Phototoxic damage to cone photoreceptors can be independent of the visual pigment: the porphyrin hypothesis
Phototoxic damage to cone photoreceptors can be independent of the visual pigment: the porphyrin hypothesis"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Lighting is rapidly changing with the introduction of light-emitting diodes (LEDs) in our homes, workplaces, and cities. This evolution of our optical landscape raises major
concerns regarding phototoxicity to the retina since light exposure is an identified risk factor for the development of age-related macular degeneration (AMD). In this disease, cone
photoreceptors degenerate while the retinal pigment epithelium (RPE) is accumulating lipofuscin containing phototoxic compounds such as A2E. Therefore, it remains unclear if the
light-elicited degenerative process is initiated in cones or in the RPE. Using purified cone photoreceptors from pig retina, we here investigated the effect of light on cone survival from
390 to 510 nm in 10 nm steps, plus the 630 nm band. If at a given intensity (0.2 mW/cm²), the most toxic wavelengths are comprised in the visible-to-near-UV range, they shift to blue-violet
light (425–445 nm) when exposing cells to a solar source filtered by the eye optics. In contrast to previous rodent studies, this cone photoreceptor phototoxicity is not related to light
absorption by the visual pigment. Despite bright flavin autofluorescence of cone inner segment, excitation–emission matrix of this inner segment suggested that cone phototoxicity was instead
caused by porphyrin. Toxic light intensities were lower than those previously defined for A2E-loaded RPE cells indicating cones are the first cells at risk for a direct light insult. These
results are essential to normative regulations of new lighting but also for the prevention of human retinal pathologies since toxic solar light intensities are encountered even at high
latitudes. SIMILAR CONTENT BEING VIEWED BY OTHERS PHOTOTOXICITY OF LOW DOSES OF LIGHT AND INFLUENCE OF THE SPECTRAL COMPOSITION ON HUMAN RPE CELLS Article Open access 21 March 2024 WEEKLONG
IMPROVED COLOUR CONTRASTS SENSITIVITY AFTER SINGLE 670 NM EXPOSURES ASSOCIATED WITH ENHANCED MITOCHONDRIAL FUNCTION Article Open access 24 November 2021 PATHOGENIC MECHANISMS CONTRIBUTING TO
THE VULNERABILITY OF AGING HUMAN PHOTORECEPTOR CELLS Article 02 June 2021 INTRODUCTION Lighting is rapidly changing with the introduction of light-emitting diodes (LEDs) in our homes,
workplaces, and cities. These lighting modifications raise major concerns regarding phototoxicity to the retina and other ocular compartments1,2. In fact, all external tissues of the body
including skin and ocular structures (conjunctiva, cornea, lens, and retina) are daily exposed to natural and artificial lights. Light is a well-known environmental factor to cause diseases
of the skin with associated ocular symptoms3. Epidemiological studies have also indicated that light, and more precisely blue light, is an identified risk factor for the development of
age-related macular degeneration (AMD)4. Blue light, whatever its origin (natural sunlight or artificial lightings), carries the highest energy in the visible range and can thus be very
deleterious to cells. In addition, many natural molecules like the visual pigment derivative A2E are present in retinal cells and absorb light in the blue range rendering cells highly
photosensitive to blue light5,6. To further understand the etiology of AMD, different animal and cell models were used to uncover the mechanisms underlying blue light toxicity. In rodents,
rhodopsin and cone pigments have been suggested as the death-mediating chromophores to photoreceptors7. More precisely, photoreceptor degeneration was shown to be caused by prolonged or
constitutive opsin activation by light8,9. Opsin regeneration is required without the need for complete phototransduction cascade8,10,11. Consistent with this hypothesis, intermittent blue
light exposure induced the death of short wavelength sensitive cones in nonhuman primates whereas green light induced a transient loss of green light sensitive cones12. In vitro light damage
to cones was only examined using the 661W cell line, which has lost its typical morphology of photoreceptors13,14,15. With these cells, light was found to be more toxic in the presence of
all-trans retinal, which is obtained by light conversion of the natural opsin chromophore, the 11-cis retinal13. Because artificial and natural light toxicities were never examined on
primary isolated cone photoreceptors, we here investigated the effect of 10 nm wavelength bands onto freshly purified porcine cone photoreceptors16. We applied either the same light
intensity at all wavelengths to define the most toxic or at intensities of a solar source filtered by the eye optics. Results were interpreted based on autofluorescence examinations of cones
and of cone inner segment emission-excitation matrix. Finally, we measured sun light intensities in Paris to compare them with those used in this study. RESULTS PHOTOTOXICITY TO ISOLATED
CONE PHOTORECEPTORS To investigate if phototoxicity onto cone photoreceptors could be an early event in ocular diseases and acute light burns, we measured light toxicity on primary isolated
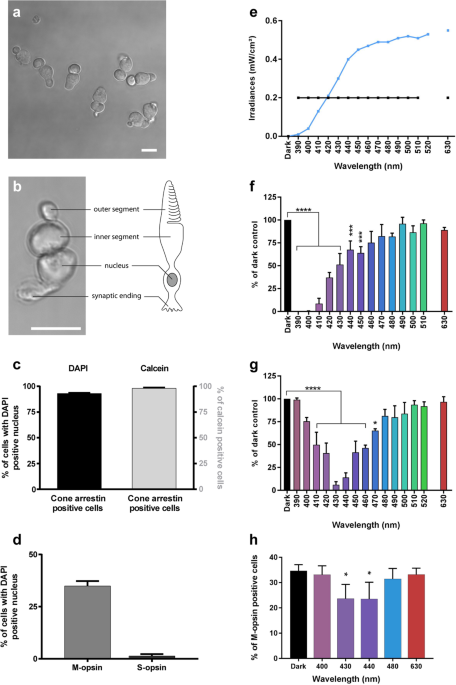
cone photoreceptors from pig retinas (Fig. 1a, b) using our dedicated light-emitting device delivering 10 nm-wide bands of light5. We first characterized the cell preparation regarding the
cell morphology, viability and purity with cone specific markers such as cone arrestin and opsins. Cells exhibited a typical cone photoreceptor morphology with inner and outer segment, cell
