Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs
Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A comprehensive understanding of the cellular heterogeneity and molecular mechanisms underlying the development, homeostasis, and disease of human intervertebral disks (IVDs)
remains challenging. Here, the transcriptomic landscape of 108 108 IVD cells was mapped using single-cell RNA sequencing of three main compartments from young and adult healthy IVDs,
including the nucleus pulposus (NP), annulus fibrosus, and cartilage endplate (CEP). The chondrocyte subclusters were classified based on their potential regulatory, homeostatic, and
effector functions in extracellular matrix (ECM) homeostasis. Notably, in the NP, a PROCR+ resident progenitor population showed enriched colony-forming unit-fibroblast (CFU-F) activity and
trilineage differentiation capacity. Finally, intercellular crosstalk based on signaling network analysis uncovered that the PDGF and TGF-β cascades are important cues in the NP
microenvironment. In conclusion, a single-cell transcriptomic atlas that resolves spatially regulated cellular heterogeneity together with the critical signaling that underlies homeostasis
will help to establish new therapeutic strategies for IVD degeneration in the clinic. SIMILAR CONTENT BEING VIEWED BY OTHERS SINGLE-CELL SEQUENCING REVEALS CELLULAR HETEROGENEITY OF NUCLEUS
PULPOSUS IN INTERVERTEBRAL DISC DEGENERATION Article Open access 08 November 2024 UNVEILING THE ROLE OF TCF19 IN INTERVERTEBRAL DISC DEGENERATION WITH SINGLE-CELL AND BULK RNA SEQUENCING
Article Open access 08 May 2025 FIBROCYTE ENRICHMENT AND MYOFIBROBLASTIC ADAPTATION CAUSES NUCLEUS PULPOSUS FIBROSIS AND ASSOCIATES WITH DISC DEGENERATION SEVERITY Article Open access 20
January 2025 INTRODUCTION Degenerative disc disease (DDD) is regarded as the primary cause of low back pain, resulting in a global healthcare burden and significant socioeconomic costs.1 It
may lead to a severe impact on the quality of life of patients.2 The current treatment of DDD, mainly including bed rest, rehabilitation, medication, interventional therapy, and surgery,3
provides only symptomatic relief but fails to reestablish the homeostasis of the intervertebral disc (IVD).4 Furthermore, the deterioration of the health of the compromised spine cannot be
prevented.5 Thus, the unrelenting threat posed by DDD to human health has motivated the search for an increased understanding of human IVD physiology and pathology. The IVD has a
well-confined structure, including three components: the central hydrated nucleus pulposus (NP), the surrounding lamellar annulus fibrosus (AF), and the cartilage endplate (CEP) that is
adjoining to the vertebra.6 The confined structure of the IVD plays a part in the mechanical function.7 Unfortunately, alterations in the cellular composition and microenvironment cause the
IVD to undergo a slow but relentless program that causes the confined structure to be compromised during the degenerative process.8,9,10 The origin of the IVD is heterologous, where the NP
is believed to be derived from the notochord,11,12 and the AF and CEP are derived from the sclerotome.13,14 Consequently, the cells in the IVD are also heterogeneous, composed of NP cells,
and notochord cells in the NP, AF cells in the AF, and chondrocytes in the CEPs.15 However, classification based on spatial location cannot uncover the highly heterogeneous cell populations
in regard to phenotype and function. Although previous studies have revealed phenotypes of IVD cells by bulk RNA sequencing,16,17,18 the search for molecular mechanisms underlying
degeneration has been complicated by the large amount of heterogeneity in cellular compositions and the subsequently highly complex cellular microenvironment of the IVD. To further examine
the cellular heterogeneity, some efforts were made to distinguish the critical cell types in IVD. The hypothesis regarding cellular heterogeneity in the IVD was initially supported by Hunter
CJ et al., as evidenced by the existence of large vacuolar notochordal cells in the NP and small rounded chondrocytes.12,19 Notochordal cells are thought to disappear starting in
adolescence in the human IVD,20,21 which has been questioned because _brachyury (TBXT)_, a notochord lineage marker, continued to be expressed in the IVD.22 Thus, notochord cells are thought
to be the precursors of all NP cells regardless of variations in morphology and size at different stages.23 In addition, mesenchymal stem cells (MSCs) are thought to exist in the IVD due to
the expression of the MSC markers _ENG_ (CD105), _CD44_, _THY1_ (CD90), _NT5E_ (CD73), and _NGFR_ (CD271).24,25 NP progenitor cells are characterized by clonogenicity, pluripotency, and NP
reorganization properties.26 However, the different lineages remain largely unknown due to the lack of high-precision and unbiased resolution for distinguishing cell populations in the human
IVD, although its importance is widely acknowledged. Single-cell RNA sequencing (scRNA-seq) is considered as a powerful tool for resolving cellular heterogeneity and hierarchical factors
forming a complicated cell niche.27,28 Here, we performed scRNA-seq to obtain an unbiased picture of IVD cell populations. Our findings provide a better understanding of the inherent
heterogeneity and reshape the existing classifications of chondrocytes in the IVD. Notably, we also confirmed the existence of progenitor cells in the IVD marked by _PDGFRA_ and _PROCR_.
Thus, our study reveals the cellular landscape of the human IVD and provides insights that could help to identify therapeutic targets for human DDD. RESULTS COMPREHENSIVE SCRNA-SEQ ANALYSES
RESOLVE THE MAJOR CELL TYPES IN THE HUMAN IVD To determine the cellular composition of the human IVD, we employed droplet-based single-cell transcriptomic profiling (10X Genomics Chromium
System) of cells from the NP, AF, and CEP from five healthy human IVDs (Pfirrmann I) (Fig. 1a and Supplementary Table 1), as evaluated by magnetic resonance imaging (MRI) according to the
Pfirrmann grading system29 (Supplementary Fig. 1a). The integrity of the IVD was confirmed because the sagittal cross-section showed that it met the criteria of high hydration and ordered
organization with increased deposition of chondroitin sulfate based on hematoxylin & eosin and safranin O/fast green staining (Supplementary Fig. 1a). Because it was difficult to
distinguish the boundary between the NP and inner AF, we harvested gelatinous tissue from the central region as the NP. Thus, the tissue origins of harvested cells were identified clearly
due to the strict criteria of sampling. A total of 128 833 individual human IVD cells were profiled, and 108 108 cells were retained for subsequent analysis after rigorous quality control
and doublet exclusion (Supplementary Table 1). The resulting cells were sequenced to a median depth of 5 367 unique molecular identifiers (UMIs) per cell, with a median of 1 569 genes
detected per cell (Supplementary Fig. 1b and Supplementary Table 1). Similarities between samples determined by Pearson’s correlations and the sequencing depth suggested that all samples
were comparable (Supplementary Fig. 1c). We performed fastMNN30 to correct batch effects among different data sets. Unbiased clustering based on t-distributed stochastic neighbor embedding
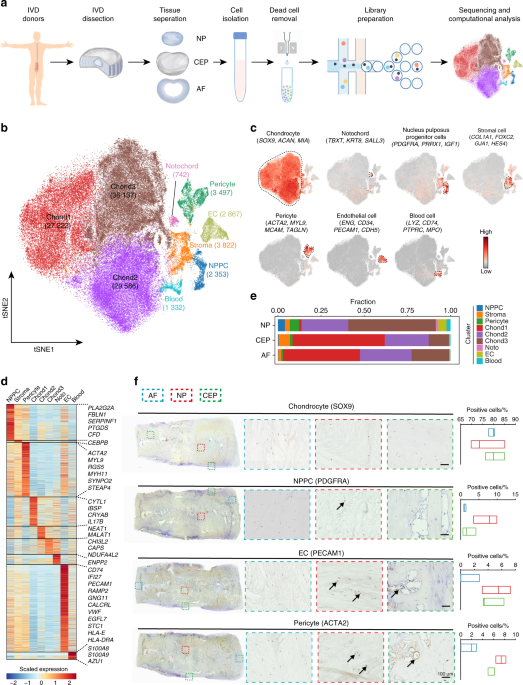
(tSNE) identified nine putative root clusters in the healthy human IVD (Fig. 1b and Supplementary Fig. 1d), including (1–3) three clusters of _SOX9_+ chondrocytes (Chond1, Chond2, and
Chond3); (4) notochord cells; (5) stromal cells; (6) pericytes; (7) endothelial cells (ECs); (8) nucleus pulposus progenitor cells (NPPCs); and (9) blood cells. The chondrogenic marker gene
_SOX9_+ and chondrocyte-specific ECM genes (_COL2A1_ and _ACAN_) were ubiquitously expressed in the three chondrocyte clusters (Fig. 1c, d). The notochord origin marker gene, _TBXT_,31 was
dominantly expressed in the notochord cell cluster, along with notochord-derived cytokeratin genes, such as _KRT8_32 (Fig. 1c, d). _FOXC2_, _GJA1_, and _HES4_, which are essential for
stromal cell differentiation,33,34,35 that were mainly expressed in the stromal cell cluster. Pericyte and EC clusters were identified by feature gene expression (_ACTA2_, _TAGLN_, and
_MCAM_ for pericytes36,37,38 and _PECAM1_, _CD34_, _CDH5_, _ERG_, and _VWF_ for EC39,40,41,42) (Fig. 1c, d and Supplementary Table 2). We found that _PDGFRA_ (the mesenchymal progenitor
marker),43 _PRRX1_ (which is restricted to the mesodermal origin and regulator of mesenchymal precursors)44 and _IGF1_ (a growth factor that effectively differentiates MSCs into NP-like
cells)45 were specifically expressed in the NPPC clusters (Fig. 1c, d). Thus, we speculated that _PDGFRA_+ NPPCs could be a mesoderm-derived progenitor cell cluster in the IVD. A total of 2
651 differentially expressed genes (DEGs) were identified that distinguished human IVD cell populations (Fig. 1d and Supplementary Table 2). Spatially, chondrocytes and stromal cells were
abundant in the NP, CEP, and AF, while notochord cells were mainly found in the NP (Fig. 1e and Supplementary Fig. 1e). The expression of some widely reported marker genes of the IVD was
also detected in these cell populations (Supplementary Fig. 1f). We then performed an immunohistochemistry assay to validate the spatial distribution of major cell types (Fig. 1f). We found
that most SOX9+ chondrocytes were detected in the NP, AF, and CEP, as expected. PDGFRA+ NPPCs were mainly distributed in the NP and rarely found in the AF and CEP. ACTA2+ pericytes and
PECAM1+ ECs were sporadically distributed in the NP and were present in the tube-like CEP, in line with previous findings on capillaries in the CEP.46 Moreover, immunofluorescence staining
of the human IVD (Pfirrmann I and II) validated the presence of scattered PECAM1+CD34+ cells and ACTA2+ cells in the IVD (Supplementary Fig. 1g). Pairwise correlation analysis clearly
distinguished the chondrocyte and nonchondrocyte subsets (Supplementary Fig. 1h). Gene ontology (GO) analysis revealed distinct functional enrichment in these cell types (Supplementary Fig.
