Slamf7 (cd319) on activated cd8+ t cells transduces environmental cues to initiate cytotoxic effector cell responses
Slamf7 (cd319) on activated cd8+ t cells transduces environmental cues to initiate cytotoxic effector cell responses"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT CD8+ T-cell responses are meticulously orchestrated processes regulated by intercellular receptor:ligand interactions. These interactions critically control the dynamics of CD8+
T-cell populations that is crucial to overcome threats such as viral infections or cancer. Yet, the mechanisms governing these dynamics remain incompletely elucidated. Here, we identified a
hitherto unknown T-cell referred function of the self-ligating surface receptor SLAMF7 (CD319) on CD8+ T cells during initiation of cytotoxic T-cell responses. According to its cytotoxicity
related expression on T effector cells, we found that CD8+ T cells could utilize SLAMF7 to transduce environmental cues into cellular interactions and information exchange. Indeed, SLAMF7
facilitated a dose-dependent formation of stable homotypic contacts that ultimately resulted in stable cell-contacts, quorum populations and commitment to expansion and differentiation.
Using pull-down assays and network analyses, we identified novel SLAMF7-binding intracellular signaling molecules including the CRK, CRKL, and Nck adaptors, which are involved in T-cell
contact formation and may mediate SLAMF7 functions in sensing and adhesion. Hence, providing SLAMF7 signals during antigen recognition of CD8+ T cells enhanced their overall magnitude,
particularly in responses towards low-affinity antigens, resulting in a significant boost in their proliferation and cytotoxic capacity. Overall, we have identified and characterized a
potent initiator of the cytotoxic T lymphocyte response program and revealed advanced mechanisms to improve CD8+ T-cell response decisions against weak viral or tumor-associated antigens,
thereby strengthening our defense against such adversaries. SIMILAR CONTENT BEING VIEWED BY OTHERS A DYNAMIC CD2-RICH COMPARTMENT AT THE OUTER EDGE OF THE IMMUNOLOGICAL SYNAPSE BOOSTS AND
INTEGRATES SIGNALS Article 14 September 2020 LAG3 ASSOCIATES WITH TCR–CD3 COMPLEXES AND SUPPRESSES SIGNALING BY DRIVING CO-RECEPTOR–LCK DISSOCIATION Article 18 April 2022 LFA-1 NANOCLUSTERS
INTEGRATE TCR STIMULATION STRENGTH TO TUNE T-CELL CYTOTOXIC ACTIVITY Article Open access 09 January 2024 INTRODUCTION Recognition and subsequent elimination of cancerous cells by cytotoxic
CD8+ T lymphocytes is an essential part of immune surveillance and provides the basis for T-cell mediated immune control. Due to their endogenous origin, tumor-associated antigens are
typically subject to tolerance mechanisms and thus only can activate a CD8+ T cell repertoire of low-affinity T cells [1]. Hence, understanding and enhancing the T-cell responsiveness to
these antigens to induce durable anti-tumoral responses is a major goal of cancer immunotherapy. A cascade of signals is required to achieve full-fledged cytotoxic T cells, including TCR
recognition of cognate antigens, costimulatory signals, and danger cues provided by inflammatory cytokines [2, 3]. Further, priming of CD8+ T cells typically lasts for several days and
consists of a series of events and phases resulting in populations of expanded antigen-specific T-cell clones [4]. These phases require contact-dependent information exchange [5] embedded in
the formation of CD8+ T-cell populations that according to the Quorum hypothesis determine the outcome of a response through collective decisions rather than an individual activation of
single cells [6,7,8]. These dynamics within CD8+ T-cell populations are well described and follow a remarkably reproducible and robust sequence of contact formations, involving motile
kinapses and CD8-dependent stable synapses [9, 10]. Ultimately, this program could lead to ICAM-1-mediated T-cell:T-cell interactions that result in commitment to CD8+ T-cell expansion and
differentiation [11,12,13,14]. Furthermore, these stable CD8+ T-cell populations facilitate the communication via intercellular IFN-γ exchange, a critical modulator of CD8+ T-cell
differentiation [15,16,17]. CD8+ T-cell Quorum decisions are thought to increase the robustness and the quality of responses and can be regulated by intercellular receptor-interactions such
as the interplay between CD28, B7 (CD80/CD86), and CTLA-4 [18]. In particular, the immuno-regulatory receptor CTLA-4 plays a pivotal role in integrating cellular contacts and the size of
cell populations to regulate CD8+ T-cell differentiation [19]. To identify the underlying mechanisms of CTLA-4 as an immune-checkpoint, analyses of downstream targets of CTLA-4 in CD8+ T
cells revealed novel putative regulators such as the self-ligating surface receptor SLAMF7 [20]. Because of its binding characteristics, SLAMF7 (CD319) may enable hetero- and homotypic
interactions to regulate the communication and interactions between different and same types of immune cells during the complex process of an immune response [21, 22]. Various functions of
SLAMF7 have been reported, such as regulation of phagocytosis and activation of macrophages, activation and degranulation of NK cells, as well as modulation of B-cell signaling
[23,24,25,26,27,28]. In T cells, SLAMF7 serves as a surrogate marker for cytotoxic subsets [29, 30]. Hence, its signals may promote cytotoxicity in the cytotoxic CD4+ subset or reverse the
defective phenotype of CD8+ cytotoxic T lymphocytes of patients with systemic lupus erythematosus [31, 32]; however, in cancer-specific settings its expression is also related to exhaustion
or a suppressive capacity of CD8+ T cells [33, 34]. This versatility of effects could result from multiple SLAMF7 signaling modes depending on the intracellular molecules that bind via Src
homology-2 (SH-2) domains to the two phospho-tyrosine motifs within the cytoplasmic tail of SLAMF7, such as EAT-2, SHP-1 and SHP-2, SHIP1, Csk, Fyn, or PLC-γ [24, 27]. So far, albeit SLAMF7
has become a clinically relevant target of novel cancer therapies, its function in CD8+ T-cells, especially during initiation of cytotoxic T-cell responses, remains elusive. In this study,
we investigated the particular function of SLAMF7 after antigen-recognition of CD8+ T cells and characterized whether its signals promote or impede their effector differentiation. METHODS
SAMPLES AND T-CELL ACTIVATION All experiments were performed in accordance with institutional, state, and federal guidelines. An approval has been obtained by the Clinical Research Ethics
Committee of the University of Magdeburg (certificate 53/19); all donors provided written informed consent in accordance with the declaration of Helsinki (see Supplementary Methods).