body and synaptic ending (Fig. 1a, b). The cell preparation was highly enriched with cone photoreceptors since 93% of cells with a DAPI-stained nucleus were immunopositive with a cone
arrestin antibody (Figs. 1c, 2a–f). Furthermore, when assessing the cell viability of our preparation with calcein green, we counted that 98% of viable cells were cone-arrestin
immunopositive (Figs. 1c, 2a–f). To further characterize the isolated cone photoreceptors, we used cone opsin antibodies against either the blue S-opsin or the green M-opsin. Among isolated
cells with a DAPI stained nucleus, 35% of them exhibited an outer segment immunopositive for M-opsin (green cones), only 1.3% of cells exhibited S-opsin immunopositive outer segment (blue
cones) (Figs. 1d, 3i–p). Because 98% of purified cells were cone photoreceptors as indicated by their immunolabelling for the cone arrestin protein, the opsin-negative cells, 63% of purified
cells, were most likely cone photoreceptors that had lost their outer segment during the dissociation procedure without losing their viability. We first exposed these purified cones to
light at a given irradiance (0.2 mW/cm²) for 15 h for each tested wavelength band (390–510 in 10 nm steps, plus the 630 nm band) to characterize their photosensibility to artificial light
(Fig. 1e, f). Blue-violet light (390–450 nm) emerges as highly toxic, with a maximum toxicity between 390 and 410 nm and a toxicity decreasing with increasing radiation wavelength up to 450
nm (Fig. 1f). These data demonstrate the potential toxicity of monochromatic LED light within the near-UV and blue-violet range. In a second step, we normalized light irradiances to account
for eye optic absorption and to evaluate effect of sunlight levels reaching the retina, as previously described5. Under these conditions, the most toxic bands were shifted to the 410–460 nm
range with a peak of toxicity at 430 and 440 nm (Fig. 1e, g). Indeed, at 430 nm, the survival rate decreased to 6% of the number of cone photoreceptor surviving in darkness. No significant
toxicity was observed with green light (Fig. 1g) although the preparation contained 35% M-opsin-positive (green) cones (Fig. 1d). To investigate if the cell toxicity was related to opsin
absorption, we quantified the number of surviving cells which were immunopositive for the M-opsin. Surprisingly, the ratio of M-opsin positive cells in surviving cones was decreased with a
statistical significance under blue light (430 and 440 nm, Fig. 1h), indicating that the relative loss of these green cones was not correlated to the amplitude of the M-opsin absorption
spectrum; the toxicity was greater at 430–440 nm than at 480 nm (Fig. 1h). We attributed this greater blue toxicity onto green-sensitive cones to their cell morphological integrity and the
likely light sensitivity of the inner segment (see below). Indeed, the green M-opsin immunolabelling is restricted to the outer segment, therefore it can only be seen in cone photoreceptors
with intact outer/inner segments. The absence of greater green light toxicity onto green cones suggests that, in our experimental conditions, light toxicity was independent of the visual
pigment. NONVISUAL PIGMENTS AND AUTOFLUORESCENCE IN CONE PHOTORECEPTORS To further understand the origin of this phototoxicity, we investigated the presence of other photosensitive pigments
in cone photoreceptors. On ex vivo alive retinas, we localized a bright autofluorescence in cone inner segments across species from pigs to nonhuman primates and even humans (Fig. 4a). This
bright fluorescence remained on vertical sections that were prepared by vibratome sectioning from the fresh tissue (Fig. 4b, d). Finally, in purified primary cone photoreceptors, the bright
fluorescence of their inner segments confirmed the high purity of our preparations from pig, nonhuman primate and even from postmortem human tissue (Fig. 4e, g, h, j). By contrast, rod inner
segments generate a weaker autofluorescence, slightly visible on the peripheral flat-mounted retina of the nonhuman primate (Fig. 4b, d). An intense autofluorescence was also localized in
cell bodies of retinal ganglion cells (Fig. 5a) and in their axon bundles (Fig. 5b). This autofluorescence in all these structures was attributed to mitochondria because it colocalized to
the mitochondrial ATP synthase immunolabelling (Figs. 6, 7). EXCITATION/EMISSION SPECTRA OF CONE AUTOFLUORESCENCE The autofluorescence in cone photoreceptor inner segment was bright (I488)
upon 488 nm excitation, weak upon blue excitation at 405 nm (≈33% of I488), and below detection thresholds (<1% of I488) upon red (594 nm) and far red (644 nm) excitation wavelengths. To
investigate the nature of this autofluorescent chromophore, we measured its fluorescence emission spectrum on freshly isolated pig retinas excited by a 475 nm laser source (Fig. 8a, b). Data
showed a high fluorescence emission peak centered at 550 nm in the cone inner segment (Fig. 8a, b). This chromophore is unlikely to provoke the light toxicity on cone photoreceptors, as one
would have expected to see a peak of toxicity at the absorption peak (488 nm) under the equal illumination irradiances (Fig. 1f). To further define the spectral signatures of chromophores
in cones, an excitation–emission matrix was measured in their inner segments (Fig. 8c). Cone inner segments were exposed to wavelengths ranging from 300 to 490 nm in 15 nm increments, and
the corresponding emission spectra were recorded. The resulting excitation–emission matrix was normalized to compensate for variations in the intensity of the excitation source (Fig. 8c).