1i). For example, Chond1 was enriched for signaling regulation and stimulus-response, while Chond2 was enriched for ECM synthesis and organization. As expected, pericyte and ECs were
enriched for genes involved in regulating vasculature development, cell adhesion, and junctions. Interestingly, the NPPC cluster was enriched for terms that regulated skeletal development
and ossification. To validate the conserved cell heterogeneity of the IVD across species, we compared the transcriptome of IVD cells between humans and rats by reanalyzing the scRNA-seq data
from a recent rat study.47 As expected, most of the cell clusters identified in the human IVD were also found in the rat IVD and showed gene expression conservation across cell types,
including NPPCs, ECs, and pericytes (Supplementary Fig. 2a, b). In particular, NPPCs in rats also highly expressed _PDGFRA_, _PRRX1_, and _IGF1_ and shared distinct gene expression patterns
with their counterparts in humans (Supplementary Fig. 2c, d). Overall, these results revealed the cellular diversity in the human IVD, and we identified a set of markers that can potentially
be used to recognize the cell clusters in the human IVD. THE FUNCTIONAL DEFINITION OF CHONDROCYTE SUBPOPULATIONS IN THE IVD As chondrocytes are known to play a pivotal role in ECM
homeostasis and the degeneration of the IVD,48 we sought to determine their composition. Each of the three chondrocyte clusters was divided into two subclusters (Fig. 2a). The distribution
of subclusters exhibited apparent distinctions in the three compartments of the IVD (Fig. 2b). The subclusters of C1 and C2 were mostly located in the AF and CEP, while C5 was mainly located
in the NP. Subclusters of C3, C4, and C6 were relatively evenly distributed in the NP, AF, and CEP. A total of 912 DEGs were found among the six chondrocyte subclusters (Fig. 2c and
Supplementary Table 3). We found that C1 preferentially expressed growth factor (GF) genes such as _BMP2, TGFB1_, and _FGF2_. Subclusters C3 and C4 preferentially expressed the genes of the
main ECM components of the IVD, such as _ACAN_ and _COL2A1_. Subclusters C5 and C6 preferentially expressed _PRG4_ and _CNMD_, suggesting that they may play a protective role and stabilize
the chondrocyte phenotype.49,50 To better understand the specific characteristics of IVD chondrocytes, we compared the transcriptomic differences between these chondrocyte subclusters and
articular cartilage chondrocytes at different stages of osteoarthritis (stages 0-4) (Fig. 2d).51 There was no characteristic correspondence among subclusters C1, C2, and articular
chondrocytes. We found that subclusters C1 and C2 shared some DEGs with articular regulatory chondrocytes (RegCs), including _CKS2_ and _HMOX1_ (Fig. 2e), and highly expressed _IBSP and
CYTL1_, which are NP-negative biomarkers (Supplementary Table 3).17,52 Moreover, subclusters C1 and C2 showed a higher percentage of cells arrested in the G2/M phase than that in others,
which was indicative of relatively higher proliferative activity (Fig. 2f). We also evaluated these subclusters using a gene set related to chondrocyte function (Fig. 2g). The results showed
that oxidative phosphorylation played a role in the metabolic pattern of C1 and C2, which could be explained by the fact that C1 and C2 were mainly located in the vascularized AF and CEP.
Notably, subcluster C1 was enriched for genes related to chondrogenic differentiation. Therefore, we hypothesized that subclusters C1 and C2 represent regulatory chondrocytes that stimulated
surrounding cells by secreting GFs. Pairwise correlation analysis revealed close relationships among C3, C4, and homeostatic chondrocytes (HomCs, Fig. 2d) with a similar pattern of gene
expression as articular HomCs, such as _CCNL1_ and _WSB1_ (Fig. 2e).51 In contrast with regulatory chondrocytes C1 and C2, fewer cells in C3 and C4 were arrested in the G2/M phase (Fig. 2f).
In particular, both C3 and C4 exhibited strong enrichment of circadian regulation genes and moderate enrichment of chondrogenic differentiation (Fig. 2g). C3 was also enriched for cellular
adhesion genes, which are critical for forming chondrocyte clonal columns within an ordered, three-dimensional cell array.53 Considering that they preferentially expressed ECM-related genes,
we chose to classify C3 and C4 as homeostatic chondrocytes, which function in maintaining ECM homeostasis and circadian rhythm. The C6 subcluster was relatively similar to hypertrophic
chondrocytes (HTCs) and prehypertrophic chondrocytes (preHTCs, Fig. 2d). _COL5A1_ and _EPYC_ were expressed in the subclusters of C5 and C6 (Fig. 2e), similar to articular HTCs and
preHTCs.51 We also found that C5 and C6 highly expressed genes reflecting protective characteristics (_KLF2_ and _CHI3L1_) (Fig. 2e)54,55 and existed in the dormant stage of proliferation
(Fig. 2f). Interestingly, subclusters C5 and C6 preferentially performed metabolic processes, including oxidative phosphorylation and glycolysis, showing the traits of a high metabolism
(Fig. 2g) and the characteristics of articular effector chondrocytes.51 Unlike the resident quiescent chondrocytes with low metabolism,56 these subclusters were possibly adapted to anaerobic
metabolism because C5 was mainly located in the NP, which has an avascular and hypoxic microenvironment, consistent with previous study showing that the NP that is predominantly glycolytic
due to vigorous _HIF1_ activity.57 Collectively, we inferred that C5 and C6 were effector chondrocytes with high metabolic rates and protective/repair functions. To reveal the core function
of chondrocytes in modulating ECM homeostasis, we detected the expression of matrisome-related genes. Matrisome genes were categorized into the core matrisome (collagens, proteoglycans, and
ECM glycoproteins) and matrisome-associated (ECM regulators, ECM affiliation, and secreted factors) according to a matrisome classification database (matrisomeproject.mit.edu).58 We first
evaluated the average expression of six modules in eight clusters (Supplementary Fig. 3a) and compared the expression of matrisome genes distinctly expressed in the NP, CEP, and AF
(Supplementary Fig. 3b and Supplementary Table 4). Correlation analysis of matrisome-related genes in chondrocytes revealed two patterns: the core matrisome and matrisome-associated (Fig.
2h). To clarify the primary function of matrisome-related gene subsets in six chondrocyte subclusters, we compared the expression abundance of these genes (Fig. 2i, j). We found that
secreted factors were predominantly expressed in the regulatory C1 subset, while the homeostatic C3 subset preferentially expressed genes of the core matrisome (Fig. 2i, j). In contrast, the
effector C5 subset exhibited high expression of ECM regulators, reflecting its regulatory role in ECM homeostasis (Fig. 2i, j). Taken together, these data add to the knowledge on the
functions of chondrocyte subclusters in human IVD. DELINEATING NUCLEUS PULPOSUS PROGENITOR CELLS AND THEIR SIGNATURE GENES NP progenitor/stem cells are critical in the physiological and
pathological processes of the IVD.59,60 We identified NPPC-enriched genes related to bone development, bone morphogenesis, connective tissue development, and endochondral bone growth
(Supplementary Fig. 1i). To better understand the role of the NPPC cluster, we sought to determine their composition in the human IVD. We partitioned NPPCs into four subclusters (Fig. 3a).
The localization of the discogenic marker _PAX1_ confirmed the physical presence of the NPPC-1 subcluster. _PAX1_ is expressed in the sclerotome, which is critical for the formation of
vertebrae and IVDs,61 indicating the potential role of discogenic differentiation in NPPCs. Subcluster NPPC-2 specifically expressed _ANGPT1_, which is critical for the survival of nucleus
pulposus cells.26 _PRG4_, the signature gene of NPPC-3, was also highly expressed in articular cartilage progenitor cells.62 _SOX9_ expression indicated the chondrogenic priming of NPPC-4
(Fig. 3b). These NPPC subclusters were also distinguished by the indicated DEGs (Supplementary Fig. 4a and Supplementary Table 5). GO analysis of these DEGs showed that NPPC-1 and NPPC-3
were enriched for genes regulating ECM organization, while NPPC-4 was enriched for genes involved in mRNA catabolic metabolism (Supplementary Fig. 4b). Gene set enrichment analysis (GSEA)
showed that NPPC-1 was enriched for the calcium signaling pathway, which played a vital role in modulating NP homeostasis by regulating _AQP2_.63 NPPC-2 was enriched for the MAPK signaling
pathway, potentially playing a protective role in cell survival in the NP.64 NPPC-3 preferentially expressed the _SMAD2/3_ pathway, and NPPC-4 was enriched for _NOTCH_ signaling, which plays
a role in cell growth (Supplementary Fig. 4c).65 To explore the regulatory networks that determine cell fate specification in the NPPC subclusters, we utilized single-cell regulatory
network inference and clustering (SCENIC) to infer the regulatory activity (regulon) from the coexpression of transcription factors (TFs) and their downstream target genes.66 We filtered 21