OVA-specific TCRtg mice (OT-I), C57BL/6, and SLAMF7−/− mice were bred under specific pathogen-free conditions in the central animal facility of the University of Magdeburg Medical Faculty
(licence 42502-2-1743 Uni MD) (Magdeburg, Germany). SLAMF7−/− mice (B6/JGpt-_Slamf7__em9Cd5012_/Gpt) were obtained from GemPharmatech (San Diego, CA, U.S.A.). Sex- (both gender) and
age-matched (age 6-12 weeks) mice were used for all experiments. All mice have been back-crossed for several generations to the C57BL/6JRj strain. Isolation of APCs or naive CD8+ CD44low
CD62Lhigh T cells from spleens, inguinal- and axillary-lymph nodes was performed with magnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s
instructions. Isolated WT C57BL/6 CD8+ T cells were seeded in 96 well flat bottom plates and activated with 0.4 µg/ml αCD3ε (#130-092-973) and 0.8 µg/ml αCD28 (#130-093-182) antibodies (both
Miltenyi Biotec), coupled to microspheres composed of sulfate polysterene (ThermoFisher Scientific, Waltham, MA, U.S.A.) at a ratio of 3:2. Varying antibody concentrations are otherwise
indicated. In some experiments, cells were treated with 10 µM STAT1 inhibitor (Fludarabine), 100 µM STAT4 inhibitor (Lisofylline), 5 µM cyclosporine A (all ThermoFisher Scientific), 1 µM Akt
inhibitor II, 10 µM PKA inhibitor (14-22 amide), or 5 µM rottlerin (all Merck, Darmstadt, Germany). For antigen-specific activation TCR-transgenic CD8+ T cells from OT-1 mice were either
activated with APCs (CD90 depleted splenocytes from WT or SLAMF7−/− mice) or antigen presenting spheres consisting of 0.1 µg/ml peptide-pulsed MHC I complexes (DimerX I H-2Kb:Ig fusion
protein, BD Biosciences, Franklin Lakes, NJ, U.S.A.) with or without 0.5 µg/ml recombinant CD80-Fc (Biolegend, San Diego, CA, U.S.A.) coupled to microspheres, respectively. WT APCs either
were pulsed with 0.2 μg/mL SIINFEKL (N4, OVA257–264) peptide (Invivogen, San Diego, CA, U.S.A.) or with 0.2 μg/mL V4 (SIIVFEKL) peptide (DGpeptides, Hangzhou, China), respectively. SLAMF7−/−
APCs were pulsed with 0.2 μg/mL V4 peptide. Antigen-presenting spheres were pulsed with 0.2 μg/mL N4, T4 (SIITFEKL) (AnaSpec, Fremont, CA, U.S.A.), or V4 peptides as indicated. To induce
SLAMF7 activation 3 µg/ml αSLAMF7 antibodies (Biolegend, #152002) was added to αCD3/αCD28 on microspheres or 4 µg/ml αSLAMF7 antibodies was added to antigen presenting spheres or otherwise
as indicated. An IgG1, κ isotype antibody (Biolegend, #400402) was used as control. Cells were cultured with complete medium (RPMI, with 100 U/ml Penicillin, 100 μg/ml Streptomycin (Thermo
Fisher Scientific), and 10% FCS (Biochrom, Berlin, Germany) and polarized under Tc1-inducing conditions by adding 3 ng/ml IL-12 (Biolegend) or under Tc17-inducing conditions by adding 20
ng/ml IL-23, 40 ng/ml IL-6 (both Miltenyi Biotec), 2 ng/ml TGF-β (R&D Systems, Minneapolis, MN, U.S.A.), and 20 µg/ml anti-IFN-γ (Biolegend, #505834). Co-cultures of CD8+ T cells and
SLAMF7−/− or WT control APCs were cultivated with X-VIVO 15 medium (Lonza, Basel, Switzerland) supplemented with 3 ng/ml IL-12. MELC MICROSCOPY Tissue sections from C57BL/6 spleen snap
frozen in liquid nitrogen and stored at −80 °C. After embedding the biopsy in Tissue-Tek® O.C.T.™ Compound (Sakura Finetek, Umkirch, Germany) cryosections of 10 μm thickness were sliced and
applied on silane (Merck) coated coverslips. The coverslips were stored at −20 °C. To prepare the samples for the MELC procedure, the tissue sections were rehydrated and fixed with 2%
paraformaldehyde solution in PBS (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) for 15 min and permeabilized with 0,2% Triton X-100 (Carl Roth, Karlsruhe, Germany), diluted in PBS, for 10
min at room temperature. Than sections were blocked with 1% Bovine Serum Albumin (Merck), diluted in PBS, for 30 min. MELC process: Fc receptor blocking reagent (Miltenyi Biotec), propidium
iodide (Merck) as a nucleic acid dye and fluorescence-labelled antibodies against the following epitopes were used: αCD3 (#100203), αCD8α (#100706), αSLAMF7 (#152006, all Biolegend). Slides
with the prepared tissue sections were placed on the stage of an inverted wide-field fluorescence microscope (Leica DMi 8; HC PL APO 20x/0.80 NA lens, Leica, Wetzlar, Germany). Antibodies
and PBS as washing solution were added and removed robotically by the Toponome Imaging Cycler (MelTec). By a cyclic robotic process, tissue sections were incubated with one of the given
antibodies for 15 min and subsequently rinsed with PBS. Afterwards, phase contrast and fluorescence signals were imaged by the ORCA-Fusion scientific CMOS camera (C14440-20UP, 2304 × 2304
pixels, Hamamatsu, Herrsching, Germany). To delete the specific signal of the given antibody before pipetting the consecutive one, a bleaching step was performed sequentially for each marker
and a post-bleaching image was taken. The fully automated process of antibody incubation, repositioning (including autofocus), fluorescence imaging and bleaching process is controlled by an
adapted version of BioDecipher® Device control software (BioDecipher, Magdeburg, Germany). Fluorescence images produced by each marker were aligned pixel-wise using the corresponding phase
contrast images. Images were corrected for illumination faults using flat-field correction. The post-bleaching images were subtracted from the previous fluorescence images to increase the
specificity of the visualization of each marker. All processing steps were performed with MATLAB based software developed at the Institute for Molecular and Clinical Immunology at the
University of Magdeburg. IMMUNOSPOT ANALYSIS AND FLOW CYTOMETRY For analysis of T-cell populations and proliferation, cells were labeled with CFSE (Merck) prior activation. After 24 h the
number and size of formed populations were detected by ImmunoSpot S6 analyzer (CTL, Cleveland, OH, USA). Cytometric measurements were performed on a FACS-Canto II or LSRFortessa X-20 (BD
Biosciences) and analyzed with FlowJo (BD Biosciences) software. Surface and intracellular molecules of CD8+ T cells were stained with fluorochrome-labeled antibodies. Prior to intracellular
cytokine analysis, the cells were incubated with brefeldin A for 4 h. Intracellular staining was performed after the cells were fixed with 2% paraformaldehyde (Merck) in PBS for 20 min on
ice and permeabilized in 0.5% saponine (Merck) in PBS/BSA. Following antibodies were used: αCD8 (#100751), αCD44 (#103039), αSLAMF7 (#152003), and αIFN-γ (#505826, all Biolegend). WESTERN
BLOT ANALYSIS AND PULL-DOWN ASSAYS Cellular extracts of activated T cells were separated on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes. Blots were either probed with
antibodies against phospho-Akt (S473, #9271), phospho-Akt (T308, #4056), Akt (pan, #4691), phospho-Erk1/2 (#9101), p44 MAPK (#4695), CDK6 (#3136), Cyclin D3 (#2936), β-Actin (#3700, all Cell
Signaling Technology, Danvers, MA, USA), or GAPDH (Abcam, Cambridge UK), then stained with IRDye 800CW (#926-32210) and 680RD secondary antibodies (# 926-68071, both LI-COR, Lincoln, NE,
U.S.A.) and visualized as well as quantified using the Odyssey scanner and software (LI-COR). Western Blot experiments were performed in two independent replicates and the original western
blots are provided as supplemental information (Fig. S2). SLAMF7 tyrosine domain Y261 and Y281 pull-down experiments were performed using the synthetic peptides CLEENAD(p)YDTIPYTE and
CAPNTF(p)YSTVQIPK with the indicated tyrosine either phosphorylated or non-phosphorylated to identify interacting signaling molecules. The N-terminal cysteine in each peptide is not part of
the original sequence but was added to allow for cysteine-mediated covalent peptide coupling to agarose beads according to the manual (SulfoLink Beads, Thermo Fisher Scientific). 1 × 107
CD8+ T cells activated with 0.75 µg/ml αCD3 and 1.5 µg/ml αCD28 antibodies (both Miltenyi Biotec, Germany) coupled to microspheres were lysed and the soluble fraction of the lysate was
incubated with the agarose-bound peptides. After washing with lysis buffer, bound proteins were eluted and separated by SDS-PAGE. Protein digestion and in-gel 16O/ 18O-labeling was performed
as described [35]. Each peptide pull-down assay was performed in 2 independent replicates using a reverse labeling strategy. LC-MS analysis was subsequently performed by a reversed-phase
capillary nanoliquid chromatography system (Ultimate 3000, Thermo Scientific) connected to an Orbitrap Q Exactive HF mass spectrometer. Identification of proteins was done by searching
against the protein sequence database of mouse (Swiss Prot) and 16O/18O-based quantification was done using the Mascot Distiller software (version 2.7.1.0). Network and GO:BP enrichment
analysis of identified interaction proteins was performed with the NetworkAnalyst software [36]. CYTOTOXICITY ASSAY TCR-transgenic OT-I CD8+ T cells were pre-activated in X-VIVO 15 media
supplemented with 3 ng/ml IL-12 for 36 h with Antigen-presenting spheres consisting of 0.1 µg/ml peptide-pulsed MHC I complexes (DimerX I) with or without 0.5 µg/ml recombinant CD80-Fc
coupled to microspheres, respectively. Spheres were pulsed with 0.2 μg/mL N4, T4, or V4 antigens, respectively. Target and control cells were obtained from CD90 depleted WT splenocytes.
