Cytokines in clinical cancer immunotherapy
Cytokines in clinical cancer immunotherapy"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cytokines are soluble proteins that mediate cell-to-cell communication. Based on the discovery of the potent anti-tumour activities of several pro-inflammatory cytokines in animal
models, clinical research led to the approval of recombinant interferon-alpha and interleukin-2 for the treatment of several malignancies, even if efficacy was only modest. These early
milestones in immunotherapy have been followed by the recent addition to clinical practice of antibodies that inhibit immune checkpoints, as well as chimeric antigen receptor T cells. A
renewed interest in the anti-tumour properties of cytokines has led to an exponential increase in the number of clinical trials that explore the safety and efficacy of cytokine-based drugs,
not only as single agents, but also in combination with other immunomodulatory drugs. These second-generation drugs under clinical development include known molecules with novel mechanisms
of action, new targets, and fusion proteins that increase half-life and target cytokine activity to the tumour microenvironment or to the desired effector immune cells. In addition, the
detrimental activity of immunosuppressive cytokines can be blocked by antagonistic antibodies, small molecules, cytokine traps or siRNAs. In this review, we provide an overview of the novel
trends in the cytokine immunotherapy field that are yielding therapeutic agents for clinical trials. SIMILAR CONTENT BEING VIEWED BY OTHERS HARNESSING CYTOKINES AND CHEMOKINES FOR CANCER
THERAPY Article 07 January 2022 CYTOKINES THAT TARGET IMMUNE KILLER CELLS AGAINST TUMORS Article 10 June 2020 EMERGING PRINCIPLES OF CYTOKINE PHARMACOLOGY AND THERAPEUTICS Article 21
September 2022 INTRODUCTION Cytokines are polypeptides or glycoproteins with a molecular weight usually below 30 kDa that provide growth, differentiation and inflammatory or
anti-inflammatory signals to different cell types. Cytokines are most often released during a defined period in response to a stimulus, and the extent of their action is short-lived due to
their limited half-life in the circulation. As a result, cytokines normally exert an autocrine or paracrine effect. As an exception to the general rule, cytokines such as interleukin (IL)-7
or haematopoietic growth factors are produced homeostatically in a continuous fashion. Cytokine target cells express high-affinity receptors on their cellular membrane. Following cytokine
binding, the receptors trigger intracellular signalling which leads to modifications in gene transcription. Cytokines thereby modify proliferation and differentiation and induce or modify
particular cell functions. Target cells expressing the corresponding sets of receptors integrate the information derived from the concentration and timing of exposure to different cytokines.
Thus, synergy or antagonism among different cytokines is a common characteristic, with high degrees of complexity. CYTOKINES AS A MONOTHERAPY Several cytokines limit tumour cell growth by a
direct anti-proliferative or pro-apoptotic activity, or indirectly by stimulating the cytotoxic activity of immune cells against tumour cells. A paradigmatic case is interferon-alpha
(IFN-α), first discovered in 1957 as a result of its antiviral properties.1 After 13 years, Gresser and Bourali2 described the anti-tumour activity of IFN-α against different tumour cell
lines inoculated in mice. This discovery of the ability of cytokines to potentiate immune responses against cancer in conjunction with the development of recombinant DNA technologies led, in
the 1980s and 1990s, to intense preclinical and clinical investigation of the potential anti-tumour activity of several recombinant cytokines. However, results from clinical trials failed
to meet the high expectations raised in preclinical models and highlighted the limitations of approaches based on unmodified recombinant proteins. These limitations include the short
half-life of most cytokines and narrow therapeutic windows with only modest anti-tumour efficacy, at least as monotherapies. Only two cytokines, IL-2 and IFN-α, demonstrated mild clinical
benefit and consequently received The Food and Drug Administration (FDA) approval for the treatment of several malignant diseases. IL-2 was approved for the treatment of advanced renal cell
carcinoma (RCC)3 and metastatic melanoma,4 whereas IFN-α was approved for the treatment of hairy cell leukaemia,5 follicular non-Hodgkin lymphoma,6 melanoma7 and AIDS-related Kaposi’s
sarcoma.8 The clinical use of these cytokines marked a milestone in cancer immunotherapy, as it was the first demonstration that immunotherapy could favourably tilt the balance between
cancer and the anti-tumour immune response, leading to durable objective responses. However, the low response rate and high toxicity associated with high-dose IL-2 and IFN-α administration
have relegated the clinical use of these cytokines in favour of targeted therapy and immune checkpoint inhibitors.9,10 POTENTIATING THE EFFECTS OF IMMUNOTHERAPIES Immune checkpoint
inhibitors represent a revolution in cancer immunotherapy. Clinical immunotherapy with monoclonal antibodies to block the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) or programmed
cell death protein 1 (PD-1)–PD-1 ligand (PD-L1) axes have been FDA-approved for the treatment of several malignancies such as melanoma, non-small cell lung cancer, RCC, Hodgkin lymphoma,
Merkel cell carcinoma, head and neck cancer and carcinoma of the bladder. These novel therapies have yielded long-lasting responses in a fraction of patients. In contrast to recombinant IL-2
or IFN-α as mentioned above, immune checkpoint inhibitors have a more favourable safety profile. Any adverse effects are mainly autoimmune-like syndromes that can usually be controlled by
treatment with corticosteroids or other anti-inflammatory drugs, such as infliximab.11 The synergistic combination of anti-CTLA-4 and anti-PD-1 monoclonal antibodies has been approved for
the treatment of advanced melanoma, microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer and advanced RCC by the FDA and has improved the overall
response rate of patients.12,13,14 The clinical success of this combination provides the rationale for new combination approaches in immunotherapy.15 In this context, cytokines are being
incorporated into combination clinical trials, mainly in conjunction with anti-PD-1 and anti-PD-L1 monoclonal antibodies. Adoptive T cell therapies are also coming of age due to impressive
efficacy results and FDA approval of anti-CD19 chimeric antigen receptor (CAR) T cells,16 and the use of cultures of tumour-infiltrating T lymphocytes (TILs).17 Notably, these approaches are
totally dependent on cytokines for in vitro expansion and in vivo persistence of transferred T cells. These cellular immunotherapies can be optimised by the incorporation of cytokine genes
into the lentiviral vector that encodes the CARs.18 The search for the next generation of cytokine-based drugs is based on three concepts. The first concept is synergistic combinations, such
that the approved anti-PD-1–PD-L1 monoclonal antibodies and CAR19 T cells provide a suitable basis for combining with cytokines. The second concept looks at improved pharmacokinetics. The
systemic administration of cytokines requires optimisation of the pharmacokinetic profile to increase the half-life in circulation (surpassing the kidney filtration threshold) and to
increase the cytokine concentration in the tumour microenvironment (TME). This aim can be achieved by conjugating polyethylene glycol (PEG) to the cytokine or by constructing a fusion
protein with antibodies, Fc domains, apolipoprotein A-I, albumin or the latent peptide of transforming growth factor-β (TGF-β). The third concept is that of local administration. An
alternative strategy to achieve high local concentrations of cytokines in the TME is to directly inject the recombinant protein19 or intratumoural gene therapy vectors that encode the
cytokine20,21 into the TME. Clinical trials are currently making use of oncolytic viruses, plasmid electroporation and intratumoural injection of lipid nanoparticles loaded with modified
messenger RNAs. In this review, we describe how these blueprint concepts are being applied to several cytokine-based compounds already undergoing clinical trials. Among the immunostimulatory
cytokines that potentiate immune responses against cancer, we focus our review on IFN-α, the IL-2 family, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-10. In the
case of immunosuppressive cytokines, we describe strategies to block the activity of tumour necrosis factor-alpha (TNF-α), TGF-β and cytokines that nurture tumour-associated myeloid cells,
such as colony-stimulating factor-1 (CSF-1). Finally, we discuss the key concepts that must be considered for future development of cytokine-based immunotherapy. POTENTIATING THE IMMUNE
RESPONSE Pro-inflammatory cytokines can contribute to cancer immunotherapy, acting on every phase of the cancer immunity cycle.22,23 Thus, cytokines can improve antigen priming, increase the
number of effector immune cells in the TME and enhance their cytolytic activity. However, making drugs based on cytokines requires fine-tuning of their pharmacological properties using
biotechnological strategies. INTERFERON-Α Since the first approval of IFN-α for the treatment of hairy cell leukaemia in 1986, this family of cytokines has been used for the treatment of
several haematological malignancies and solid tumours at high doses to exploit their direct pro-apoptotic/anti-proliferative activity on tumour cells. These high doses of IFN-α also exert
anti-tumour activity due to effects on the tumour vasculature as IFN-α displays a potent antiangiogenic activity.24 PEGylated IFN-α was also approved for the adjuvant treatment of
melanoma.25 This variant consists of a chemical modification that increases its half-life in circulation and therefore prolongs the exposure of the tumour cells to high IFN-α concentrations.