core regulons out of 227 regulons that were used to discriminate the four NPPC clusters (Fig. 3c, Supplementary Fig. 4d, and Supplementary Table 6). The highly enriched regulons in NPPC-1
included _HOXA10_ and _HOXA7_. The _SOX4_, _RARA_, and _MEIS1_ regulons were specific to NPPC-2. NPPC-3 exhibited strong enrichment of _ZFP14_ and _SMAD3_. NPPC-4 was enriched for regulons
such as _GLI1_, _EGR2_, and _NR2F1_ (Fig. 3c and Supplementary Table 6). Some important regulons, including _HOXA10_, _SOX4_, _SMAD3_, and _GLI1_, together with their downstream target
genes, such as the abovementioned _PAX1_, _PRG4_, and _ANGPT_, had the potential to regulate the function of NPPCs (Fig. 3d and Supplementary Table 5). Specifically, _HOXA10_ is a critical
regulator of osteogenesis.67 _SOX4_ is highly expressed in osteoblast progenitors, and its expression is increased during osteoblast differentiation.68 _GLI1_ marks mesenchymal progenitors
responsible for bone formation and fracture repair and regulates chondrocyte differentiation.69 _SMAD3_, the downstream target of _TGF-β_, plays a dominant role in chondrogenesis and
maintaining the phenotype of chondrocytes.70 To immunophenotype these NPPC subclusters, we screened for cell surface marker genes that were differentially expressed among the four NPPC
subclusters. Among them, _PDGFRA_ showed higher expression in NPPC-1, NPPC-2, and NPPC-3 than in NPPC-4. Interestingly, we found that NPPC-3 preferentially expressed _PROCR_ (Fig. 3e), a
widely reported signature gene for progenitor cells in multiple organs, including the hematopoietic and vascular systems,71,72,73,74 pancreas,75 ovaries,76 etc. Thus, the specific expression
of _PROCR_ suggested the potential stemness capacity of NPPC-3. We then combined the expression of the membranous marker genes _PDGFRA_ and _PROCR_ and the transcription factor _PRRX1_ as a
signature for the identification of NPPC-3 (Fig. 3f and Supplementary Fig. 4e) and performed immunofluorescence staining to examine their coexpression (Fig. 3g). Immunostaining of a healthy
IVD (Pfirrmann I, Supplementary Table 1) showed that PDGFRA+PROCR+ NPPCs were mainly located in the NP zone (Supplementary Fig. 4f). To assess the proportion of PDGFRA+PROCR+ NPPCs in the
NP, primary PDGFRA+PROCR+ cells were flow cytometrically sorted from the human IVD (Pfirrmann II, Supplementary Table 1). The results showed that the frequency of PDGFRA+PROCR+ cells was
0.36% (Fig. 3h), and PDGFRA was enriched in almost all PROCR+ cells in the IVD. To test the clonogenicity of NPPC-3, primary PROCR+ cells were sorted by flow cytometry for a colony-forming
unit-fibroblast (CFU-F) colony formation assay. The counts of typical colonies derived from primary PROCR+ cells were 25.9 ± 3.3 per 1 000 cells, which was comparable to that for PDGFRA+
MSCs77 and significantly higher than that for PROCR− cells (2.9 ± 1.4 per 1 000 cells) (Fig. 3i), indicating that NPPCs exhibited enhanced colony-formation ability. To verify the in silico
finding of the enriched regulatory activity of SMAD3 in PROCR+ NPPCs, we detected the expression level of p-SMAD3 in P2 PROCR+ and PROCR− cells from the human IVD (Pfirrmann II,
Supplementary Table 1). As expected, the expression of p-SMAD3 in the nucleus was higher in PROCR+ cells than in PROCR− cells (Fig. 3j). These results indicated that SMAD3 was highly
activated in PROCR+ cells, suggesting the potential role of PROCR+ cells in the chondrogenesis of IVD. Taken together, these data elucidated the cellular heterogeneity in NPPCs, which was
highly regulated and comprised the population with clonogenicity that could be enriched by PDGFRA and PROCR. RECONSTRUCTION OF THE BILINEAGE TRAJECTORY OF PDGFRA+PROCR+ NPPCS Connective
tissue comprised stromal cells with phenotypic and functional complexity,78 which provided support during NP development and repair.79 We collected 1 372 stromal cells from the NP that were
divided into six subclusters (Supplementary Fig. 5a and Supplementary Table 7), including three subclusters of fibroblasts (Fib1, Fib2, and Fib3) that expressed high levels of fibroblast
signature genes, such as _CEMIP_, _AKR1C1_, _MGP_, _COMP_, _DNER_, and _MELTF_,80,81 two subclusters of neurogenic cells (Neu1 and Neu2) with high expression of the neurogenic markers
_SOX2_, _NGFR_, _NCMAP_, and _CLDN19_,82,83,84,85 and osteogenic cells that expressed high levels of the osteogenic regulators _RUNX2_ and _DLX5_ (Supplementary Fig. 5b).86,87,88 To further
verify the existence of the cell clusters in the IVD, immunofluorescence staining of human IVDs (Pfirrmann I and II) showed a few RUNX+SP7+ cells and SOX2+ cells in the IVD (Supplementary
Fig. 5c), consistent with the findings from the scRNA-seq analysis. We next sought to investigate the differentiation trajectories that determined the cellular hierarchy in NP cells. All NP
cells, including four subclusters of NPPCs, three subclusters of fibroblasts, three subclusters of chondrocytes, and osteogenic cells, were involved in reconstructing the differentiation
trajectories using Monocle 3 (Fig. 4a), an algorithm for the reconstruction of lineage programs based on similarity at the transcriptional level.89 We set NPPC-3 as the starting point of the
differentiation trajectories due to its high expression of pluripotent genes and progenitor potential identified above, and then computed pseudotime for cells along the inferred
developmental axis (Fig. 4a, b). More specifically, NPPC-3 was predicted to differentiate into two distinct cell lineages, including the chondrogenic branch, which includes NPPC-3, NPPC-1,
Chond2, and Chond3, and the osteogenic branch, which includes NPPC-3, NPPC-2, NPPC-4, osteogenic cells, and Fib3 (Fig. 4a, b). To explore gene expression dynamics along the trajectories, we
grouped genes that varied between cell clusters into 16 modules using Louvain community analysis (Supplementary Table 8). A heatmap showed the aggregated expression in each module across
cell clusters (Fig. 4c). We found that the expression of genes in module 4 was downregulated along both trajectories, such as the differential regulator genes _TWIST1_ and _FOXP1_,90,91
which were enriched for genes related to ECM organization (Fig. 4d–f). In contrast, the expression of chondrogenic genes was gradually elevated along the chondrogenic trajectory, such as
_COL2A1_ and _ACAN_ in module 7, and remained at high expression levels until terminal differentiation (Fig. 4d, e), which was also evidenced by the expression of module 9 (e.g., the
chondrogenic _CNMD and FGFBP2_) (Supplementary Fig. 5d, e). The expression of the osteogenic gene set was elevated along the osteogenic trajectory, such as _RUNX2_ and _ALPL_ in module 11
(Fig. 4d, e) and the expression of _SP7_, _BGLAP_, and _MMP11_ in module 16 (Supplementary Fig. 5d, e). According to the prediction of the bifurcating differentiation trajectories of NPPC-3,
we tested the trilineage differentiation of PROCR+ cells (cells that were expanded from CFU-F colonies) ex vivo and found that they efficiently underwent osteogenic, chondrogenic, and
adipogenic differentiation (Fig. 4g). Taken together, these data depicted the trajectories of NP cells, in which PROCR+ cells were enriched for multipotent NPPCs that generate three
lineages, consequently revealing the successive activation of transcriptional programs in NP homeostasis. PUTATIVE SIGNALING NETWORK FOR THE INTERCELLULAR CROSSTALK REGULATING THE
HOMEOSTASIS OF THE NP To seek further insights into the critical factors involved in the NP cell niche of the human IVD, we investigated the signaling network among the main cell types in
the NP. CellChat analysis of these 14 subclusters in the NP identified the signaling network for intercellular crosstalk. Relative active bidirectional signaling interactions among these
cell subclusters revealed highly regulated cellular communications (Fig. 5a and Supplementary Table 9). ECs, pericytes, fibroblasts, and neurogenic cells identified as niche components in
the NP played distinct roles in signaling interactions to regulate the differential process. To determine the important factors, we further analyzed the intercellular signaling networks of
VEGF, TGFB, PDGF, and FGF (Fig. 5b–e). Interestingly, Fib3 was involved in VEGF signaling, both autocrine and paracrine (Fig. 5b, f). ECs were the leading receiver of VEGF signals, as
expected, and NPPC subclusters functioned as regulators of the communication (Fig. 5b). Moreover, the TGF-β pathway was involved in many signaling interactions among chondrocyte subclusters
and NPPC clusters via _TGFB3-TGFBR_ or _TGFB3-ACVR1_ (Fig. 5c, f). As shown above, NPPC-3 was enriched for _SMAD3_, the key downstream target of TGF-β, which prompted us to further
investigate the role of TGF-β3 in chondrogenesis in NPPCs. The results showed that 10 ng·mL−1 TGF-β3 effectively induced chondrogenesis and the formation of dense cartilage extracellular
matrix (ECM) compared with that in the negative control group after 28 days of differentiation. However, supplementation with 10 μmol·L−1 SB505124, a TGF-β receptor inhibitor, blocked the
chondrogenesis of PROCR+ cells both with and without TGF-β3 (Fig. 5g). The results demonstrated that the TGF-β family plays an important role in the chondrogenic regulation of PROCR+ cells.
