No evidence of disease versus residual disease in long-term responders to first-line her2-targeted therapy for metastatic breast cancer
No evidence of disease versus residual disease in long-term responders to first-line her2-targeted therapy for metastatic breast cancer"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Long-term response to HER2-targeted therapies is infrequent in metastatic breast cancer (MBC). We evaluated clinical characteristics of HER2-positive MBC patients with no
evidence of disease (NED) vs residual disease (RES) experiencing long-term response to first-line HER2-targeted therapy. METHODS Patients receiving first-line chemotherapy-trastuzumab (CT)
or taxane-trastuzumab-pertuzumab (THP) with response duration ≥2-fold higher than in phase II/III trials (CT [18.2 months]; THP [40.4 months]) were included. Clinical characteristics and
radiographic review for NED or RES was evaluated by Cox-regression (hazard ratio; HR) or Kaplan–Meier (log-rank). Characteristics associated with NED were evaluated by logistic regression
(Odds; OR). RESULTS From 01/2005-01/2016, _N_ = 103 (4.6%) patients were identified. In multivariate analyses, NED (_N_ = 46) showed improved progression-free (PFS) and overall survival (OS)
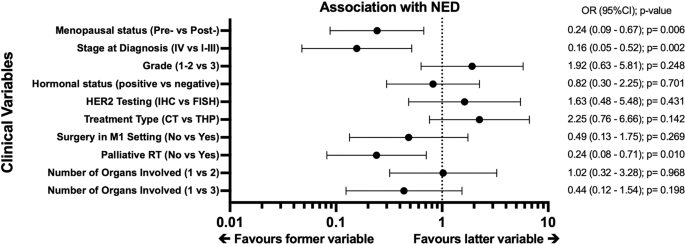
[_p_ < 0.001] versus RES (_N_ = 57), with high 5-year PFS/OS for NED (93.2%/97.4%) relative to RES (10.6%/61.3%). Premenopausal status (_p_ = 0.006), de-novo metastases (_p_ = 0.002),
and no palliative radiotherapy (_p_ = 0.01) were associated with NED. Overall, 6/7 (85.7%) patients with NED were alive and disease-free after discontinuing HER2 treatment (≥1 year) versus
1/17 (5.9%) with RES. CONCLUSIONS Long-term responders with NED have better survival compared to RES. Premenopausal status and de novo metastatic disease are associated with NED. Prospective
studies of HER2 therapy discontinuation with NED in MBC are warranted. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 24 print issues and online access $259.00 per year only $10.79 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PATHOLOGIC COMPLETE RESPONSE AND SURVIVAL AFTER NEOADJUVANT
CHEMOTHERAPY IN CT1-T2/N0 HER2+ BREAST CANCER Article Open access 12 May 2022 SHORTER DURATION OF FIRST-LINE CHEMOTHERAPY REFLECTS POORER OUTCOMES IN PATIENTS WITH HER2-NEGATIVE ADVANCED
BREAST CANCER: A MULTICENTER RETROSPECTIVE STUDY Article Open access 02 November 2021 HER2DX _ERBB2_ MRNA SCORE IN FIRST-LINE ADVANCED HER2-POSITIVE BREAST CANCER TREATED WITH CHEMOTHERAPY,
TRASTUZUMAB, AND PERTUZUMAB Article Open access 25 April 2025 DATA AVAILABILITY The data that support the findings of this study are available upon request from the corresponding author. The
data are not publicly available due to privacy and ethical restrictions. REFERENCES * Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’
follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205.
https://doi.org/10.1016/S0140-6736(16)32616-2. Article CAS PubMed PubMed Central Google Scholar * Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, et al. Trastuzumab plus
adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol.
2014;32:3744–52. https://doi.org/10.1200/JCO.2014.55.5730. Article CAS PubMed PubMed Central Google Scholar * Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al.
Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl J Med. 2015;372:724–34. https://doi.org/10.1056/NEJMoa1413513. Article CAS PubMed PubMed Central
Google Scholar * Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med.
2019; https://doi.org/10.1056/nejmoa1914609. * Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in
HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2020;38:3138–49.
https://doi.org/10.1200/JCO.20.00147. Article CAS Google Scholar * Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated
HER2-positive breast cancer. N. Engl J Med. 2020;382:610–21. https://doi.org/10.1056/NEJMoa1914510. Article CAS PubMed Google Scholar * Swain SM, Miles D, Kim SB, Im YH, Im SA,
Semiglazov V, et al. End-of-study analysis from the phase III, randomized, double-blind, placebo (Pla)-controlled CLEOPATRA study of first-line (1L) pertuzumab (P), trastuzumab (H), and
docetaxel (D) in patients (pts) with HER2-positive metastatic breast cancer (MBC). J Clin Oncol. 2019;37:1020–1020. https://doi.org/10.1200/JCO.2019.37.15_suppl.1020. Article Google Scholar
* Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast
cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–35. https://doi.org/10.1016/S1470-2045(19)30328-6. Article CAS PubMed Google Scholar * Butterbaugh
ST, Patel R, Romond EH, Mathew A. Trastuzumab use in patients with durable complete response in HER2-amplified metastatic breast cancer: to continue or not to continue. Ann Oncol.
