Diaphragmatic electromyography in infants: an overview of possible clinical applications
Diaphragmatic electromyography in infants: an overview of possible clinical applications"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Preterm infants often experience breathing instability and a hampered lung function. Therefore, these infants receive cardiorespiratory monitoring and respiratory support. However,
the current respiratory monitoring technique may be unreliable for especially obstructive apnea detection and classification and it does not provide insight in breathing effort. The latter
makes the selection of the adequate mode and level of respiratory support difficult. Electromyography of the diaphragm (dEMG) has the potential of monitoring heart rate (HR) and respiratory
rate (RR), and it provides additional information on breathing effort. This review summarizes the available evidence on the clinical potential of dEMG to provide cardiorespiratory
monitoring, to synchronize patient-ventilator interaction, and to optimize the mode and level of respiratory support in the individual newborn infant. We also try to identify gaps in
knowledge and future developments needed to ensure widespread implementation in clinical practice. IMPACT * Preterm infants require cardiorespiratory monitoring and respiratory support due
to breathing instability and a hampered lung function. * The current respiratory monitoring technique may provide unreliable measurements and does not provide insight in breathing effort,
which makes the selection of the optimal respiratory support settings difficult. * Measuring diaphragm activity could improve cardiorespiratory monitoring by providing insight in breathing
effort and could potentially have an important role in individualizing respiratory support in newborn infants. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS RESPIRATORY MUSCLE FUNCTION IN THE NEWBORN: A NARRATIVE REVIEW Article Open access 19 April 2021 DIAPHRAGMATIC ELECTROMYOGRAPHY DURING A SPONTANEOUS BREATHING
TRIAL TO PREDICT EXTUBATION FAILURE IN PRETERM INFANTS Article Open access 06 May 2022 DIAPHRAGMATIC ACTIVITY AND NEURAL BREATHING VARIABILITY DURING A 5-MIN ENDOTRACHEAL CONTINUOUS
POSITIVE AIRWAY PRESSURE TRIAL IN EXTREMELY PRETERM INFANTS Article 17 September 2020 INTRODUCTION Preterm infants often experience difficulties in breathing due to their immature
respiratory system leading to an unstable breathing pattern with frequent apnea and a hampered lung function.1,2 For this reason, most infants need cardiorespiratory monitoring to detect
breathing instability and respiratory support to restore lung function.1,3 The current respiratory monitoring technique used in daily clinical practice, chest impedance (CI), is not always
reliable in detecting and classifying apnea of prematurity.1,2,3,4 Furthermore, CI does not provide insight in breathing effort, which makes selecting the optimal mode and level of
respiratory support difficult in newborn infants.1,2,3,4 Over the last decade, measuring the electrical activity of the diaphragm, the main breathing muscle, with electromyography (dEMG) has
been investigated as an alternative technique for cardiorespiratory monitoring. dEMG has the potential to provide data on heart rate (HR) and respiratory rate (RR), but also breathing
effort.5 This review will summarize the current evidence on the use of dEMG as a cardiorespiratory monitor in newborn infants to highlight its potential for future clinical application, to
identify knowledge gaps, and to direct future research. METHOD This narrative review was written based on a comprehensive literature search in PubMed from 1968 (first dEMG article in PubMed)
up to 2023. Search terms included: ‘diaphragm’, ‘electromyography’, ‘transcutaneous electromyography’, ‘esophageal electromyography’, ‘newborns’, ‘neonates’, ‘preterm infants’, ‘term
infants’. In contrast to a systematic review, we have only included studies within the scope of our narrative review, being potential clinical dEMG applications. As in the past years other
reviews have been published describing the use of esophageal dEMG in newborn infants, we also referred to these reviews instead of redoing their work.6,7 DIAPHRAGMATIC ACTIVITY The diaphragm
is the main breathing muscle and consists of a costal and crural part, which are innervated by the left and right phrenic nerve.8 During normal breathing, the respiratory control center in
the brainstem receives and combines information on the efficacy of gas exchange and lung stretch, which in turn sends an action potential via the phrenic nerves towards the diaphragm.
Consecutively, its muscle fibers are depolarized followed by the formation of action potentials that facilitate diaphragmatic contraction.8,9,10,11 As the diaphragm flattens, the chest
volume increases and the intrapleural pressure decreases, resulting in an influx of air into the lungs.8 With dEMG, a summation of the electrical activity of the innervated muscle fibers
near the measurement electrodes can be measured. Since the electrical activity of the cardiac muscle is detected as well, it can also provide data on cardiac activity.12 In newborn infants,
diaphragm activity is measured using either a transcutaneous or an esophageal route. Transcutaneous dEMG uses adhesive skin electrodes placed bilaterally in the frontal midclavicular line at
the costal margin and one reference electrode on the sternum.5,13 Recently, dry electrodes incorporated in a belt have also been used.14 Transcutaneous dEMG mainly reflects the activity of
the costal diaphragm. The dorsal diaphragm activity can also be measured by placing two additional electrodes on the back, at the same level as the frontal electrodes.13 Changing the lead
configuration, these five electrode positions can also be used to compare the electrical activity of the left and right diaphragmatic hemisphere.15 Esophageal dEMG mainly records the crural
diaphragmatic activity and is measured with a special naso- or orogastric tube containing multiple electrodes and is positioned at the level of the gastroesophageal junction.7,16
Irrespective of the differences between the two measurement methods, a good correlation between both was observed in adults on assisted ventilation and in healthy adults during spontaneous
