Effect of topiramate on eating behaviours in prader-willi syndrome: toprader double-blind randomised placebo-controlled study
Effect of topiramate on eating behaviours in prader-willi syndrome: toprader double-blind randomised placebo-controlled study"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prader–Willi Syndrome (PWS) is a rare genetic syndrome leading to severe behavioural disorders and mild cognitive impairment. The objective of this double-blind randomised
placebo-controlled trial was to study the efficacy and tolerance of topiramate on behavioural disorders in patients with PWS. Participants (aged 12–45 years) had genetically confirmed PWS
and severe irritability/impulsivity, eating disorders and/or obesity, and skin picking. Thirty-two participants received a placebo (PBO), and 30 participants received topiramate (TOP)
(50–200 mg/day) for 8 weeks. The primary outcome was the rate of responders using the Clinical Global Impression-Improvement (CGI-I) scale. The secondary outcome measures included the
Aberrant Behaviour Checklist, the Dykens Hyperphagia Questionnaire (DHK), the Self-Injurious Behaviour Scale (SIBS) and the body mass index (BMI). We found no significant difference in the
primary outcome (the CGI-I): 9 (30%) patients were very much or much improved in the TOP group compared to 7 (22.6%) patients in the PBO group. However, the DHK behaviour and severity scores
improved significantly more over time in patients treated with topiramate versus those receiving a placebo, with a significant dose–effect relationship. DHK scores were also significantly
associated with genetic subtypes and hospitalisation status. The effects of topiramate on eating behaviours remained significant after adjusting for genetic subtype and hospitalisation.
Topiramate had therefore a significant effect on eating disorders, with a dose–effect relationship. Given the burden of eating disorders in PWS, we believe that topiramate may become the
first psychotropic option within the global care of obesity in individuals with PWS. SIMILAR CONTENT BEING VIEWED BY OTHERS WHAT PHARMACOLOGICAL INTERVENTIONS ARE EFFECTIVE IN BINGE-EATING
DISORDER? INSIGHTS FROM A CRITICAL EVALUATION OF THE EVIDENCE FROM CLINICAL TRIALS Article 07 January 2022 BODY DYSMORPHIC DISORDER Article 05 December 2024 KLEPTOMANIA ON THE
IMPULSIVE–COMPULSIVE SPECTRUM. CLINICAL AND THERAPEUTIC CONSIDERATIONS FOR WOMEN Article Open access 06 March 2025 INTRODUCTION Prader-Willi syndrome (PWS) is a rare genetic syndrome
occurring in 1/21000 newborns in the general population1. This genetic abnormality is located in the 15q11-q13 region, a region with genomic imprinting, and lead to an absence of expression
of a paternal gene due to a micro-deletion (65%) or a maternal disomy (30%). Very few cases are due to a defect of the imprinting centre (5%). The clinical presentation is heterogeneous, but
some trends can be observed. First, the syndrome follows a developmental course. This is particularly clear for feeding disorders: infants with PWS have poor feeding and social skills along
with severe hypotonia that switches to uncontrolled hyperphagia around the age of 2 years. Second, cognitive impairments (mild intellectual disabilities) and psychiatric impairments are
common. Third, endocrine dysfunctions are likely to occur via growth hormone (GH) deficiency and hypogonadism. Adults with PWS exhibit numerous challenging behaviours (irritability,
aggression, hyperphagia, alimentary compulsions and skin lesions) and psychiatric disorders (mood disorders, obsessive-compulsive disorder, brief psychotic episodes, hallucinations and
delusional ideas)2. Feeding disorders are severe in 2/3 of cases, and the consequences of them can include obesity, somatic and metabolic disorders and temper outbursts caused by frustration
over alimentation or misunderstandings of social situations. Irritability and aggression are particularly challenging for families and institutions. Skin lesions due to skin picking can
lead to infections or chronic inflammation and severe anaemia. The therapeutic approach to PWS has seen tremendous improvements during the last two decades and includes (i) the early use of
GHs to improve short stature and body composition3, (ii) strict behavioural measures during childhood to control food access and food intake to prevent obesity4, and (iii) the possible use
of oxytocin for infant feeding deficits, which have shown promising results5. However, despite the prevalence of psychiatric disorders and challenging behaviours among those with PWS, to
date, no specific treatments have been approved, and very little research has been conducted6. Antidepressants (IRS) and antipsychotics seem to be the most frequently used medications6.