In the PDGF signaling network, the NPPC clusters acted as critical contributors by secreting PDGFA ligand, leading to the paracrine activity of NPPCs to osteogenic cells, pericytes, and Fib1
and the autocrine activity of NPPCs to themselves. Specifically, NPPC-3 was the key population that dominated the PDGF signaling network (Fig. 5d, f). Previous studies have reported that
PDGF-AA is involved in the regulation of cell proliferation.92,93 Therefore, we explored the effect of PDGF-AA on the proliferation of PROCR+ cells from the human IVD (Fig. 5h). The results
showed that 20 ng·mL−1 PDGF-AA significantly promoted the proliferation of PROCR+ cells on the 10th day of expansion, and 100 nmol·L−1 crenolanib, a PDGFR α/β inhibitor, significantly
inhibited the proliferation of PROCR+ cells in the presence or absence of PDGF-AA after treatment for 10 days. The FGF signaling network exhibited intensive exchanges among almost all the
cell types with _FGF_ ligands that were mainly secreted by Fib3 (Fig. 5e, f). By comprehensively predicting signaling networks for intercellular crosstalk, large numbers of ligand-receptor
pairs participated in ligand-receptor pairs of _VEGF_, _TGFB_, _SEMA3_, _PDGF_, _NGF_, _LAMC_, _FGF_, _BMP_, and _ANGPT_ between NPPCs and other cell types (Fig. 5f). Interestingly, Fib3 was
involved in almost all the above pathways, suggesting its significance in NP homeostasis. We further revealed that NPPC-1, NPPC-2, NPPC-3, and pericytes sent communications to other cells
via _IGF_ and _PDGF_ (Supplementary Fig. 6a). As expected, Fib3 was exclusively dominated by _FN1_ in regard to outgoing communication. In addition, Fib3 and Chond1 received incoming
communication by _BMP_, _GDF_, and _ANGPT_, which reportedly played a prominent essential role in the IVD (Supplementary Fig. 6b).26,94,95 _EGF_, used by NPPC-2, NPPC-4, and Neu1 for
incoming signaling, could be a protective factor in IVD regeneration (Supplementary Fig. 6b).96 CellChat analysis of NP, AF, and CEP cells also revealed a large number of signaling networks
among cell subclusters from the three substructures of the IVD (Supplementary Fig. 6c). For example, NPPCs interacted with Chond1 from the AF and CEP. In particular, the GAS signaling
pathways were intensively regulated between NPPCs and Chond3 from the AF (Supplementary Fig. 6d), possibly protecting the IVD from inflammatory factors.97 _SPP1_, an osteogenesis-related
factor, was highly involved in the interaction among NPPCs and stromal cells in the CEP (Supplementary Fig. 6d). This was in line with our above hypothesis that osteogenic cells might play a
role in IVD homeostasis and/or degenerative processes. However, these proposed signaling pathways should be considered as multiple biological cascades rather than a sole event because the
three substructures always work as a whole. Taken together, these results indicated that there is a complicated relationship among the distinct cell types and described a cellular crosstalk
network with a hierarchical signaling pathway that regulates NP homeostasis in a coordinated manner. DISCUSSION The severe threat of DDD to human health prompted us to seek an innovative
treatment that reestablishes IVD homeostasis. Inadequate knowledge of IVD physiology, and pathology poses a challenge to the development of novel treatment strategies. Due to the cellular
heterogeneity and resulting complex microenvironment in the human IVD, an in-depth understanding of specific markers and their roles in IVD homeostasis is urgently needed. Here, we resolved
the cellular diversity at a single-cell level using transcriptomic profiling and identified the cell types with a set of specific markers in the human IVD. We classified IVD chondrocytes
into three subtypes based on their potential roles in ECM homeostasis. Notably, we identified new subtypes of progenitor cells with signature genes, spatial distribution in situ, and
progenitor potential. Moreover, we analyzed the intercellular crosstalk based on the signaling network and uncovered key factors, such as the PDGF and TGF-β cascades, as important cues for
regulating the NP microenvironment. Together with previous studies,12,98,99 a better understanding of the cellular heterogeneity of the human IVD is developing, with the aim of contributing
to new therapeutic strategies for DDD. The cellular heterogeneity of IVD cells has been a long-debated controversy due to the complexity of the IVD ontogeny, a tricomponent organization with
distinct origins.100 Multiple developmental origins lead to the inhomogeneity of the cell composition. Although some scholars have attempted to examine the IVD at the single-cell level, a
highly precise and unbiased description of cell populations in the human IVD remains to be elucidated.52,98 Previously, notochord cells and chondrocytes were recognized in the NP, which was
regarded as the notochordal lineage, evidenced by the constant expression of _TBXT_.101 In line with previous findings, we found a minor cluster that expressed high levels of the markers
_TBXT_ and _KRT8_, which could be a rare but distinct notochord cell cluster. As expected, we found three major clusters of chondrocytes, which are always regarded as core players in ECM
homeostasis in the human IVD. Although the expression of _TBXT_ was not detected, another notochord marker, _NOG_, was expressed in the majority of chondrocytes (Supplementary Fig. 1f). This
interesting finding coincides with a previous theory that distinctive cellular morphology in the NP is due to the various phases along the notochord lineage during aging and
degeneration.5,102,103,104,105 Apart from the leading role of notochord lineage cells, the supporting role of minor cell clusters is more notable because of their unclear function, which has
been infrequently reported. First, _SOX2__+__NGFR__+_ neurogenic cells, one of the stromal subclusters, were also found in the NP (Supplementary Fig. 5a–c). Although the healthy disc was
regarded as an aneural tissue,106 the pattern of nerve endings has been previously confirmed in healthy and degenerative IVDs,107,108,109,110 which were small in diameter and relatively
sparse.111 Thus, sporadic SOX2+ neurogenic cells were probably related to neural ingrowth. Furthermore, _RUNX2_ played a part in postnatal IVD development and regulated the notochordal
transition into chondrocyte-like cells.112 Upregulated _RUNX2_ expression was also found in the degenerated IVD, which led to IVD calcification.113,114 In addition, the stem cells in the IVD
exhibited osteogenic potential during ex vivo culture.25 These studies may have indicated that the homeostasis of bone formation is important for the physiological and pathological
processes of IVD. Our scRNA-seq analysis and immunofluorescence staining revealed the existence of a rare cell cluster that differentially expressed the osteogenic genes _RUNX2_, _DLX5_, and
_SP7_,86,87,88 which were defined as osteogenic cells (Supplementary Fig. 5a–c). This finding suggested that osteogenic cells exist in healthy IVDs. We hypothesized that osteogenic cells
likely contribute to the homeostasis of the IVD or are involved in the pathological process of early degeneration, which began as early as during the teenage years.115,116 Finally, the
dynamics of vascularization, represented by ECs and pericytes, play a role in disc homeostasis. Previous studies showed that blood vessels penetrated the AF and CEP during the early
postnatal years but regressed later, leaving an avascular microenvironment, which accounted for the poor ability for remodeling and repair in IVDs.117,118,119 However, blood vessels are
present in the human IVD until even the third decade of life.120 During the slow process of vascular regression, it is reasonable that some remnants are left behind, such as ECs. A recent
study reported that cross bridges after vascular regression are indeed present in both healthy and degenerated human disks. The cross-bridges of the IVD stained positively for PECAM1 in
adult sheep, although the PECAM1+ cross-bridges declined with aging.121 In line with scRNA-seq analysis, ACTA2+MCAM+ pericytes and PECAM1+CD34+ ECs were scattered in the IVD (Fig. 1b–f and
Supplementary Fig. 1g). Our data showed that ECs and pericytes communicated with NPPCs via the VEGF, PDGF, and TGF-β signaling pathways, suggesting that they played a role in NP homeostasis
(Fig. 5). Notably, MCAM is regarded as a classical surface marker of pericytes/MSCs.122 Previously, periosteal and meniscal MCAM+ cells were shown to exhibit canonical features of
skeletogenesis,123,124 and MCAM+ or ACTA2+ cells were also detected in the disc.47,125,126,127 Interestingly, MCAM was specifically expressed in the cell population with migration and
repopulating potential in degenerative IVDs.125 The functional characteristics of these cell types should be investigated in future studies. The highly conserved cellular heterogeneity
across cell clusters between human and rat IVDs (Supplementary Fig. 2) suggested that the rat is an ideal animal model to study the role of the above cell clusters in IVD homeostasis. Cells
in the IVD are generally referred to as “chondrocyte-like” cells or “IVD chondrocytes”. Traditionally, chondrocytes in the IVD are classified into NP, AF, and CEP chondrocytes based on their
spatial distribution. However, the spatial-based classification of the cell population was insufficient because of the cellular heterogeneity and possible cell migration among the three
sites of the IVD.128 Thus, the precise roles of IVD chondrocytes in ECM homeostasis are still largely unknown.16,17,18,129 Therefore, a deeper understanding of the roles of IVD chondrocytes
in ECM homeostasis is necessary. Taking advantage of the high throughput nature of analysis at the single-cell level with scRNA-seq, we were able to identify six subclusters of IVD
chondrocytes with three functional patterns (Fig. 2). First, we identified a new population of regulatory chondrocytes with active GF expression and chondrogenic pathway regulators, implying
its regulatory role in chondroid ECM homeostasis. In contrast, homeostatic chondrocytes showed high similarity to classical chondrocytes, which were quiescent, fully differentiated, and
responsible for ECM deposition.130 Interestingly, homeostatic chondrocytes were enriched in circadian regulation genes, which involved key pathways regulating the homeostasis of IVDs.131
This finding suggests that homeostatic chondrocytes could be a potential therapeutic target for circadian rhythm in the human IVD. It is noteworthy that the effector chondrocytes were
metabolically active, which is important in maintaining the ECM biogenesis of the IVD.132 In addition, the high expression of _PRG4_ (lubricin) also implies that they play a protective role
in reducing shear stress and inflammation and keeping the joint healthy.133 In contrast, effector chondrocytes were characterized by ossification and shared expression patterns with
articular HTCs.51 Thus, the definitive function of effector chondrocytes is certainly worth future investigation. Overall, the six transcriptomically defined populations of chondrocytes
exhibited distinct roles in ECM homeostasis, providing new perspectives for exploring the mechanism of IVD chondrocytes. The IVD possesses the capability of spontaneous regeneration, as
evidenced by self-healing after disc degeneration,134 probably due to the presence of in situ progenitor cells. Progenitor cells expressing different marker gene sets existed in three
compartments of the IVD.59,60 The progenitor cells exhibited certain plasticity and the ability to slow down disc degeneration.135,136 Thus, it is a promising strategy to activate endogenous
progenitor cells or transplant exogenous progenitor cells for DDD therapy. However, a comprehensive understanding of their in vivo characteristics, including discriminable identity,
lineage, spatial distribution, and functional role, is still lacking. We sought to help to increase the understanding of progenitor cells at a single-cell resolution. Surprisingly, we found
a cluster of cells that exclusively expressed _PDGFRA_, a signature of MSCs,77,137,138 and was mainly distributed in the NP (Supplementary Fig. 4). Notably, the PDGFRA+PROCR+ NPPC subcluster
was enriched for genes in the SMAD3 signaling pathway and exhibited higher activation of p-SMAD3 (Fig. 3), which determines the TGF-β-induced chondrogenesis139 and cell fate decisions of
stem cells by participating in the cell-cycle process and binding of m6A methyltransferase.140,141 Moreover, _PROCR_ was used to sort rare progenitor/stem cells with high efficacy. For
example, _PROCR_ (encoding CD201) was used as a sorting marker to harvest isolated 1% of islet cells, which robustly formed islet-like organoids.75 Applications in the hematopoietic system
showed that _PROCR_ enriched T1 prehematopoietic stem cells at a resolution of 68 parts per million and functional HSCs in the human fetal liver.71,73 In this study, we identified an NPPC
cluster that highly expressed _PROCR_, which exhibited pluripotency with colony-formation capacity and osteochondrogenic potentials (Figs. 3 and 4), similar to the characteristics of
multipotent mesenchymal stromal cells.142 Thus, we characterized these cells as resident progenitor cells in the human IVD. It is possible that the alternative cell fate in NPPCs determines
the outcome of the IVD when a degenerative program is initiated. On the one hand, the chondrogenic fate could help rebalance IVD homeostasis via cell replenishment.143 On the other hand, the
osteogenic fate could lead to DDD by inducing heterotopic ossification.144 Accordingly, these results have new implications for innovative therapeutic strategies targeting NPPCs. The two
branches of the cell fate of NPPCs motivated us to explore the key regulatory factors. Resident progenitor cells are exhausted or altered during degeneration,26,145 indicating that the
microenvironment has a significant influence on cell fate. To identify the key factors regulating the fate of NPPCs, CellChat analysis was used to dissect the intercellular crosstalk based
on the signaling network in the human IVD (Fig. 5). We found that GF-related signaling pathways were involved in the crosstalk network, mainly including the previously reported FGF
family,143,146 TGF-β family,147,148 BMP family,149,150 and PDGF family.151 Among them, TGF-β was important due to the high activation of SMAD3 in NPPCs. TGF-β directs embryonic matrix
development within the notochord and promotes the differentiation of the sclerotome into the AF,61,152,153 suggesting that it is an inherent regulator of the human IVD. Previous studies have
shown that the TGF-β family plays an important role in the development and protection of the IVD, especially in maintaining the phenotype of chondrocytes.154 Moreover, the loss of TGF-β
signaling in growth plate chondrocytes and inner AF cells led to the loss of matrix tissue and endplate cartilage cells and abnormal growth plate cartilage morphology in Tgfbr2 conditional
knockout mice.155 The critical role of TGF-β was also evidenced by the observation that the knockout of SMAD3, the key downstream target of TGF-β, led to the spontaneous development of IVD
degeneration in 30-day-old mice.156 In addition, TGF-β has been shown to have a beneficial effect on chondrogenic anabolism in MSCs.157 In this study, _TGFB_ was involved in regulating
NPPCs, as evidenced by TGF-β3 promoting the chondrogenesis of PROCR+ cells (Fig. 5). Meanwhile, the secretory role of chondrocyte clusters on TGF-β should not be neglected in the human IVD
(Fig. 5). Furthermore, _PDGF_ was found to engage in regulating NPPCs, probably due to the exclusive expression of its receptor gene _PDGFRA_ in NPPCs. Previous studies have reported that
PDGF-AA is involved in the regulation of cell proliferation.92,93 In line with the CellChat analysis, we found that PDGF-AA significantly promoted the proliferation of PROCR+ cells (Fig. 5).