breathing with a predetermined inspiratory load.17,18 To obtain a respiration waveform, the acquired signal must first be processed to remove the cardiac interference.12 The resulting
waveform can be used to observe the breathing pattern and extract the RR. The waveform can also be used to assess breathing effort, by extracting several dEMG-related parameters, such as the
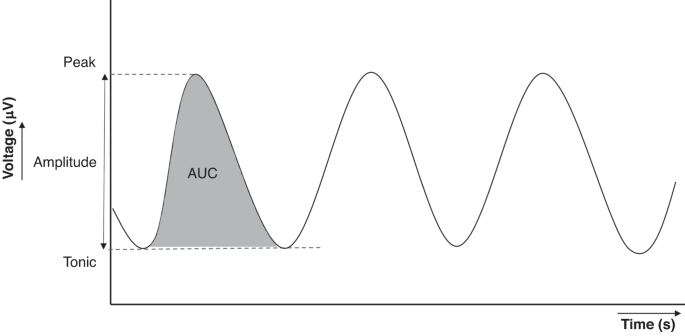
amplitude, peak activity, tonic (end-expiratory) activity and area under the curve (AUC) (see Fig. 1).12 dEMG is measured in µV and its signal strength depends on technical factors (e.g.,
electrode placement, skin-electrode contact and the used hardware and software) as well as factors determined by the patient (e.g., skin type, subcutaneous tissue and chest wall anatomy).12
This results in the lack of normative dEMG parameter values for transcutaneous dEMG and a wide range in peak activity measured with esophageal dEMG, being generally between 6 and 13 µV.7
Therefore, we recommend comparing the measured dEMG within an infant (e.g., over time, left versus right hemisphere of the diaphragm, pre- versus post-intervention) instead of the absolute
µV values. REPORTED CLINICAL APPLICATIONS OF DEMG CARDIORESPIRATORY MONITORING Several studies have assessed dEMG as a technique for cardiorespiratory monitoring in newborn infants. These
studies mainly examined the agreement of the HR and RR between dEMG and the electrocardiogram (ECG)/CI. Both in the delivery room (DR) and the neonatal intensive care unit (NICU),
transcutaneous dEMG provided a high level of HR-agreement with ECG. However, the agreement in RR between dEMG and CI was less robust in both settings.5,19 It has been suggested that this
suboptimal agreement in RR is caused by the differences in measuring techniques (impedance versus electrical activity) and the impact of patient handling and movement on both signals. The
latter is supported by the observation that RR agreement significantly improved when solely stable dEMG and CI data were used for the analysis.5 Similar results were observed when using
esophageal dEMG for HR and RR monitoring.20,21,22 Next to measuring HR and RR, transcutaneous and esophageal dEMG also enable apnea detection, which is of great importance in the preterm
population. Several studies have shown that both dEMG techniques are able to detect apnea and one study also suggested that transcutaneous dEMG resulted in an improved classification of
central and obstructive apnea compared to CI. In addition, a study using esophageal dEMG showed the ability to detect congenital central hypoventilation syndrome (central apnea during
sleep).6,7,22,23,24 DEMG AS A MONITOR FOR BREATHING EFFORT As previously mentioned, dEMG also has the ability to quantify the amount of electrical activity delivered by the diaphragm.
Studies in adults and children have shown that electrical effort of the diaphragm measured with esophageal dEMG was linearly related to the work of breathing (WOB) based on esophageal
pressure and airway pressure during expiratory occlusion.25,26 More recently, a study in preterm infants showed a modest correlation between peak activity measured with transcutaneous dEMG
and conventional WOB measured with volume and esophageal pressure recordings.27 Other studies provided more indirect evidence that the electrical activity of the diaphragm reflects changes
in WOB, showing that deterioration in the infant’s respiratory status or an increased demand for respiratory support led to an increase in peak diaphragmatic activity measured with
esophageal dEMG.7 However, it should be noted that the relationship between dEMG and breathing effort or WOB depends on the neuro-muscular efficiency (i.e., the measured electrical activity
of the diaphragm in µV and the consecutively generated airway pressure). This efficiency could be lowered due to sedation (iatrogenic) or in patients with neuromuscular disease (e.g., spinal
muscular atrophy).28,29,30 These studies suggest that dEMG provides important insight in breathing effort, which could also be used to optimize respiratory support, as discussed in the
following paragraphs. DEMG FOR PATIENT-VENTILATOR SYNCHRONIZATION During invasive and non-invasive mechanical ventilation, the spontaneous breathing effort of the patient is supported with
mechanical inflations. Ideally, these mechanical inflations are synchronized with the onset and the end of a spontaneous breath. Historically, changes in airway pressure, airway flow and
volume have been used to detect the start of inspiration. However, during invasive mechanical ventilation this requires placement of a pressure or flow sensor in the patient circuit which
can increase physiological dead space which is a potential problem in infants requiring small tidal volumes. Furthermore, leakage around the endotracheal tube compromises the accuracy of
triggering.4 Air leakage is an even bigger problem when trying to synchronize non-invasive mechanical ventilation, with leaks via the mouth and around the nasal interface. This leads to
patient-ventilator asynchrony (PVA) which is associated with less effective support, prolonged mechanical ventilation, increased WOB, dynamic hyperinflation and an increased need for
sedation.4,31 The ability of dEMG to monitor the breathing cycles also provides the opportunity to use its signal to trigger the onset and offset of a mechanical inflation in response to a
spontaneous breath.6,7 The dEMG technique may reduce the delay time between the onset of the spontaneous breath and the start of the mechanical inflation as diaphragm contraction precedes
thoracic expansion and the subsequent change in flow and pressure.32 Furthermore, the dEMG measurement does not necessarily require a flow or pressure sensor in the patient circuit and is
not impacted by leakage at the airway opening. Although, in case of using dEMG to trigger respiratory support, a flow/pressure sensor could be used to enable flow/pressure based triggering
during periods of signal noise in the dEMG recording. If not suitable to have an additional flow/pressure sensor, one could activate back-up ventilation during dEMG signal noise periods.