However, antipsychotics can lead to an increase appetite7 and are responsible for a worsening of weight gain and irritability as well as temper outbursts related to eating disorders. So far,
only two double-blind randomised placebo-controlled studies have been conducted, but they had small sample sizes (_n_ = 15). The first trial reported on the efficacy of fenfluramine
compared to a placebo on improving eating and aggressive behaviours (skin picking) and inducing weight loss8. However, this treatment was withdrawn from the market because of serious cardiac
side effects. The second trial reported on the efficacy of rimonabant on eating behaviours compared to a placebo9. This treatment was also withdrawn from the market due to its serious
psychiatric side effects (anxiety, dysthymia and delusion). An alternative therapeutic approach has been proposed with topiramate (TOP), an antiepileptic drug that has also been used as an
anti-impulsive and mood stabiliser10. In addition, this antiepileptic treatment, unlike other ones, does not promote weight gain and instead results in a decrease in appetite and,
consequently, weight loss10. Topiramate has previously been prescribed for the treatment of eating disorders. In a meta-analysis that pooled five randomised controlled trials of bulimia
nervosa (_n_ = 128) and binge eating disorder (_n_ = 528) patients, topiramate was more efficient in reducing the quantity of binges, the frequency of ‘loss of control’ (binging), and weight
compared with a placebo11. Two prospective open trials that included 8 patients with PWS each explored the efficacy of topiramate. The first reported an improvement in skin picking and
aggressive behaviours but no change in eating behaviours12. The second reported improvements in eating behaviours, skin picking and aggressive behaviours, and no side effects were
reported13. Up to now, no double-blind randomised placebo-controlled trials have been conducted in patients with PWS. The objective of this double-blind randomised placebo-controlled trial
was to study the efficacy of topiramate on eating disorders, skin picking, and irritability/impulsivity in patients with PWS and its tolerance by patients. Given the large spectrum of
symptoms, we chose the Clinical Global Impression Scale’s Improvement measure as the primary variable. Secondary variables included symptomatic scales focusing specifically on eating
behaviours, skin picking or irritability/impulsivity. METHODS ETHICS AND REGULATIONS The TOPRADER study was approved by the local ethical committee of the principal investigator (_Comité de
Protection des Personnes d’Ile de France VI_ under number CPP/104-11). It was also registered with the French regulatory authorities (_Agence Nationale des Produits de Santé_ under the
number EUDRACT: 2011-003432-32) and the ClinicalTrials.gov international registry under number NCT02810483. All of the subjects received complete information regarding the protocol before
enrolment and gave written consent. Regarding minors or adults under guardianship, either the parents or legal guardian also received this information and gave written consent STUDY DESIGN
The TOPRADER study was a multicentre double-blind randomised placebo-controlled trial. The objective was to evaluate the efficacy and tolerance of topiramate on irritability/impulsivity,
eating disorders and self-mutilation in patients with PWS over an 8-week period. The subjects were randomly allocated into two groups, one taking topiramate, and one taking a placebo. The
dosage of topiramate was 50 mg/day initially with increases of 50 mg per week up to 200 mg/day. Visits for inclusion and monitoring occurred at inclusion, baseline and at 2, 4, 6 and 8 weeks
(the endpoint). PARTICIPANTS Participants were outpatients and inpatients from the French reference centre for PWS, which encompasses 3 sites (Pitié-Salpêtrière University Hospital in
Paris, Marin Hospital in Hendaye and Children University Hospital in Toulouse), and the French reference centre for rare psychiatric disorders (Pitié-Salpêtrière University Hospital in
Paris). The inclusion criteria were as follows: a genetically confirmed diagnosis of PWS, being between 12 and 45 years of age inclusive, being over 50 kg in weight and presenting with one
of the following symptoms1: irritability/impulsivity2, eating disorders and/or obesity, or3 self-harm. The exclusion criteria were as follows: the presence of hallucinations, meeting the
diagnostic criteria for schizophrenia according to the DSM IV, suicidal risk, severe depression, comorbid organic conditions (epilepsy, the use of anticonvulsants or mood stabilisers,
unbalanced diabetes (HbA1C > 10%), type 2 diabetes treated with metformin or gibenclamide, a history of nephrolithiasis or glaucoma, hereditary fructose intolerance problems, glucose