Despite a measurement uncertainty below 400 nm due to the lower power of our excitation source, this analysis indicated that the green autofluorescence (540 nm) had two excitation peaks at
350 and 470 nm, which are highly reminiscent of the flavin spectral properties, supporting further a previous proposition for cone in vivo autofluorescence rather than other proposed
alternatives17. In this analysis, we observed an additional red autofluorescence (610 nm) with an excitation peak at 350 nm (Fig. 8c), which could be attributed to porphyrins18,19,20. This
absorption by porphyrins extending in the blue range is consistent with the observed spectrum of phototoxicity on cone photoreceptors (Supplementary information 2). The potential implication
of porphyrins in cone phototoxicity is further supported by their known photosensitization18. DISCUSSION Phototoxicity of mouse rod photoreceptors was previously attributed to light
absorption by rhodopsin10,11. We here observed a high toxicity of visible-to-near-UV light to all cone photoreceptors independently of their spectral sensitivity. Blue light was also shown
to produce irreversible damage exclusively to short-wavelength sensitive cones in nonhuman primates but green light did not irreversibly damage middle-wavelength cones12. This specific blue
light damage of short-wavelength cones could be related to their greater vulnerability compared to other cones21. The spectrum of cone damage is consistent with the presence of a
photosensitive chromophore with a spectral sensitivity in the visible-near-UV range. As cone cell damage was also observed in cones having lost their outer segment, this photosensitive
chromophore is likely located outside this specific cell compartment. The measured cone excitation–emission matrix is consistent with high levels of both flavins and porphyrins in cone
photoreceptor inner segment. Their location in cone inner segment is in agreement with their high concentrations in mitochondria22,23 and with the greater density of these organelles in the
cone inner segment24. The absence of increased phototoxicity at the blue peak of flavin absorption indicates that the phototoxicity is more likely induced by porphyrins. By contrast, the
overlap between published absorption spectra of porphyrins and the light toxicity on cone photoreceptors which we measured at equal irradiance (Supplementary information 2) supports the
porphyrin implication. In addition, the phototoxicity of porphyrin derivatives is widely known and taken to advantage in photodynamic therapy to erase cancer cells by light25,26,27. Blue
light has been defined as a risk factor in AMD, which is characterized by the macular loss of cone photoreceptors4. Here, normalizing the light irradiance to the solar spectrum reaching the
retina, we found out that the most toxic range is located within blue light at 425–445 nm because most of the near-UV light is filtered by the anterior segment of the eye. This toxicity
range was evaluated using moderate irradiances (0.39 mW/cm² at 440 nm) and overlaps the toxic light range previously determined on RPE aging models using higher irradiances5 (1.09 mW/cm² at
440 nm). A direct cone insult may therefore prevail over the RPE cell damage in the early phases of AMD. Filtering out these blue wavelengths selectively may efficiently protect from AMD
development while barely affecting color vision and circadian rhythms. These findings could be extended to other tissues daily exposed to blue-violet light such as the skin and ocular
surface and may explain symptoms in several diseases with an increased sensitivity to photo-oxidative stress3. Even if most of the injury is induced by UV-light, our study with a constant
spectral irradiance (Fig. 1f) indicates that blue-violet light and thus LED light exposure can contribute to cell damages as reported previously on skin cells28. Indeed, visible light
penetrates deeper than UV light, and blue-violet light can strongly contribute to oxidative stress in skin cells28. In corneal cells at the eye surface, both UV and blue-violet light
exposures can contribute to the development of pathologies like the dry-eye syndrome29,30. To investigate the relevance of these results to human pathologies, we compared our toxic light
irradiances to daily light levels. The irradiance of 0.39 mW/cm² over a 10 nm light band centered at 440 nm used in vitro corresponds approximately to 0.93 mW/cm² in the same spectral band
at the corneal level for a 40-year-old person. Comparatively, on a sunny morning (11:00 a.m.) in Paris during summer (August 29th, 2017), the irradiance level reached 0.46 mW/cm² (Table 1)
over the 10 nm-wide band centered at 440 nm when pointing 15° downward a calibrated spectroradiometer, towards low-reflecting surfaces on the fifth floor of a building with an almost clear
view, East orientation (Supplementary information 1). Over this very narrow band (440 ± 5 nm), the irradiance measured on a bright morning at the relatively high latitude of Paris (Table 1)
is only half the irradiance causing in vitro toxicity in our experiments. Taking into account the effect of other toxic wavelength bands (410–460 nm), lower latitudes, or higher albedos, a
pedestrian can encounter toxic blue light levels on a sunny summer day. Fortunately, the 15-h exposition inflicted on isolated cone photoreceptors is unlikely, but cumulative effects over a