Studies have shown that esophageal dEMG can be used to initiate and cycle off mechanical inflations during invasive and non-invasive respiratory support6,7, in a wide range of newborns,
including preterm and low birth weight infants, infants with unilateral diaphragmatic paralysis or congenital diaphragmatic hernia.6,33,34,35,36,37 Using esophageal dEMG to synchronize
(non-)invasive support in newborn infants, reduces PVA compared to other triggering modes.6,7,38 Synchronizing inflations with transcutaneous dEMG has not yet been studied. PROPORTIONAL
ASSIST BASED ON DEMG Some experts believe that respiratory support should unload the disease-related increase in WOB, also referred to as proportional assist ventilation. Given the ability
of dEMG to provide insight in breathing effort, diaphragm activity might be used as the input signal to determine the level of pressure needed to unload the inspiratory WOB of an individual
infant.4 Until now, proportional assist ventilation using dEMG is only available on one commercial ventilator, which uses an esophageal catheter to measure diaphragmatic activity. During
this so-called neurally adjusted ventilatory assist (NAVA) ventilation, the peak inflation pressure is proportional to the electrical activity of the diaphragm. The clinician sets the level
of pressure delivered per microvolt of diaphragm activity (“gain” or “NAVA-level”).6,7 Studies have shown that the concept of NAVA is feasible in newborn infants during both invasive and
non-invasive support.6,7 Regarding the long-term effects of NAVA, a retrospective study and a randomized controlled trial in extremely low birth weight preterm infants compared nasal CPAP
(nCPAP) to non-invasive NAVA. These studies observed among others a lower reintubation rate and a shorter duration of mechanical ventilation in the non-invasive NAVA group, and no difference
in the incidence of bronchopulmonary dysplasia and death.39,40 The duration of invasive ventilation did not differ in preterm infants on NAVA compared to conventional mechanical
ventilation.41 WEANING OF RESPIRATORY SUPPORT The ability of dEMG to provide insight in breathing effort can also be used to assess if the level or mode of (non-)invasive respiratory support
is sufficient for the individual patient. Most of the studies on dEMG have looked at its role in weaning patients of respiratory support. WEANING FROM INVASIVE RESPIRATORY SUPPORT Assessing
extubation readiness in newborns is challenging with a reported extubation failure rate varying between 13 and 46%.42,43,44,45,46,47 This may have serious consequences as continuing
invasive mechanical ventilation too long could lead to ventilator-induced lung injury and diaphragm dysfunction. On the other hand extubating too soon with subsequent failure is associated
with increased mortality, health care costs and length of hospital stay.48,49,50,51 Several studies investigated whether diaphragm activity measured before extubation could predict
extubation readiness. One study that measured transcutaneous dEMG showed a higher diaphragm activity (peak and tonic activity) before extubation in the infants that failed compared to the
infants successfully extubated.46 However, three other studies, two using esophageal and one using transcutaneous dEMG, observed no difference in diaphragm activity before extubation in
infants successfully extubated and those failing.47,52,53 Studies also investigated if measuring dEMG during a spontaneous breathing trial (SBT) could improve the prediction of extubation
failure. During a SBT the patient is switched from mechanical ventilation to endotracheal continuous positive airway pressure (CPAP) to challenge the patients’ ability to maintain adequate
gas exchange in absence of mechanical inflations.54 Two studies, one using esophageal and the other using transcutaneous dEMG, reported a significant increase in diaphragm activity in all
newborn infants during the SBT compared to mechanical ventilation.44,55 However, only one study using transcutaneous dEMG observed a significantly higher median increase in diaphragmatic
activity (expressed with the AUC) during the SBT in the group that failed extubation compared to those successfully extubated.55 Some studies assessed the change in diaphragm activity before
and after extubation, aiming to identify patients who are going to fail as soon as possible after extubation, thereby avoiding significant clinical deterioration on non-invasive support.
These studies reported an overall increase in diaphragm activity after extubation, which is probably caused by the reduction in level of support.46,53 When comparing the group of patients
successfully extubated and those failing, a relative increase in diaphragmatic activity was observed in the success cases. However, the group that showed higher absolute values of
diaphragmatic activity after extubation differed between the studies.46,53 Although the abovementioned studies show the potential of dEMG to assess extubation readiness and reduce infants
failing extubation, they also show that the results are inconsistent. Differences in measurement period, sample size, and mode of support after extubation may explain some differences
between the reported studies. Furthermore, an increase in diaphragmatic activity following extubation may be physiological to a certain level and promote successful extubation, while the
inability to do so may lead to extubation failure. On the other hand, requiring supraphysiological levels of diaphragmatic activity for a longer period of time might also be the cause of
extubation failure. Normative data collected in future studies might enable the clinician to differentiate between these two scenarios. The role of dEMG in weaning mechanical ventilation
settings (e.g., tidal volume, airway pressure) was examined in one study which described the effect of weaning positive end-expiratory pressure (PEEP) during mechanical ventilation on
diaphragmatic activity.56 Applying zero PEEP in intubated infants led to a significant increase in tonic activity of the diaphragm measured with esophageal dEMG, which was immediately
reversed after reapplying the PEEP. Although zero PEEP is usually not applied in clinical practice, this study does show the potential of dEMG to guide weaning of mechanical ventilation in
the future as PEEP seems to influence the tonic activity measured with dEMG. WEANING OF NON-INVASIVE RESPIRATORY SUPPORT In most institutions weaning non-invasive respiratory support is not
protocolized and left to the discretion of the attending physician who uses a trial and error approach, based on his/her own experience and clinical condition of the patient. A more
objective measure to wean non-invasive support is urgently needed and several studies investigated the potential role of dEMG for this application. One study, including stable preterm
infants on nCPAP, assessed the effect of changing the nCPAP level between 2, 4, and 6 cmH2O on diaphragmatic activity. They reported no significant changes in most of the dEMG parameters.27
Other studies, applying different high flow nasal cannula (HFNC) levels (4, 6 and 8 L/min) or weaning HFNC flow in steps of 1 L/min, also reported no significant differences in diaphragmatic
activity between different flows.57,58,59 As most of the infants did not show clinical deterioration during the weaning steps, these results could indicate that many infants were probably
overtreated and could be weaned more rapidly. Studies also assessed the effect of switching between different non-invasive support modalities on diaphragmatic activity. A cross-over study
between nCPAP (mean 5 cmH2O) and HFNC (mean 5 L/min) using esophageal dEMG, found no significant differences in diaphragm activity.60 Similarly, switching from nCPAP to HFNC in a 1:1
pressure-to-flow ratio also did not lead to changes in diaphragm activity measured with transcutaneous dEMG.61 As in most of these studies the intent was probably to switch between
modalities and not so much wean the level of support, the dEMG findings confirm that this was successful. Some studies measured changes in diaphragmatic activity when switching to a mode
considered less supportive or even no support at all. Two studies, measuring dEMG via the transesophageal route, reported an increase in peak activity, amplitude and neural RR when
transferring infants from HFNC to room air or low flow nasal cannula (LFNC).57,62 One of these studies also reported a trend towards higher diaphragm activity in the success group compared
to the group of infants that failed the weaning attempt.57 Another study showed that weaning from nCPAP from a median level of 3 cmH2O to a LFNC level of 1 L/min in stable preterm infants