Target cells were labeled with 10 µM CellTrace Violet (CTV, ThermoFisher Scientific) and pulsed with N4-peptide whereas control cells were labeled with 0.1 µM CTV and left unpulsed. Primed T
cells then were co-cultured for 16 h with CTVhi target cells or CTVlo control cells at an effector to target cell ratio of 1:1. Cytotoxic activity was assessed by flow cytometry and
calculated according to the ratio of target to control cells. STATISTICAL ANALYSIS Statistical analyses were performed with Prism 9 as indicated (Dotmatics, Boston, MA, U.S.A.). Sample sizes
were chosen according to respective statistical tests and represent number of animals that are indicated as data points, respectively. No variances between groups could be detected by F
test and normal distribution was checked by Q-Q plot and KS test. _P_-values were calculated using linear regression analysis or two-way ANOVA with Sidak’s multiple comparison test.
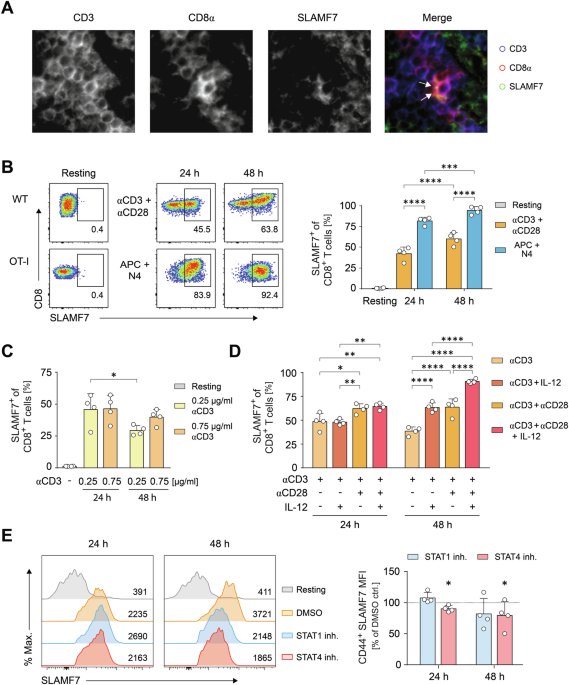
Statistical significance is indicated as followed: *_p_ < 0.05, **_p_ < 0.01, ***_p_ < 0.001, and ****_p_ < 0.0001. RESULTS SLAMF7 EXPRESSION ON CD8+ T CELLS REFLECTS ACTIVATING
AND ENVIRONMENTAL CUES To characterize SLAMF7 on CD8+ T cells, we initially assessed SLAMF7+ T cells under physiological steady-state conditions in secondary lymphoid organs, using spleen
tissue sections. Multi-epitope-ligand cartography (MELC) revealed that SLAMF7 was strongly and polarized expressed along with high CD8α expression within the contact zone between CD8+ T
cells (Fig. 1A). Since the CD8 co-receptor is necessary for the assembly of stable contacts of CD8+ T cells via immunological synapses, we hypothesized that SLAMF7 functions in the
communication and regulation of CD8+ T cells during their activation. To elucidate the function of SLAMF7 in this context, we analyzed the expression of SLAMF7 after priming CD8+ T cells
with different stimuli. Wildtype (WT) CD8+ T cells were stimulated using anti-CD3 and anti-CD28-coupled microspheres or Ag-specific OT-I TCR-transgenic CD8+ T cells were stimulated of using
cognate OVA peptide loaded on APCs (Fig. 1B). As a result of physiological cell-cell-interactions, antigen-specific activated CD8+ T cells showed a more than 50% increased SLAMF7 expression
when compared to anti-CD3 plus anti-CD28-activated T cells (Fig. 1B). Anti-CD3 stimulation alone resulted in increased SLAMF7 expression that was diminished by using lower activation
strength (Fig. 1C). Both co-stimulation with anti-CD28 and the Tc1-inducing cytokine IL-12, enhanced CD3-driven expression of SLAMF7 on CTLs over time (Fig. 1D) whereas IL-2 did not show any
impact (Fig. S1A). Co-stimulation with CD28 potentiated the levels of SLAMF7 on CD8+ T cell after both 24 h and 48 h by up to 64%, and IL-12 increased TCR-induced SLAMF7 expression after 48
h (Fig. 1D). However, the combination of three signals- TCR activation, co-stimulation by CD28, and IL-12 cytokine signaling- led to a maximum frequency of 91% SLAMF7-expressing CD8+ T
cells (Fig. 1D). As cytokines could indirectly impinge on SLAMF7 expression via increased activation, we monitored CD44 surface expression as this receptor serves as a surrogate marker of
T-cell activation [37]. Of note, CD44 expression was only enhanced after 48 h by CD28 signaling, but not affected by IL-12 (Fig. S1B). To further investigate whether IL-12 directly promotes
SLAMF7 expression via STAT4 or indirectly by IL-12-induced T-cell intrinsic IFN-γ production via STAT1, we applied chemical inhibitors of STAT1 and STAT4 during antibody-specific stimulation
(Fig. 1E). To exclude any interference of the inhibitors with activation events, we analyzed SLAMF7 intensities within the activated CD44hi T-cell compartment. Our experiments revealed that
inhibiting STAT4, at least partly, curtailed SLAMF7 expression, whereas blocking STAT1 had no effect (Fig. 1E). In line, antibody-mediated blockade of IFN-γ did not affect SLAMF7 expression
(Fig. S1C). This suggests IL-12 primarily up-regulated SLAMF7 in a direct manner. To dissect the key signaling molecules that are responsible for the initiation of SLAMF7 expression on
activating CD8+ T-cells, we further applied pharmacological inhibitors and again assessed SLAMF7 molecule intensities in combination with CD44 expression. Herein, a strong significant loss
of SLAMF7 was detected after administration of cyclosporine A that impinges on the transcription factor NFAT, whereas inhibition of PKCθ by rottlerin also showed reduced SLAMF7, but neither
the inhibition of Akt nor PKA did (Fig. S1D). Taken together, SLAMF7 expression occurs immediately after the onset of T-cell activation and is initiated in an NFAT-dependent manner by TCR
triggering and further increased by CD28 co-stimulation, and IL-12 signaling. By these direct relations, SLAMF7 possesses the capability to transduce activating cues into the outcome of a
cytotoxic CD8+ T-cell response. SLAMF7 EXPRESSION IS LINKED TO THE CYTOLYTIC CD8+ T-CELL RESPONSE PROGRAM To further delineate the role of SLAMF7 during initiation of cytotoxic CD8+ T-cell
activation, we analyzed SLAMF7 expression at varying overall signal strengths by titrating tandem triggering of the TCR complex and co-stimulation by CD28 using dilutions of the indicated