However, the advent of targeted therapies and novel immunotherapies with superior safety and efficacy profiles has reduced clinical use of IFN-α in oncohaematology. This situation might be
reverted with new cytokine modifications that exploit the immunostimulatory properties of IFN-α, as this cytokine is critical for the maturation of dendritic cells (DCs) and for the
acquisition of effector function by T lymphocytes.26 One strategy to unplug the cytotoxic activity of IFN-α from its immunostimulatory activity involves its fusion to apolipoprotein A-I.27
The apolipoprotein A-I moiety incorporates the cytokine into high-density lipoproteins, improving the pharmacokinetics and anti-tumour activity of IFN-α.28,29 AcTakines, activity-on-Target
cytokines, can also minimise the toxicity of IFN-α and maximise its immunostimulatory activity. This strategy is based on the fusion of a mutated cytokine that shows reduced affinity for its
receptor to a cell-specific targeting domain. IFN-α fused to single domain antibodies targeting Clec9A, a molecule expressed on DCs specialised in cross-priming, displays a potent
anti-tumour effect.30 Finally, IFN-α immunocytokines have been demonstrated to exert an anti-tumour effect mediated by the activation of immune system cells.31,32 In summary, type I IFN
immunobiology is likely to be exploited in the near future but is not currently in the frontline oncology armamentarium. INTERLEUKIN-2 IL-2 is viewed as a key cytokine in promoting the
expansion of natural killer (NK) cells and T lymphocytes. Thus, it is widely used in protocols of adoptive transfer for both expanding lymphocytes in culture and increasing the persistence
of transferred cells in cancer patients. The infusion of this cytokine at high doses is currently approved for the treatment of metastatic RCC and metastatic melanoma. However, the systemic
administration of this cytokine at the recommended dose is hampered by its toxic profile, which includes frequent grade 3 and 4 adverse effects. Second-generation IL-2-based therapies with
improved pharmacokinetic and pharmacodynamic profiles are being developed. Improvement of the pharmacokinetic profile is achieved through covalent binding of IL-2 to moieties that increase
the half-life in circulation, such as the Fc domains of immunoglobulins or PEG molecules, or by chimerisation with antibodies that target the cytokine to the TME. Improvement of the
pharmacodynamic properties is attained by using biotechnology tricks to reduce binding to the high-affinity IL-2 receptor while maintaining binding to the medium-affinity IL-2 receptor to
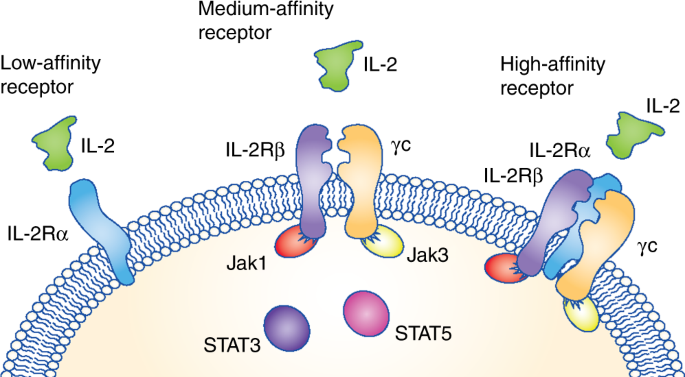
increase the amount of cytokine that is available to stimulate NK and T cells. The IL-2 receptor is composed of three different complexes formed by three chains (Fig. 1). The low-affinity
receptor is composed of the IL-2Rα chain alone but does not trigger an intracellular signalling cascade. Thus, the two receptors that induce signalling are the medium-affinity and the
high-affinity receptors. The medium-affinity receptor is composed of the IL-2Rβ chain and the common γ chain; when the IL-2Rα chain is also present in the receptor complex, IL-2 is bound
with high affinity. IL-2Rα is highly expressed on T regulatory (Treg) cells and therefore the high-affinity IL-2 receptor skews IL-2 activity towards the expansion of Treg cells, while
limiting the bioavailability of the cytokine to stimulate anti-tumour effector NK and T lymphocytes.9 Several of these second-generation IL-2-based compounds engineered to avoid binding to
IL-2Rα/CD25 have reached clinical trials. NKTR-214 is composed of recombinant IL-2 together with multiple molecules of PEG. Directed PEGylation generates an inactive cytokine with a long
half-life in circulation. The PEG groups are progressively released, yielding IL-2 molecules with double or single PEGylation that can interact with the medium-affinity IL-2 receptor but not
with the high-affinity IL-2 receptor.33 This modified cytokine is being evaluated in clinical trials in combination with the immune checkpoint inhibitors atezolizumab (NCT03138889),
nivolumab (NCT02983045, NCT03282344 and NCT03435640) and nivolumab plus ipilimumab (NCT02983045). As reported at the American Society of Clinical Oncology (ASCO) annual meeting in 2018,
NKTR-214 has undergone dose-escalation studies and has also been used in combination with nivolumab to treat 214 patients, showing promising response rates in immunotherapy-naive patients
suffering from melanoma, RCC or NSCLC. In terms of safety, the combination was tolerated, and comparative randomised studies will be performed to confirm benefit over nivolumab single-agent
therapy.34 Another strategy to avoid signalling through the high-affinity receptor uses an engineered mutated IL-2 variant that shows reduced binding to IL-2Rα. This mutant is fused to
antibodies to target the cytokine to the TME. In the case of cergutuzumab amunaleukin, the IL-2 variant is fused to an antibody that targets it to the carcinoembryonic antigen.35 A phase I
clinical trial is evaluating this fusion protein in combination with atezolizumab (NCT02350673). In the case of RO6874281, the mutated cytokine is fused to an antibody that targets the
fibroblast activation protein which is expressed by cancer-associated fibroblasts. This fusion protein is being combined in clinical trials with the epidermal growth factor receptor
inhibitors trastuzumab or cetuximab (NCT02627274), with atezolizumab (NCT03386721 and NCT03063762) as well as with bevacizumab, a monoclonal antibody that targets vascular endothelial growth
factor (VEGF) (NCT03063762). INTERLEUKIN-15 IL-15 is mainly produced by activated myeloid cells as a membrane-bound heterodimer associated with IL-15Rα in such a way that it is
trans-presented to NK cells and T cells expressing IL-2/IL-15Rβ and the common γ chain receptor.36,37 Importantly, IL-15 is critically needed for the ontogeny of NK cells and CD8+ T
cells38,39 and also induces the proliferation, cytotoxic action and the release of other cytokines such as IFN-γ38,40 from these cells, highlighting its role in potentiating the immune
response. Preclinical observations strongly support the potential anti-tumour activity of IL-15 mediated by NK cells and T lymphocytes.41,42 Unlike IL-2, IL-15 does not stimulate Treg cells,
a subset that might reduce the anti-tumour activity of NK and T cells. This is due to the fact that IL-15 does not bind to the IL-2Rα chain (also known as CD25).43 First-in-human clinical
trials using recombinant aglycosylated IL-15 produced in _Escherichia coli_ consisted of intravenous bolus administration to patients with advanced melanoma or RCC. Patients showed an
expansion of NK and CD8+ T cells in peripheral blood, but there were severe adverse events and dose-limiting toxicities, including high fever, hypotension and thrombocytopenia.44 This trial
stopped due to dose-limiting toxicity at 1.0 μg/kg per day. There were no clinical responses by Response Evaluation Criteria In Solid Tumours (RECIST) v1.1 criteria, but 5 out of 18 patients
with malignant melanoma or RCC showed a reduction of between 10% and 30% in their marker lesion. In another clinical trial reported recently, IL-15 was subcutaneously administered to
patients with melanoma, RCC, non-small cell lung cancer (NSCLC) or squamous cell head and neck carcinoma.45 The maximum tolerated dose of IL-15 administered subcutaneously was significantly
higher than the dose that was feasible by intravenous bolus injection, and reached 3.0 μg/kg per day. The number of circulating NK cells dramatically increased with IL-15 administration in a
dose-dependent fashion. There were no objective clinical responses in this trial, but several patients had disease stabilisation, including a patient with RCC whose disease was stable for
over 2 years.45 In vitro and in vivo preclinical studies indicate that IL-15 is more bioactive when trans-presented adsorbed onto the IL-15Rα receptor subunit.46,47,48 Recombinant IL-15 is
quickly eliminated from the blood due to its small molecular size (it has an in vivo half-life of 2.5 h), and several approaches have therefore focused on designing more stable protein
constructs encompassing IL-15 and IL-15Rα that display a longer half-life and better biodistribution parameters. The different therapeutic forms of IL-15 and its signalling receptor are
described below and schematically represented in Fig. 2. The recombinant protein RLI encompasses the binding domain of IL-15Rα (the so-called sushi domain) bound to IL-15 by a flexible
linker; this fusion protein displays superagonistic activity towards the IL-15R–β/γ complex and exerts anti-tumour properties in in vivo models.49 Some variations of the original structure
have been made to redirect IL-15 to the tumour, such as the addition of anti-CD20 or anti-GD2 antibody domains.50,51 Our group has constructed a recombinant protein termed Sushi-IL15-Apo, a
triple fusion protein comprising human IL-15, the binding domain of IL-15Rα and apolipoprotein A-I. Apolipoprotein A-I, the major component of high-density lipoproteins, binds to its main
receptor, SR-BI, which is overexpressed on liver and tumour cells, thereby effectively targeting IL-15 activity within the recombinant protein to the tumour.52,53,54 ALT-803, another variant
of IL-15 that encompasses the sushi domain and a mutated IL-15 fused to an IgG1 Fc domain, has been shown to promote strong IL-15 activity in mouse models55 and has been tested in clinical
trials. In the dose-escalation phase I clinical study with this recombinant protein, 33 patients with haematological cancer received ALT-803 after disease relapse following bone marrow
allotransplantation.56 Sixteen subjects received the treatment intravenously and 17 received it subcutaneously. The treatment was well tolerated in both cohorts. All patients showed an
increase in the levels of circulating NK and CD8+ T cells (the increase was more pronounced in the subcutaneous treatment cohort), and 19% of the subjects met criteria for clinical benefit.