malabsorption or sucrose-isomaltase insufficiency), current use of an effective dose of topiramate for a sufficient time without efficacy, the introduction or change in dose of a
psychotropic medication within the previous 3 months, hypersensitivity to sulphonamides or to any of the components of topiramate or its placebo, the use of a medication with St John’s Wort,
pregnancy or breastfeeding, a lack of effective contraception in females of childbearing age, and no informative adult to provide feedback on subjects’ behaviour. Exclusion criteria also
included biological abnormalities indicating renal failure (serum creatinine greater than 1.5× normal), hepatic impairment (alanine aminotransferase greater than 2× normal), anaemia
(haemoglobin < 12 g/dl (female) or <13 g/dl (male), hyperammonaemia (above laboratory standards), and decreased serum bicarbonates (below laboratory standards). EFFICACY ASSESSMENTS
The primary outcome measure was the rate of responders to the Clinical Global Impression-Improvement (CGI-I) scale with a response defined as a patient obtaining a score of 1 or 2 (very much
or much improved) after 8 weeks of treatment. The CGI-I is a 7-item scale14. The secondary outcome measures included1 the Aberrant Behaviour Checklist, which includes sub-scores to monitor
irritability, lethargy, inappropriate speech, hyperactivity and stereotypies2,15; the Dykens Hyperphagia Questionnaire, which includes sub-scores to monitor hyperphagic behaviour, drive and
severity3,16; the Self-Injurious Behaviour Scale (SIBS), which monitors self-injury behaviours in low-functioning patients with or without autism17 (given PWS phenotypes, we focused on
self-injurious behaviours to the skin); and4 body mass index (BMI), for which weight and size were monitored. SAFETY AND TOLERABILITY EVALUATIONS Specific attention was given to potential
psychiatric adverse effects given that topiramate is known to cause adverse events and that psychiatric symptoms are common in PWS phenotype. At each visit, we monitored patients using the
following assessments: the Schizophrenia Positive Symptoms with the Scale for the assessment of positive symptoms18, the Brief Psychiatric Rating Scale19, the Hamilton Anxiety Scale20 and
the Columbia Classification Algorithm of Suicide Assessment21. Biological parameters were also monitored: NFS, serum electrolytes, creatinine, ammonia plasma, serum bicarbonate, hepatic
measures (AST, ALT, and GGT), total fasting ghrelin, fasting glucose, lipid profile, insulin, leptin, triglycerides and HbA1c. We also monitored topiramate plasmatic concentrations at
baseline, 4 weeks and 8 weeks to explore a possible dose–effect response in patients exposed to topiramate. STATISTICAL ANALYSIS Categorical variables were expressed as frequencies and
percentages. Continuous variables were expressed as means (standard deviation, SD) and medians (inter quartiles, IQR). The comparison of the primary outcome between the 2 treatment groups
was performed using a Pearson's chi-squared test. Regarding secondary outcomes, which included continuous variables measured at baseline, 2, 4, 6 and 8 weeks, we performed an analysis
of repeated measurements using linear mixed models (LMMs). In this model, the fixed effects were time, treatment groups and group-by-time interaction. We introduced subject as random effect.
We also conducted an exploratory analysis to assess possible modulators such as the study site (Hendaye _vs_. Toulouse vs. Paris), hospitalisation status (inpatient _vs_. outpatient),
genetic subtype (disomy _vs_. deletion) and topiramate plasmatic concentration (in µg/mL at baseline, 4 weeks and 8 weeks). We used a logistic regression model for the CGI-I response.
Modulators were studied separately using the following formula: [CGI = Treatment + Modulator + Treatment*Modulator]. We used LMMs for secondary continuous variables by adding the effect of
the modulator to the model with the following formula: [Secondary variable = Treatment + Time + Treatment *Time + Modulator]. For secondary variables, we only performed the modulator effect
analysis in cases of significant effects in group-by-time interactions. All statistical tests were performed with a 2-tailed α level of 0.05. The data were analysed using R version 3.3.3 (R
Core Team, R foundation for Statistical Computing, Vienna, Austria, 2017, URL https://www.R-project.org/). RESULTS SOCIO-DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE SAMPLE The flowchart
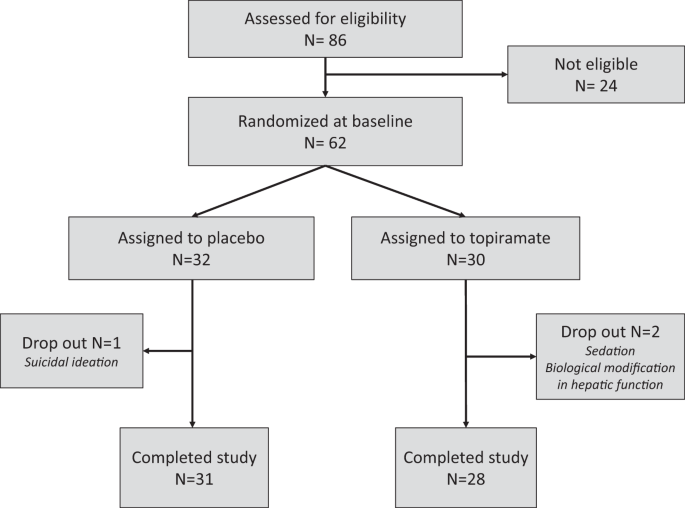
of the study is presented in Fig. 1. Between December 2012 and September 2016, 86 subjects were screened for inclusion in this trial. Twenty-four were not eligible or refused to participate.