agonistic antibodies coupled to microspheres under Tc1 skewing conditions. Increasing the overall activation strength resulted in an up to four-fold increase in the frequencies of
SLAMF7-expressing CD8+ T cells in a dose dependent manner (Fig. 2A). Since the strength of the interaction of the TCR with the peptide-MHC complex represents a critical factor for the
functional outcome of the CD8+ T-cell response [1], we next analyzed the impact of TCR-affinity on SLAMF7 expression. Therefore we stimulated naive TCRtg-CD8+ T cells with cognate OVA
antigen SIINFEKL (N4) compared to the low affinity OVA peptide SIITFEKL (T4) pulsed on MHC I bearing antigen-presenting spheres (Fig. 2B). Similarly, weak TCR-antigen interactions were able
to induce SLAMF7 expression but cognate TCR activation further led to a significant increment (Fig. 2B). Thus, SLAMF7 expression is connected to the strength of initial cytotoxic CD8+ T-cell
activation and thereby could function to discriminate between antigen affinities. As cytolytic molecules such as granzyme and perforin have been found to be restricted to SLAMF7 expressing
CD8+ T cells [32], we subsequently determined co-expression of surface SLAMF7 along with the regulatory Tc1 cytokine IFN-γ. Remarkably, both after polyclonal αCD3 and αCD28- or
antigen-specific-activation, SLAMF7 was evident on over 90% of the IFN-γ-expressing effector cells (Fig. 2C, D). Importantly, there was a strong correlation (R2 = 0.92, _p_ < 0.0001)
between the frequencies of SLAMF7 surface expression and IFN-γ producers (Fig. 2E). To further investigate the connection between SLAMF7 expression and cytotoxic CD8+ T-cell activation, we
polarized CD8+ T-cells by both Tc1 and Tc17 conditions, as the latter strongly prevents the induction of IFN-γ production and cytolytic functions [38]. In line, albeit being similarly
activated as monitored by CD44 expression, CD8+ T cells polarized under Tc17 conditions failed to up-regulate SLAMF7 on their surface (Fig. S1E). Thus, activation-dependent expression of
SLAMF7 on CD8+ T cells is hardwired within the induction of the cytotoxic CD8+ T-cell effector program. SLAMF7 PROMOTES FORMATION OF ACTIVATION-INDUCED CD8+ T-CELL POPULATIONS Since SLAMF7
is immediately expressed after initial antigen-recognition of CD8+ T cells and its surface expression is a marker for their commitment to execute cytotoxic responses, we sought to identify
the particular role of SLAMF7 in the initiation of these response programs. As these processes are defined by a sequential program that involves cellular contact dynamics, we firstly
detected a strong correlation (R2 = 0.934, _p_ < 0.0001) of SLAMF7 expression with the T-cell receptor CD44, involved in adhesion, migration, and effector function [39, 40], suggesting a
possible role of SLAMF7 in this context (Fig. 3A). To identify the SLAMF7-mediated effects directly on CD8+ T cells, we employed an in vitro system that ensures unidirectional SLAMF7
signaling and thereby excludes possible SLAMF7 effects mediated by CD8+ T cells in APCs. The latter may occur in vivo by the ability of SLAMF7 to act as a self-ligand that enables
bidirectional communication between interacting cells. Therefore, we activated SLAMF7 signaling by cross-linking anti-SLAMF7 antibodies that were concomitantly coupled to APC mimicking
microspheres alongside TCR- and CD28-activating ligands or antibodies, respectively. The concentrations of the SLAMF7 engaging antibodies were adjusted according to previous studies [31,
32]. Further, these spheres were devoid of ligands of adhesion molecules, such as ICAM-1 or LFA-1. Because of the detected correlation of SLAMF7 expression with the activation status of CD8+
T cells, we firstly sought to investigate, whether SLAMF7 signals itself may enhance initial T-cell activation events. However, neither phosphorylation of Akt and Erk signaling molecules,
nor expression of CD44 were increased in SLAMF7-engaged CD8+ T cells when compared to those T cells activated without additional SLAMF7-crosslinking antibodies (Fig. 3B, C). Nevertheless,
when considering CD8+ T cells on the supra-cellular level, SLAMF7 signals were strikingly able to increase the formation of stable T-cell populations by two-fold independent of the TCR and
CD28 activation strength (Fig. 3D). This effect mediated by SLAMF7 was not only restricted to the amount of T-cell populations visible as numbers of clusters, but it further increased the
size of T-cell populations in the context of strong activating stimuli (Fig. 3E). Hence, SLAMF7 signaling caused that more T cells initiate stable contacts resulting in homotypic
interactions and bigger populations. Next, we applied this mode of SLAMF7-activation to antigen-presenting spheres and found that SLAMF7-signals facilitated the formation of stable T-cell
populations also in response to antigen-specific activation that was highly significant even in responses towards the lowest affinity OVA peptide SIIVFEKL (V4) (Fig. 3F). Consequently, we
conclude that SLAMF7 plays an important role in the contact formation and communication between CD8+ T cells as indicated by MELC analysis of secondary lymphoid tissue sections (Fig. 1A).