Another trial has been performed with ALT-803 in 21 patients with metastatic NSCLC, testing escalating doses via subcutaneous administration in combination with anti-PD-1 therapy. The
recombinant protein drug was well tolerated, no dose-limiting toxicities were recorded and 29% of patients achieved an objective response. A phase II continuation clinical trial is ongoing
(NCT02989844).57 It has also been reported that ALT-803 is well tolerated in combination with intravesical bacillus Calmette-Guérin therapy for high-risk localised bladder cancer, achieving
sustained complete responses for 24 months in 9 out of 9 patients.58 These data have led the FDA to grant fast-track designation to this treatment for patients with non-muscle invasive
bladder cancer. Other clinical studies involving IL-15 proteins as experimental agents are ongoing. Most also include administering IL-15 in combination with other agents. Some trials are
testing IL-15 as an adjuvant in T cell or NK cell adoptive cell therapies (NCT01875601, NCT02465957 and NCT01385423). Other clinical studies have been designed to test the ability of IL-15
to increase antibody-dependent cellular cytotoxicity (ADCC) effects, using IL-15 in combination with tumour-targeting monoclonal antibodies such as alemtuzumab (NCT02689453) or rituximab
(NCT02384954). INTERLEUKIN-21 IL-21 is another cytokine from the IL-2-family that is also being actively tested in cancer clinical trials alone (NCT00095108, NCT00514085 and NCT00336987) or
in combination with ipilimumab (NCT01489059), nivolumab (NCT01629758), sunitinib (NCT00617253), rituximab (NCT00347971), sorafenib (NCT00389285) or doxorubicin (NCT00523380). In a
dose-escalation phase I trial, there were pharmacodynamic effects of anti-tumour immunity upregulation, and 3 out of 26 patients experienced objective partial responses.59 In non-Hodgkin
lymphoma patients, recombinant IL-21 has been tested in combination with rituximab, achieving clinical responses in 8 out of 19 patients.60 There is also interest in using IL-21 to
potentiate adoptive T cell therapy.61 However, the clinical development of this cytokine is still in its infancy and progress is likely to occur in the form of combinations. INTERLEUKIN-10
IL-10 is released by innate and adaptive immune cells to fine-tune the activity of pro-inflammatory cytokines.62 IL-10 is considered to be an immunosuppressive cytokine as it can decrease
the antigen-presenting activity of DCs63 and inhibit the cytotoxic and cytokine-release functions performed by T and NK lymphocytes.64 However, recent reports point to a context-dependent
outcome of IL-10 activity.65 In chronic infections and cancer, autocrine IL-10 activity on CD8+ T lymphocytes has been shown to be crucial for inhibiting antigen-induced CD8+ T cell
apoptosis, thereby prolonging the effector activity of these cytotoxic lymphocytes.66,67 This concept has been evaluated in a phase I clinical trial in advanced, treatment-refractory tumours
(NCT02009449) using IL-10 conjugated with PEG to increase its half-life. Administration of the PEGylated cytokine (termed pegilodecakin) is well tolerated, and grade 3–4 immune-related
adverse effects were detected in only 15% of patients. Partial responses were observed in patients with uveal melanoma, RCC and colorectal cancer.68 At ASCO 2018, it was reported that the
combination of pegilodecakin with nivolumab or pembrolizumab was tolerable in 38 patients with RCC. The clinical activity of pegilodecakin looks promising, with 50% of patients achieving a
RECIST 1.1 response, 9% of which were complete.69 INTERLEUKIN-12 The IL-12 family comprises unique heterodimeric cytokines that include IL-12, IL-23, IL-27 and IL-35. The heterodimeric 70
kDa biologically active form of IL-12 (p35–p40) is composed of two independently produced subunits, α (IL12p35) and β (IL12p40), linked by disulphide bonds. The α subunit (IL12p35) shares
sequence homology with IL-6 and can also be part of IL-23 (p19–p40), whereas the β subunit (IL12p40) is shared with IL-35 (p35/Ebi3).70 IL-12 is mainly produced by activated
antigen-presenting cells such as DCs, macrophages, monocytes and B cells. IL-12 production is a tightly controlled process, regulated mainly at the transcriptional level. Production is
initiated by activation of pathogen recognition receptors such as Toll-like receptors in antigen-presenting cells upon sensing of pathogen-associated molecular patterns) or damage-associated
molecular patterns.71 In addition, cytokine stimulation and direct immune cell–cell contact, including CD40–CD40L interactions, induce IL-12 production. This latter CD40-dependent mechanism
is likely to be the principal mechanism for the production of IL-12 in cancer.72 IL-12 binds to the IL-12 receptor (IL-12R), expressed as a high-affinity heterodimer of IL-12Rβ1 and
IL-12Rβ2 subunits mostly on T cells and NK cells. The β1 subunit is constitutively expressed on immune cells, whereas the β2 subunit is upregulated in T cells and NK cells upon activation.73
IL-12R activation results in the recruitment of the kinases Janus kinase (JAK2) and Tyk2 and subsequent phosphorylation of signal transducer and activator of transcription 4 (STAT4).
Dimerisation and nuclear translocation of phosphorylated STAT4 ultimately leads to IFN-γ production, transcriptional reprogramming of CD4+ and CD8+ T cells towards type 1 T helper (Th1) cell
differentiation and maturation of NK cells. IFN-γ causes a FAS-ligand-dependent collapse of the myeloid compartment and M1 macrophage polarisation, paving the way for an optimal T cell
infiltration and cytolytic function.74,75 Furthermore, the potent antiangiogenic properties of IL-12 also render it an attractive cytokine for cancer treatment.71,76 The antiangiogenic
effects are mediated by the production of IFN-γ which alters extracellular matrix remodelling and the expression of adhesion molecules on endothelial cells.77 An interesting antiangiogenic
loop involving IL-12, IFN-γ and CXC chemokine ligand 10 (CXCL10) has been discovered and involves CXCR4 expression on proliferating vascular endothelial cells.78 In preclinical studies,
systemic administration of recombinant IL-12 induced potent anti-tumour efficacy in xenograft mouse models.75,79 However, in clinical trials, the short half-life of the recombinant protein
in serum meant that high and multiple doses were required, which resulted in dose-related toxicities, mainly caused by elevated levels of circulating IFN-γ.80 To overcome the toxicities,
different approaches have been proposed, focusing on local delivery of IL-12 to avoid toxic systemic exposure. Several preclinical studies exploiting local gene transfer using viral
vectors,81 liposomes82 or in vivo electroporation of an IL-12-encoding plasmid in accessible tumour lesions83 have demonstrated strong anti-tumour activity with no apparent toxicity.