Interestingly, all the minor clusters in the NP are involved in interacting with NPPCs, suggesting their potential role in regulating NPPCs and subsequently maintaining IVD homeostasis.
Moreover, further investigations need to elucidate their roles and establish an innovative strategy to optimize the microenvironment and benefit IVD stem/progenitor cells. Although we
validated the existence of identified cell populations by flow cytometry, immunofluorescence staining, and scRNA-seq evidence from the rat IVD, we surprisingly found that Sample 1 was from a
16-year-old donor who suffered from vertebral fracture exhibited obvious variability in the proportion of cell clusters (Supplementary Fig. 1e). Acute trauma has been shown to stimulate
resident cells to regenerate in previous studies.158,159,160 Interestingly, a recent study reported that NP cells derived from trauma patients showed higher adipogenic and chondrogenic
potential than those derived from degenerated IVDs.161 Thus, we are more inclined to hypothesize that ECs, pericytes, and NPPCs are rare in the IVD, and acute trauma may induce local
regeneration, which accounts for the unwanted distribution variability across donors. Due to the scarcity of desirable samples of healthy disks from young patients with vertebral fractures,
this needs to be explored in future studies. In summary, our study described the cell atlas of the human IVD, providing a valuable resource for further investigation of IVD homeostasis at
the mechanistic level. The cellular heterogeneity and signaling network we uncovered help to increase the understanding of the human IVD at a single-cell level and provide crucial clues for
establishing new therapeutic strategies for DDD treatment. MATERIALS AND METHODS For full methods, see the Supplementary Methods. HUMAN IVD TISSUE SPECIMENS This study was approved by the
Institutional Ethics Review Board of Daping Hospital [Ethics Committee (2019-127)] and the Chinese Clinical Trial registry (ChiCTR1900028201). All procedures were performed in accordance
with the ethical standards of the committee responsible for human experimentation and with the Declaration of Helsinki of 1975, as revised in 2000. Informed consent was obtained from all
patients for inclusion in the study. Eleven human IVDs were carefully dissected from nine donors in this study (Supplementary Table 1). The gelatinous tissue from the central region was
harvested as the NP. The peripheral lamellar structure of the outer IVD was harvested as AF. The superior and inferior homogeneous cartilage tissue was harvested as CEP. The sampling areas
of NP, AF, and CEP are indicated (Supplementary Fig. 1a). SINGLE-CELL RNA SEQUENCING The cells were washed with PBS three times and concentrated to 700–1 200 cells per μL. The suspension was
then loaded on a Chromium Controller (10X Genomics). For scRNA-seq library construction, a Chromium Single Cell 3′ Library and Gel Bead Kit V2 (10X Genomics, PN120237) was utilized to
generate single-cell gel beads in emulsion (GEM) within barcoded, full-length cDNA from polyadenylated mRNA. The captured cells were lysed in GEM, and the released RNA was
reverse-transcribed with primers containing poly-T, a barcode, UMIs, and the read 1 primer sequence, in that order. Barcoded, full-length cDNA was PCR amplified for library construction.
After enzymatic fragmentation, an adapter ligation reaction was performed to add a sample index and read 2 primer sequences to the cDNA fragment. After quality control, the libraries were
sequenced on an Illumina NovaSeq 6000 platform to generate 150-bp paired-end reads, according to the manufacturer’s instructions (Berry Genomics). DATA AVAILABILITY All data from the study
are available in online supplementary files. The scRNA-seq data have been deposited in GEO (GSE160756). All other relevant data from this study are available from the corresponding authors
upon reasonable request. CHANGE HISTORY * _ 23 AUGUST 2021 In Supplementary Information section, Supplementary figures 1-6 have been added. Supplementary Methods and Supplementary Table 1
have been updated. _ REFERENCES * Katz, J. N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. _J. Bone Jt. Surg. Am._ 88, 21–24 (2006). Google Scholar *
Colombier, P., Camus, A., Lescaudron, L., Clouet, J. & Guicheux, J. Intervertebral disc regeneration: a great challenge for tissue engineers. _Trends Biotechnol._ 32, 433–435 (2014).
Article CAS PubMed Google Scholar * Lewis, R. A. et al. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. _Spine J._
15, 1461–1477 (2015). Article PubMed Google Scholar * Cloyd, J. M. et al. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based
hydrogels and tissue engineering scaffolds. _Eur. Spine J._ 16, 1892–1898 (2007). Article PubMed PubMed Central Google Scholar * Risbud, M. V. & Shapiro, I. M. Notochordal cells in
the adult intervertebral disc: new perspective on an old question. _Crit. Rev. Eukaryot. Gene Expr._ 21, 29–41 (2011). Article CAS PubMed PubMed Central Google Scholar * Humzah, M. D.
& Soames, R. W. Human intervertebral disc: structure and function. _Anat Rec_. 220, 337–356 (1988). * Johannessen, W. & Elliott, D. M. Effects of degeneration of the biphasic
material properties of human nucleus pulposus in confined compression. _Spine_ 30, E724–E729 (2005). * Antoniou, J. et al. The human lumbar endplate. Evidence of changes in biosynthesis and
denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. _Spine_ 21, 1153–1161 (1996). Article CAS PubMed Google Scholar * Smith, L. J., Nerurkar, N.
L., Choi, K. S., Harfe, B. D. & Elliott, D. M. Degeneration and regeneration of the intervertebral disc: lessons from development. _Dis. Model Mech._ 4, 31–41 (2011). Article PubMed
CAS Google Scholar * Roughley, P. J. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. _Spine_ 29, 2691–2699 (2004). Article PubMed Google
Scholar * Fleming, A., Keynes, R. J. & Tannahill, D. The role of the notochord in vertebral column formation. _J. Anat._ 199, 177–180 (2001). Article CAS PubMed PubMed Central
Google Scholar * Hunter, C. J., Matyas, J. R. & Duncan, N. A. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. _J. Anat._ 205,
357–362 (2004). Article PubMed PubMed Central Google Scholar * Bagnall, K. M., Higgins, S. J. & Sanders, E. J. The contribution made by cells from a single somite to tissues within a
body segment and assessment of their integration with similar cells from adjacent segments. _Development_ 107, 931–943 (1989). Article CAS PubMed Google Scholar * Goldstein, R. S. &
Kalcheim, C. Determination of epithelial half-somites in skeletal morphogenesis. _Development_ 116, 441–445 (1992). Article CAS PubMed Google Scholar * Pattappa, G. et al. Diversity of
intervertebral disc cells: phenotype and function. _J. Anat._ 221, 480–496 (2012). Article CAS PubMed PubMed Central Google Scholar * Sakai, D., Nakai, T., Mochida, J., Alini, M. &
Grad, S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. _Spine_ 34, 1448–1456 (2009).
Article PubMed Google Scholar * Minogue, B. M., Richardson, S. M., Zeef, L. A., Freemont, A. J. & Hoyland, J. A. Transcriptional profiling of bovine intervertebral disc cells:
implications for identification of normal and degenerate human intervertebral disc cell phenotypes. _Arthritis Res. Ther._ 12, R22 (2010). Article PubMed PubMed Central CAS Google
Scholar * Minogue, B. M., Richardson, S. M., Zeef, L. A., Freemont, A. J. & Hoyland, J. A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker
gene expression to define adult stem cell differentiation. _Arthritis Rheum._ 62, 3695–3705 (2010). Article PubMed Google Scholar * Hunter, C. J., Matyas, J. R. & Duncan, N. A. The
three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. _J. Anat._ 202, 279–291 (2003). Article PubMed
PubMed Central Google Scholar * Trout, J. J., Buckwalter, J. A. & Moore, K. C. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. _Anat. Rec._ 204,
307–314 (1982). Article CAS PubMed Google Scholar * Pazzaglia, U. E., Salisbury, J. R. & Byers, P. D. Development and involution of the notochord in the human spine. _J. R. Soc.
Med._ 82, 413–415 (1989). Article CAS PubMed PubMed Central Google Scholar * Gilson, A., Dreger, M. & Urban, J. P. Differential expression level of cytokeratin 8 in cells of the
bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. _Arthritis Res. Ther._ 12, R24 (2010). Article PubMed PubMed Central CAS Google Scholar *
Risbud, M. V., Schaer, T. P. & Shapiro, I. M. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. _Dev. Dyn._ 239,
2141–2148 (2010). Article CAS PubMed PubMed Central Google Scholar * Erwin, W. M. et al. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural
repair. _Spine_ 38, 211–216 (2013). Article PubMed Google Scholar * Risbud, M. V. et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. _Spine_ 32,
2537–2544 (2007). Article PubMed Google Scholar * Sakai, D. et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. _Nat. Commun._
3, 1264 (2012). Article PubMed CAS Google Scholar * Wen, L. & Tang, F. Boosting the power of single-cell analysis. _Nat. Biotechnol._ 36, 408–409 (2018). Article CAS PubMed Google
Scholar * Zeng, Y. et al. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. _Cell Res._ 29, 881–894 (2019). Article CAS PubMed PubMed
Central Google Scholar * Pfirrmann, C. W., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. _Spine_ 26,
1873–1878 (2001). Article CAS PubMed Google Scholar * Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by
matching mutual nearest neighbors. _Nat. Biotechnol._ 36, 421–427 (2018). Article CAS PubMed PubMed Central Google Scholar * José-Edwards, D. S. et al. Brachyury, Foxa2 and the
cis-regulatory origins of the notochord. _PLoS Genet._ 11, e1005730 (2015). Article PubMed PubMed Central CAS Google Scholar * Rodrigues-Pinto, R. et al. Human notochordal cell
transcriptome unveils potential regulators of cell function in the developing intervertebral disc. _Sci. Rep._ 8, 12866 (2018). Article PubMed PubMed Central CAS Google Scholar * Lin,
F. X. et al. Naringin promotes osteogenic differentiation of bone marrow stromal cells by up-regulating Foxc2 expression via the IHH signaling pathway. _Am. J. Transl. Res._ 8, 5098–5107
(2016). CAS PubMed PubMed Central Google Scholar * Zappitelli, T., Chen, F. & Aubin, J. E. Up-regulation of BMP2/4 signaling increases both osteoblast-specific marker expression and
bone marrow adipogenesis in Gja1Jrt/+ stromal cell cultures. _Mol. Biol. Cell_ 26, 832–842 (2015). Article CAS PubMed PubMed Central Google Scholar * Cakouros, D. et al. Novel basic
helix-loop-helix transcription factor hes4 antagonizes the function of twist-1 to regulate lineage commitment of bone marrow stromal/stem cells. _Stem Cells Dev._ 24, 1297–1308 (2015).