Augmenting SLAMF7 signals during initial CD8+ T-cell activation facilitated tight interactions of CD8+ T-cells that increased the amount and size of CD8+ T-cell populations. SLAMF7 FUNCTION
INVOLVES PROTEINS THAT REGULATE CELLULAR CONTACTS As formation of tight homotypic T-T contacts within CD8+ T-cell populations are dependent on ICAM-1 (CD54), we analyzed the interplay of
SLAMF7 with ICAM-1. Firstly, we detected similar ICAM-1 expression levels on SLAMF7-activated and stimulated control CD8+ T cells and therefore excluded that SLAMF7 promotes population
formation by increasing ICAM-1 abundances (Fig. 4A). Furthermore, antibody-mediated blockade of ICAM-1 during priming had no impact on SLAMF7 expression (data not shown). However, ICAM-1
blockade abolished the tight contact formation of stable T-cell populations enhanced by SLAMF7 signals (Fig. 4B). Since the activating antibody-coupled microspheres were devoid of either
ICAM-1 or LFA-1, their delivered SLAMF7 signals could not impinge on the adhesion between the T cells and the microspheres itself. Therefore, the SLAMF7 engaging signals had to be transduced
by the T cells to initiate and maintain tight homotypic cell contacts based on the interaction between ICAM-1 and LFA-1 on the CD8+ T cells. So far, how the receptor SLAMF7 intracellularly
transmits signals has been mostly derived from data using innate cytotoxic NK cells that function without variant antigen-specific receptors. CD8+ T cells differ from NK cells in their
function of TCR sensing of antigens; therefore, we sought to identify the signaling molecules affected by SLAMF7 ligation by combining pull-down assays with mass spectrometry baiting with
phosphorylated and non-phosphorylated SLAMF7 tyrosine-motifs Y261 or Y281, respectively (Fig. 4C). For discrimination, we used stable isotope labeling by light and heavy water (H216O/H218O)
during the tryptic digestion. By plotting the heavy/light (H/L) and light/heavy (L/H) isotope ratios of proteins identified in two independent experiments with reversed labeling we found
several signaling molecules to be highly enriched exclusively by the phosphorylated SLAMF7 sequences (Fig. 4C). Among the detected molecules, the adaptor proteins CRK and CRKL that are
involved in T-cell adhesion and mechanosensing [41, 42], interacted with both phosphorylated SLAMF7 tyrosine domains. Further, we identified an interaction between SLAMF7 tyrosine domain
p-Y281 and the TCR-related adaptors Nck1 and Nck2. Interestingly, the prototypic SLAM-adapter SAP (SH21DA) possessed the ability to associate to the SLAMF7 p-Y281 domain. Other detected
signaling proteins such as EAT-2, SHP-1 and -2, SHIP1, Csk, Fyn, or PLC-γ confirm similar binding modalities compared to NK cells. To extract further information from the dataset, we applied
interaction network modeling with GO:BP enrichment analysis that predicted further interacting proteins that could be allocated to GO biological processes of cell shape and cytoskeleton
organization regulation as well as MAPK signaling (Fig. 4D). Network analysis of the identified p-Y261 and p-Y281 binding partners revealed the potential SLAMF7-activated signaling pathways
in detail (Fig. 4D). This supports the idea that SLAMF7 actually affects how CD8+ T cells adhere and respond. SLAMF7 SIGNALS LOWER THE THRESHOLD FOR CD8+ T CELLS TO INITIATE CYTOTOXIC
RESPONSES To determine whether SLAMF7 induced formation of stable contacts is a prerequisite for initiating CD8+ T-cell response programs, we labeled CD8+ T cells with CFSE to monitor
proliferation prior their activation in the presence of agonistic SLAMF7 signals. Therefore, we added increasing concentrations of SLAMF7-crosslinking antibodies coupled to microspheres
alongside TCR- and CD28-activating antibodies (Fig. 5A). SLAMF7 ligation by a high anti-SLAMF7 antibody concentration doubled CD3/CD28-induced proliferation 48 h after the onset of
stimulation in a dose-dependent manner (Fig. 5A). These induced SLAMF7 signals led to an increased expression of cell cycle proteins that mediate the transition through G1 phase, including
cyclin D3 by 55% at 48 h, as determined by western blot (Fig. 5B). In addition, levels of CDK6 that could act independently of IL-2 [43], were more than two-times higher with cross-linking
of SLAMF7 than without additional SLAMF7 signals (Fig. 5B). Next, we investigated the impact of SLAMF7 on the proliferative response to different antigen affinities as the TCR-ligand
specificity crucially affects the expansion outcome. CD8+ T cells were stimulated using MHC I complexes, CD80, and anti-SLAMF7 antibodies (or isotype as control) coupled to microspheres
mimicking antigen-presenting cells, and pulsed with lower (V4, T4) or high (N4) affinity ligands, respectively. Intriguingly, SLAMF7 signals led to a more than 5-fold increased expansion of
CD8+ T cells 72 h after priming with very low affinity V4 antigen (Fig. 5C). However, whilst still almost doubling the proliferation in response to low affinity T4 peptide, no SLAMF7 effect
was detected in the setting with high affinity N4-peptide (Fig. 5C right panel). To physiologically validate the effect of these unidirectional SLAMF7 signals that occur during the
initiation of CD8+ T-cell responses, we activated TCR-tg CD8+ T cells either with V4-pulsed APCs from WT or SLAMF7−/− mice and assessed the proliferative response (Fig. 5D–F). Whereas the
lack of SLAMF7 on APCs during priming did not impair SLAMF7 expression of the CD8+ T cells (Fig. 5D), it consequently resulted in a marked decrease in CD8+ T-cell expansion (Fig. 5E, F). To
assess the impact of SLAMF7-mediated signals on functional outcomes of T-cell differentiation, we stimulated Ag-specific T cells using low (V4) and intermediate affinity peptides (T4).
Expression of IFN-γ—a primary CD8+ T cell differentiation and effector cytokine of CD8+ T cells [17]—was analyzed and frequencies of IFN-γ producers were determined by flow cytometry. On day
2 after beginning of the stimulation, CD8+ T cells primed with peptides V4 or T4 showed a 2.5- to 7-fold increase in the frequency of cytokine producers, respectively, compared to cells
that did not receive a SLAMF7 signal (Fig. 5G). Ultimately, to proof the enhancing effect of SLAMF7 signals in a cytotoxicity assay, we primed CD8+ T cells with V4, T4, or N4 pulsed antigen
presenting spheres that were loaded with CD80, anti-SLAMF7, or isotype control antibodies, or a combination of both, respectively. The primed CD8+ T cells were then subsequently incubated
with N4-pulsed high fluorescent target cells together with low fluorescent control cells. By analyzing the relative decline of target cells in comparison to control cells, we detected an up
to two-fold increased cytotoxic activity in V4-primed and SLAMF7-activated CD8+ T cells that, of note, was dependent on CD28 signals (Fig. 5H). Similarly, a high amount of lysed target cells
occurred after SLAMF7-activation with T4 priming whereas both activated control cells and SLAMF7 engaged CD8+ T cells showed similar cytotoxic capacities after N4 pre-activation (Fig. 5I).
Thus, the observed boost by SLAMF7 in cytotoxicity was restricted to CD8+ T cells primed with low-affinity antigens. As these affinities typically apply to self-peptides such as
tumor-associated antigens we assessed the capability of SLAMF7 signals to enhance the cytotoxic responses of human CD8+ T cells, activated with NY-ESO-1-pulsed HLA-A2 of antigen-presenting
spheres that delivered SLAMF7 signals by anti-SLAMF7 antibodies, recombinant SLAMF7 molecules, or not [44, 45]. In line, SLAMF7 signals during initiation of CD8+ T-cell activation strongly
increased IFN-γ and Granzyme B secretion as analyzed by ELISpot (Fig. S1F lower panel). Of note, albeit to much lower extend, this effect of SLAMF7 was also detected in CD8+ T-cell responses
against high affinity antigens of infectious pathogens (Fig. S1F upper panel). In summary, the SLAMF7-enhanced formation of stable CD8+ T-cell populations after antigen recognition and
initiation of cytotoxic CD8+ T-cell programs profoundly improved their expansion as well as their effector functions resulting in superior responses. In particular, responses towards low
affinity antigens were enhanced by SLAMF7, implicating a crucial role for SLAMF7 to regulate the decision of CD8+ T cells to commit to executing cytotoxic responses. DISCUSSION Here, we
found that agonistic SLAMF7-stimuli at the early steps of initial CD8+ T-cell activation strongly enhanced the commitment to expand and execute cytotoxic effector functions of CD8+ T cells.