initially increased peak and tonic diaphragmatic activity, measured with transcutaneous dEMG, followed by a gradual decrease over time. Infants failing this weaning attempt tended to have a
higher diaphragm activity than those successfully weaned.63 These studies again suggest that dEMG could potentially contribute to selecting the adequate mode and level of non-invasive
respiratory support. EFFECT OF OTHER RESPIRATORY INTERVENTIONS ON DEMG Studies have also used dEMG to assess the effect of other respiratory interventions on diaphragmatic activity, often to
clarify the working mechanism of these interventions on the respiratory system. One of these interventions is the administration of caffeine, a breathing stimulating drug often given to
preterm infants. Studies using transcutaneous and esophageal dEMG have shown that an intravenous loading dose of caffeine in spontaneously breathing infants or mechanically ventilated
infants results in an increased diaphragm activity.64,65,66 This increase of diaphragmatic activity resulted in an increased tidal volume and minute ventilation.65 In contrast, doxapram,
another breathing stimulant, had no effect on transcutaneous and esophageal measured diaphragm activity, indicating that this drug influences neural drive or breathing pattern rather than
effort.67,68 The effect of minimally invasive surfactant administration on dEMG was also studied. The authors reported a reduction of diaphragm activity in the majority of infants in the
first hour after surfactant treatment, which probably reflects the improved lung compliance resulting in less WOB.69 dEMG has also been used to assess respiratory interventions in the DR. A
study reporting that an initial high fraction of inspired oxygen (FiO2) increased minute volume compared to a low FiO2 in preterm infants, showed that this increased minute volume was
accompanied by a higher diaphragm activity measured with transcutaneous dEMG. This could suggest an oxygen dependent increase in diaphragm activity leading to improved ventilation.70,71 When
measuring dEMG and airway flow simultaneously, the active and passive phase of expiration can be discriminated. The active phase, also called post-inspiratory activity (PIA), describes the
remaining diaphragm activity during expiratory flow, while the passive phase could consist of a plateau in the dEMG waveform whilst expiratory flow remains present (or at least no
inspiratory flow is measured). After the passive expiratory phase, the diaphragm activity increases before inspiratory flow occurs, which is called the ramp inspiratory activity and
represents diaphragmatic contraction.72 A study showed that during hypercapnia the PIA as measured with transcutaneous dEMG was prolonged, suggesting that mechanisms to increase
end-expiratory lung volume via expiratory braking are intensified during hypercapnia in preterm infants. In addition to the PIA, the peak activity during inspiration was also increased
during hypercapnia.73 Another study showed that using a helium-oxygen mixture compared to air-oxygen during invasive ventilation, resulted in a reduction in diaphragm activity measured with
esophageal dEMG.7,74 This reduction was explained by the lower density and increased laminar flow of the helium-oxygen mixture compared to air, both reducing airway resistance and thereby
WOB.75 Lastly, certain sedatives also seem to influence diaphragm activity. For example, children on mechanical ventilation who received a bolus of propofol showed a mean reduction in
diaphragm activity measured with esophageal dEMG of 32%.76 Beck et al. also described that a reduction in sedation in newborn infants resulted in an increase in esophageal dEMG.7
DIAPHRAGMATIC DYSFUNCTION Diaphragm dysfunction caused by abnormal development or phrenic nerve damage can cause serious breathing problems.8 Currently, chest X-ray and ultrasound are used
to, respectively, show diaphragm elevation or reduced or absent diaphragmatic movement. However, these techniques have disadvantages such as exposure to radiation, the occurrence of
false-negative results caused by paradoxical movements of the diaphragm, or not being applicable at the bedside.77 Several case reports have indicated that dEMG can play an important role in
diagnosing diaphragmatic dysfunction in newborn infants. Absent or reduced diaphragmatic activity, measured with transcutaneous dEMG, has been reported in unilateral diaphragmatic
paresis.15 Unlike transcutaneous dEMG, in esophageal dEMG the detection of unilateral diaphragmatic dysfunction may be less clear compared to bilateral dysfunction because this technique is
unable to measure the electrical activity of the left and right hemisphere separately. In addition to the diagnosis of diaphragmatic dysfunction, repeated measurements or continuous
monitoring of transcutaneous or esophageal dEMG may also provide insight in spontaneous recovery over time.78 In case of neuromuscular disorders, the dEMG technique could identify aberrant
respiratory patterns in pediatric intensive care unit (PICU) patients that could not be observed clinically and, as mentioned before, a lowered neuro-muscular efficiency could be observed.29
The neuro-muscular efficiency is also lowered in critically ill PICU patients on mechanical ventilation and further decreases over time during mechanical ventilation, which could be due to
inspiratory muscle atrophy.28 Moreover, dEMG can detect diaphragmatic inactivity during mechanical ventilation, which could be suggestive for a surplus of assistance or sedation.79 KNOWLEDGE
GAPS In this narrative review, the potential of measuring diaphragm activity in preterm and term infants has been described and several knowledge gaps have been identified. First, normative
values of diaphragm activity representing an (in)adequate breathing effort are lacking. Second, the relation between diaphragm activity and WOB requires further research. Third, the ability
to trigger ventilator inflations and to provide proportional assist with transcutaneous dEMG should be studied. Finally, using dEMG to predict weaning readiness, to early detect weaning
failure and to individualize the mode and level of respiratory support require further research. FUTURE DIRECTIONS More information on these knowledge gaps could be obtained when widespread
routine dEMG monitoring is enabled as this would result in big (continuous) data recorded with a standardized measurement set-up. Nowadays, usage of dEMG is limited as only the esophageal
technique is applied in clinical practice. Esophageal dEMG is mainly used to trigger ventilator inflations and to provide proportional assist. When used during non-invasive support, it can
also monitor breathing and detect apnea. However, the esophageal dEMG technique is invasive, expensive and only available on one commercial ventilator. To further increase the accessibility
of dEMG, it would be beneficial if the transcutaneous technique would become available in clinical practice and could be enabled in combination with commercially available ventilators and/or
patient monitors. Before implementation can occur, this technique needs to be optimized. Transcutaneous dEMG should be measured without the need for additional electrodes and hardware. A
study has already shown that diaphragmatic activity can be measured using ECG/CI electrodes and the same hardware.80 The dEMG technique can be further improved by using a wireless and
non-adhesive interface to increase user- and patient-friendliness. For this purpose, a novel wireless and non-adhesive cardiorespiratory monitoring belt based on dEMG has been developed.14
When cardiorespiratory monitoring is performed with this belt, big data on dEMG can be obtained. In general, we do believe that incorporating the dEMG technique in already used hardware
(i.e., no additional electrodes are required and an existing connection to the cardiorespiratory monitor), would be preferable.80 The resulting big data could be used to assess the influence
of technical factors and factors determined by the patient on the variability of the measured signal strength. In addition, this data could contribute to obtaining normative dEMG values
(absolute µV or delta values) in infants or in subgroups of infants (e.g., different age or birth weight groups). When transcutaneous dEMG is introduced on a larger scale for
cardiorespiratory monitoring in clinical practice, data on neural breathing effort can be measured in a larger group of infants. These data could be used to obtain normative values by
grouping infants, to assess the relation with other WOB related measures, and to determine cut-off values that indicate weaning readiness and failure. Routine dEMG monitoring could also
stimulate further research on the role of dEMG in individualizing respiratory support by observing (changes in) diaphragm activity when changing the mode and level of respiratory support.