Finally, 62 subjects were randomly allocated to receive topiramate (_n_ = 30, TOP) or a placebo (_n_ = 32, PBO). Socio-demographic and clinical characteristics of the participants are
presented in Table 1. The final randomised sample included 32 females and 30 males, and the mean age was 23.8 (8.3) years [range 12-43]. Of those patients, 33 (53.2%) were outpatients, and
29 (46.8%) were inpatients. Regarding PWS genetic subtypes, 42 (67.7%) subjects had a deletion, 18 (29%) had a maternal disomy and 2 (3.2%) had a defect of the imprinting centre. The mean
BMI was 40.74 (12.69), which is categorised as very severely obese (obese class II). At the endpoint, the mean dosage of topiramate in the patients exposed to the active compound was 8.3
(5.83) μg/mL [range: 0.8–22.6]. EFFICACY OF TOPIRAMATE Regarding the main outcome (CGI-I), we found no significant difference between the number of responders in patients treated with
topiramate and in patients treated with the placebo. A total of 9 (30%) patients were very much or much improved in the TOP group compared to 7 (22.6%) in the PBO group (_p_ = 0.51). The
secondary outcome variables at baseline and the endpoint are shown in Table 2. For most variables, there was significant improvement over time. However, we found a significant interaction
between treatment group and time for the Dykens Hyperphagia Questionnaire behaviour and severity scores, meaning that these scores improved significantly more over time in patients treated
with topiramate versus those receiving a placebo (Table 2). The effect of topiramate versus a placebo was also significant using the Hyperphagia Questionnaire for use in PWS Clinical Trials
(HQ-CT, data not shown). This recent scale was validated in PWS during our trial and only differs from the Dykens Hyperphagia Questionnaire by the removal of 3 items22. There was no
significant interaction between group and time for any other secondary variable (sub-scores of the ABC and SIBS-skin picking) except for the ABC-lethargy sub-score: although lethargy
improved in both groups, it appeared that the decrease was significantly lower over time in the TOP group versus the PBO group. Finally, a trend was observed for a decrease in BMI in the
topiramate group versus the placebo group (40.4 to 38.7 in the TOP group _vs_. 41.0 to 40.5 in the PBO group), but without a significant effect for the interaction between time and group in
the statistical model (see Table 2). EXPLORATORY ANALYSIS REGARDING MODULATORS As explained in the Methods section, we aimed to explore 4 possible modulators of the topiramate response: the
study site (Hendaye _vs_. Toulouse vs. Paris), hospitalisation status (inpatient _vs_. outpatient), genetic subtype (disomy _vs_. deletion) and topiramate plasmatic concentration (in µg/mL
at baseline, 4 weeks and 8 weeks). Detailed analyses are reported in the supplemental materials (table S1 to S12). For the CGI response, we found no modulating effect of the variables we
explored. For the Dykens Hyperphagia Questionnaire behaviour and severity scores and the ABC-lethargy sub-score, we found four significant modulator effects. The Dykens Hyperphagia
Questionnaire behaviour score was significantly associated with the genetic subtype, with patients with disomy showing lower scores than patients with a deletion (ß estimate = −2.15, _p_ =
0.011). The Dykens Hyperphagia Questionnaire severity score was significantly associated with the hospitalisation status and study site. Inpatients had lower scores than outpatients (ß
estimate = −1.11, _p_ = 0.017), and patients in Hendaye had lower scores than patients at other sites (ß estimate = −1.62, _p_ = 0.002). To explore the modulating effect of the topiramate
plasmatic concentration, we only used the HQ-CT score, since the models only included participants exposed to topiramate. Patients with higher topiramate plasmatic concentrations had lower
scores for eating disorder behaviours (ß estimate = −0.32, _p_ = 0.0029). The effect remained significant after adjusting for BMI, genetic subtype and study site (ß estimate = −0.31, _p_ =
0.0032; ß estimate = −0.32, _p_ = 0.003; ß estimate = −0.33, _p_ = 0.002, respectively). Finally, the ABC-lethargy sub-score was significantly associated with the hospitalisation status;
inpatients had lower scores than outpatients (ß estimate = −3.06, _p_ = 0.004). SAFETY Adverse events are shown in Table 3. There were only three cases of severe adverse events leading to a
patient’s withdrawal: 1 for suicidal ideation in the PBO group, 1 for hepatic dysfunction and 1 for excessive sedation in the TOP group. Overall, 18 subjects presented with at least one
adverse event, and the total number of adverse events was 23. We observed 7 biological modifications in hepatic function, 6 cases of hyperammonaemia, 1 skin rash, 2 infectious episodes, 4