Therefore, we not only affirm the relation of SLAMF7 as a marker for cytotoxic T cells [29, 30] but further provide a novel function of SLAMF7 and propose a coherent model that connects
SLAMF7 expression as a transducer of activating and environmental cues. SLAMF7 mediated signals then support stable CD8+ T-cell-T-cell contacts leading to CD8+ T-cell populations that allow
Quorum decisions and response commitment (Fig. 6). Ultimately, this response decision serves as a threshold that has to be passed in order to execute cytotoxic CD8+ T-cell response programs
that involve expansion, effector differentiation, and population intrinsic regulatory mechanisms (Fig. 6) [8, 10,11,12,13]. Therefore, the role of SLAMF7 within initial CD8+ T-cell
activation could be a part of a missing link that connects two activating mechanisms that do not mutually exclude each other, namely single stimuli, such as antigen avidity, co-stimulation,
or inflammatory cues, with the process of T-cell quorum formation [7]. In line, this novel function of SLAMF7 could also explain the observed effect of IL-12 in augmenting of CD8+ T-cell
population formation [11], as IL-12 signaling strongly boosted SLAMF-7 expression. Similarly inducing SLAMF7 signals promoted SLAMF7-SLAMF7 interactions also between other cell types, such
as NK cells [46]. Hence, these SLAMF7-mediated stable T-cell populations increase the reliability and reproducibility of cytotoxic CD8+ T-cell responses by collective decisions that promote
expansion and effector differentiation to determine their biological outcome [6, 18]. Importantly, these identified effects are related to the early processes within the initiation of CD8+
T-cell responses starting with antigen-recognition of CD8+ T-cells. Of note, further and later activation of CD8+ T cells might regulate SLAMF7 expression and function by other mediators or
mechanisms. Here, we show that the SLAMF7-mediated clustering of CD8+ T-cell populations depend on ICAM-1 interactions between T cells. Because of the nature of SLAMF7 to act as a
self-ligand that offers a multiplicity of interactions and feedback loops, agonistic SLAMF7-mediated effects on CD8+ T cells observed in our study could differ from those described in SLAMF7
ko mouse models. In these models, the phenotype of the T cells primarily was strongly affected by the lack of SLAMF7 on other cell types, such as tumor-associated macrophages or B cells,
giving space for further co-stimulation or signaling/inhibition by other surface molecules [26, 34]. Even though findings on the role of SLAMF7 in CD8+ T-cell exhaustion used sole SLAMF7
crosslinking in the absence of TCR activation [34] our results may explain these later effects: Activated cytotoxic CD8+ T cells express SLAMF7 and, as continuous stimulation drives
exhaustion, the same cells still expressing SLAMF7 may present now an exhaustive phenotype [33, 47]. There limited responsiveness may be due to intracellular usage of a different adapter for
SLAMF7 as shown for NK cells, such as the Csk or Shp phosphatases [27]. By performing pull-down experiments, putative SH-2 domain containing SLAMF7-interacting signaling molecules were
identified that partially differ from those analyzed in NK cells where positive signaling relies on the adapter EAT-2 [24, 27]. The newly identified binders such as Nck, CRK and CRKL can
serve to explain the observed effects. By recruiting Nck adaptors to the synapse SLAMF7 signals could modulate and lower the threshold of CD8+ T-cell responsiveness [48, 49]. The profound
improvement of adhesion could be mediated by the CRK and CRKL adapters [50]. Furthermore, these adapters are critical sensors of inflammation that are important regulators of CD8+ T-cell
migration and invasion [41, 42]. Moreover, to support this novel function of SLAMF7 in T-cell adhesion and communication an immunoreceptor coupling and organization motif (ICOM) has been
recently described for the SLAMF7 transmembrane domain [51]. These conserved leucine zipper motifs could allow SLAMF7 to laterally interact with other transmembrane proteins to form
complexes that regulate their activity. In this regard, the SLAMF7 ICOM is closely related to that of other adhesion molecules, such as cadherins, and thereby SLAMF7 could directly enhance
T-cell contact formation [51]. However, SLAMF7 on CD8+ T cells does not merely function as a molecule that promotes adhesion. Furthermore, it combines the aspects of both cellular contacts
and communication and acts as a co-transducer that integrates antigenic and environmental cues in the context of T-cell populations into the outcome of a CD8+ T-cell response. Intriguingly,
the findings of this study are especially related to the process of CD8+ T-cell responses towards low-affinity antigens. By augmenting SLAMF7 signals during CD8+ T-cell priming, we were able
to induce an effective response to the very low affinity V4 antigen that is about 700-fold less potent than the adequate N4 peptide [52] and towards the tumor-associated self-antigen
NY-ESO-1 that is shared among different tumor entities [45]. By enhancing the T-cell population dynamics the subsequent IFN-γ exchange might promote the expansion of the low avidity T cells
[53]. This relation could also be important to defend viral antigens and widen the window of response when the antigen availability is restricted and only few host cells are exploited for
viral replication. Further, targeting of SLAMF7 on CD8+ T cells could serve as a mean to regulate the formation of ICAM-1-LFA-1 dependent CTL populations within the tumor tissue [54], and by
enrichment of tumor-specific CD8+ T cells, SLAMF7 signals could improve the tumor control. As SLAMF7 and its function within the initiation of CD8+ T-cell responses could be addressed by
immune-checkpoint inhibitors, such as CTLA-4 and PD-1, it has been shown that these inhibitory checkpoint receptors strongly impinge on contact formation and migration behavior of CD8+
T-cells, too [55, 56]. Especially CTLA-4 expression on CD8+ T cells is tightly connected to ICAM-1 as well as homotypic T-cell interactions and functions as a critical regulator of T-cell
population dynamics that are decisive for the differentiation and the magnitude of CD8+ T-cell responses [11, 18, 19]. Therefore, as SLAMF7 is targeted by CTLA-4 [20] we presume that CTLA-4
could utilize SLAMF7 mediated interactions between CD8+ T cells to communicate via IFN-γ exchange that ultimately increase the robustness of T-cell response dynamics. In this regard, these
population-intrinsic feedback loops serve to adjust the overall outcome of a T-cell response [18] and may function as a mechanism to compensate inappropriate activation of a small fraction
of T-cells within the population, that e.g. individually differ in the expression of an immune-checkpoint receptor. Therefore, these regulatory circuits might also explain the physiological
outcome of the T-cell response in mixed bone marrow chimeras of CTLA-4 competent and deficient mice by Quorum sensing at the population level [57]. The results of this study support a novel
role for SLAMF7 on CD8+ T cells, which upon expression renders these cells to be committed to exert their cytotoxic effector response. Therefore, SLAMF7-expressing T cells may be good
candidates for autologous cell therapy for tumor rejection, as it potentially enhances collective cell decisions to improve CD8+ T-cell responses against weak antigens. In this regard,
targeting SLAMF7 with agonistic signals could serve as an advanced mechanism to induce or at least strongly support CD8+ T-cell responses during immunotherapy. DATA AVAILABILITY The datasets
generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Hoffmann MM, Slansky JE. T-cell receptor affinity
in the age of cancer immunotherapy. Mol Carcinog. 2020;59:862–70. Article PubMed PubMed Central CAS Google Scholar * Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal
for T cell activation. Curr Opin Immunol. 2010;22:333–40. Article PubMed PubMed Central CAS Google Scholar * Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et
al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. Article PubMed CAS Google Scholar * Obst R. The Timing of T Cell
Priming and Cycling. Front Immunol. 2015;6:563. Article PubMed PubMed Central Google Scholar * Mempel TR, Henrickson SE, Andrian UH, von. T-cell priming by dendritic cells in lymph nodes
occurs in three distinct phases. Nature. 2004;427:154–9. Article PubMed CAS Google Scholar * Butler TC, Kardar M, Chakraborty AK. Quorum sensing allows T cells to discriminate between
self and nonself. Proc Natl Acad Sci USA. 2013;110:11833–8. Article PubMed PubMed Central CAS Google Scholar * Al-Yassin GA, Bretscher PA. Does T Cell Activation Require a Quorum of
Lymphocytes? J Immunol. 2018;201:2855–61. Article PubMed CAS Google Scholar * Gérard A, Khan O, Beemiller P, Oswald E, Hu J, Matloubian M, et al. Secondary T cell-T cell synaptic
interactions drive the differentiation of protective CD8+ T cells. Nat Immunol. 2013;14:356–63. Article PubMed PubMed Central Google Scholar * Potter TA, Grebe K, Freiberg B, Kupfer A.
Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA.