Focusing on targeted protein delivery, a fusion protein encompassing single-chain IL-12 coupled to an extracellular double-stranded-DNA binding antibody (which thereby targets tumour
necrotic areas) showed enhanced anti-tumour activity in mouse models.84 IL-12-based local treatment can be synergistically combined with adoptive T cell transfer. In relation to this
approach, the use of transgenic tumour-specific T cells engineered to secrete IL-12 in the TME in response to TCR-mediated antigen stimulation was evaluated in mouse models, and showed very
promising efficacy.85 However, leakage during production of the inducible IL-12 by the adoptively transferred TILs was reported in a subsequent clinical trial, leading to unacceptable
toxicity, with one lethal case.86 Local IL-12 delivery and the use of immunostimulatory monoclonal antibodies can generate synergistic results. In line with this, combining the local
delivery of IL-12 with the blockade of PD-1–PD-L1 has been shown to eradicate large established tumours in preclinical models.81 An in vivo electroporation approach is currently undergoing
clinical trials for local delivery of IL-12 as a single agent (NCT01579318, NCT00323206, NCT01502293 and NCT02345330)87 and in combination with pembrolizumab (NTC02493361 and NTC03132675).
GRANULOCYTE-MACROPHAGE COLONY-STIMULATING FACTOR GM-CSF (CSF-2) is a clinically available recombinant cytokine used to promote myeloid reconstitution after bone marrow transplantation or
following induction chemotherapy in patients with acute myelogenous leukaemia. This cytokine promotes the expansion and activation of myeloid cells such as DCs and macrophages. For this
reason, it provides an adjuvant effect for various types of vaccines. As a serious drawback, however, GM-CSF also promotes the differentiation and accumulation of tumour-associated myeloid
cells which support tumour growth. Subcutaneous recombinant GM-CSF given in combination with ipilimumab improved the overall survival and reduced ipilimumab-related toxicity in patients with
advanced melanoma.88 The adjuvant activity of GM-CSF is exploited in the FDA-approved drug talimogene laherparepvec (T-VEC). This genetically modified herpes simplex virus encodes GM-CSF
and, upon intratumoural injection, has demonstrated overall response benefit over subcutaneous GM-CSF in advanced melanoma patients.89 GM-CSF gene transfection into autologous or allogeneic
tumour cells has been the basis for GVAX products which, in the allogeneic setting, have not met satisfactory endpoints in prostate cancer phase III trials, but hold promise in the
autologous setting and in combination regimens in pancreatic cancer.90,91 INHIBITING THE IMMUNOSUPPRESSIVE ACTIVITY Cytokines might be released or activated in the TME by tumour cells or
infiltrating immune cells to promote all phases of tumourigenesis. In this case, strategies to neutralise the pathogenic activity of such cytokines can be developed to enhance cancer
immunotherapy. These strategies not only include the use of antagonistic antibodies but also polypeptides, cytokine traps, small interfering RNA (siRNA) and small molecules that inhibit
signal transduction from cytokine receptors. However, it must be kept in mind that, like double-edged swords, some of these cytokines may exert pro- and anti-tumour activities, depending on
context and elements in the TME. TNF-Α TNF-α is a pro-inflammatory cytokine produced mainly by myeloid-derived cells such as monocytes, macrophages and DCs, although many other cells such as
T lymphocytes, endothelial cells, adipocytes and fibroblasts can also produce this cytokine under stressful conditions. TNF-α is recognised by two receptors with a broad tissue
distribution: TNFR1 and TNFR2.92 TNF-α is a key pathogenic mediator of several autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic
arthritis and ankylosing spondylitis. TNF-α activates macrophages at the inflammation site inducing the release of other pro-inflammatory cytokines that exacerbate inflammation. The activity
of TNF-α on epithelial cells impairs barrier function and promotes permeability to commensal bacteria. The activity of TNF-α on fibroblasts leads to the expression of metalloproteinases and
the synthesis of collagen, thereby promoting tissue fibrosis. Finally, TNF-α acts on endothelial cells to increase the adhesion molecules expressed on blood vessels, subsequently leading to
increases in leucocyte infiltration; it might also induce endothelial cell apoptosis. The relevance of this cytokine in autoimmune diseases led to the generation and approval of several
TNF-α antagonists such as infliximab, adalimumab and etanercept.92 Infliximab is also included in the guidelines for the treatment of several autoimmune-like syndromes that are associated
with immune checkpoint inhibitor treatment that are refractory to corticosteroid treatment.11 In cancer immunotherapy, TNF-α is mainly considered as a mediator of anti-tumour immune
responses, and several immunotherapies have shown depleted anti-tumour efficacy when co-administered with TNF-α antagonists.93,94 It is likely that acute immune responses are boosted by
TNF-α release. However, chronic exposure to TNF-α can promote tumour growth by mediating activation-induced cell death of effector T lymphocytes.95 In mouse models, TNF-α has been shown to
have a detrimental effect on immunotherapies based on the blockade of the PD-1 pathway. The proposed mechanism involves the TNF-α-mediated upregulation of the secondary checkpoint component
TIM-3 in CD8+ T lymphocytes, induced by anti-PD-1 antibody therapy.96 Safety of the triple combination of ipilimumab, nivolumab and an antibody to block TNF-α (infliximab or certolizumab) is
being evaluated in a phase I clinical trial (NCT03293784). TGF-Β TGF-β plays a dual role in the tumourigenic process. During the initial stages of tumourigenesis, TGF-β inhibits tumour
development due to cell-cycle blockade in cells undergoing transformation. However, tumour cells develop resistance mechanisms to the anti-proliferative activity of TGF-β,97 and in later
stages of tumour development, TGF-β acts both on tumour cells and on cells of the TME to promote tumour progression. In tumour cells, TGF-β is a key mediator of the epithelial–mesenchymal
transition. In the tumour stromal compartments, this cytokine promotes the release of angiogenic factors, such as VEGF, and the recruitment of Treg cells and myeloid cells with a pro-tumour
polarisation, such as neutrophils, macrophages, myeloid-derived suppressor cells (MDSCs) and tolerogenic DCs. Moreover, TGF-β decreases the activity of NK cells and CD8+ T lymphocytes.98 The
relevance of the immunosuppressive activity of this cytokine in tumours has been conducive to the development of several small molecules, peptides, antisense oligonucleotides, cytokine
traps and antibodies to block the TGF-β pathway in cancer.98 Although their efficacy as monotherapy agents has been disappointing, their activity in combination with anti-PD-1 or anti-PD-L1
agents has renewed interest in inhibitors tackling the functions of this cytokine. TGF-β might be the main driver of immune cell exclusion in several tumours.99 Numerous clinical trials are
testing the safety and anti-tumour activity of the combined blockade of TGF-β using small molecules, such as galunisertib, or antagonistic monoclonal antibodies, such as fresolimumab, with
PD-1–PD-L1 such as nivolumab or durvalumab (NCT02423343 and NCT02734160). Additionally, M7824, a compound designed to simultaneously block both of these targets, is undergoing clinical
testing (NCT03451773 and NCT03451773). This construct combines a chimeric version of an anti-PD-L1 monoclonal antibody (avelumab) with a fragment of TGF-βR4 to entrap active TGF-β.100
Inhibiting this cytokine could be especially relevant in tumours treated with radiotherapy, as radiotherapy activates the latent form of TGF-β and induces its transcription.101,102 CSF-1 AND
OTHER CYTOKINES THAT PROMOTE MYELOID CELLS Immune cells differentiated from haematopoietic myeloid precursors are key components of the immune response. The myeloid cells that can be found
in tumours are tolerogenic DCs, tumour-associated macrophages (TAMs), tumour-associated neutrophils and MDSCs, all of which comprise a heterogenous population of immature myeloid precursors
arrested in their differentiation process.76,103 Tumour-released factors promote the expansion, recruitment and polarisation of these myeloid cell populations, which reciprocally support
tumour development through the release of anti-inflammatory cytokines, pro-angiogenic mediators and growth factors. Furthermore, these cells express immune checkpoint ligands such as PD-L1
on their surface, as well as enzymes that promote metabolic alterations in the TME. All these mechanisms reduce the release of cytokines by, and cytotoxic activity of, NK and T lymphocytes.