week could become toxic since mitochondrial renewal is not finalized in a day: complete mitochondrial fusion–fission cycles were reported to take 16 days in the adult mouse31. These data
strongly indicate that cone phototoxicity can occur even at moderate latitudes, or under artificial lighting. This cell toxicity mechanism can easily explain retinal damage observed when
staring at the sun during solar eclipses32 or following indirect ground reflection in more exposed countries33,34. This photosensitization could also accelerate the degenerative process in
the context of retinal diseases like retinitis pigmentosa showing greater sensitivity of cones to oxidative stress24. Because the described phototoxicity of cone photoreceptors can affect
all the population including children, it is further reinforcing the concerns raised by the use of high-intensity LEDs with high blue contents1,2. MATERIALS AND METHODS MAMMALIAN RETINAS
Porcine eyes were bought at a local slaughterhouse in agreement with the local regulatory department and the slaughterhouse veterinarians (agreement FR75105131). This procedure adheres to
the European initiative for restricting animal experimentation because not a single animal was euthanized for our experimentation. Eyes were taken from animals daily sacrificed for food
production. Nonhuman primate retinas were prepared using eyes received from adult macaques (_Macaca fascicularis_) that were terminally anesthetized for unrelated studies. All animal
experiments and procedures were ethically approved by the French “Ministère de l’Education, de l’Enseignement Supérieur et de la Recherche” and were carried out according to institutional
guidelines in adherence with the National Institutes of Health guide for the care and use of laboratory animals as well as the Directive 2010/63/EU of the European Parliament. Postmortem
human ocular globes from donors were acquired from the School of Surgery (Ecole de Chirurgie, Assistance Publique Hôpitaux de Paris, Paris, France, CODECOH DC-2015–2400). The protocol was
approved by the IRBs of the School of Surgery and the Quinze-Vingts National Ophtalmology Hospital (Paris, France). All experiments on postmortem human retinal explants were performed
according to the local regulations, as well as the guidelines of the Declaration of Helsinki. TISSUE PREPARATION Eyes were cleaned up from muscles and incubated during 2 min in
Pursept-AXpress (Thermo Fisher Scientific, Waltham, MA, USA) for disinfection. The anterior segment was cut along the limbus to remove the cornea, lens and vitreous. The retina was carefully
removed from the eyecup and placed into CO2 independent medium (Life Technologies, Carlsbad, CA, USA). A 1 cm² piece was cut and either immediately flat-mounted in Permafluor medium (Thermo
Fisher Scientific) or included in 2–4% low melting agarose dissolved in a pre-warmed (+37 °C) custom modified Neurobasal A medium without any photosensitizer such as phenol red, riboflavin,
folic acid and aromatic amino acids (modified NBA, Life Technologies) for retinal sectioning. Transversal slices of 100 µm thickness were cut using a vibrating microtome (Leica, Wetzlar,
Germany) in modified NBA medium and then mounted in Permafluor medium (Thermo Fisher Scientific). Images were acquired without further delays. CELL ISOLATION Cone photoreceptors were
purified by lectin-panning selection as previously described16 and seeded in black clear bottom 96-well plates in a custom modified Neurobasal A medium without any photosensitizer such as
phenol red, riboflavin, folic acid and aromatic amino acids (modified NBA, Life Technologies) for light exposure. For live imaging, cells were isolated on specific 35 mm Petri dish with high
optical quality bottom for microscopy (IBIDI, Munich, Germany). LIGHT CONDITIONS Cells were exposed to 10 nm-wide light bands produced by a custom-made LED-based fibered illumination device
for 15 h as previously described by5. Each light band is designated by its central wavelength. Central wavelengths of the narrow light bands were equally distributed from 390 to 520 nm in
10 nm increments (14 narrow bands available). A 15th band with a central wavelength set at 630 nm was added. To model physiological light conditions reaching the retina, irradiances for each
band of light were calibrated according to a normalized daylight spectrum obtained by applying the natural ocular media filtering of a 40-year-old eye (CIE 203:2012) onto the referenced
solar spectrum (ASTM, G173-03, International standard ISO 9845-1, 19925,35). Accordingly, the 10-nm irradiances varied from 0.01 mW/cm² at 390 nm to 0.56 mW/cm² at 630 nm, with 0.39 mW/cm²
at 440 nm (Supplementary Fig. S6). Irradiances and spectra were measured using the calibrated spectroradiometer JAZ (Ocean Optics, Dunedin, USA). After a 4-h rest period following seeding in
a 96-well plate, cone photoreceptors were either exposed to the physiological solar spectrum or to 0.2 mW/cm² for each band of light. After light exposure, cells were maintained in darkness
for 24 h before viability measurement or fixation for immunocytochemistry. Irradiance measurements in real life at the corneal level were assessed using the spectroradiometer JAZ oriented
downward with a −15° toward a low-reflecting ground (to mimic the head inclination of a person walking) on the fifth floor of a building (GPS coordinates: 48.84983 and 2.372486) with an