2017;28:3098–9. https://doi.org/10.1093/annonc/mdx532. Article CAS PubMed Google Scholar * Wong Y, Raghavendra AS, Hatzis C, Irizarry JP, Vega T, Horowitz N, et al. Long‐term survival of
de novo stage IV human epidermal growth receptor 2 (HER2) positive breast cancers treated with HER2‐targeted therapy. Oncologist. 2019;24:313–8.
https://doi.org/10.1634/theoncologist.2018-0213. Article CAS PubMed Google Scholar * Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs
trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32.
https://doi.org/10.1016/j.annonc.2021.08.2087. * Datta J, Fracol M, McMillan MT, Berk E, Xu S, Goodman N, et al. Association of depressed anti-HER2 T-helper type 1 response with recurrence
in patients with completely treated HER2-positive breast cancer: role for immune monitoring. JAMA Oncol. 2016;2:242–6. https://doi.org/10.1001/jamaoncol.2015.5482. Article PubMed Google
Scholar * Datta J, Berk E, Xu S, Fitzpatrick E, Rosemblit C, Lowenfeld L, et al. Anti-HER2 CD4+ T-helper type 1 response is a novel immune correlate to pathologic response following
neoadjuvant therapy in HER2-positive breast cancer. Breast Cancer Res. 2015;17:71 https://doi.org/10.1186/s13058-015-0584-1. Article PubMed PubMed Central Google Scholar * Griguolo G,
Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer. 2019;7:90
https://doi.org/10.1186/s40425-019-0548-6. Article PubMed PubMed Central Google Scholar * Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus
trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371–82.
https://doi.org/10.1016/S1470-2045(18)30812-X. Article CAS PubMed Google Scholar * Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim SB, et al. Trastuzumab emtansine plus
atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet
Oncol. 2020;21:1283–95. https://doi.org/10.1016/S1470-2045(20)30465-4. Article CAS PubMed Google Scholar * Witzel I, Müller V, Abenhardt W, Kaufmann M, Schoenegg W, Schneeweis A, et al.
Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—results from the HER-OS patient registry. BMC Cancer. 2014;14:806
https://doi.org/10.1186/1471-2407-14-806. Article PubMed PubMed Central Google Scholar * Yardley DA, Tripathy D, Brufsky AM, Rugo HS, Kaufman PA, Mayer M, et al. Long-term survivor
characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer. 2014;110:2756–64. https://doi.org/10.1038/bjc.2014.174. Article CAS PubMed PubMed Central Google
Scholar * Moilanen T, Mustanoja S, Karihtala P, Koivunen JP. Retrospective analysis of HER2 therapy interruption in patients responding to the treatment in metastatic HER2+ breast cancer.
ESMO Open. 2017;2:e000202 https://doi.org/10.1136/esmoopen-2017-000202. Article PubMed PubMed Central Google Scholar * Gullo G, Zuradelli M, Sclafani F, Santoro A, Crown J. Durable
complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol. 2012;23:2204–5. https://doi.org/10.1093/annonc/mds221. Article CAS PubMed
Google Scholar * Niikura N, Shimomura A, Fukatsu Y, Sawaki M, Ogiya R, Yasojima H, et al. Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis.
Breast Cancer Res Treat. 2018;167:81–87. https://doi.org/10.1007/s10549-017-4489-9. Article PubMed Google Scholar * Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER
kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–94. https://doi.org/10.1038/nature25475. Article CAS PubMed PubMed Central Google Scholar *
Altundag K. Duration of trastuzumab in HER2-positive metastatic breast cancer after complete remission: still debatable issue? Breast Cancer Res Treat. 2018;167:815–815.
https://doi.org/10.1007/s10549-017-4536-6. Article PubMed Google Scholar * Viel E, Arbion F, Barbe C, Bougnoux P. Prolonged complete response after treatment withdrawal in
HER2-overexpressed, hormone receptor-negative breast cancer with liver metastases: the prospect of disappearance of an incurable disease. BMC Cancer. 2014;14:690
https://doi.org/10.1186/1471-2407-14-690. Article PubMed PubMed Central Google Scholar * Lambertini M, Vaz-Luis I. Is HER2-positive metastatic breast cancer still an incurable disease?