Moreover, research can be done on the ability to trigger ventilator inflations and to provide proportional assist based on the transcutaneous technique. CONCLUSION This review underlines the
potential of measuring diaphragmatic activity in clinical practice, using either esophageal or transcutaneous electrodes, in newborn infants. Electromyography provides accurate and direct
information on HR, RR, and breathing pattern, including the detection of apnea. In contrast to currently used monitoring techniques (ECG and CI), dEMG also provides information on (neural)
breathing effort. Although more evidence is needed, studies have shown that the characteristics of dEMG provide a great potential to use it for cardiorespiratory monitoring, triggering of
mechanical ventilation and selection of the optimal mode and level of respiratory support. Further development of the interface (non-adhesive and/or wireless), hardware and software will
allow implementation on a large clinical scale. REFERENCES * Donn, S. M., Mammel, M. C. & van Kaam, A. H. L. C. _Manual of Neonatal Respiratory Care_ 5 edn (Springer, 2022). * Di Fiore,
J. M., Poets, C. F., Gauda, E., Martin, R. J. & MacFarlane, P. Cardiorespiratory events in preterm infants: etiology and monitoring technologies. _J. Perinatol._ 36, 165–171 (2016).
Article PubMed Google Scholar * Di Fiore, J. M. Neonatal cardiorespiratory monitoring techniques. _Semin Neonatol._ 9, 195–203 (2004). Article PubMed Google Scholar * van Kaam, A. H.
et al. Modes and strategies for providing conventional mechanical ventilation in neonates. _Pediatr. Res._ 90, 957–962 (2021). Article PubMed Google Scholar * Kraaijenga, J. V., Hutten,
G. J., de Jongh, F. H. & van Kaam, A. H. Transcutaneous electromyography of the diaphragm: a cardio-respiratory monitor for preterm infants. _Pediatr. Pulmonol._ 50, 889–895 (2015).
Article PubMed Google Scholar * Stein, H., Beck, J. & Dunn, M. Non-invasive ventilation with neurally adjusted ventilatory assist in newborns. _Semin. Fetal Neonatal Med._ 21, 154–161
(2016). Article PubMed Google Scholar * Beck, J. & Sinderby, C. Neurally adjusted ventilatory assist in newborns. _Clin. Perinatol._ 48, 783–811 (2021). Article PubMed Google
Scholar * Barrow, A. & Pandit, J. J. Lung ventilation and the physiology of breathing. _Surg. (Oxf.)_ 35, 227–233 (2017). Article Google Scholar * Di Fiore, J. M., Martin, R. J. &
Gauda, E. B. Apnea of prematurity-perfect storm. _Respir. Physiol. Neurobiol._ 189, 213–222 (2013). Article PubMed Google Scholar * Hopkins, P. M. Skeletal muscle physiology. _Contin.
Educ. Anaesth. Crit. Care Pain._ 6, 1–6 (2006). Article Google Scholar * Blackburn, S. T. _Maternal, Fetal, Neonatal Physiology—A Clinical Perspective_ 5th Edition edn (Elsevier, 2018). *
van Leuteren, R. W. et al. Processing transcutaneous electromyography measurements of respiratory muscles, a review of analysis techniques. _J. Electromyogr. Kinesiol_ 48, 176–186 (2019).
Article PubMed Google Scholar * van Leuteren, R. W. et al. Diaphragmatic electromyography in preterm infants: the influence of electrode positioning. _Pediatr. Pulmonol._ 55, 354–359
(2020). Article PubMed Google Scholar * Scholten, A. W. J. et al. Feasibility of wireless cardiorespiratory monitoring with dry electrodes incorporated in a belt in preterm infants.
_Physiol. Meas._ 43, 1–8 (2022). * Kraaijenga, J. V., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Diagnosis of hemidiaphragmatic paresis in a preterm infant with transcutaneous
electromyography: a case report. _Neonatology_ 108, 38–41 (2015). Article PubMed Google Scholar * Jonkman, A. H. et al. Proportional modes of ventilation: technology to assist physiology.
_Intensive Care Med._ 46, 2301–2313 (2020). Article PubMed PubMed Central Google Scholar * Bellani, G. et al. Measurement of diaphragmatic electrical activity by surface
electromyography in intubated subjects and its relationship with inspiratory effort. _Respir. Care_ 63, 1341–1349 (2018). Article PubMed Google Scholar * Lin, L., Guan, L., Wu, W. &
Chen, R. Correlation of surface respiratory electromyography with esophageal diaphragm electromyography. _Respir. Physiol. Neurobiol._ 259, 45–52 (2019). Article PubMed Google Scholar *
van Leuteren, R. W. et al. Cardiorespiratory monitoring in the delivery room using transcutaneous electromyography. _Arch. Dis. Child Fetal Neonatal Ed._ 106, 352–356 (2021). Article PubMed
Google Scholar * Burgin, C. et al. Multichannel esophageal signals to monitor respiratory rate in preterm infants. _Pediatr. Res_ 91, 572–580 (2022). Article CAS PubMed Google Scholar
* Simmen, P. et al. Multichannel esophageal heart rate monitoring of preterm infants. _IEEE Trans. Biomed. Eng._ 68, 1903–1912 (2021). Article PubMed Google Scholar * Beck, J. et al.