almost clear view, East orientation, in a sunny morning (11:00 a.m.) in Paris during the summer (August 29th, 2017). Spectral irradiances (mW/cm²) were measured with a 1 nm step and then
integrated over 10 nm bandwidths to compare to the in vitro irradiances. VIABILITY MEASUREMENT To assess viability, calcein (Life Technologies) was added to the cell culture medium 24 h
after the end of light exposure and incubated during 1 h. The number of positive viable cells was then counted with an automated microscope equipped with a ×10 objective (Arrayscan, Thermo
Scientific, Rockford, IL, USA). Figure 1f_n_ = 3 for 390, 460, 470, 510 nm, _n_ = 4 for 410, 420, 450, 490, 500 nm, _n_ = 5 for 400, 430, 440, 480, 630. Figure 1g_n_ = 5 for 390, 460, 470,
510, 520 nm, _n_ = 7 for all other wavelengths. Experiments were excluded when surviving cells in the dark control condition were 25% below the number of viable seeded cells, indicating a
poor quality of the provided retina. Mean of the surviving cells observed in dark control was 50% of seeded cells. IMMUNOCYTOCHEMISTRY Cone photoreceptors were either fixed in the 96-well
plates or Petri dish with 4% paraformaldehyde after cell isolation during 15 min at room temperature (PFA, Sigma-Aldrich, St Louis, MO, USA) or 24 h after the end of light exposure during 1
h at room temperature. Cells were then washed three times with phosphate buffer saline (PBS) before being permeabilized with PBS 0.1% Triton X-100 (Sigma) for 3 min at room temperature and
washed again three times in PBS. To prevent nonspecific binding cells were incubated during 2 h at room temperature in a blocking buffer solution containing 1% BSA (Bovine Serum Albumin,
Sigma-Aldrich), 0.05% Tween 20 (Sigma-Aldrich) in PBS. Cells were immuno-stained overnight at +4 °C with a rabbit polyclonal antibody raised against S-opsin (Merck-Millipore, Billerica, MA,
USA), a rabbit polyclonal antibody raised against M-opsin (Merck-Millipore), with a rabbit polyclonal antibody directed against the human cone arrestin (LUMIf-hCAR/human cone arrestin
(ARR4), Cheryl Mae Craft, University of Southern California Roski Eye Institute, Los Angeles, CA36,37) or with a mouse antibody raised against ATP synthase subunit b (Life Technologies).
After primary immunostaining, cells were washed several times with PBS and incubated for 2 h at room temperature with either anti-rabbit Alexa 594 or anti-mouse Alexa 594 secondary
antibodies (Life Technologies). All antibodies were diluted in the blocking buffer solution. Nuclei were counterstained with 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI,
Sigma-Aldrich). IMMUNOHISTOCHEMISTRY For opsin labeling, porcine eyes without anterior segment were fixed with 4% paraformaldehyde overnight at +4 °C. Eyes were cryoprotected in successive
solutions of PBS containing 10, 20, and 30% sucrose at +4 °C before being embedded in NEG50 (Microm, Francheville, France). Retinal cryosections (12 µm) were cut using a cryostat (Microm).
For retinal flatmount immuno-labeling, freshly isolated retina were fixed with 4% paraformaldehyde during 1 h at room temperature. Retinal piece or sections were incubated 2 h at room
temperature in blocking buffer solution containing PBS, 1% Triton X-100, 5% BSA, 0.5% Tween 20. Retinal tissues or cryosections were immune-stained overnight at +4 °C with a rabbit
polyclonal antibody raised against S-opsin (AB5407, Merck-Millipore, Billerica, MA, USA), a rabbit polyclonal antibody raised against M-opsin (AB5405, Merck-Millipore), or with a mouse
antibody raised against ATP synthase subunit b (A-21351, Life technologies). All antibodies were diluted in the blocking buffer solution. After primary immunostaining, retinal tissues and
cryosections were washed several times with PBS and incubated for 2 h at room temperature with either anti-rabbit Alexa 594 or anti-mouse Alexa 594 secondary antibodies (Life Technologies).
Nuclei were counterstained with 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI, Sigma-Aldrich). Retinal tissues and cryosections were then mounted in Permafluor medium (Thermo Fisher
Scientific). OPSIN QUANTIFICATION After immunocytochemistry, M-opsin and S-opsin positive cells were quantified among purified cone photoreceptors in 96 well-plates using an automated
microscope equipped with a ×10 objective (Arrayscan, Thermo Scientific). CONFOCAL IMAGING Imaging was performed either on freshly isolated cells or tissues without any fixation step or on
immunolabelled samples using an Olympus FV1200 (or FV1000) laser-scanning confocal microscope. DAPI counterstaining, autofluorescence, and AlexaFluor-594 were excited using 405 nm laser
diode, 488 nm argon ion laser, and 559 nm laser diode lines, respectively. Selections of excitation wavelengths and emission wavelengths were controlled by appropriate filters: dichroic
miror (405/488/559/635), emission beam splitters SDM490 (or spectral detection), SDM560 (or spectral detection), SDM640, and barrier filters BA430–470, BA505–540, BA575–675, and BA655–755,
respectively. The objectives used were water dipping objective LUMPLFLN 40X NA 0.8-WD 3.3, oil immersion PLAPON 60X SC NA1.4-WD 0.12, UPLSAPO 100X NA 1.4 WD 0.13. Control of the microscope
and image acquisition were conducted using Olympus Fluoview software version 4.2. Image acquisition was conducted at a resolution of 1024 × 1024 pixels, with a scan rate of 10 μs pixel−1.