Lancet Oncol. 2020;21:471–2. https://doi.org/10.1016/S1470-2045(20)30058-9. Article PubMed Google Scholar * Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab
emtansine for HER2-positive advanced breast cancer. N. Engl J Med. 2012;367:1783–91. https://doi.org/10.1056/NEJMoa1209124. Article CAS PubMed PubMed Central Google Scholar * Chan A,
Martin M, Untch M, Gil MG, Guillem-Porta V, Wojtukiewicz M, et al. Vinorelbine plus trastuzumab combination as first-line therapy for HER 2-positive metastatic breast cancer patients: an
international phase II trial. Br J Cancer. 2006;95:788–93. https://doi.org/10.1038/sj.bjc.6603351. Article CAS PubMed PubMed Central Google Scholar * Urruticoechea A, Rizwanullah M, Im
SA, Ruiz ACS, Láng I, Tomasello G, et al. Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor
2–positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therap. J Clin Oncol. 2017;35:3030–8. https://doi.org/10.1200/JCO.2016.70.6267.
Article CAS PubMed Google Scholar * Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth
factor receptor 2–overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25:3246–50. https://doi.org/10.1200/JCO.2006.09.6826. Article CAS
PubMed Google Scholar * Murthy P, Kidwell KM, Schott AF, Merajver SD, Griggs JJ, Smerage JD, Van Poznak CH, Wicha MS, Hayes DF, Henry NL. Clinical predictors of long-term survival in
HER2-positive metastatic breast cancer. Breast cancer Res Treat. 2016;155:589–95. https://doi.org/10.1007/s10549-016-3705-3. Article PubMed PubMed Central Google Scholar * Printz C. NCI
launches exceptional responders initiative: researchers will attempt to identify why some patients respond to treatment so much better than others. Cancer. 2015;121:803–4.
https://doi.org/10.1002/cncr.29311. Article PubMed Google Scholar * Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1:276–90.
https://doi.org/10.1038/s43018-020-0043-5. Article CAS PubMed Google Scholar * Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in
neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021;32:229–39. https://doi.org/10.1016/j.annonc.2020.11.007. Article CAS PubMed Google Scholar * Tokudome N,
Hayes DF. Analysis of circulating tumor DNA to monitor metastatic breast cancer. Breast Dis: A Year Book Q. 2013;24:350–2. https://doi.org/10.1016/j.breastdis.2013.10.034. Article Google
Scholar * Guan X, Liu B, Niu Y, Dong X, Zhu X, Li C, et al. Longitudinal HER2 amplification tracked in circulating tumor DNA for therapeutic effect monitoring and prognostic evaluation in
patients with breast cancer. Breast. 2020;49:261–6. https://doi.org/10.1016/j.breast.2019.12.010. Article PubMed Google Scholar * Ma F, Zhu W, Guan Y, Yang L, Xia X, Chen S, et al. ctDNA
dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget. 2016;7:66020–31. https://doi.org/10.18632/oncotarget.11791. Article
PubMed PubMed Central Google Scholar * Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 2020;18. https://doi.org/10.6004/jnccn.2020.0016. * Perez EA, Cortés J, Gonzalez-Angulo AM, Bartlett JMS. HER2 testing: current status and future
directions. Cancer Treat Rev. 2014;40:276–84. https://doi.org/10.1016/j.ctrv.2013.09.001. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Princess Margaret Cancer
Centre and Cancer Care Alberta would like to acknowledge the patients and families for their contribution to this research. This study was presented at the American Society of Clinical
Oncology (ASCO) annual meeting in Chicago, USA, in June 2019. FUNDING No funding was procured for this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medical Oncology and
Hematology, St Michael’s Hospital, Toronto, ON, Canada Zachary Veitch * Department of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Toronto, ON, Canada Zachary Veitch,
Philippe L. Bedard & David W. Cescon * Medical Oncology Department, Institute of Oncology Ljubljana, Ljubljana, Slovenia Domen Ribnikar * Cancer Care Alberta, Alberta Health Services,
Calgary, AB, Canada Derek Tilley * Department of Medical Oncology, Tom Baker Cancer Centre, Calgary, AB, Canada Patricia A. Tang & Sasha Lupichuk * Department of Medical Oncology, Cross
Cancer Institute, University of Alberta, Edmonton, AB, Canada Karen King Authors * Zachary Veitch View author publications You can also search for this author inPubMed Google Scholar * Domen
Ribnikar View author publications You can also search for this author inPubMed Google Scholar * Derek Tilley View author publications You can also search for this author inPubMed Google
Scholar * Patricia A. Tang View author publications You can also search for this author inPubMed Google Scholar * Karen King View author publications You can also search for this author
inPubMed Google Scholar * Philippe L. Bedard View author publications You can also search for this author inPubMed Google Scholar * Sasha Lupichuk View author publications You can also
search for this author inPubMed Google Scholar * David W. Cescon View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualisation—ZWV,
PAT, DR, KK, SL and DC. Data curation—ZWV, SL, KK and DR. Formal analyses—ZWV and DT. Funding acquisition—None. Investigation—ZWV and DR, Methodology—ZWV, PAT and SL. Project
administration—ZWV, DWC and PLB. Resources—ZWV, DT and DWC. Software—ZWV and DT. Supervision—SL and DWC. Validation—Not applicable. Visualisation—ZWV. Writing—Original draft—ZWV.