cases of sedative effects or psychomotor slowdowns, 1 hospitalisation for a suicidal attempt, 1 case of anxiety and tears and 1 case of suicidal ideation. Of these, the following 14 were
reported as occurring in the TOP group: 4 cases of sedative effects or psychomotor slowdowns, 4 biological modifications in hepatic function, 4 cases of hyperammonaemia, and 2 infectious
episodes (bronchitis asthma and sinusitis). This low number of AEs did not allow for a statistical analysis. However, it appeared that most of the AEs occurred in both groups, except for
sedation, which occurred only in the TOP group. We found no changes in Schizophrenia Positive Symptoms and the Hamilton Anxiety Scale. DISCUSSION The TOPRADER study is the first randomised
placebo-controlled trial assessing improvement of behavioural impairments in PWS. There was no significant effect in the topiramate group compared to the placebo group on our primary
outcome, the CGI-I. However, the results strongly support that topiramate is significantly effective on hyperphagia and challenging eating behaviour but not on the other behavioural
dimensions. The Clinical Global Impression scale is a global scale that covers eating behaviours, irritability/aggression and skin picking in PWS patients. The finding that the effect of
topiramate was significant for eating behaviours but not for the other dimensions may explain why the CGI was not significant between the two groups. Indeed, an important result from the
study is the significant effect of topiramate on reducing challenging eating disorders in PWS patients combined with overall good short-term safety. Up to now, only one prospective open
trial reported improvements in eating behaviours13 in PWS, but it was not a double-blind randomised placebo-controlled trial. Eating disorders are very challenging in PWS. It has been found
that controlling food intake is very effective when established early on1,4. However, very few data exist concerning pharmacological therapeutic approaches6. In this trial, the significant
positive effects of topiramate over time on eating behaviours in PWS was observed in two sub-scores of the Dykens Hyperphagia Questionnaire16: hyperphagic behaviours and their severity.
These hyperphagic behaviours are the most challenging behaviours in PWS. Indeed, they start early (6 years old in our sample), and their consequences are dramatic both physically and
psychologically through obesity (and its morbidity) and behavioural disorders due to alimentary frustrations (irritability, aggression and temper outbursts)2,23. These behaviours can
frequently lead to exclusion from institutions and an escalation in the use of pharmacological drugs with potential secondary aggravating effects (e.g., second-generation antipsychotics are
frequently used and can lead to increased appetite and metabolic disorders). Regarding improvements in eating disorder severity over time, we found two interesting modulator effects. To
interpret our results, it is important to note that study sites and hospitalisation status were highly correlated since the Hendaye site is a hospitalisation unit dedicated to PWS patients
and proposing systematic behavioural food control. The reduction in eating behaviour severity was greater when there was a concomitant effect from topiramate treatment and
behavioural/educational methods through hospitalisation. The second interesting significant modulation was the dose-response effect we observed in patients exposed to topiramate. Patients
with higher topiramate plasmatic concentrations had lower Dykens Hyperphagia Questionnaire behaviour scores. The effect remained significant after adjusting for BMI, genetic subtype and
study site. Although we did not find a significant effect on other challenging behaviours and BMI, it is likely that the positive effect on eating behaviours should also have a positive
effect on temper outbursts related to food frustrations and BMI in the longer term. The fact that the trial lasted 8 weeks, with only 5 weeks with full posology, can explain the absence of a
significant effect over time for BMI. Longer follow-up studies are warranted. Regarding safety, 23 AEs were reported, including 14 AEs among patients receiving topiramate. Concerning
psychiatric events, sedative effects or psychomotor slowdowns were reported in four patients (28.6%). These data are confirmed by lethargy over time as measured by the ABC scale, which was
significantly increased in patients in the TOP versus the placebo group. Other adverse events in patients with topiramate concerned biological (hyperammonaemia, modifications in hepatic
function) and organic disorders (infectious episodes). No isolated fever was reported. In PWS, thermal dysregulation with fever can be observed and hypohidrosis and hyperthermia were
described in a few case reports of patients treated by topiramate24. We should thus be careful about this possible side effect in this population. In the topiramate group, only two patients