Images were acquired sequentially, line by line, in order to reduce excitation and emission crosstalk, and step size was defined according to the Nyquist–Shannon sampling theorem. Exposure
settings that minimized saturated pixels in the final images were used. Twelve bit images were then processed with ImageJ or FIJI, Z-sections were projected on a single plane using maximum
intensity under Z-project function and finally converted to 24 bits RGB color images. Figures were then assembled by using Adobe Photoshop CC and Adobe Illustrator software (Adobe, San Jose,
CA, USA). FLUORESCENCE EXCITATION AND EMISSION SPECTRA To define the spectral signature of chromophore in cone photoreceptors, freshly isolated retinas were transversally sectioned using a
vibrating microtome as described above, a section was place between slide and coverslip in Permafluor mounting medium to expose cone inner segments to wavelengths ranging from 300 to 490 nm
in 15 nm increments. The corresponding emission spectra were recorded using focused excitation, a high-λ-pass filter to remove the excitation, and confocal detection with an Andor Shamrock
SR303i spectrometer and Andor Ixon 3 EM-CCD (Andor Technology, Belfast, Northern Ireland). The resulting excitation–emission matrix was normalized by the excitation intensity to compensate
its spectral variations, although this increases noises below 400 nm, where the available excitation intensity is weaker. STATISTICS Experiments were repeated at least three times. Results
for cell viability and cell type quantifications after light exposure were therefore the averaged values of at least three independent experiments (represented biological replicates). For
cell viability measurement, each experiment was the mean of at least three technical replicates. The data were normalized to dark control and represented as mean ± SEM. Statistical analyzes
were performed using GraphPad Prism 7.03 Software (GraphPad Prism, La Jolla, CA, USA). One-way ANOVA followed by Dunnett’s post hoc tests were used to compare variances of all groups.
Differences between samples and dark control were considered to be significant when _p_ < 0.05 (*), _p_ < 0.01 (**), _p_ < 0.001 (***) or _p_ < 0.0001 (****). REFERENCES *
Behar-Cohen, F. et al. Light-emitting diodes (LED) for domestic lighting: any risks for the eye? _Prog. Retin. Eye Res._ 30, 239–257 (2011). Article CAS Google Scholar * Scientific
Committee on Health, E. a. E. R. S. Opinion on potential risks to human health of Light Emitting Diodes (LEDs).
https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_011.pdf (2018). * Rambhatla, P. V., Brescoll, J., Hwang, F., Juzych, M. & Lim, H. W.
Photosensitive disorders of the skin with ocular involvement. _Clin. Dermatol._ 33, 238–246 (2015). Article Google Scholar * Sui, G. Y. et al. Is sunlight exposure a risk factor for
age-related macular degeneration? A systematic review and meta-analysis. _Br. J. Ophthalmol._ 97, 389–394 (2013). Article Google Scholar * Arnault, E. et al. Phototoxic action spectrum on
a retinal pigment epithelium model of age-related macular degeneration exposed to sunlight normalized conditions. _PLoS ONE_ 8, e71398 (2013). Article CAS Google Scholar * Sparrow, J. R.,
Nakanishi, K. & Parish, C. A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. _Investig. Ophthalmol. Vis. Sci._ 41, 1981–1989
(2000). CAS Google Scholar * Noell, W. K., Walker, V. S., Kang, B. S. & Berman, S. Retinal damage by light in rats. _Investig. Ophthalmol._ 5, 450–473 (1966). CAS Google Scholar *
Reme, C. E. The dark side of light: rhodopsin and the silent death of vision the proctor lecture. _Investig. Ophthalmol. Vis. Sci._ 46, 2671–2682 (2005). Article Google Scholar * Fain, G.
L. & Lisman, J. E. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice. _Investig. Ophthalmol. Vis. Sci._ 40, 2770–2772
(1999). CAS Google Scholar * Organisciak, D. T. & Vaughan, D. K. Retinal light damage: mechanisms and protection. _Prog. Retin Eye Res._ 29, 113–134 (2010). Article Google Scholar *
Grimm, C. et al. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. _Nat. Genet._ 25, 63–66 (2000). Article CAS Google Scholar *
Sperling, H. G., Wright, A. A. & Mills, S. L. Color vision following intense green light exposure: data and a model. _Vis. Res._ 31, 1797–1812 (1991). Article CAS Google Scholar *
Kanan, Y., Moiseyev, G., Agarwal, N., Ma, J. X. & Al-Ubaidi, M. R. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line.