Writing—Review and editing—ZWV, DR, DT, PAT, KK, PLB, SL and DWC. CORRESPONDING AUTHOR Correspondence to Zachary Veitch. ETHICS DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE This
study was approved by the Princess Margaret Cancer Centre Research Ethics Board (NCT03740503) and the Health and Research Ethics Board of Alberta (Cancer Committee). CONSENT TO PUBLISH Not
applicable. COMPETING INTERESTS ZWV has received honoraria from Pfizer, Exact Sciences, Knight Pharmaceuticals, and Novartis. DWC reports consultancy and advisory fees from AstraZeneca,
Exact Sciences, Gilead, GlaxoSmithKline, Merck, Novartis, Pfizer, and Roche; research funding to their institution from GlaxoSmithKline, Inivata, Merck, Pfizer and Roche; is a member of a
trial steering committee for Merck; and holds a patent (US62/675,228) for methods of treating cancers characterised by a high expression level of spindle and kinetochore associated complex
subunit 3 (ska3) gene. PLB has received research funding from Bristol-Myers Squibb, Sanofi, AstraZeneca, Genentech/Roche, SERVIER, GlaxoSmithKline, Novartis, SignalChem, PTC Therapeutics,
Nektar, Merck, Seattle Genetics, Mersana, Immunomedics, Eli-Lilly, Zymeworks, and VelosBio. Uncompensated advisory for Lilly, Seagen, Merck, Genentech/Roche, Bristol-Myers Squibb, and
Gilead. PAT has honoraria from Teva, Eisai, Pfizer, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca and Roche. No conflicts of interest: The following authors have declared no conflicts
of interest: DR, DT, KK and SL. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENT REPRODUCIBILITY CHECKLIST STROBE CHECKLIST RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Veitch, Z., Ribnikar,
D., Tilley, D. _et al._ No evidence of disease versus residual disease in long-term responders to first-line HER2-targeted therapy for metastatic breast cancer. _Br J Cancer_ 126, 881–888
(2022). https://doi.org/10.1038/s41416-021-01676-4 Download citation * Received: 22 October 2021 * Revised: 27 November 2021 * Accepted: 10 December 2021 * Published: 20 December 2021 *
Issue Date: 01 April 2022 * DOI: https://doi.org/10.1038/s41416-021-01676-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Deeleview charolais dispersal hits €9,000 - farmers weeklyThe Dispersal sale of the Deeleview Charolais herd on behalf of Ian Maxwell, Convoy, Co Donegal hit a high of €9,000 whe...
Review: in stephen adly guirgis' 'den of thieves,' deft writing steals the showIf he had been born in another generation, Stephen Adly Guirgis could have been a gag writer on “Your Show of Shows.” Gu...
“ORGANIZATIONS FOR PEOPLE”“ORGANIZATIONS FOR PEOPLE”Clip: 1/28/2020 | 12m 16sVideo has Closed Captions | CC“ORGANIZATIONS FOR PEOPLE”Aired 01/28/2...
Chinese artist ai weiwei on his memoir, his persecution and 'why autocracy fears art'On the Shelf 1000 Years of Joys and Sorrows By Ai Weiwei, trans. by Allan H. Barr Crown: 400 pages, $32 _If you buy book...
In thailand, santa delivers presents on elephantsIn Thailand, Santa delivers presents on elephants | WTVB | 1590 AM · 95.5 FM | The Voice of Branch County Close For the ...
Latests News
No evidence of disease versus residual disease in long-term responders to first-line her2-targeted therapy for metastatic breast cancerABSTRACT BACKGROUND Long-term response to HER2-targeted therapies is infrequent in metastatic breast cancer (MBC). We ev...
Lewis hamilton drops indy 500 hint after trying out winner's ringHamilton watched Sebastian Vettel disappear from his mirrors in the early stages of the Japanese Grand Prix as his title...
Kelly thomas: fullerton loses bid to keep officers' files secretFullerton must turn over portions of the personnel files of two former officers charged in the death of Kelly Thomas aft...
Cross-dressers in film - Los Angeles TimesEntertainment & Arts Cross-dressers in film ‘Mrs. Doubtfire’ In this 1993 family comedy, divorced daddy Daniel Hillard -...
Comcast beats revenue estimates on boost from theme parks, studiosComcast beats revenue estimates on boost from theme parks, studios | WTVB | 1590 AM · 95.5 FM | The Voice of Branch Coun...