Characterization of neural breathing pattern in spontaneously breathing preterm infants. _Pediatr. Res_ 70, 607–613 (2011). Article PubMed PubMed Central Google Scholar * Kraaijenga, J.
V. et al. Classifying apnea of prematurity by transcutaneous electromyography of the diaphragm. _Neonatology_ 113, 140–145 (2018). Article PubMed Google Scholar * Sinclair, R., Teng, A.,
Jonas, C. & Schindler, T. Congenital central hypoventilation syndrome: a pictorial demonstration of absent electrical diaphragmatic activity using non-invasive neurally adjusted
ventilatory assist. _J. Paediatr. Child Health_ 54, 200–202 (2018). Article PubMed Google Scholar * Essouri, S. et al. Relationship between diaphragmatic electrical activity and
esophageal pressure monitoring in children. _Pediatr. Crit. Care Med._ 20, e319–e325 (2019). Article PubMed Google Scholar * Bellani, G. et al. Estimation of patient’s inspiratory effort
from the electrical activity of the diaphragm. _Crit. Care Med._ 41, 1483–1491 (2013). Article PubMed Google Scholar * van Leuteren, R. W., de Waal, C. G., Hutten, G. J., de Jongh, F. H.
& van Kaam, A. H. Transcutaneous monitoring of diaphragm activity as a measure of work of breathing in preterm infants. _Pediatr. Pulmonol._ 56, 1593–1600 (2021). Article PubMed PubMed
Central Google Scholar * Crulli, B. et al. Evolution of inspiratory muscle function in children during mechanical ventilation. _Crit. Care_ 25, 229 (2021). Article PubMed PubMed Central
Google Scholar * Fine-Goulden, M. R., Puppala, N. K. & Durward, A. Mechanisms of ventilator dependence in children with neuromuscular and respiratory control disorders identified by
monitoring diaphragm electrical activity. _Intensive Care Med._ 38, 2072–2079 (2012). Article PubMed Google Scholar * Martin, S., Feder, J., Ducharme-Crevier, L., Savy, N. & Emeriaud,
G. Diaphragm electrical activity target during nava: one size may not fit all. _Pediatr. Pulmonol._ 57, 1358–1360 (2022). Article PubMed Google Scholar * Epstein, S. K. How often does
patient-ventilator asynchrony occur and what are the consequences? _Respir. Care_ 56, 25–38 (2011). Article PubMed Google Scholar * de Waal, C. G., Kraaijenga, J. V., Hutten, G. J., de
Jongh, F. H. & van Kaam, A. H. Breath detection by transcutaneous electromyography of the diaphragm and the Graseby capsule in preterm infants. _Pediatr. Pulmonol._ 52, 1578–1582 (2017).
Article PubMed Google Scholar * Soreze, Y., Motte, E., Dell’Orto, V., Yousef, N. & De Luca, D. Use of neurally adjusted ventilator assist in postsurgical hemidiaphragmatic paralysis.
_Arch. Dis. Child Fetal Neonatal Ed._ 103, F86–F87 (2018). Article PubMed Google Scholar * Kurland, Y. et al. Neurally adjusted ventilatory assist in neonates with congenital
diaphragmatic hernia. _J. Perinatol._ 41, 1910–1915 (2021). Article CAS PubMed PubMed Central Google Scholar * Gentili, A. et al. Neurally adjusted ventilatory assist in weaning of
neonates affected by congenital diaphragmatic hernia. _J. Matern Fetal Neonatal Med._ 26, 598–602 (2013). Article PubMed Google Scholar * Durrani, N. U., Chedid, F. & Rahmani, A.
Neurally adjusted ventilatory assist mode used in congenital diaphragmatic hernia. _J. Coll. Physicians Surg. Pak._ 21, 637–639 (2011). PubMed Google Scholar * Amin, R. & Arca, M. J.
Feasibility of non-invasive neurally adjusted ventilator assist after congenital diaphragmatic hernia repair. _J. Pediatr. Surg._ 54, 434–438 (2019). Article PubMed Google Scholar * Lee,
J. et al. Non-invasive neurally adjusted ventilatory assist in preterm infants: a randomised phase II crossover trial. _Arch. Dis. Child Fetal Neonatal Ed._ 100, F507–F513 (2015). Article
PubMed Google Scholar * Yagui, A. C. et al. Nasal continuous positive airway pressure (NCPAP) or noninvasive neurally adjusted ventilatory assist (NIV-NAVA) for preterm infants with
respiratory distress after birth: a randomized controlled trial. _Pediatr. Pulmonol._ 54, 1704–1711 (2019). Article PubMed Google Scholar * Yagui, A. C. et al. Is noninvasive neurally
adjusted ventilatory assistance (Niv-Nava) an alternative to ncpap in preventing extubation failure in preterm infants? _J. Matern Fetal Neonatal. Med._ 34, 3756–3760 (2021). Article PubMed
Google Scholar * Kallio, M. et al. Neurally adjusted ventilatory assist (Nava) in preterm newborn infants with respiratory distress syndrome-a randomized controlled trial. _Eur. J.
Pediatr._ 175, 1175–1183 (2016). Article PubMed Google Scholar * Kamlin, C. O., Davis, P. G. & Morley, C. J. Predicting successful extubation of very low birthweight infants. _Arch.