_Investig. Ophthalmol. Vis. Sci._ 48, 40–51 (2007). Article Google Scholar * Krishnamoorthy, R. R. et al. Photo-oxidative stress down-modulates the activity of nuclear factor-kappaB via
involvement of caspase-1, leading to apoptosis of photoreceptor cells. _J. Biol. Chem._ 274, 3734–3743 (1999). Article CAS Google Scholar * Natoli, R. et al. The role of pyruvate in
protecting 661W photoreceptor-like cells against light-induced cell death. _Curr. Eye Res._ 41, 1473–1481 (2016). Article CAS Google Scholar * Balse, E. et al. Purification of mammalian
cone photoreceptors by lectin panning and the enhancement of their survival in glia-conditioned medium. _Investig. Ophthalmol. Vis. Sci._ 46, 367–374 (2005). Article Google Scholar *
Sharma, R., Williams, D. R., Palczewska, G., Palczewski, K. & Hunter, J. J. Two-photon autofluorescence imaging reveals cellular structures throughout the retina of the living primate
eye. _Investig. Ophthalmol. Vis. Sci._ 57, 632–646 (2016). Article CAS Google Scholar * Boulton, M., Rozanowska, M. & Rozanowski, B. Retinal photodamage. _J. Photochem. Photobiol. B_
64, 144–161 (2001). Article CAS Google Scholar * Monici, M. Cell and tissue autofluorescence research and diagnostic applications. _Biotechnol. Annu. Rev._ 11, 227–256 (2005). Article
CAS Google Scholar * Croce, A. C. & Bottiroli, G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. _Eur. J. Histochem._ 58, 2461 (2014). CAS
PubMed PubMed Central Google Scholar * Kam, J. H. et al. Mitochondrial absorption of short wavelength light drives primate blue retinal cones into glycolysis which may increase their pace
of aging. _Vis. Neurosci._ 36, E007 (2019). Article Google Scholar * Papayan, G., Petrishchev, N. & Galagudza, M. Autofluorescence spectroscopy for NADH and flavoproteins redox state
monitoring in the isolated rat heart subjected to ischemia-reperfusion. _Photodiagnosis Photodyn. Ther._ 11, 400–408 (2014). Article CAS Google Scholar * Benson, R. C., Meyer, R. A.,
Zaruba, M. E. & McKhann, G. M. Cellular autofluorescenc—is it due to flavins? _J. Histochem. Cytochem._ 27, 44–48 (1979). Article CAS Google Scholar * Narayan, D. S., Wood, J. P.,
Chidlow, G. & Casson, R. J. A review of the mechanisms of cone degeneration in retinitis pigmentosa. _Acta Ophthalmol._ 94, 748–754 (2016). Article Google Scholar * dos Santos, A. F.,
de Almeida, D. R. Q., Terra, L. F., Baptista, M. S. & Labriola, L. Photodynamic therapy in cancer treatment—an update review. _J. Cancer Metastasis Treat._ 5, 25,
https://doi.org/10.20517/2394-4722.2018.83 (2019). * Kou, J., Dou, D. & Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. _Oncotarget_ 8, 81591–81603
(2017). Article Google Scholar * Sternberg, E. D, D. & C, B. Porphyrin-based photosensitizers for use in photodynamic therapy. _Tetrahedron_ 54, 4151–4202 (1998). Article CAS Google
Scholar * Vandersee, S., Beyer, M., Lademann, J. & Darvin, M. E. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of
free radicals. _Oxid. Med. Cell Longev._ 2015, 579675 (2015). Article Google Scholar * Lee, J. B. et al. Blue light-induced oxidative stress in human corneal epithelial cells: protective
effects of ethanol extracts of various medicinal plant mixtures. _Investig. Ophthalmol. Vis. Sci._ 55, 4119–4127 (2014). Article CAS Google Scholar * Kaido, M. et al. Reducing
short-wavelength blue light in dry eye patients with unstable tear film improves performance on tests of visual acuity. _PLoS ONE_ 11, e0152936 (2016). Article Google Scholar * Chen, Y.,
Liu, Y. & Dorn, G. W. II Mitochondrial fusion is essential for organelle function and cardiac homeostasis. _Circ. Res._ 109, 1327–1331 (2011). Article CAS Google Scholar * Young, R.