Strategies to counteract these tumour-promoting activities involve depleting or repolarising myeloid cells to restore efficient activity of anti-tumour effector immune cells such as NK
cells, Th1 CD4+ T lymphocytes and CD8+ cytotoxic T lymphocytes, as well as their infiltration into the malignant tissue.76 CSF-1 receptor, the receptor for CSF-1 and IL-34, is expressed on
TAMs and maintains the homoeostasis of myeloid cells. By contrast, M1 macrophages with anti-tumour activity are not dependent on the CSF-1 receptor, making this pathway an excellent target
for small molecules, such as pexidartinib, or monoclonal antibodies, such as cabiralizumab. CSF-1R blockade leads to the alteration of macrophage polarisation in the TME, which shows lower
myeloid cell content associated with a certain degree of tumour regression in preclinical animal models.104,105 The accumulation of myeloid cells in the TME is orchestrated by chemokine
gradients. The most relevant cytokines in myeloid cell recruitment are CXCL8 (also known as IL-8), CC chemokine ligand 2 (CCL2), CCL3 and CCL5.106 CXCL8/IL-8 is produced by tumour cells, and
elevations in the serum concentrations of this chemokine reflect tumour growth dynamics and have been proposed as a biomarker that can predict the response to anti-PD-1 monoclonal
antibodies in melanoma and NSCLC.107 An antibody to block the interaction of CXCL8/IL-8 with its receptors on myeloid cells, CXCR1 and CXCR2, has been tested in a phase I clinical trial in
patients with metastatic or unresectable, locally advanced stage solid tumours (NCT02536469) and is now entering into a phase Ia/II study in combination with nivolumab (NCT03400332).108,109
CCR2 is the receptor for CCL2, while CCR5 binds both to CCL3 and CCL5. CCR2/CCR5 inhibitors are being developed to synergistically block the activity of these chemokines, thereby inhibiting
myeloid cell recruitment, in combination with either chemotherapy or nivolumab in patients with metastatic colorectal cancer or pancreatic cancer (NCT03184870). Both CCR2 and CCR5 are
involved in the recruitment of Treg cells, TAMs and MDSCs. CCR2 is also involved in the bone marrow egress of myeloid cells. In addition, CCR5 blockade can repolarise TAMs from a
pro-tumourigenic phenotype to an M1 phenotype.110 Finally, several small molecules and monoclonal antibodies targeting the VEGF pathway have been approved for the treatment of several types
of cancer. These compounds were originally developed to interfere with the angiogenic activity of VEGF-A, but it is now known that this pleiotropic cytokine is also involved in the
recruitment of immunosuppressive myeloid cells to the TME.111 The relevance of this concept in the clinic has been highlighted by the results of the phase II study of atezolizumab with or
without bevacizumab in patients with untreated metastatic RCC. Patients who showed tumour immune signatures of T effector cells and myeloid cell infiltration were refractory to treatment
with atezolizumab alone but responded to the combination of bevacizumab and atezolizumab.112 Moreover, a combination of bevacizumab plus atezolizumab plus chemotherapy provides evidence for
activity against NSCLC as reported in ASCO 2018.113 The combination of bevacizumab and atezolizumab has also been tested in a phase I trial for advanced hepatocellular carcinoma patients,
showing good tolerability and a very promising 62% overall response rate.114 CONCLUSIONS Cytokines are potent but complex immune mediators. Making cytokine-based drugs is a formidable
challenge that requires a profound knowledge of cytokine biology and contemporary biotechnology to exploit their anti-tumour activity while keeping toxicity to a minimum. The approved
monoclonal antibodies against the PD-1–PD-L1 axis provide a tool to reinvigorate effector lymphocytes but primary and acquired resistance mechanisms limit the fraction of patients who
benefit from these novel immunotherapies. Cytokines will be key molecules to overcome such resistance mechanisms due to their ability to expand and reactivate effector NK and T lymphocytes
and promote tumour infiltration by lymphocytes as well as their persistence in the TME. Current excitement surrounding the immunobiology of cytokines has resulted in a number of ongoing
clinical trials (Table 1), the results of which are eagerly expected. The possibility of blocking cytokines and chemokines that mediate the recruitment of Treg cells and tumour-associated
myeloid cells might expose an as yet under exploited Achilles’ heel in tumours, against which we can deploy promising combination strategies. In future developments, two key aspects are to
be considered: confining the effects of the cytokines to the site of action to avoid systemic pro-inflammatory effects, and including these treatments in combination immunotherapy
strategies.15 For the first point, approaches based on targeting agents to the TME or intratumoural administration of the proteins or their encoding genes can be envisioned. These
tumour-targeted approaches would also be relevant for the neutralisation of immunosuppressive cytokines. Cytokines in the realms of gene therapy, cell therapy and monoclonal antibody-based
therapies might become formidable partners in elegant synergistic strategies (Fig. 3), whose anti-tumour efficacy only the future will reveal. REFERENCES * Isaacs, A. & Lindenmann, J.
Virus interference. I. The interferon. _Proc. R. Soc. Lond. Ser. B Biol. Sci._ 147, 258–267 (1957). Article CAS Google Scholar * Gresser, I. & Bourali, C. Antitumor effects of
interferon preparations in mice. _J. Natl. Cancer Inst._ 45, 365–376 (1970). CAS PubMed Google Scholar * Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell
carcinoma who received high-dose recombinant interleukin-2 therapy. _J. Clin. Oncol._ 13, 688–696 (1995). Article CAS PubMed Google Scholar * Atkins, M. B. et al. High-dose recombinant
interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. _J. Clin. Oncol._ 17, 2105–2116 (1999). Article CAS PubMed Google
Scholar * Golomb, H. M. et al. Alpha-2 interferon therapy of hairy-cell leukemia: a multicenter study of 64 patients. _J. Clin. Oncol._ 4, 900–905 (1986). Article CAS PubMed Google
Scholar * Solal-Celigny, P. et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes
de l’Adulte. _New Engl. J. Med._ 329, 1608–1614 (1993). Article CAS PubMed Google Scholar * Kirkwood, J. M. et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous
melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. _J. Clin. Oncol._ 14, 7–17 (1996). Article CAS PubMed Google Scholar * Groopman, J. E. et al. Recombinant alpha-2
interferon therapy for Kaposi’s sarcoma associated with the acquired immunodeficiency syndrome. _Ann. Intern. Med._ 100, 671–676 (1984). Article CAS PubMed Google Scholar * Waldmann, T.
A. Cytokines in cancer immunotherapy. _Cold Spring Harb. Perspect. Biol_. https://doi.org/10.1101/cshperspect.a028472 (2017). Article Google Scholar * Sharma, P. & Allison, J. P. The
future of immune checkpoint therapy. _Science_ 348, 56–61 (2015). Article CAS PubMed Google Scholar * Haanen, J. et al. Management of toxicities from immunotherapy: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. _Ann. Oncol._ 28, iv119–iv142 (2017). Article CAS PubMed Google Scholar * Motzer, R. J. et al. Nivolumab plus Ipilimumab
versus Sunitinib in Advanced Renal-Cell Carcinoma. _New Engl. J. Med._ 378, 1277–1290 (2018). Article CAS PubMed Google Scholar * Larkin, J. et al. Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. _New Engl. J. Med._ 373, 23–34 (2015). Article PubMed CAS Google Scholar * Overman, M. J. et al. Durable clinical benefit with nivolumab plus
ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. _J. Clin. Oncol._ 36, 773–779 (2018). Article CAS PubMed Google Scholar *
Melero, I. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. _Nat. Rev. Cancer_ 15, 457–472 (2015). Article CAS PubMed Google Scholar * June, C. H.,
O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. _Science_ 359, 1361–1365 (2018). Article CAS PubMed Google Scholar *
Hinrichs, C. S. & Rosenberg, S. A. Exploiting the curative potential of adoptive T cell therapy for cancer. _Immunol. Rev._ 257, 56–71 (2014). Article CAS PubMed PubMed Central
Google Scholar * Chmielewski, M. & Abken, H. TRUCKs: the fourth generation of CARs. _Expert Opin. Biol. Ther._ 15, 1145–1154 (2015). Article CAS PubMed Google Scholar * Jackaman, C.
et al. IL-2 intratumoral immunotherapy enhances CD8+T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. _J. Immunol._ 171,
5051–5063 (2003). Article CAS PubMed Google Scholar * Sangro, B. et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors.