2001;98:12624–9. Article PubMed PubMed Central CAS Google Scholar * Mayya V, Judokusumo E, Abu Shah E, Peel CG, Neiswanger W, Depoil D, et al. Durable Interactions of T Cells with T
Cell Receptor Stimuli in the Absence of a Stable Immunological Synapse. Cell Rep. 2018;22:340–9. Article PubMed PubMed Central CAS Google Scholar * Zumwalde NA, Domae E, Mescher MF,
Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J Immunol. 2013;191:3681–93. Article PubMed CAS Google
Scholar * Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–22. Article PubMed
PubMed Central CAS Google Scholar * van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and
differentiation. Nat Immunol. 2001;2:423–9. Article PubMed Google Scholar * Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, et al. Functional anatomy of T cell
activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. Article PubMed PubMed Central CAS Google Scholar * Krummel MF, Mahale JN, Uhl LFK, Hardison EA, Mujal AM, Mazet JM,
et al. Paracrine costimulation of IFN-γ signaling by integrins modulates CD8 T cell differentiation. Proc Natl Acad Sci USA. 2018;115:11585–90. Article PubMed PubMed Central CAS Google
Scholar * Curtsinger JM, Agarwal P, Lins DC, Mescher MF. Autocrine IFN-γ promotes naive CD8 T cell differentiation and synergizes with IFN-α to stimulate strong function. J Immunol.
2012;189:659–68. Article PubMed CAS Google Scholar * Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and
cytotoxicity. Cell Death Dis. 2017;8:e2836. Article PubMed PubMed Central CAS Google Scholar * Zenke S, Palm MM, Braun J, Gavrilov A, Meiser P, Böttcher JP, et al. Quorum Regulation via
Nested Antagonistic Feedback Circuits Mediated by the Receptors CD28 and CTLA-4 Confers Robustness to T Cell Population Dynamics. Immunity. 2020;52:313–27.e7. Article PubMed CAS Google
Scholar * Thaventhiran, Hoffmann JED, Magiera A, La Roche L, de M, Lingel H, et al. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc
Natl Acad Sci USA. 2012;109:E2223–9. Article PubMed PubMed Central CAS Google Scholar * Lingel H, Wissing J, Arra A, Schanze D, Lienenklaus S, Klawonn F, et al. CTLA-4-mediated
posttranslational modifications direct cytotoxic T-lymphocyte differentiation. Cell Death Differ. 2017;24:1739–49. Article PubMed PubMed Central CAS Google Scholar * Boles KS, Mathew
PA. Molecular cloning of CS1, a novel human natural killer cell receptor belonging to the CD2 subset of the immunoglobulin superfamily. Immunogenetics. 2001;52:302–7. Article PubMed CAS
Google Scholar * Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol.
2001;167:5517–21. Article PubMed CAS Google Scholar * Chen J, Zhong M-C, Guo H, Davidson D, Mishel S, Lu Y, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via
Mac-1 integrin. Nature. 2017;544:493–7. Article PubMed PubMed Central CAS Google Scholar * Cruz-Munoz M-E, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family
receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10:297–305. Article PubMed CAS Google Scholar * Gutierrez-Guerrero A, Mancilla-Herrera I,
Maravillas-Montero JL, Martinez-Duncker I, Veillette A, Cruz-Munoz ME. SLAMF7 selectively favors degranulation to promote cytotoxicity in human NK cells. Eur J Immunol. 2022;52:62–74.
Article PubMed CAS Google Scholar * O’Connell P, Blake MK, Godbehere S, Amalfitano A, Aldhamen YA. SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS
autoimmunity. J Neuroinflammation. 2022;19:241. Article PubMed PubMed Central Google Scholar * Tassi I, Colonna M. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the
PI3K and phospholipase Cgamma signaling pathways in human NK cells. J Immunol. 2005;175:7996–8002. Article PubMed CAS Google Scholar * Wu Y, Wang Q, Li M, Lao J, Tang H, Ming S et al.
SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis. J Clin Investig. 2023;133:e150224. * Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E,
Wallace ZS, et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138:825–38. Article PubMed PubMed Central CAS
Google Scholar * Loyal L, Warth S, Jürchott K, Mölder F, Nikolaou C, Babel N, et al. SLAMF7 and IL-6R define distinct cytotoxic versus helper memory CD8+ T cells. Nat Commun. 2020;11:6357.
Article PubMed PubMed Central CAS Google Scholar * Cachot A, Bilous M, Liu Y-C, Li X, Saillard M, Cenerenti M et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human
cancer. Sci Adv. 2021;7:eabe3348. * Comte D, Karampetsou MP, Yoshida N, Kis-Toth K, Kyttaris VC, Tsokos GC. Signaling Lymphocytic Activation Molecule Family Member 7 Engagement Restores
Defective Effector CD8+ T Cell Function in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69:1035–44. Article PubMed PubMed Central CAS Google Scholar * Awwad MHS, Mahmoud A,
Bruns H, Echchannaoui H, Kriegsmann K, Lutz R, et al. Selective elimination of immunosuppressive T cells in patients with multiple myeloma. Leukemia. 2021;35:2602–15. Article PubMed PubMed
Central CAS Google Scholar * O’Connell P, Hyslop S, Blake MK, Godbehere S, Amalfitano A, Aldhamen YA. SLAMF7 Signaling Reprograms T Cells toward Exhaustion in the Tumor Microenvironment.
J Immunol. 2021;206:193–205. Article PubMed Google Scholar * Lange S, Sylvester M, Schümann M, Freund C, Krause E. Identification of phosphorylation-dependent interaction partners of the
adapter protein ADAP using quantitative mass spectrometry: SILAC vs (18)O-labeling. J Proteome Res. 2010;9:4113–22. Article PubMed CAS Google Scholar * Zhou G, Soufan O, Ewald J,
Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–41. Article PubMed
PubMed Central CAS Google Scholar * Guan H, Nagarkatti PS, Nagarkatti M. Role of CD44 in the differentiation of Th1 and Th2 cells: CD44-deficiency enhances the development of Th2
effectors in response to sheep RBC and chicken ovalbumin. J Immunol. 2009;183:172–80. Article PubMed CAS Google Scholar * Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K,
et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–25. Article PubMed CAS Google Scholar * Misra S, Hascall
VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front Immunol. 2015;6:201. Article PubMed
PubMed Central Google Scholar * Mrass P, Kinjyo I, Ng LG, Reiner SL, Puré E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor
immunity. Immunity. 2008;29:971–85. Article PubMed PubMed Central CAS Google Scholar * Huang Y, Clarke F, Karimi M, Roy NH, Williamson EK, Okumura M, et al. CRK proteins selectively
regulate T cell migration into inflamed tissues. J Clin Invest. 2015;125:1019–32. Article PubMed PubMed Central Google Scholar * Roy NH, MacKay JL, Robertson TF, Hammer DA, Burkhardt JK.
Crk adaptor proteins mediate actin-dependent T cell migration and mechanosensing induced by the integrin LFA-1. Sci Signal. 2018;11:eaat3178. * Brunner MC, Chambers CA, Chan FK-M, Hanke J,
Winoto A, Allison JP. CTLA-4-Mediated Inhibition of Early Events of T Cell Proliferation. J Immunol. 1999;162:5813–20. Article PubMed CAS Google Scholar * Schultz-Thater E, Noppen C,
Gudat F, Dürmüller U, Zajac P, Kocher T, et al. NY-ESO-1 tumour associated antigen is a cytoplasmic protein detectable by specific monoclonal antibodies in cell lines and clinical specimens.
Br J Cancer. 2000;83:204–8. Article PubMed PubMed Central CAS Google Scholar * Valmori D, Dutoit V, Liénard D, Rimoldi D, Pittet MJ, Champagne P, et al. Naturally occurring human
lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499–506. PubMed CAS Google Scholar * Pazina T, James
AM, Colby KB, Yang Y, Gale A, Jhatakia A, et al. Enhanced SLAMF7 Homotypic Interactions by Elotuzumab Improves NK Cell Killing of Multiple Myeloma. Cancer Immunol Res. 2019;7:1633–46.