W. Solar radiation and age-related macular degeneration. _Surv. Ophthalmol._ 32, 252–269 (1988). Article CAS Google Scholar * Sliney, D. H. Exposure geometry and spectral environment
determine photobiological effects on the human eye. _Photochem. Photobiol._ 81, 483–489 (2005). Article CAS Google Scholar * Pastuszka, M. et al. Ocular findings in Polish Armed Forces in
Iraq and Afghanistan, a review of medical examinations by The Military Medical Commission in Lodz. _Klin. Ocz._ 115, 296–299 (2013). Google Scholar * Marie, M. et al. Light action spectrum
on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. _Cell Death Dis._ 9, 287 (2018). Article Google Scholar * Zhang, Y. et al. in _Retinal
Degeneration Diseases and Experimental Therapy_ (eds Hollyfield, J. G., Anderson, R. E., & LaVail, M. M.) 309–318 (Kluwer Academic: Plenum Publishers, 2001). * Craft, C. M., Huang, J.,
Possin, D. E. & Hendrickson, A. Primate short-wavelength cones share molecular markers with rods. _Adv. Exp. Med. Biol._ 801, 49–56 (2014). Article Google Scholar Download references
ACKNOWLEDGEMENTS We thank Arthur Planul for artwork, Kate Grieve for carefully reading the manuscript, Anaïs Potey and Elisabeth Dubus for technical assistance. We thank the cell culture,
imaging and HTS platforms at the Institut de la Vision. We thank the School of Surgery (Ecole de Chirurgie, Assistance Publique Hôpitaux de Paris) for postmortem human ocular globes. We
thank Cheryl Mae Craft (University of Southern California ROSKI Eye Institute, Los Angeles, CA) for providing the antibody (LUMIf-hCAR) for cone arrestin. This work was supported by French
state funds managed by the Agence Nationale de la Recherche within the Investissements d’Avenir program, RHU LIGHT4DEAF [ANR-15-RHU-0001], LABEX LIFESENSES [ANR-10-LABX-65], IHU FOReSIGHT
[ANR-18-IAHU-0001], [ANR-11-IDEX-0004–02]. It received a grant from the Agence National pour la Recherche for the CHRONOMOFOS project [ANR-12-TECS-0013] and a grant from Essilor
International. AUTHOR INFORMATION Author notes * These authors contributed equally: Mélanie Marie, Valérie Forster AUTHORS AND AFFILIATIONS * Sorbonne Université, INSERM, CNRS, Institut de
la Vision, 17 rue Moreau, 75012, Paris, France Mélanie Marie, Valérie Forster, Stéphane Fouquet, Pascal Berto, José-Alain Sahel, Gilles Tessier & Serge Picaud * Université de Paris,
Campus Saint Germain, 45 rue des Saints Pères, 75006, Paris, France Pascal Berto * Essilor International R&D, 147 Rue de Paris, 94220, Charenton-Le-Pont, France Coralie Barrau &
Camille Ehrismann * The University of Pittsburgh School of Medicine, 3550 Terrace Street, Pittsburgh, PA, 15213, USA José-Alain Sahel * CHNO des Quinze-Vingts, DHU Sight Restore, INSERM-DGOS
CIC 1423, 28 Rue de Charenton, 75012, Paris, France José-Alain Sahel Authors * Mélanie Marie View author publications You can also search for this author inPubMed Google Scholar * Valérie
Forster View author publications You can also search for this author inPubMed Google Scholar * Stéphane Fouquet View author publications You can also search for this author inPubMed Google
Scholar * Pascal Berto View author publications You can also search for this author inPubMed Google Scholar * Coralie Barrau View author publications You can also search for this author
inPubMed Google Scholar * Camille Ehrismann View author publications You can also search for this author inPubMed Google Scholar * José-Alain Sahel View author publications You can also
search for this author inPubMed Google Scholar * Gilles Tessier View author publications You can also search for this author inPubMed Google Scholar * Serge Picaud View author publications
You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Serge Picaud. ETHICS DECLARATIONS Patent by M.M., V.F., C.B., C.E., J-A.S., and S.P. In
addition, C.B. and C.E. are Essilor employees. S.P. received honoraria for participating to a meeting organized by Essilor. S.F., P.B., and G.T. declare no competing interests. The authors
declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. Edited by N. Bazan SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1 SUPPLEMENTARY INFORMATION 2 SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Marie, M., Forster, V., Fouquet, S.
_et al._ Phototoxic damage to cone photoreceptors can be independent of the visual pigment: the porphyrin hypothesis. _Cell Death Dis_ 11, 711 (2020).
https://doi.org/10.1038/s41419-020-02918-8 Download citation * Received: 12 March 2020 * Revised: 01 August 2020 * Accepted: 03 August 2020 * Published: 29 August 2020 * DOI:
https://doi.org/10.1038/s41419-020-02918-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Photocaged 5′ cap analogues for optical control of mrna translation in cellsABSTRACT The translation of messenger RNA (mRNA) is a fundamental process in gene expression, and control of translation...
Short course antibiotic therapy: when is no difference the same?TO THE EDITOR: In the most recent issue of _the Journal of Perinatology_, Sanchez et al. report their results of a retro...
How one chauffeur took down a corrupt brazilian politicianIt's election season in Brazil, and a group of young women hold up placards outside the Cuiaba airport in support o...
Body mass index and contralateral ratio predict outcome following unilateral adrenalectomy in primary aldosteronismABSTRACT The effect of unilateral adrenalectomy on blood pressure (BP) outcome in primary aldosteronism (PA) is diverse....
Self-assembly of polyoxometalate clusters into two-dimensional clusterphene structures featuring hexagonal poresABSTRACT Two-dimensional (2D) structures have been shown to possess interesting and potentially useful properties. Becau...
Latests News
Phototoxic damage to cone photoreceptors can be independent of the visual pigment: the porphyrin hypothesisABSTRACT Lighting is rapidly changing with the introduction of light-emitting diodes (LEDs) in our homes, workplaces, an...
The institutions strike back | thearticlePutin’s ideas of a Ukraine quickly crushed under his jackboot have all but evaporated. The West has tried to facilitate ...
The belt and road initiative and china’s strategic rebalanceThe Belt and Road Initiative and China’s Strategic Rebalance | Carnegie Endowment for International Peace Fri. April 22n...
These new bond etfs will let investors target specific parts of the corporate marketA new group of bond funds aims to give investors a way to bet on specific segments of the corporate bond market, potenti...
Princess kate wins prestigious award for delightful portrait of queen camillaIn the photo, Queen Camilla is pictured beaming at the camera while sitting on a bench in the grounds of garden clutchin...