_J. Clin. Oncol._ 22, 1389–1397 (2004). Article CAS PubMed Google Scholar * Hu, J. et al. T cell homing therapy for reducing regulatory T cells and preserving effector T cell function in
large solid tumors. _Clin. Cancer Res._ 24, 2920–2934 (2018). Article CAS PubMed PubMed Central Google Scholar * Chen, D. S. & Mellman, I. Oncology meets immunology: the
cancer-immunity cycle. _Immunity_ 39, 1–10 (2013). Article PubMed CAS Google Scholar * Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. _Nature_
541, 321–330 (2017). Article CAS PubMed Google Scholar * Spaapen, R. M. et al. Therapeutic activity of high-dose intratumoral IFN-beta requires direct effect on the tumor vasculature.
_J. Immunol._ 193, 4254–4260 (2014). Article CAS PubMed Google Scholar * Herndon, T. M. et al. U.S. Food and Drug Administration Approval: peginterferon-alfa-2b for the adjuvant
treatment of patients with melanoma. _Oncologist_ 17, 1323–1328 (2012). Article CAS PubMed PubMed Central Google Scholar * Hervas-Stubbs, S. et al. Direct effects of type I interferons
on cells of the immune system. _Clin. Cancer Res._ 17, 2619–2627 (2011). Article CAS PubMed Google Scholar * Fioravanti, J. et al. Anchoring interferon alpha to apolipoprotein A-I
reduces hematological toxicity while enhancing immunostimulatory properties. _Hepatology_ 53, 1864–1873 (2011). Article CAS PubMed Google Scholar * Fioravanti, J. et al. The fusion
protein of IFN-alpha and apolipoprotein A-I crosses the blood-brain barrier by a saturable transport mechanism. _J. Immunol._ 188, 3988–3992 (2012). Article CAS PubMed Google Scholar *
Vasquez, M. et al. Antitumor effect of an adeno-associated virus expressing apolipoprotein A-1 fused to interferon alpha in an interferon alpha-resistant murine tumor model. _Oncotarget_ 8,
5247–5255 (2017). Article PubMed Google Scholar * Cauwels, A. et al. Delivering type I interferon to dendritic cells empowers tumor eradication and immune combination treatments. _Cancer
Res._ 78, 463–474 (2018). Article CAS PubMed Google Scholar * Cauwels, A. et al. A safe and highly efficient tumor-targeted type I interferon immunotherapy depends on the tumor
microenvironment. _Oncoimmunology_ 7, e1398876 (2018). Article PubMed Google Scholar * Yang, X. et al. Targeting the tumor microenvironment with interferon-beta bridges innate and
adaptive immune responses. _Cancer Cell_ 25, 37–48 (2014). Article PubMed PubMed Central CAS Google Scholar * Charych, D. et al. Modeling the receptor pharmacology, pharmacokinetics,
and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. _PLoS ONE_ 12, e0179431 (2017). Article PubMed PubMed Central CAS
Google Scholar * Diab, A. et al. NKTR-214 (CD122-biased agonist) plus nivolumab in patients with advanced solid tumors: Preliminary phase 1/2 results of PIVOT. _J. Clin. Oncol._ 36,
3006–3006 (2018). Article Google Scholar * Klein, C. et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy:
overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. _Oncoimmunology_ 6, e1277306 (2017). Article PubMed PubMed Central CAS Google Scholar * Grabstein, K.
H. et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. _Science_ 264, 965–968 (1994). Article CAS PubMed Google Scholar * Bamford,
R. N. et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T cell proliferation and the induction of
lymphokine-activated killer cells. _Proc. Natl. Acad. Sci. USA_ 91, 4940–4944 (1994). Article CAS PubMed PubMed Central Google Scholar * Kennedy, M. K. et al. Reversible defects in
natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. _J. Exp. Med._ 191, 771–780 (2000). Article CAS PubMed PubMed Central Google Scholar * Lodolce, J. P. et
al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. _Immunity_ 9, 669–676 (1998). Article CAS PubMed Google Scholar * Di Scala, M. et al.
Identification of IFN-gamma-producing T cells as the main mediators of the side effects associated to mouse interleukin-15 sustained exposure. _Oncotarget_ 7, 49008–49026 (2016). Article
PubMed PubMed Central Google Scholar * Evans, R., Fuller, J. A., Christianson, G., Krupke, D. M. & Troutt, A. B. IL-15 mediates anti-tumor effects after cyclophosphamide injection of
tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. _Cell. Immunol._ 179, 66–73 (1997). Article CAS PubMed Google Scholar * Klebanoff,
C. A. et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8(+) T cells. _Proc. Natl. Acad. Sci. USA_ 101, 1969–1974 (2004). Article CAS PubMed PubMed Central Google
Scholar * Marshall, D., Sinclair, C., Tung, S. & Seddon, B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. _J. Immunol._ 193,
5525–5533 (2014). Article CAS PubMed Google Scholar * Conlon, K. C. et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production
during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. _J. Clin. Oncol._ 33, 74–82 (2015). Article CAS PubMed Google Scholar * Miller, J. S.
et al. A first-in-human phase i study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. _Clin. Cancer Res._ 24, 1525–1535 (2018). Article CAS
PubMed Google Scholar * Ochoa, M. C. et al. Antitumor immunotherapeutic and toxic properties of an HDL-conjugated chimeric IL-15 fusion protein. _Cancer Res._ 73, 139–149 (2013). Article
CAS PubMed Google Scholar * Lucas, M., Schachterle, W., Oberle, K., Aichele, P. & Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15.
_Immunity_ 26, 503–517 (2007). Article CAS PubMed PubMed Central Google Scholar * Rubinstein, M. P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. _Proc.
Natl. Acad. Sci. USA_ 103, 9166–9171 (2006). Article CAS PubMed PubMed Central Google Scholar * Mortier, E. et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a
selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. _J. Biol. Chem._ 281, 1612–1619 (2006). Article CAS PubMed
Google Scholar * Vincent, M., Quemener, A. & Jacques, Y. Antitumor activity of an immunocytokine composed of an anti-GD2 antibody and the IL-15 superagonist RLI. _Oncoimmunology_ 2,
e26441 (2013). Article PubMed PubMed Central Google Scholar * Liu, B. et al. Evaluation of a novel CD20-targeted IL-15 immunotherapeutic with potent activity against B cell lymphoma. _J.
Immunother. Cancer_ 2, P122–P122 (2014). Article PubMed Central Google Scholar * Ochoa, M. C. et al. Interleukin-15 in gene therapy of cancer. _Curr. Gene Ther._ 13, 15–30 (2013).
Article CAS PubMed Google Scholar * Ochoa, M. C., Melero, I. & Berraondo, P. High-density lipoproteins delivering interleukin-15. _Oncoimmunology_ 2, e23410 (2013). Article PubMed
PubMed Central Google Scholar * Ochoa, M. C. et al. Enhancement of antibody-dependent cellular cytotoxicity of cetuximab by a chimeric protein encompassing interleukin-15. _Oncoimmunology_
7, e1393597 (2018). Article PubMed Google Scholar * Rhode, P. R. et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. _Cancer
Immunol. Res._ 4, 49–60 (2016). Article CAS PubMed Google Scholar * Romeem R. et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse
after transplantation. _Blood_ 131,2515–2527 (2018). Article CAS PubMed PubMed Central Google Scholar * Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with
nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. _Lancet Oncol._ 19, 694–704 (2018). Article CAS PubMed PubMed Central
Google Scholar * Rosser, C. J., Nix, J., Ferguson, L., Hernandez, L. & Wong, H. C. Phase Ib trial of ALT-803, an IL-15 superagonist, plus BCG for the treatment of BCG-naïve patients
with non-muscle-invasive bladder cancer. _J. Clin. Oncol._ 36, 510–510 (2018). Article Google Scholar * Schmidt, H. et al. Safety and clinical effect of subcutaneous human interleukin-21
in patients with metastatic melanoma or renal cell carcinoma: a phase I trial. _Clin. Cancer Res._ 16, 5312–5319 (2010). Article CAS PubMed Google Scholar * Timmerman, J. M. et al. A
phase I dose-finding trial of recombinant interleukin-21 and rituximab in relapsed and refractory low grade B-cell lymphoproliferative disorders. _Clin. Cancer Res._ 18, 5752–5760 (2012).