dropped out of the study (for excessive sedation and hepatic dysfunction). This pharmacological treatment seems to be well tolerated in the short term, especially with regard to psychiatric
side effects; no hallucinations were reported. Suicidal ideation (_n_ = 1) and an attempt (_n_ = 1) were reported in the placebo group. Regarding biological side effects, regular biological
monitoring should be performed, as with most antiepileptic drugs, with particular attention paid to ammonia levels, as they can induce long-term neurotoxicity. However, ammonia levels can
fluctuate and be difficult to measure, particularly with temperature sensitive dosages. This may explain the cases of hyperammonaemia observed in the placebo group. Up to now, only case
reports of encephalopathy induced by the interaction of valproate and topiramate have been reported in the literature25. The results of this study should be interpreted considering both its
strengths and limitations. The trial strengths include it being the first double-blind randomised placebo-controlled study of PWS, the reporting of a substantial sample (_n_ = 62)
considering that PWS is a rare disease, and the use of the Dykens Hyperphagia Questionnaire behaviour scale, which is commonly used and pertinent in the PWS population26,27. The trial also
has several limitations: first, we chose a primary outcome measure that was overly broad, the CGI-I. This was based on the limited literature regarding PWS psychopharmacology. We balanced
this with the literature on topiramate that retains two main indications: epilepsy28 and bulimia nervosa/binge eating11. Second, the study was short in duration (only 8 weeks, with only 5 at
a stable posology). Therefore, we may have missed some long-term efficacy effects as well as long-term AEs. Specifically, we cannot exclude that the reassuring absence of hallucinations and
severe psychiatric symptomatology is due to ending the study before the occurrence of such AEs. A longer safety study should be conducted to monitor topiramate use in PWS. CONCLUSIONS PWS
is a rare developmental syndrome that includes cognitive impairment, eating and behavioural disorders, learning disabilities and an increased prevalence of psychiatric disorders. This first
double-blind randomised placebo-controlled trial reported a significant efficacy of topiramate on eating disorders. Given the burden of eating disorders in PWS, we believe that this effect
on eating disorders may offer new therapeutic opportunities for individuals with PWS. REFERENCES * Bar, C. et al. Early diagnosis and care is achieved but should be improved in infants with
Prader–Willi syndrome. _Orphanet. J. Rare. Dis._ 12, 118 (2017). Article Google Scholar * Thuilleaux, D. et al. A model to characterize psychopathological features in adults with
Prader–Willi syndrome. _Am. J. Med. Genet. A._ 176, 41–47 (2018). Article Google Scholar * Deal, C. L. et al. GrowthHormone Research Society workshop summary: consensus guidelines for
recombinant human growth hormone therapy in Prader–Willi syndrome. _J. Clin. Endocrinol. Metab._ 98, E1072–E1087 (2013). Article CAS Google Scholar * Goldstone, A. P., Holland, A. J.,
Hauffa, B. P., Hokken-Koelega, A. C. & Tauber, M., speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis
and management of Prader-Willi syndrome. _J. Clin. Endocrinol. Metab._ 93, 4183–4197 (2008). Article CAS Google Scholar * Tauber, M. et al. The use of oxytocin to improve feeding and
social skills in infants with Prader–Willi syndrome. _Pediatrics_ 139, e20162976 (2017). Article Google Scholar * Bonnot, O. et al. Psychotropic treatments in Prader–Willi syndrome: a
critical review of published literature. _Eur. J. Pediatr._ 175, 9–18 (2016). Article CAS Google Scholar * Cohen, D., Bonnot, O., Bodeau, N., Consoli, A. & Laurent, C. Adverse effects
of second-generation antipsychotics in children and adolescents: a Bayesian meta-analysis. _J. Clin. Psychopharmacol._ 32, 309–316 (2012). Article CAS Google Scholar * Selikowitz, M.,
Sunman, J., Pendergast, A. & Wright, S. Fenfluramine in Prader–Willi syndrome: a double blind, placebo controlled trial. _Arch. Dis. Child._ 65, 112–114 (1990). Article CAS Google
Scholar * Motaghedi, R. et al. Psychiatric adverse effects of rimonobant in adults with Prader–Willi syndrome. _Eur. J. Med. Genet._ 54, 14–18 (2011). Article Google Scholar * Arnone, D.
Review of the use of Topiramate for treatment of psychiatric disorders. _Ann. Gen. Psychiatry_ 4, 5 (2005). Article Google Scholar * Arbaizar, B., Gomez-Acebo, I. & Llorca, J. Efficacy
of topiramate in bulimia nervosa and binge-eating disorder: a systematic review. _Gen. Hosp. Psychiatry_ 30, 471–475 (2008). Article Google Scholar * Shapira, N. A., Lessig, M. C., Lewis,
M. H., Goodman, W. K. & Driscoll, D. J. Effects of topiramate in adults with Prader–Willi syndrome. _Am. J. Ment. Retard._ 109, 301–309 (2004). Article Google Scholar * Smathers, S.
