellent services provided by the Microscopy Laboratory supported by NIH-shared instrumentation grant 1S10OD024976-01 in the Department of Genetics, Bone Histomorphometry Core
Laboratory, North Campus Flow Cytometry and Cellular Imaging Core Facility, Advanced Technology Genomics Core, Small Animal Imaging Facility and Functional Genomics Core at MDACC. FUNDING
This work was supported by RO1 grant from National Institute of Health (AR049072 to B.d.C.) and by Swim Across America (to R. Gorlick). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division
of Pediatrics, University of Texas MD Anderson Cancer Center, Houston, TX, 77030, USA Xin Zhou, Zhaohui Xu & Richard Gorlick * Thomas Scientific, Swedesboro, NJ, USA Allyson Beilter *
Houston Methodist Research Institute, Houston, TX, USA Ruli Gao * Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX, 77030, USA Shunbin Xiong, Adriana
Paulucci-Holthauzen, Guillermina Lozano & Benoit de Crombrugghe Authors * Xin Zhou View author publications You can also search for this author inPubMed Google Scholar * Allyson Beilter
View author publications You can also search for this author inPubMed Google Scholar * Zhaohui Xu View author publications You can also search for this author inPubMed Google Scholar * Ruli
Gao View author publications You can also search for this author inPubMed Google Scholar * Shunbin Xiong View author publications You can also search for this author inPubMed Google Scholar
* Adriana Paulucci-Holthauzen View author publications You can also search for this author inPubMed Google Scholar * Guillermina Lozano View author publications You can also search for this
author inPubMed Google Scholar * Benoit de Crombrugghe View author publications You can also search for this author inPubMed Google Scholar * Richard Gorlick View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS X.Z. designed, carried out, analyzed the experiments, and wrote the manuscript. A.B. and Z.X. performed the experiments.
R.G. did RNA-seq bioinformatic analysis. A.P.H. provided imaging support. S.X. provided technical support. G.L. provided critical insights. B.d.C. and R.G. provided funding, supervised the
project, and finalized the manuscript. CORRESPONDING AUTHORS Correspondence to Xin Zhou or Richard Gorlick. ETHICS DECLARATIONS ETHICS STATEMENT Not applicable. CONFLICT OF INTEREST The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. Edited by M. Agostini SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE LEGENDS SUPPLEMENTARY FIGURE S1 SUPPLEMENTARY FIGURE S2 SUPPLEMENTARY FIGURE S3 SUPPLEMENTARY FIGURE S4
SUPPLEMENTARY FIGURE S5 SUPPLEMENTARY FIGURE S6 SUPPLEMENTARY FIGURE S7 SUPPLEMENTARY FIGURE S8 SUPPLEMENTARY FIGURE S9 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhou, X., Beilter, A., Xu, Z. _et al._ Wnt/ß-catenin-mediated p53
suppression is indispensable for osteogenesis of mesenchymal progenitor cells. _Cell Death Dis_ 12, 521 (2021). https://doi.org/10.1038/s41419-021-03758-w Download citation * Received: 08
February 2021 * Revised: 20 April 2021 * Accepted: 22 April 2021 * Published: 21 May 2021 * DOI: https://doi.org/10.1038/s41419-021-03758-w SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative
Trending News
North sea ‘taxed to death’, warns sir jim ratcliffeAdam Mawardi 15 May 2023 11:00pm BST Sir Jim Ratcliffe has warned that taxing the North Sea oil and gas industry “to dea...
Imagine, if you will, a valentine for your heart's contentH_ ow do they love each other? Let them count the ways: By the way they dress, the little things they say and do, the tr...
Jet airways shares plunge 22. 5 per cent, hit 1-year lowShares of Jet Airways plunged 22.5 per cent on May 2, 2019  NEW DELHI: Shares of Jet Airways plunged 22.5 per c...
[withdrawn] data protection if there’s no brexit dealGuidance DATA PROTECTION IF THERE’S NO BREXIT DEAL How the collection and use of personal data would change if the UK le...
How playing with a fractured hand once, made kedar jadhav the batsman he is todayKedar Jadhav came down the wicket to pacers and dealt with the slower bowlers with equal ease. His 12 fours and four six...
Latests News
Wnt/ss-catenin-mediated p53 suppression is indispensable for osteogenesis of mesenchymal progenitor cellsABSTRACT The developmental origins of mesenchymal progenitor cells (MPCs) and molecular machineries regulating their fat...
Van nistelrooy gives update on leicester city transfers amid coulibaly questionLeicester City boss Ruud van Nistelrooy kept his cards close to his chest regarding the club's January transfer bus...
The Ray Society† | NatureABSTRACT THE Council, in presenting their thirtieth Annual Report, congratulate the members upon the continued prosperit...
Rspb denies organic threat to birds - farmers weekly3 January 2001 RSPB denies organic threat to birds _BY FWI STAFF_ THE RSPB has distanced itself from claims that organic...
Error 404Error 404 No encontramos la página que buscas....