Dis. Child Fetal Neonatal Ed._ 91, F180–F183 (2006). Article CAS PubMed PubMed Central Google Scholar * Currie, A., Patel, D. S., Rafferty, G. F. & Greenough, A. Prediction of
extubation outcome in infants using the tension time index. _Arch. Dis. Child Fetal Neonatal Ed._ 96, F265–F269 (2011). Article PubMed Google Scholar * Latremouille, S., Bhuller, M., Rao,
S., Shalish, W. & Sant’Anna, G. Diaphragmatic activity and neural breathing variability during a 5-min endotracheal continuous positive airway pressure trial in extremely preterm
infants. _Pediatr. Res._ 89, 1810–1817 (2021). Article PubMed Google Scholar * Khan, N., Brown, A. & Venkataraman, S. T. Predictors of extubation success and failure in mechanically
ventilated infants and children. _Crit. Care Med._ 24, 1568–1579 (1996). Article CAS PubMed Google Scholar * van Leuteren, R. W. et al. Diaphragm activity pre and post extubation in
ventilated critically ill infants and children measured with transcutaneous electromyography. _Pediatr. Crit. Care Med._ 22, 950–959 (2021). Article PubMed Google Scholar * Hunt, K. A.,
Hunt, I., Ali, K., Dassios, T. & Greenough, A. Prediction of extubation success using the diaphragmatic electromyograph results in ventilated neonates. _J. Perinat. Med._ 48, 609–614
(2020). Article PubMed Google Scholar * Xue, Y., Yang, C. F., Ao, Y., Qi, J. & Jia, F. Y. A prospective observational study on critically Ill children with diaphragmatic dysfunction:
clinical outcomes and risk factors. _BMC Pediatr._ 20, 422 (2020). Article CAS PubMed Google Scholar * Dreyfuss, D. & Saumon, G. Ventilator-induced lung injury: lessons from
experimental studies. _Am. J. Respir. Crit. Care Med._ 157, 294–323 (1998). Article CAS PubMed Google Scholar * Rothaar, R. C. & Epstein, S. K. Extubation failure: magnitude of the
problem, impact on outcomes, and prevention. _Curr. Opin. Crit. Care_ 9, 59–66 (2003). Article PubMed Google Scholar * Laham, J. L., Breheny, P. J. & Rush, A. Do clinical parameters
predict first planned extubation outcome in the pediatric intensive care unit? _J. Intensive Care Med._ 30, 89–96 (2015). Article PubMed Google Scholar * Singh, N., McNally, M. J. &
Darnall, R. A. Does diaphragmatic electrical activity in preterm infants predict extubation success? _Respir. Care_ 63, 203–207 (2018). Article PubMed Google Scholar * Iyer, N. P. et al.
Neural breathing pattern in newborn infants pre- and postextubation. _Acta Paediatr._ 106, 1928–1933 (2017). Article PubMed Google Scholar * Keszler, M. & Suresh Gautham, K.
_Goldsmith’s Assisted Ventilation of the Neonate_, Vol. 7, 83–84 (Elsevier, 2022). * Williams, E. E. et al. Diaphragmatic electromyography during a spontaneous breathing trial to predict
extubation failure in preterm infants. _Pediatr. Res._ 92, 1064–1069 (2022). * Emeriaud, G., Beck, J., Tucci, M., Lacroix, J. & Sinderby, C. Diaphragm electrical activity during
expiration in mechanically ventilated infants. _Pediatr. Res._ 59, 705–710 (2006). Article PubMed Google Scholar * Naples, R., Fenton, A. C., Brodlie, M., Harigopal, S. & O’Brien, C.
Diaphragm electrical activity during weaning of nasal high-flow therapy in preterm infants. _Arch. Dis. Child Fetal Neonatal Ed_. 108, 237–243 (2023). * Hough, J. L., Shearman, A. D.,
Jardine, L. & Schibler, A. Nasal high flow in preterm infants: a dose-finding study. _Pediatr. Pulmonol._ 55, 616–623 (2020). Article PubMed Google Scholar * Jeffreys, E., Hunt, K.
A., Dassios, T. & Greenough, A. Diaphragm electromyography results at different high flow nasal cannula flow rates. _Eur. J. Pediatr._ 178, 1237–1242 (2019). Article PubMed PubMed
Central Google Scholar * Nasef, N. et al. High-flow nasal cannulae are associated with increased diaphragm activation compared with nasal continuous positive airway pressure in preterm
infants. _Acta Paediatr._ 104, e337–e343 (2015). Article CAS PubMed Google Scholar * de Waal, C. G., Hutten, G. J., Kraaijenga, J. V., de Jongh, F. H. & van Kaam, A. H. Electrical
activity of the diaphragm during nCPAP and high flow nasal cannula. _Arch. Dis. Child Fetal Neonatal Ed._ 102, F434–F438 (2017). Article PubMed Google Scholar * Oda, A., Parikka, V.,
Lehtonen, L., Porres, I. & Soukka, H. Nasal high-flow therapy decreased electrical activity of the diaphragm in preterm infants during the weaning phase. _Acta Paediatr._ 108, 253–257
(2019). Article PubMed Google Scholar * Kraaijenga, J. V., de Waal, C. G., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Diaphragmatic activity during weaning from respiratory
support in preterm infants. _Arch. Dis. Child Fetal Neonatal Ed._ 102, F307–F311 (2017). Article PubMed Google Scholar * Williams, E. E. et al. Electrical activity of the diaphragm
following a loading dose of caffeine citrate in ventilated preterm infants. _Pediatr. Res._ 87, 740–744 (2020). Article CAS PubMed Google Scholar * Kraaijenga, J. V., Hutten, G. J., de
Jongh, F. H. & van Kaam, A. H. The effect of caffeine on diaphragmatic activity and tidal volume in preterm infants. _J. Pediatr._ 167, 70–75 (2015). Article CAS PubMed Google Scholar
* Parikka, V. et al. The effect of caffeine citrate on neural breathing pattern in preterm infants. _Early Hum. Dev._ 91, 565–568 (2015). Article CAS PubMed Google Scholar * de Waal,
C. G., Hutten, G. J., Kraaijenga, J. V., de Jongh, F. H. & van Kaam, A. H. Doxapram treatment and diaphragmatic activity in preterm infants. _Neonatology_ 115, 85–88 (2019). Article
PubMed Google Scholar * Araki, R. et al. Effect of doxapram on the electrical activity of the diaphragm waveform pattern of preterm infants. _Pediatr. Pulmonol._ 57, 1483–1488 (2022).
Article PubMed Google Scholar * de Waal, C. G., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. The effect of minimally invasive surfactant therapy on diaphragmatic activity.