Article CAS PubMed PubMed Central Google Scholar * Li, Y., Bleakley, M. & Yee, C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell
response. _J. Immunol._ 175, 2261–2269 (2005). Article CAS PubMed Google Scholar * O’Garra, A. & Vieira, P. T(H)1 cells control themselves by producing interleukin-10. _Nat. Rev.
Immunol._ 7, 425–428 (2007). Article PubMed CAS Google Scholar * Llopiz, D. et al. IL-10 expression defines an immunosuppressive dendritic cell population induced by antitumor
therapeutic vaccination. _Oncotarget_ 8, 2659–2671 (2017). Article PubMed Google Scholar * Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the
interleukin-10 receptor. _Annu. Rev. Immunol._ 19, 683–765 (2001). Article CAS PubMed Google Scholar * Gao, B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver
disease. _J. Gastroenterol. Hepatol._ 27(Suppl. 2), 89–93 (2012). Article CAS PubMed PubMed Central Google Scholar * Fioravanti, J. et al. Effector CD8(+) T cell-derived interleukin-10
enhances acute liver immunopathology. _J. Hepatol._ 67, 543–548 (2017). Article CAS PubMed PubMed Central Google Scholar * Mumm, J. B. et al. IL-10 elicits IFNgamma-dependent tumor
immune surveillance. _Cancer Cell_ 20, 781–796 (2011). Article CAS PubMed Google Scholar * Naing, A. et al. Safety, antitumor activity, and immune activation of pegylated recombinant
human interleukin-10 (AM0010) in patients with advanced solid tumors. _J. Clin. Oncol._ 34, 3562–3569 (2016). Article CAS PubMed PubMed Central Google Scholar * Tannir, N. M. et al.
Pegilodecakin with nivolumab (nivo) or pembrolizumab (pembro) in patients (pts) with metastatic renal cell carcinoma (RCC). _J. Clin. Oncol._ 36, 4509–4509 (2018). Article Google Scholar *
Choi, J., Leung, P. S., Bowlus, C. & Gershwin, M. E. IL-35 and autoimmunity: a comprehensive perspective. _Clin. Rev. Allergy Immunol._ 49, 327–332 (2015). Article CAS PubMed Google
Scholar * Berraondo, P., Prieto, J. & Gonzalez-Aseguinolaza, G. Advances in interleukin-12 gene therapy for acquired liver diseases. _Curr. Gene Ther._ 9, 62–71 (2009). Article CAS
PubMed Google Scholar * Kelsall, B. L., Stuber, E., Neurath, M. & Strober, W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T cell responses.
_Ann. N.Y. Acad. Sci._ 795, 116–126 (1996). Article CAS PubMed Google Scholar * Szabo, S. J., Dighe, A. S., Gubler, U. & Murphy, K. M. Regulation of the interleukin (IL)-12R beta 2
subunit expression in developing T helper 1 (Th1) and Th2 cells. _J. Exp. Med._ 185, 817–824 (1997). Article CAS PubMed PubMed Central Google Scholar * Ibe, S., Qin, Z., Schuler, T.,
Preiss, S. & Blankenstein, T. Tumor rejection by disturbing tumor stroma cell interactions. _J. Exp. Med._ 194, 1549–1559 (2001). Article CAS PubMed PubMed Central Google Scholar *
Medina-Echeverz, J. et al. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. _J. Immunol._ 186,
807–815 (2011). Article CAS PubMed Google Scholar * Medina-Echeverz, J., Aranda, F. & Berraondo, P. Myeloid-derived cells are key targets of tumor immunotherapy. _Oncoimmunology_ 3,
e28398 (2014). Article PubMed PubMed Central Google Scholar * Del Vecchio, M. et al. Interleukin-12: biological properties and clinical application. _Clin. Cancer Res._ 13, 4677–4685
(2007). Article PubMed Google Scholar * Romagnani, P. et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. _J. Clin.
Investig._ 107, 53–63 (2001). Article CAS PubMed PubMed Central Google Scholar * Brunda, M. J. et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. _J.
Exp. Med._ 178, 1223–1230 (1993). Article CAS PubMed Google Scholar * Leonard, J. P. et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and
interferon-gamma production. _Blood_ 90, 2541–2548 (1997). CAS PubMed Google Scholar * Quetglas, J. I. et al. Virotherapy with a Semliki Forest virus-based vector encoding IL12 synergizes
with PD-1/PD-L1 blockade. _Cancer Immunol. Res._ 3, 449–454 (2015). Article CAS PubMed Google Scholar * Rodrigo-Garzon, M., Berraondo, P., Ochoa, L., Zulueta, J. J. &
Gonzalez-Aseguinolaza, G. Antitumoral efficacy of DNA nanoparticles in murine models of lung cancer and pulmonary metastasis. _Cancer Gene Ther._ 17, 20–27 (2010). Article CAS PubMed
Google Scholar * Lucas, M. L., Heller, L., Coppola, D. & Heller, R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10
melanoma. _Mol. Ther._ 5, 668–675 (2002). Article CAS PubMed Google Scholar * Fallon, J. et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. _Oncotarget_ 5, 1869–1884
(2014). Article PubMed PubMed Central Google Scholar * Kerkar, S. P. et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. _J. Clin.
Investig._ 121, 4746–4757 (2011). Article CAS PubMed PubMed Central Google Scholar * Zhang, L. et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene
encoding interleukin-12 for the immunotherapy of metastatic melanoma. _Clin. Cancer Res._ 21, 2278–2288 (2015). Article CAS PubMed PubMed Central Google Scholar * Daud, A. I. et al.
Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. _J. Clin. Oncol._ 26, 5896–5903 (2008). Article CAS PubMed PubMed Central Google Scholar *
Hodi, F. S. et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. _Jama_ 312, 1744–1753 (2014). Article CAS PubMed
PubMed Central Google Scholar * Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. _J. Clin. Oncol._ 33, 2780–2788 (2015).
Article CAS PubMed Google Scholar * Le, D. T. et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic
pancreatic cancer. _J. Clin. Oncol._ 33, 1325–1333 (2015). Article CAS PubMed PubMed Central Google Scholar * Le, D. T. et al. Evaluation of ipilimumab in combination with allogeneic
pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. _J. Immunother._ 36, 382–389 (2013). Article CAS PubMed PubMed Central Google Scholar *
Palladino, M. A., Bahjat, F. R., Theodorakis, E. A. & Moldawer, L. L. Anti-TNF-alpha therapies: the next generation. _Nat. Rev. Drug Discov._ 2, 736–746 (2003). Article CAS PubMed
Google Scholar * Zhao, L., Ching, L. M., Kestell, P. & Baguley, B. C. The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. _Br. J.
Cancer_ 87, 465–470 (2002). Article CAS PubMed PubMed Central Google Scholar * van Horssen, R., Ten Hagen, T. L. & Eggermont, A. M. TNF-alpha in cancer treatment: molecular
insights, antitumor effects, and clinical utility. _Oncologist_ 11, 397–408 (2006). Article PubMed Google Scholar * Zheng, L. et al. Induction of apoptosis in mature T cells by tumour
necrosis factor. _Nature_ 377, 348–351 (1995). Article CAS PubMed Google Scholar * Bertrand, F. et al. TNFalpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. _Nat.