A., Wilson, J. G. & Nigro, M. A. Topiramate effectiveness in Prader–Willi syndrome. _Pediatr. Neurol._ 28, 130–133 (2003). Article Google Scholar * Guy, W. in _Assessment Manual for
Psychopharmacology Revised_ (ed ECDEU) (National Institute for Mental Health, Rockville, MD, 1976) * Aman, M. G., Singh, N. N., Stewart, A. W. & Field, C. J. The aberrant behavior
checklist: a behavior rating scale for the assessment of treatment effects. _Am. J. Ment. Defic._ 89, 485–491 (1985). CAS PubMed Google Scholar * Dykens, E. M., Maxwell, M. A., Pantino,
E., Kossler, R. & Roof, E. Assessment of hyperphagia in Prader–Willi syndrome. _Obes. (Silver Spring)._ 15, 1816–1826 (2007). Article Google Scholar * Tordjman S., Cohen D., Haag G.
Manual OIBS-SIBS for the Other-Injurious Behavior Scale (OIBS), (eds Echelle des Conduites HétéroAgressives (ECHA) and the Self-Injurious Behavior Scale (SIBS), Echelle des Consuites
AutoAgressives (ECAA). (ECPA) LEdCdPA) (Paris, 2008). * Andreasen N. _Scale for the assesment of positive symptoms (SAPS)_ (University of Iowa Press, Iowa City, 1981) (french translation by
Lecrubier Y. and Boyer P.) 1981. * Rhoades, H. M. & Overall, J. E. The semistructured BPRS interview and rating guide. _Psychopharmacol. Bull._ 24, 101–104 (1988). CAS PubMed Google
Scholar * Bruss, G. S., Gruenberg, A. M., Goldstein, R. D. & Barber, J. P. Hamilton anxiety rating scale interview guide: joint interview and test-retest methods for interrater
reliability. _Psychiatry Res._ 53, 191–202 (1994). Article CAS Google Scholar * Posner, K., Oquendo, M. A., Gould, M., Stanley, B. & Davies, M. Columbia Classification Algorithm of
Suicide Assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. _Am. J. Psychiatry_ 164, 1035–1043 (2007). Article
Google Scholar * Fehnel S., et al. Development of the hyperphagia questionnaire for use in Prader–Willi syndrome clinical trials. In _Meeting ItAI_ (NC, 2015). * Diene, G., Postel-Vinay,
A., Pinto, G., Polak, M. & Tauber, M. [The Prader–Willi syndrome]. _Ann. Endocrinol. (Paris)_ 68, 129–137 (2007). Article CAS Google Scholar * Incecik, F., Herguner, M. O. &
Altunbasak, S. Hypohidrosis and hyperthermia during topiramate treatment in children. _Turk. J. Pediatr._ 54, 515–518 (2012). PubMed Google Scholar * Tantikittichaikul, S., Johnson, J.,
Laengvejkal, P. & DeToledo, J. Topiramate-induced hyperammonemic encephalopathy in a patient with mental retardation: a case report and review of the literature. _Epilepsy Behav. Case
Rep._ 4, 84–85 (2015). Article Google Scholar * Holland, A. et al. The European Prader–illi Syndrome Clinical Research Database: an aid in the investigation of a rare genetically
determined neurodevelopmental disorder. _J. Intellect. Disabil. Res._ 53, 538–547 (2009). Article CAS Google Scholar * Dykens, E. M. & Roof, E. Behavior in Prader–Willi syndrome:
relationship to genetic subtypes and age. _J. Child Psychol. Psychiatry_ 49, 1001–1008 (2008). Article Google Scholar * Pulman, J. et al. Topiramate add-on for drug-resistant partial
epilepsy. _Cochrane Database Syst. Rev._ 25, CD001417 (2014). Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Département de Psychiatrie de l’Enfant et de
l’Adolescent, AP-HP, Groupe-Hospitalier Pitié-Salpêtrière, Paris, France Angèle Consoli, Marie Raffin & David Cohen * GRC-15, Approche dimensionnelle des épisodes psychotiques de
l’enfant et de l’adolescent, Faculté de Médecine, UPMC, Sorbonne Universités, Paris, France Angèle Consoli & Marie Raffin * Unité d’Endocrinologie, Obésité, Maladies Osseuses, Génétique
et Gynécologie Médicale. Centre de Référence du Syndrome de Prader-Willi, Hôpital des Enfants, Toulouse, France Sophie Çabal Berthoumieu & Maïthé Tauber * Assistance-Publique Hôpitaux de
Paris (AP-HP), Hopital Marin de Hendaye, French Reference Center for Prader-Willi Syndrome, Hendaye, France Denise Thuilleaux * Assistance Publique-Hôpitaux de Paris (APHP),
Pitié-Salpêtrière Hospital, French Reference Center for Prader-Willi Syndrome, Nutrition Department, CRNH Ile de France, F-75013, Paris, France Christine Poitou & Muriel Coupaye *
Sorbonne Université, INSERM, Nutriomics team, F-75013, Paris, France Christine Poitou * Assistance-Publique Hôpitaux de Paris (AP-HP), Necker Enfants Malades Hospital University Hospital,
Pediatric Endocrinology, Diabetology and Gynecology Department, F-75015, Paris, France Graziella Pinto * Assistance-Publique Hôpitaux de Paris (AP-HP), Pitié-Salpêtrière Hospital, Department