Article PubMed PubMed Central CAS Google Scholar * Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. Article PubMed PubMed
Central CAS Google Scholar * Roy E, Togbe D, Holdorf AD, Trubetskoy D, Nabti S, Küblbeck G, et al. Nck adaptors are positive regulators of the size and sensitivity of the T-cell
repertoire. Proc Natl Acad Sci USA. 2010;107:15529–34. Article PubMed PubMed Central CAS Google Scholar * Hartl FA, Ngoenkam J, Beck-Garcia E, Cerqueira L, Wipa P, Paensuwan P et al.
Cooperative Interaction of Nck and Lck Orchestrates Optimal TCR Signaling. Cells. 2021;10:834. * Braiman A, Isakov N. The Role of Crk Adaptor Proteins in T-Cell Adhesion and Migration. Front
Immunol. 2015;6:509. Article PubMed PubMed Central Google Scholar * Reth M. Discovering immunoreceptor coupling and organization motifs. Front Immunol. 2023;14:1253412. Article PubMed
PubMed Central CAS Google Scholar * Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–4. Article PubMed PubMed
Central CAS Google Scholar * Uhl LFK, Cai H, Oram SL, Mahale JN, MacLean AJ, Mazet JM, et al. Interferon-γ couples CD8+ T cell avidity and differentiation during infection. Nat Commun.
2023;14:6727. Article PubMed PubMed Central CAS Google Scholar * Yanguas A, Garasa S, Teijeira Á, Aubá C, Melero I, Rouzaut A. ICAM-1-LFA-1 Dependent CD8+ T-Lymphocyte Aggregation in
Tumor Tissue Prevents Recirculation to Draining Lymph Nodes. Front Immunol. 2018;9:2084. Article PubMed PubMed Central Google Scholar * Brunner-Weinzierl MC, Rudd CE. CTLA-4 and PD-1
Control of T-Cell Motility and Migration: Implications for Tumor Immunotherapy. Front Immunol. 2018;9:2737. Article PubMed PubMed Central Google Scholar * Li K, Yuan Z, Lyu J, Ahn E,
Davis SJ, Ahmed R, et al. PD-1 suppresses TCR-CD8 cooperativity during T-cell antigen recognition. Nat Commun. 2021;12:2746. Article PubMed PubMed Central CAS Google Scholar * Homann D,
Dummer W, Wolfe T, Rodrigo E, Theofilopoulos AN, Oldstone MBA, et al. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J Virol.
2006;80:270–80. Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by German Research Foundation (DFG) Br1860/12 and SFB854
B14 and Sander Foundation 2024.003.1. The authors’ own work cited in this review was also supported by the DFG inter alia in the context of the Collaborative Research Center CRC 854. For
mass spectrometry, we would like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. Further, we would like to thank the German Red Cross for providing of
blood samples and Edward A. Clark (University of Washington) for the valuable input during drafting the manuscript. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Experimental Paediatrics, University Hospital, Otto-von-Guericke-University, Magdeburg, Germany Holger Lingel, Laura Fischer, Sven
Remstedt, Irina Han, Jan-Erik Sander, Aditya Arra & Monika C. Brunner-Weinzierl * Health Campus Immunology, Infectiology and Inflammation, Otto-von-Guericke-University, Magdeburg,
Germany Holger Lingel, Sven Remstedt, Lars Philipsen, Irina Han, Jan-Erik Sander, Aditya Arra & Monika C. Brunner-Weinzierl * Institute of Chemistry and Biochemistry, Freie Universität
Berlin, Berlin, Germany Benno Kuropka & Christian Freund * Multi-parametric bioimaging and cytometry (MPBIC) core facility, University Hospital, Otto-von-Guericke-University, Magdeburg,
Germany Lars Philipsen * Institute of Cellular and Molecular Immunology, University Hospital, Otto-von-Guericke-University, Magdeburg, Germany Lars Philipsen Authors * Holger Lingel View
author publications You can also search for this author inPubMed Google Scholar * Laura Fischer View author publications You can also search for this author inPubMed Google Scholar * Sven
Remstedt View author publications You can also search for this author inPubMed Google Scholar * Benno Kuropka View author publications You can also search for this author inPubMed Google
Scholar * Lars Philipsen View author publications You can also search for this author inPubMed Google Scholar * Irina Han View author publications You can also search for this author
inPubMed Google Scholar * Jan-Erik Sander View author publications You can also search for this author inPubMed Google Scholar * Christian Freund View author publications You can also search
for this author inPubMed Google Scholar * Aditya Arra View author publications You can also search for this author inPubMed Google Scholar * Monika C. Brunner-Weinzierl View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS HL, BK, and MCB-W designed research; HL, LF, BK, LP, IH, and JS performed research; HL, LF, SR, BK, and
LP analyzed data; SR, BK, LP, and CF contributed new reagents/analytic tools; HL and MCB-W wrote the paper; LF, BK, LP, and AA edited the manuscript. CORRESPONDING AUTHOR Correspondence to
Monika C. Brunner-Weinzierl. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that the research was conducted in the absence of any commercial or financial relationships that
could be construed as a potential conflict of interest. LP is shareholder and CEO of M04PB UG, Kiel, Germany and CEO of BioDecipher GmbH, Magdeburg, Germany. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Lingel, H., Fischer, L., Remstedt, S. _et al._ SLAMF7 (CD319) on activated CD8+ T cells transduces environmental cues to initiate cytotoxic effector cell responses. _Cell Death
Differ_ 32, 561–572 (2025). https://doi.org/10.1038/s41418-024-01399-y Download citation * Received: 21 November 2023 * Revised: 10 September 2024 * Accepted: 02 October 2024 * Published: 10
October 2024 * Issue Date: March 2025 * DOI: https://doi.org/10.1038/s41418-024-01399-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Susannah townsend calls time on her international hockey career | england hockeyAfter playing 188 games across a 13-year career, Susannah Townsend has retired from international hockey. A double Olymp...
Video 'Big Little Lies' star Laura Dern teases possible return to 'Jurassic Park' - ABC NewsABC NewsVideoLiveShowsShopStream onLive UpdatesLive UpdatesTrump 2nd term Ukraine drone attack Trump tariffs Boulder att...
Do people still celebrate their name days in france?IT IS A FRENCH TRADITION TO SEND PEOPLE GOOD WISHES ON THEIR ‘NAME DAY’. WE LOOK AT WHERE THIS CAME FROM AND WHETHER THE...
What could happen next after jay slater's inquest dramatically haltedJAY'S FAMILY WANT TWO KEY WITNESSES BROUGHT TO COURT 20:07, 21 May 2025 The inquest into the death of Jay Slater wa...
New approaches to quantifying the spread of infectionKEY POINTS * The last decade has seen considerable advances in statistical, mathematical and computational techniques th...
Latests News
Slamf7 (cd319) on activated cd8+ t cells transduces environmental cues to initiate cytotoxic effector cell responsesABSTRACT CD8+ T-cell responses are meticulously orchestrated processes regulated by intercellular receptor:ligand intera...
Income and corporation tax: repeal of the renewals allowance* HM Revenue & Customs Policy paper INCOME AND CORPORATION TAX: REPEAL OF THE RENEWALS ALLOWANCE Published 16 March ...
Weight reduction and exercise capacity in obese adolescentsABSTRACT Physical activity is nore strenuous for obese children and adolescents compared to normalweight healthy peers. ...
Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forestsABSTRACT Soils of northern temperate and boreal forests represent a large terrestrial carbon (C) sink. The fate of this ...
'the heron's cry' chapters 16-18 | members only accessWesley’s shed was as large as a good-sized barn, built from wood, but solid. He’d once held a party there, after hours, ...