Commun._ 8, 2256 (2017). Article PubMed PubMed Central CAS Google Scholar * Massague, J. TGFbeta in cancer. _Cell_ 134, 215–230 (2008). Article CAS PubMed PubMed Central Google
Scholar * Akhurst, R. J. & Hata, A. Targeting the TGFbeta signalling pathway in disease. _Nat. Rev. Drug Discov._ 11, 790–811 (2012). Article CAS PubMed PubMed Central Google
Scholar * Tauriello, D. V. F. et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. _Nature_ 554, 538–543 (2018). Article CAS PubMed Google Scholar
* Knudson, K. M. et al. M7824, a novel bifunctional anti-PD-L1/TGFbeta Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. _Oncoimmunology_ 7,
e1426519 (2018). Article PubMed PubMed Central Google Scholar * Vanpouille-Box, C. et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. _Cancer Res._ 75,
2232–2242 (2015). Article CAS PubMed PubMed Central Google Scholar * Formenti, S. C. et al. Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. _Clin. Cancer
Res._ 24, 2493–2504 (2018) * Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. _Nature_ 454, 436–444 (2008). Article CAS PubMed Google Scholar *
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. _Nat. Med._ 19, 1264–1272 (2013). Article CAS PubMed PubMed Central Google Scholar
* Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. _Cancer Cell_ 25, 846–859 (2014). Article CAS PubMed Google
Scholar * Kitamura, T. & Pollard, J. W. Therapeutic potential of chemokine signal inhibition for metastatic breast cancer. _Pharmacol. Res._ 100, 266–270 (2015). Article CAS PubMed
PubMed Central Google Scholar * Sanmamed, M. F. et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung
cancer patients. _Ann. Oncol._ 28, 1988–1995 (2017). Article CAS PubMed PubMed Central Google Scholar * Dominguez, C., McCampbell, K. K., David, J. M. & Palena, C. Neutralization of
IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. _JCI Insight_ 2, e94296 (2017). Article PubMed Central Google Scholar *
Alfaro, C. et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. _Cancer Treat. Rev._ 60, 24–31 (2017). Article CAS PubMed Google Scholar * Zhao, Q. Dual targeting of
CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. _J. Leukoc. Biol._ 88, 41–55 (2010). Article CAS PubMed Google Scholar * Ott, P. A., Hodi, F. S. &
Buchbinder, E. I. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and
initial clinical data. _Front. Oncol._ 5, 202 (2015). Article PubMed PubMed Central Google Scholar * Powles, T. et al. IMmotion150: Novel radiological endpoints and updated data from a
randomized phase II trial investigating atezolizumab (atezo) with or without bevacizumab (bev) vs sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC). _Ann. Oncol._ 28, 1
(2017). Article Google Scholar * Socinski, M. A. et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo)+chemotherapy (chemo)±bevacizumab (bev)
vs chemo+bev in 1L nonsquamous (NSQ) NSCLC. _J. Clin. Oncol._ 36, 9002–9002 (2018). Article Google Scholar * Stein, S.et al. Safety and clinical activity of 1L atezolizumab+bevacizumab in
a phase Ib study in hepatocellular carcinoma (HCC). J. Clin. Oncol. 36, 4074–4074 . Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Worldwide
Cancer Research Grant under Grant 15–1146, Asociación Española Contra el Cancer (AECC) Foundation under Grant GCB15152947MELE, Red Temática de Investigacion Cooperativa en Cancer under
Grants RD12/0036/0040 and RD12/0036/0062, Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (FEDER) under Grants PI14/01686, PI13/00207 and PI16/00668, and H2020 PROCROP
project under Grant 635122. P.B. is supported by Miguel Servet II (CPII15/00004) contract from Instituto de Salud Carlos III and M.F.S. is supported by a Miguel Servet (C17/00196) contract.
AUTHOR CONTRIBUTIONS All authors contributed equally to the writing and editing of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Immunology and Immunotherapy Program, Center
for Applied Medical Research, CIMA, University of Navarra, Pamplona, Spain Pedro Berraondo, Miguel F. Sanmamed, María C Ochoa, Iñaki Etxeberria, Maria A. Aznar, José Luis Pérez-Gracia, María
E. Rodríguez-Ruiz, Mariano Ponz-Sarvise, Eduardo Castañón & Ignacio Melero * Navarra Institute for Health Research (IDISNA), Pamplona, Spain Pedro Berraondo, Miguel F. Sanmamed, María C
Ochoa, Iñaki Etxeberria, Maria A. Aznar, José Luis Pérez-Gracia, María E. Rodríguez-Ruiz, Mariano Ponz-Sarvise, Eduardo Castañón & Ignacio Melero * Centro de Investigación Biomédica en
Red de Cáncer (CIBERONC), Pamplona, Spain Pedro Berraondo, Miguel F. Sanmamed, María C Ochoa, Iñaki Etxeberria, Maria A. Aznar, José Luis Pérez-Gracia, María E. Rodríguez-Ruiz, Mariano
Ponz-Sarvise, Eduardo Castañón & Ignacio Melero * Department of Oncology and immunology, Clínica Universidad de Navarra, Pamplona, Spain Miguel F. Sanmamed, José Luis Pérez-Gracia, María
E. Rodríguez-Ruiz, Mariano Ponz-Sarvise, Eduardo Castañón & Ignacio Melero Authors * Pedro Berraondo View author publications You can also search for this author inPubMed Google Scholar
* Miguel F. Sanmamed View author publications You can also search for this author inPubMed Google Scholar * María C Ochoa View author publications You can also search for this author
inPubMed Google Scholar * Iñaki Etxeberria View author publications You can also search for this author inPubMed Google Scholar * Maria A. Aznar View author publications You can also search
for this author inPubMed Google Scholar * José Luis Pérez-Gracia View author publications You can also search for this author inPubMed Google Scholar * María E. Rodríguez-Ruiz View author
publications You can also search for this author inPubMed Google Scholar * Mariano Ponz-Sarvise View author publications You can also search for this author inPubMed Google Scholar * Eduardo
Castañón View author publications You can also search for this author inPubMed Google Scholar * Ignacio Melero View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHORS Correspondence to Pedro Berraondo or Ignacio Melero. ETHICS DECLARATIONS COMPETING INTERESTS I.M. is a consultant for Bristol Myers Squibb, Roche-Genentech,
MedImmune, Merck Serono, Bioncotech, F-STAR, Genemab and Tusk. He receives grants from Bristol Myers Squibb, Roche-Genentech, and Alligator. J.L.P.-G. is an advisor to Bristol Myers,
Roche-Genentech and Servier. The other authors declare no competing interests. NOTE This work is published under the standard license to publish agreement. After 12 months the work will
become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0). ADDITIONAL INFORMATION Note: This work is published under the
standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY
4.0). RIGHTS AND PERMISSIONS This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which
permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Berraondo, P., Sanmamed, M.F., Ochoa, M.C. _et al._ Cytokines in clinical cancer
immunotherapy. _Br J Cancer_ 120, 6–15 (2019). https://doi.org/10.1038/s41416-018-0328-y Download citation * Received: 19 June 2018 * Revised: 04 October 2018 * Accepted: 08 October 2018 *
Published: 09 November 2018 * Issue Date: 08 January 2019 * DOI: https://doi.org/10.1038/s41416-018-0328-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Drivers urged to pay car tax ahead of major ved changes next monthThe standard rate will increase by £10 for most cars which were first registered on or after April 1, 2017. For cars reg...
Elevation and fog-cloud similarity in tibeto-burman languagesABSTRACT Lexically, 52.99% of the Tibeto-Burman languages, the non-Sinitic branches of the Sino-Tibetan language family,...
France: new president macron is a ‘zombie catholic’Newly-elected President Emmanuel Macron, according to one of his biographers, embodies a new phenomenon in France known ...
Page Not Found很抱歉,你所访问的页面已不存在了。 如有疑问,请电邮[email protected] 你仍然可选择浏览首页或以下栏目内容 : 新闻 生活 娱乐 财经 体育 视频 播客 新报业媒体有限公司版权所有(公司登记号:202120748H)...
Closer look: venison sandwiches; allergies; and moreCloser Look with Rose Scott November 4, 2016 Friday on “Closer Look with Rose Scott and Jim Burress”: * 0:00: Atlanta Jo...
Latests News
Cytokines in clinical cancer immunotherapyABSTRACT Cytokines are soluble proteins that mediate cell-to-cell communication. Based on the discovery of the potent an...
Factors associated with the melanoma diagnostic interval in ontario, canada: a population-based studyABSTRACT BACKGROUND Protracted times to diagnosis of cancer can lead to increased patient anxiety, and in some cases, di...
Cloning and characterization of a novel gene pdrg that is differentially regulated by p53 and ultraviolet radiationABSTRACT We report the cloning and characterization of a novel p53 and DNA damage-regulated gene (PDRG). The human and m...
Salman khan gives you a glimpse of ‘bharat’ on independence daySalman Khan’s next biggie _Bharat_ has been in the news recently for Priyanka Chopra’s surprise exit from the film. Howe...
Psychology internship program | veterans affairsWe are particularly interested in developing psychologists who have an interest in working with veterans in underserved ...