of Biostatistics, F-75013, Paris, France Said Lebbah * Assistance-Publique Hôpitaux de Paris (AP-HP), Pitié-Salpêtrière Hospital, Department of Pharmacology, F-75013, Paris, France Noel
Zahr * Axe Pédiatrique du CIC 9302/INSERM. Hôpital des Enfants, Toulouse, France Maïthé Tauber * INSERM U1043, Centre de Physiopathologie de Toulouse Purpan, UPS, Toulouse, France Maïthé
Tauber * Institut des Systèmes Intelligents et de Robotiques, CNRS, UMR 7222, UPMC, Sorbonne Universités, Paris, France David Cohen * Service Universitaire de Psychiatrie de l’Enfant et de
l’Adolescent, CHU de Nantes, Nantes, France Olivier Bonnot Authors * Angèle Consoli View author publications You can also search for this author inPubMed Google Scholar * Sophie Çabal
Berthoumieu View author publications You can also search for this author inPubMed Google Scholar * Marie Raffin View author publications You can also search for this author inPubMed Google
Scholar * Denise Thuilleaux View author publications You can also search for this author inPubMed Google Scholar * Christine Poitou View author publications You can also search for this
author inPubMed Google Scholar * Muriel Coupaye View author publications You can also search for this author inPubMed Google Scholar * Graziella Pinto View author publications You can also
search for this author inPubMed Google Scholar * Said Lebbah View author publications You can also search for this author inPubMed Google Scholar * Noel Zahr View author publications You can
also search for this author inPubMed Google Scholar * Maïthé Tauber View author publications You can also search for this author inPubMed Google Scholar * David Cohen View author
publications You can also search for this author inPubMed Google Scholar * Olivier Bonnot View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to Angèle Consoli. ETHICS DECLARATIONS CONFLICT OF INTEREST Pr. Cohen reported past consultation for or the receipt of honoraria from Schering-Plough,
Bristol-Myers-Squibb, Otsuka, Shire, Lundbeck, Janssen, Sanofi-Aventis and IntegraGen. No other authors reported financial disclosure or conflict of interest. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARYMATERIALS
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Consoli, A., Çabal Berthoumieu, S., Raffin, M. _et al._ Effect of topiramate on eating behaviours in Prader-Willi syndrome: TOPRADER double-blind randomised placebo-controlled study.
_Transl Psychiatry_ 9, 274 (2019). https://doi.org/10.1038/s41398-019-0597-0 Download citation * Received: 31 July 2018 * Revised: 24 May 2019 * Accepted: 20 June 2019 * Published: 04
November 2019 * DOI: https://doi.org/10.1038/s41398-019-0597-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Liverpool boss klopp told he has accidentally found perfect formulaHe added: "We've talked about Salah not really firing on all cylinders this season but he steps up in big game...
Micromax Is Making A Comeback- Here's What You Need to KnowEnglish Edition हिंदी বাংলা தமிழ் తెలుగు മലയാളം ગુજરાતી मराठी Deutsch Française HomeVideosFeatures Micromax Is Making A ...
Himachal pradesh assembly election 2022: priyanka gandhi set to address rally in kangra district todayElection Commission announced the schedule for the assembly election in Himachal Pradesh on Friday. The state will go to...
Public school crusaders stake out rival camps in austinAustin has been a staging ground this week for two movements advocating very different visions for the future of America...
404 errorDog owners warned to look out for this plant in France to avoid pet injuries Action can be taken to help avoid these tin...
Latests News
Effect of topiramate on eating behaviours in prader-willi syndrome: toprader double-blind randomised placebo-controlled studyABSTRACT Prader–Willi Syndrome (PWS) is a rare genetic syndrome leading to severe behavioural disorders and mild cogniti...
How can i take part in the elite development programme?Players are either selected from the England U18 squads through recommendations from England Age Group head coaches or v...
Luge Capital | TechCrunchSAVE $200+ ON YOUR TECHCRUNCH ALL STAGE PASS BUILD SMARTER. SCALE FASTER. CONNECT DEEPER. JOIN VISIONARIES FROM PRECURSO...
The fashion formula | NatureAccess through your institution Buy or subscribe Performed in the bedroom, or in front of a mirror, or even at the break...
Richard 'dick' winters dies at 92; wwii heroRichard “Dick” Winters, the “Easy Company” commander whose World War II exploits were made famous by the book and televi...