Article CAS PubMed Google Scholar * Alarcon-Martinez, L. et al. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for
detection. _eLife_ 7, e34861 (2018). * Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. _Cell Stem Cell_ 3, 301–313 (2008). Article CAS PubMed
Google Scholar * Chen, J. et al. CD146 is essential for PDGFRβ-induced pericyte recruitment. _Protein Cell_ 9, 743–747 (2018). Article CAS PubMed Google Scholar * Hewett, P. W., Nishi,
K., Daft, E. L. & Clifford Murray, J. Selective expression of erg isoforms in human endothelial cells. _Int. J. Biochem. Cell Biol._ 33, 347–355 (2001). Article CAS PubMed Google
Scholar * Colás-Algora, N. & Millán, J. How many cadherins do human endothelial cells express? _Cell. Mol. Life Sci._ 76, 1299–1317 (2019). Article PubMed CAS Google Scholar * Duda,
D. G., Cohen, K. S., Scadden, D. T. & Jain, R. K. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood.
_Nat. Protoc._ 2, 805–810 (2007). Article CAS PubMed PubMed Central Google Scholar * Jaffe, E. A., Hoyer, L. W. & Nachman, R. L. Synthesis of von Willebrand factor by cultured human
endothelial cells. _Proc. Natl Acad. Sci. USA_ 71, 1906–1909 (1974). Article CAS PubMed PubMed Central Google Scholar * Harvey, T., Flamenco, S. & Fan, C. M. A Tppp3(+)Pdgfra(+)
tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. _Nat. Cell Biol._ 21, 1490–1503 (2019). Article CAS
PubMed PubMed Central Google Scholar * Martin, J. F., Bradley, A. & Olson, E. N. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple
lineages. _Genes Dev._ 9, 1237–1249 (1995). Article CAS PubMed Google Scholar * Ehlicke, F., Freimark, D., Heil, B., Dorresteijn, A. & Czermak, P. Intervertebral disc regeneration:
influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). _Int. J. Artif. Organs_ 33, 244–252 (2010). Article CAS PubMed Google Scholar * Rodriguez, A. G. et
al. Human disc nucleus properties and vertebral endplate permeability. _Spine_ 36, 512–520 (2011). Article PubMed PubMed Central Google Scholar * Wang, J. et al. Novel biomarkers of
intervertebral disc cells and evidence of stem cells in the intervertebral disc. _Osteoarthr. Cartil_. 29, 389-401 (2021). * Johnson, Z. I., Shapiro, I. M. & Risbud, M. V. Extracellular
osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. _Matrix Biol._ 40, 10–16 (2014). Article CAS PubMed PubMed Central
Google Scholar * Nugent-Derfus, G. E. et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. _Osteoarthr. Cartil._ 15, 566–574 (2007). Article
CAS Google Scholar * Zhu, S. et al. Chondromodulin-1 in health, osteoarthritis, cancer, and heart disease. _Cell. Mol. Life Sci._ 76, 4493–4502 (2019). Article CAS PubMed PubMed
Central Google Scholar * Ji, Q. et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. _Ann. Rheum. Dis._ 78, 100–110 (2019). Article CAS PubMed Google
Scholar * Fernandes, L. M. et al. Single-cell RNA-seq identifies unique transcriptional landscapes of human nucleus pulposus and annulus fibrosus cells. _Sci. Rep._ 10, 15263 (2020).
Article CAS PubMed PubMed Central Google Scholar * Romereim, S. M., Conoan, N. H., Chen, B. & Dudley, A. T. A dynamic cell adhesion surface regulates tissue architecture in growth
plate cartilage. _J. Dev._ 141, 2085–2095 (2014). Article CAS Google Scholar * Gao, X. et al. KLF2 protects against osteoarthritis by repressing oxidative response through activation of
Nrf2/ARE signaling in vitro and in vivo. _Oxid. Med. Cell. Longev._ 2019, 8564681 (2019). Article PubMed PubMed Central Google Scholar * Wang, R. et al. Inflammatory-sensitive CHI3L1
protects nucleus pulposus via AKT3 signaling during intervertebral disc degeneration. _FASEB J._ 34, 3554–3569 (2020). Article CAS PubMed Google Scholar * Goldring, M. B. Chondrogenesis,
chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. _Ther. Adv. Musculoskelet. Dis._ 4, 269–285 (2012). Article CAS PubMed PubMed Central
Google Scholar * Madhu, V. et al. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF-1α-BNIP3 axis. _J. Bone Miner. Res._ 35,
1504–1524 (2020). Article CAS PubMed Google Scholar * Naba, A. et al. The extracellular matrix: tools and insights for the “omics” era. _Matrix Biol._ 49, 10–24 (2016). Article CAS
PubMed Google Scholar * Sakai, D. & Andersson, G. B. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. _Nat. Rev. Rheumatol._ 11, 243–256 (2015). Article
PubMed Google Scholar * Lyu, F. J. et al. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. _Nat. Rev. Rheumatol._ 15, 102–112 (2019). Article PubMed
Google Scholar * Sivakamasundari, V. et al. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. _Biol. open_ 6, 187–199 (2017). CAS
PubMed Google Scholar * Kozhemyakina, E. et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. _Arthritis Rheumatol._ 67, 1261–1273 (2015).
Article CAS PubMed PubMed Central Google Scholar * Gajghate, S. et al. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of
the intervertebral disc. _J. Bone Miner. Res._ 24, 992–1001 (2009). Article CAS PubMed PubMed Central Google Scholar * Risbud, M. V., Guttapalli, A., Albert, T. J. & Shapiro, I. M.
Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. _Spine_ 30, 2503–2509 (2005). Article PubMed Google Scholar *
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. _Science_ 284, 770–776 (1999). Article CAS PubMed Google
Scholar * Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. _Nat. Methods_ 14, 1083–1086 (2017). Article CAS PubMed PubMed Central Google Scholar * da
Silva, R. A. et al. HOXA cluster gene expression during osteoblast differentiation involves epigenetic control. _Bone_ 125, 74–86 (2019). Article PubMed CAS Google Scholar * Lefebvre, V.
Roles and regulation of SOX transcription factors in skeletogenesis. _Curr. Top. Dev. Biol._ 133, 171–193 (2019). Article CAS PubMed PubMed Central Google Scholar * Tan, Z. et al.
Synergistic co-regulation and competition by a SOX9-GLI-FOXA phasic transcriptional network coordinate chondrocyte differentiation transitions. _PLoS Genet._ 14, e1007346 (2018). Article
PubMed PubMed Central CAS Google Scholar * Yang, X. et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage.
_J. Cell Biol._ 153, 35–46 (2001). Article CAS PubMed PubMed Central Google Scholar * Zhou, F. et al. Tracing haematopoietic stem cell formation at single-cell resolution. _Nature_ 533,
487–492 (2016). Article CAS PubMed Google Scholar * Yu, Q. C., Song, W., Wang, D. & Zeng, Y. A. Identification of blood vascular endothelial stem cells by the expression of protein
C receptor. _Cell Res._ 26, 1079–1098 (2016). Article CAS PubMed PubMed Central Google Scholar * Subramaniam, A., Talkhoncheh, M. S., Magnusson, M. & Larsson, J. Endothelial protein
C receptor (EPCR) expression marks human fetal liver hematopoietic stem cells. _Haematologica_ 104, e47–e50 (2019). Article CAS PubMed PubMed Central Google Scholar * Lan, Y. Procr+
stem cells: from vessel to blood. _Natl Sci. Rev._ 4, 523–524 (2017). Article CAS Google Scholar * Wang, D. et al. Long-term expansion of pancreatic islet organoids from resident procr+
progenitors. _Cell_ 180, 1198–1211.e19 (2020). Article CAS PubMed Google Scholar * Wang, J., Wang, D., Chu, K., Li, W. & Zeng, Y. A. Procr-expressing progenitor cells are responsible
for murine ovulatory rupture repair of ovarian surface epithelium. _Nat. Commun._ 10, 4966 (2019). Article CAS PubMed PubMed Central Google Scholar * Pinho, S. et al. PDGFRα and CD51
mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. _J. Exp. Med_. 210, 1351–1367 (2013). Article CAS PubMed PubMed Central
Google Scholar * Ling, C. et al. Differentiated fibrocytes assume a functional mesenchymal phenotype with regenerative potential. _Sci. Adv._ 5, eaav7384 (2019). Article CAS PubMed
PubMed Central Google Scholar * Xia, C. et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. _Free
Radic. Biol. Med._ 143, 1–15 (2019). Article CAS PubMed Google Scholar * Deroyer, C. et al. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. _Cell Death
Dis._ 10, 103 (2019). Article PubMed PubMed Central CAS Google Scholar * Marín, Y. E., Seiberg, M. & Lin, C. B. Aldo-keto reductase 1C subfamily genes in skin are UV-inducible:
possible role in keratinocytes survival. _Exp. Dermatol._ 18, 611–618 (2009). Article PubMed CAS Google Scholar * Minnone, G., De Benedetti, F. & Bracci-Laudiero, L. NGF and its
receptors in the regulation of inflammatory response. _Int. J. Mol. Sci._ 18, 1028 (2017). * Yamamoto, M., Sobue, G., Yamamoto, K., Terao, S. & Mitsuma, T. Expression of mRNAs for
neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. _Neurochem. Res._ 21,
929–938 (1996). Article CAS PubMed Google Scholar * Ellis, P. et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the
adult. _Dev. Neurosci._ 26, 148–165 (2004). Article CAS PubMed Google Scholar * Huang, R. et al. NCAM regulates temporal specification of neural progenitor cells via profilin2 during
corticogenesis. _J. Cell Biol._ 219, e201902164 (2020). * Artigas, N. et al. p53 inhibits SP7/Osterix activity in the transcriptional program of osteoblast differentiation. _Cell Death
Differ._ 24, 2022–2031 (2017). Article CAS PubMed PubMed Central Google Scholar * Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. _Histochem. Cell Biol._
149, 313–323 (2018). Article CAS PubMed Google Scholar * Hojo, H., Ohba, S., He, X., Lai, L. P. & McMahon, A. P. Sp7/Osterix is restricted to bone-forming vertebrates where it acts
as a Dlx co-factor in osteoblast specification. _Dev. Cell_ 37, 238–253 (2016). Article CAS PubMed PubMed Central Google Scholar * Cao, J. et al. The single-cell transcriptional
landscape of mammalian organogenesis. _Nature_ 566, 496–502 (2019). Article CAS PubMed PubMed Central Google Scholar * Cleary, M. A. et al. Dynamic regulation of TWIST1 expression
during chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. _Stem Cells Dev._ 26, 751–761 (2017). Article CAS PubMed Google Scholar * Li, H. et al. FOXP1
controls mesenchymal stem cell commitment and senescence during skeletal aging. _J. Clin. Investig._ 127, 1241–1253 (2017). Article PubMed PubMed Central Google Scholar *
Rivera-Gonzalez, G. C. et al. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. _Cell Stem Cell_ 19, 738–751 (2016). Article CAS PubMed PubMed Central
Google Scholar * Mizuno, M. et al. Platelet-derived growth factor (PDGF)-AA/AB in human serum are potential indicators of the proliferative capacity of human synovial mesenchymal stem
cells. _Stem Cell Res. Ther._ 6, 243 (2015). Article PubMed PubMed Central CAS Google Scholar * Than, K. D. et al. Bone morphogenetic proteins and degenerative disk disease.