_Neonatology_ 114, 76–81 (2018). Article PubMed Google Scholar * Dekker, J. et al. The effect of initial high vs. low Fio2 on breathing effort in preterm infants at birth: a randomized
controlled trial. _Front Pediatr._ 7, 504 (2019). Article PubMed PubMed Central Google Scholar * van Leuteren, R. W. et al. The effect of initial oxygen exposure on diaphragm activity in
preterm infants at birth. _Front Pediatr._ 9, 640491 (2021). Article PubMed PubMed Central Google Scholar * Hutten, G. J. et al. Relative impact of respiratory muscle activity on tidal
flow and end expiratory volume in healthy neonates. _Pediatr. Pulmonol._ 43, 882–891 (2008). Article PubMed Google Scholar * Eichenwald, E. C., Ungarelli, R. A. & Stark, A. R.
Hypercapnia increases expiratory braking in preterm infants. _J. Appl Physiol. (1985)_ 75, 2665–2670 (1993). Article CAS PubMed Google Scholar * Neumann-Klimasinska, N. et al. Effects of
heliox and non-invasive neurally adjusted ventilatory assist (Niv-Nava) in preterm infants. _Sci. Rep._ 11, 15778 (2021). Article CAS PubMed PubMed Central Google Scholar * Kass, J. E.
Heliox redux. _Chest_ 123, 673–676 (2003). Article PubMed Google Scholar * Amigoni, A. et al. Effects of propofol on diaphragmatic electrical activity in mechanically ventilated
pediatric patients. _Intensive Care Med._ 41, 1860–1861 (2015). Article PubMed Google Scholar * Gerard-Castaing, N. et al. Diaphragmatic paralysis in young children: a literature review.
_Pediatr. Pulmonol._ 54, 1367–1373 (2019). Article PubMed Google Scholar * Bordessoule, A., Emeriaud, G., Delnard, N., Beck, J. & Jouvet, P. Recording diaphragm activity by an
oesophageal probe: a new tool to evaluate the recovery of diaphragmatic paralysis. _Intensive Care Med._ 36, 1978–1979 (2010). Article PubMed Google Scholar * Emeriaud, G. et al.
Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. _Intensive Care Med._ 40, 1718–1726 (2014). Article PubMed Google Scholar * Scholten, A. W. J.,
van Leuteren, R. W., de Jongh, F. H., van Kaam, A. H. & Hutten, G. J. Simultaneous measurement of diaphragm activity, chest impedance, and ECG using three standard cardiorespiratory
monitoring electrodes. _Pediatr. Pulmonol_. 57, 2754–2762 (2022). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neonatology, Amsterdam UMC location
University of Amsterdam, Meibergdreef 9, Amsterdam, the Netherlands Anouk W. J. Scholten, Ruud W. van Leuteren, Cornelia G. de Waal, Juliette V. Kraaijenga, Frans H. de Jongh, Anton H. van
Kaam & Gerard J. Hutten * Amsterdam Reproduction & Development research institute, Amsterdam, the Netherlands Anouk W. J. Scholten, Ruud W. van Leuteren, Cornelia G. de Waal,
Juliette V. Kraaijenga, Anton H. van Kaam & Gerard J. Hutten * Faculty of Science and Technology, University of Twente, Drienerlolaan 5, Enschede, the Netherlands Frans H. de Jongh
Authors * Anouk W. J. Scholten View author publications You can also search for this author inPubMed Google Scholar * Ruud W. van Leuteren View author publications You can also search for
this author inPubMed Google Scholar * Cornelia G. de Waal View author publications You can also search for this author inPubMed Google Scholar * Juliette V. Kraaijenga View author
publications You can also search for this author inPubMed Google Scholar * Frans H. de Jongh View author publications You can also search for this author inPubMed Google Scholar * Anton H.
van Kaam View author publications You can also search for this author inPubMed Google Scholar * Gerard J. Hutten View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS A.S. wrote the first version of the manuscript. A.S., R.v.L., F.d.J., C.d.W., J.K., A.v.K., and J.H. modified the manuscript. All authors have read and agreed to the
published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Gerard J. Hutten. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or
its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Scholten, A.W.J., van Leuteren, R.W., de Waal, C.G. _et al._ Diaphragmatic electromyography in infants: an overview of possible clinical applications. _Pediatr Res_ 95, 52–58 (2024).
https://doi.org/10.1038/s41390-023-02800-1 Download citation * Received: 11 April 2023 * Revised: 19 July 2023 * Accepted: 09 August 2023 * Published: 02 September 2023 * Issue Date: January
2024 * DOI: https://doi.org/10.1038/s41390-023-02800-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Nature chemical biology - volume 13 issue 8, august 2017The cover depicts conidiophores of the fungus _Aspergillus nidulans_ carrying a fungal artificial chromosome (FAC), imag...
David beckham weighs into gary neville vs nottingham forest row with commentGARY NEVILLE HAS BEEN BANNED FROM WORKING AT THE CITY GROUND TO COVER NOTTINGHAM FOREST VS CHELSEA ON SUNDAY, PROMPTING ...
Households warned over common mistake that will damage hanging basket bloomsTHE DAYS ARE WARMING UP, THE NIGHTS ARE BALMIER BUT DON'T GET COMPLACENT, EVEN A COOL NIGHT COULD DAMAGE AN UNCOVER...
Man utd star marcus rashford's dream transfer back on as manager makes claimMANCHESTER UNITED STAR MARCUS RASHFORD IS LOOKING INCREASINGLY UNLIKELY TO JOIN ASTON VILLA ON A PERMANENT BASIS AND THE...
Ruben amorim details how bruno fernandes could stay at man utd amid exit fearsMANCHESTER UNITED'S 1-0 DEFEAT TO TOTTENHAM IN THE EUROPA LEAGUE FINAL HAS ROUNDED OFF A HORROR SEASON AND NOW THER...
Latests News
Diaphragmatic electromyography in infants: an overview of possible clinical applicationsABSTRACT Preterm infants often experience breathing instability and a hampered lung function. Therefore, these infants r...
Cancer cells express aberrant dnmt3b transcripts encoding truncated proteinsABSTRACT Cancer cells display an altered distribution of DNA methylation relative to normal cells. Certain tumor suppres...
Biden’s business will be getting the world back to business as usual | thearticleSlowly but surely, power is ebbing away from Donald Trump. The President appeared briefly this week in a vain attempt to...
Javascript support required...
Known prostate cancer risk loci—guilty by association?Access through your institution Buy or subscribe The results of the first study to assess a large number of susceptibili...