_Neurosurgery_ 70, 996–1002 (2012). Article PubMed Google Scholar * Zhu, J. et al. Sustained release of GDF5 from a designed coacervate attenuates disc degeneration in a rat model. _Acta
Biomater._ 86, 300–311 (2019). Article CAS PubMed Google Scholar * Shim, E. K. et al. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration
via paracrine interaction: an in vitro pilot study. _Cell Transplant._ 25, 1819–1832 (2016). Article PubMed Google Scholar * Xu, D. et al. Hydrogen sulfide protects against endoplasmic
reticulum stress and mitochondrial injury in nucleus pulposus cells and ameliorates intervertebral disc degeneration. _Pharm. Res._ 117, 357–369 (2017). Article CAS Google Scholar * Li,
K. et al. Potential biomarkers of the mature intervertebral disc identified at the single cell level. _J. Anat._ 234, 16–32 (2019). Article CAS PubMed Google Scholar * Chelberg, M. K.,
Banks, G. M., Geiger, D. F. & Oegema, T. R. Jr Identification of heterogeneous cell populations in normal human intervertebral disc. _J. Anat._ 186, 43–53 (1995). PubMed PubMed Central
Google Scholar * Chan, W. C., Au, T. Y., Tam, V., Cheah, K. S. & Chan, D. Coming together is a beginning: the making of an intervertebral disc. _Birth defects Res. C. Embryo Today._
102, 83–100 (2014). Article CAS PubMed Google Scholar * Richardson, S. M. et al. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human
nucleus pulposus cells through aging and degeneration. _Sci. Rep._ 7, 1501 (2017). Article PubMed PubMed Central CAS Google Scholar * Risbud, M. V. et al. Defining the phenotype of
young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. _J. Orthop. Res._ 33, 283–293 (2015). Article PubMed PubMed
Central Google Scholar * Choi, K. S., Cohn, M. J. & Harfe, B. D. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk
degeneration and chordoma formation. _Dev. Dyn._ 237, 3953–3958 (2008). Article CAS PubMed PubMed Central Google Scholar * McCann, M. R., Tamplin, O. J., Rossant, J. & Séguin, C. A.
Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. _Dis. Models Mech._ 5, 73–82 (2012). Article CAS Google Scholar * Weiler, C. et
al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. _Eur. Spine J._ 19, 1761–1770 (2010). Article PubMed PubMed Central
Google Scholar * Raj, P. P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. _Pain. Pract._ 8, 18–44 (2008). Article PubMed Google Scholar * Malinsky, J. The
ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). _Acta Anat._ 38, 96–113 (1959). Article CAS
PubMed Google Scholar * Roberts, S., Eisenstein, S. M., Menage, J., Evans, E. H. & Ashton, I. K. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides.
_Spine_ 20, 2645–2651 (1995). Article CAS PubMed Google Scholar * Cavanaugh, J. M., Kallakuri, S. & Ozaktay, A. C. Innervation of the rabbit lumbar intervertebral disc and posterior
longitudinal ligament. _Spine_ 20, 2080–2085 (1995). Article CAS PubMed Google Scholar * Yoshizawa, H., O’Brien, J. P., Smith, W. T. & Trumper, M. The neuropathology of
intervertebral discs removed for low-back pain. _J. Pathol._ 132, 95–104 (1980). Article CAS PubMed Google Scholar * McCarthy, P. W., Carruthers, B., Martin, D. & Petts, P.
Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. _Spine_ 16, 653–655 (1991). Article CAS PubMed Google Scholar * Liao, L.
et al. Runx2 is required for postnatal intervertebral disc tissue growth and development. _J. Cell. Physiol._ 234, 6679–6687 (2019). Article CAS PubMed Google Scholar * Iwata, M. et al.
Enhancement of Runx2 expression is potentially linked to β-catenin accumulation in canine intervertebral disc degeneration. _J. Cell. Physiol._ 230, 180–190 (2015). Article CAS PubMed
Google Scholar * Sato, S. et al. The distinct role of the Runx proteins in chondrocyte differentiation and intervertebral disc degeneration: findings in murine models and in human disease.
_Arthritis Rheum._ 58, 2764–2775 (2008). Article PubMed Google Scholar * Zhao, C. Q., Wang, L. M., Jiang, L. S. & Dai, L. Y. The cell biology of intervertebral disc aging and
degeneration. _Ageing Res. Rev._ 6, 247–261 (2007). Article PubMed Google Scholar * Phélip, X. Why the back of the child? _Eur. Spine J._ 8, 426–428 (1999). Article PubMed PubMed
Central Google Scholar * Nerlich, A. G., Schaaf, R., Wälchli, B. & Boos, N. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. _Eur. Spine J._ 16,
547–555 (2007). Article PubMed Google Scholar * Urban, J. P. & Roberts, S. Development and degeneration of the intervertebral discs. _Mol. Med. Today_ 1, 329–335 (1995). Article CAS
PubMed Google Scholar * Buckwalter, J. A. Aging and degeneration of the human intervertebral disc. _Spine_ 20, 1307–1314 (1995). Article CAS PubMed Google Scholar * Rudert, M. &
Tillmann, B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. _Ann. Anat._ 175, 237–242 (1993). Article CAS PubMed
Google Scholar * Smith, L. J. & Elliott, D. M. Formation of lamellar cross bridges in the annulus fibrosus of the intervertebral disc is a consequence of vascular regression. _Matrix
Biol._ 30, 267–274 (2011). Article CAS PubMed PubMed Central Google Scholar * Jiang, Y. & Tuan, R. S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. _Nat.
Rev. Rheumatol._ 11, 206–212 (2015). Article PubMed Google Scholar * Xu, J. et al. Comparison of skeletal and soft tissue pericytes identifies CXCR4+ bone forming mural cells in human
tissues. _Bone Res._ 8, 22 (2020). Article CAS PubMed PubMed Central Google Scholar * Sun, H. et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the
progression of meniscus degeneration. _Ann. Rheum. Dis._ 79, 408–417 (2020). Article CAS PubMed Google Scholar * Wangler, S. et al. CD146/MCAM distinguishes stem cell subpopulations with
distinct migration and regenerative potential in degenerative intervertebral discs. _Osteoarthr. Cartil._ 27, 1094–1105 (2019). Article CAS Google Scholar * Nakai, T. et al. CD146
defines commitment of cultured annulus fibrosus cells to express a contractile phenotype. _J. Orthop. Res._ 34, 1361–1372 (2016). Article CAS PubMed Google Scholar * Hastreiter, D.,
Chao, J., Wang, Q., Ozuna, R. M. & Spector, M. Alpha-smooth muscle actin in pathological human disc nucleus pulposus cells in vivo and in vitro. _Wound Repair Regen_. 12, 430–438 (2004).
Article PubMed Google Scholar * Kim, K. W. et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus
pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. _Spine_ 28, 982–990 (2003). Article PubMed Google Scholar * Lee, C. R. et al. A phenotypic
comparison of intervertebral disc and articular cartilage cells in the rat. _Eur. Spine J._ 16, 2174–2185 (2007). Article PubMed PubMed Central Google Scholar * Goldring, S. R. &
Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. _Nat. Rev. Rheumatol._ 12, 632–644 (2016). Article PubMed Google
Scholar * Dudek, M. et al. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. _Ann. Rheum. Dis._ 76, 576–584
(2017). Article CAS PubMed Google Scholar * Hartman, R. et al. Age-dependent changes in intervertebral disc cell mitochondria and bioenergetics. _Eur. Cells Mater._ 36, 171–183 (2018).
Article CAS Google Scholar * Teeple, E. et al. Lubricin deficiency in the murine lumbar intervertebral disc results in elevated torsional apparent modulus. _J. Biomech._ 48, 2210–2213
(2015). Article PubMed PubMed Central Google Scholar * Markolf, K. L. & Morris, J. M. The structural components of the intervertebral disc. A study of their contributions to the
ability of the disc to withstand compressive forces. _J. Bone Jt. Surg. Am._ 56, 675–687 (1974). Article CAS Google Scholar * Pettine, K. A., Murphy, M. B., Suzuki, R. K. & Sand, T.
T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. _Stem Cells_ 33, 146–156 (2015). Article CAS PubMed
Google Scholar * Shu, C. C. et al. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells
for intervertebral disc regeneration. _Int. J. Mol. Sci._ 18, 1049 (2017). * Miwa, H. & Era, T. Tracing the destiny of mesenchymal stem cells from embryo to adult bone marrow and white
adipose tissue via Pdgfrα expression. _Development_ 145, dev155879 (2018). * He, J. et al. Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional
analyses. _Cell Res._ 31, 742–757 (2021). * Furumatsu, T., Tsuda, M., Taniguchi, N., Tajima, Y. & Asahara, H. Smad3 induces chondrogenesis through the activation of SOX9 via
CREB-binding protein/p300 recruitment. _J. Biol. Chem._ 280, 8343–8350 (2005). Article CAS PubMed Google Scholar * Pauklin, S. & Vallier, L. The cell-cycle state of stem cells
determines cell fate propensity. _Cell_ 155, 135–147 (2013). Article CAS PubMed PubMed Central Google Scholar * Bertero, A. et al. The SMAD2/3 interactome reveals that TGFβ controls
m(6)A mRNA methylation in pluripotency. _Nature_ 555, 256–259 (2018). Article CAS PubMed PubMed Central Google Scholar * Dominici, M. et al. Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular Therapy position statement. _Cytotherapy_ 8, 315–317 (2006). Article CAS Google Scholar * Ellman, M. B. et al. Fibroblast
growth factor control of cartilage homeostasis. _J. Cell. Biochem._ 114, 735–742 (2013). Article CAS PubMed PubMed Central Google Scholar * Rutges, J. et al. Variations in gene and
protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. _Osteoarthr. Cartil._ 18, 416–423
(2010). Article CAS Google Scholar * Mizrahi, O. et al. Nucleus pulposus degeneration alters properties of resident progenitor cells. _Spine J._ 13, 803–814 (2013). Article PubMed
PubMed Central Google Scholar * Brown, E. A. et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. _Proc. Natl Acad. Sci. USA_ 114,
11476–11481 (2017). Article CAS PubMed PubMed Central Google Scholar * Zhang, J. et al. TGF-β1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral
disc degeneration and inflammation-related pain in a rat model. _Exp. Mol. Med._ 49, e379 (2017). Article CAS PubMed PubMed Central Google Scholar * Chen, S. et al. TGF-β signaling in
intervertebral disc health and disease. _Osteoarthr. Cartil._ 27, 1109–1117 (2019). Article CAS Google Scholar * Hiyama, A. et al. The relationship between the Wnt/β-catenin and TGF-β/BMP
signals in the intervertebral disc cell. _J. Cell. Physiol._ 226, 1139–1148 (2011). Article CAS PubMed Google Scholar * Wang, Z., Weitzmann, M. N., Sangadala, S., Hutton, W. C. &
Yoon, S. T. Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. _J. Biol.
Chem._ 288, 28243–28253 (2013). Article CAS PubMed PubMed Central Google Scholar * Paglia, D. N., Singh, H., Karukonda, T., Drissi, H. & Moss, I. L. PDGF-BB delays degeneration of
the intervertebral discs in a rabbit preclinical model. _Spine_ 41, E449–E458 (2016). Article PubMed Google Scholar * Ashley, J. W. et al. Intervertebral disc development and
disease-related genetic polymorphisms. _Genes Dis._ 3, 171–177 (2016). Article PubMed PubMed Central Google Scholar * Colombier, P., Clouet, J., Hamel, O., Lescaudron, L. & Guicheux,
J. The lumbar intervertebral disc: from embryonic development to degeneration. _Jt. Bone Spine_ 81, 125–129 (2014). Article Google Scholar * Zheng, L. et al. Ciliary parathyroid hormone
signaling activates transforming growth factor-β to maintain intervertebral disc homeostasis during aging. _Bone Res._ 6, 21 (2018). Article PubMed PubMed Central CAS Google Scholar *
Jin, H. et al. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. _FEBS Lett._ 585, 1209–1215 (2011). Article CAS PubMed PubMed Central
Google Scholar * Li, C. G. et al. A continuous observation of the degenerative process in the intervertebral disc of Smad3 gene knock-out mice. _Spine_ 34, 1363–1369 (2009). Article PubMed
Google Scholar * Tang, Q. O. et al. TGF-beta3: a potential biological therapy for enhancing chondrogenesis. _Expert Opin. Biol. Ther._ 9, 689–701 (2009). Article CAS PubMed Google
Scholar * Zeng, C. W., Kamei, Y., Shigenobu, S., Sheu, J. C. & Tsai, H. J. Injury-induced Cavl-expressing cells at lesion rostral side play major roles in spinal cord regeneration.
_Open Biol._ 11, 200304 (2021). Article CAS PubMed PubMed Central Google Scholar * Murphy, M. P. et al. Articular cartilage regeneration by activated skeletal stem cells. _Nat. Med._
26, 1583–1592 (2020). Article CAS PubMed PubMed Central Google Scholar * Morgun, E. I. & Vorotelyak, E. A. Epidermal stem cells in hair follicle cycling and skin regeneration: a
view from the perspective of inflammation. _Front. Cell Dev. Biol._ 8, 581697 (2020). Article PubMed PubMed Central Google Scholar * Croft, A. S. et al. Effect of different
cryopreservation media on human nucleus pulposus cells’ viability and trilineage potential. _JOR Spine_ 4, e1140 (2021). Article PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank Hongxia Hu and Haitao Hu from Berry Genomics for technical support with scRNA-seq. The authors acknowledge Zhigang Zhou and Feng Wei from Peking University
Third Hospital and Fei Luo, Bo Yu, Bo Huang, Qizhao Huang, Qinghua Ma, and Ruili Cai from Army Medical University for their excellent technical support with sample collection and
single-cell preparation. The authors are grateful to Wenxia Zheng and Hengsheng Tao from Olympus and Xue Yang and Qing Zhou from Army Medical University for help with section staining and
imaging. This study was supported by grants from the National Natural Science Foundation of China (81802165 and 31930054), National Key Research and Development Program of China
(2017YFA0103401 and 2019YFA0110201), Training Plan of Talents’ Innovation of Army Medical Center of PLA (2019CXJSB013), Postdoctoral Innovative Talent Support Program in Chongqing
(2019-298), and Fund for Excellent Young Scholars of the State Key Laboratory of Trauma, Burns and Combined Injury (SKLYQ201902). AUTHOR INFORMATION Author notes * These authors contributed
equally: Yibo Gan and Jian He. AUTHORS AND AFFILIATIONS * Department of Spine Surgery, Center of Orthopedics, Daping Hospital, Army Medical University (Third Military Medical University),
Chongqing, China Yibo Gan, Jun Zhu, Zhong Wang, Ou Hu & Peng Liu * State Key Laboratory of Trauma, Burns and Combined Injury, Army Medical University (Third Military Medical University),
Chongqing, China Yibo Gan & Peng Liu * State Key Laboratory of Proteomics, Academy of Military Medical Sciences, Academy of Military Sciences, Beijing, China Jian He, Zhengyang Xu, Jing
Yan, Zhijie Bai & Bing Liu * Center of Bone Metabolism and Repair, State Key Laboratory of Trauma, Burns and Combined Injury, Trauma Center, Research Institute of Surgery, Laboratory
for the Prevention and Rehabilitation of Military Training Related Injuries, Daping Hospital, Army Medical University (Third Military Medical University), Chongqing, China Lin Chen, Yangli
Xie, Min Jin & Shuo Huang * State Key Laboratory of Experimental Hematology, Institute of Hematology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, China Bing Liu * Key
Laboratory for Regenerative Medicine of Ministry of Education, Institute of Hematology, School of Medicine, Jinan University, Guangzhou, China Bing Liu Authors * Yibo Gan View author
publications You can also search for this author inPubMed Google Scholar * Jian He View author publications You can also search for this author inPubMed Google Scholar * Jun Zhu View author
publications You can also search for this author inPubMed Google Scholar * Zhengyang Xu View author publications You can also search for this author inPubMed Google Scholar * Zhong Wang View
author publications You can also search for this author inPubMed Google Scholar * Jing Yan View author publications You can also search for this author inPubMed Google Scholar * Ou Hu View
author publications You can also search for this author inPubMed Google Scholar * Zhijie Bai View author publications You can also search for this author inPubMed Google Scholar * Lin Chen
View author publications You can also search for this author inPubMed Google Scholar * Yangli Xie View author publications You can also search for this author inPubMed Google Scholar * Min
Jin View author publications You can also search for this author inPubMed Google Scholar * Shuo Huang View author publications You can also search for this author inPubMed Google Scholar *
Bing Liu View author publications You can also search for this author inPubMed Google Scholar * Peng Liu View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS P.L. and B.L. designed and supervised the study; Y.G., J.Z., and O.H. performed the sample collection and preparation with help from P.L., L.C., and Y.X.; Y.G. performed
single-cell RNA sequencing with the help of Z.X., Z.W., and J.H.; Y.G. performed the histological, immunohistochemistry, and immunofluorescence staining with the help of M.J. and S.H.; Y.G.,
J.H., J.Y., Z.B., and O.H. performed the FAC sorting, CFU-F, and trilineage differentiation experiments with the help of Z.X.; J.H. performed the bioinformatic analysis with help from B.L.,
P.L., and Y.G.; P.L., B.L., Y.G., and J.H. wrote the manuscript. All authors read and approved the manuscript. CORRESPONDING AUTHORS Correspondence to Bing Liu or Peng Liu. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY METHODS SUPPLEMENTARY TABLE 1 SUPPLEMENTARY TABLE 2 SUPPLEMENTARY TABLE 3
SUPPLEMENTARY TABLE 4 SUPPLEMENTARY TABLE 5 SUPPLEMENTARY TABLE 6 SUPPLEMENTARY TABLE 7 SUPPLEMENTARY TABLE 8 SUPPLEMENTARY TABLE 9 SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2
SUPPLEMENTARY FIGURE 3 SUPPLEMENTARY FIGURE 4 SUPPLEMENTARY FIGURE 5 SUPPLEMENTARY FIGURE 6 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution
4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gan, Y., He, J., Zhu, J. _et al._ Spatially defined single-cell transcriptional
profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs. _Bone Res_ 9, 37 (2021). https://doi.org/10.1038/s41413-021-00163-z
Download citation * Received: 19 November 2020 * Revised: 30 April 2021 * Accepted: 10 June 2021 * Published: 16 August 2021 * DOI: https://doi.org/10.1038/s41413-021-00163-z SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Quadrant Minimal | designboom.comDESIGNER'S OWN WORDS: the goal was to design the most minimal door handle possible. this developed into one design ...
1975: 50 people, happenings and events from 50 years ago | members onlyIn 1975, the counterculture became the culture. Youth culture was being commodified and camp-ified in mood rings and pet...
Juventus go joint top of serie aThe 23-year-old midfielder coolly finished off Alessandro Del Piero's delightful pass midway through the first half...
Cause of impaired driving and how to prevent itWhether a birthday, holiday or another celebration, numerous events throughout a year can lead to impaired driving. Howe...
Pulwama villagers rue lack of water supply schemePULWAMA, JULY 22: The residents of Uthoora village in south Kashmir’s Pulwama district are forced to drink contaminated ...
Latests News
Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertABSTRACT A comprehensive understanding of the cellular heterogeneity and molecular mechanisms underlying the development...
Comments on 'Style' - Los Angeles TimesIris Schneider’s account of her travels, “A Sunny Side of Siberia,” in Traveling in Style, and the accompanying photogra...
Spontaneous suprachoroidal haemorrhage associated with high myopia and aspirinMAIN Sir, Spontaneous suprachoroidal haemorrhage occurs in age-related macular degeneration (ARMD) and anticoagulants ar...
Donald trump charged: what lies in store legally and politically for the former president?Donald Trump charged: What lies in store legally and politically for the former president? | AP Donald Trump was arraign...
NYPD | The GuardianNYPDDecember 2024Police release new photo of suspect sought in killing of UnitedHealthcare CEO5 Dec 2024 18.23 CETBrian ...