Membrane metalloendopeptidase suppresses prostate carcinogenesis by attenuating effects of gastrin-releasing peptide on stem/progenitor cells
Membrane metalloendopeptidase suppresses prostate carcinogenesis by attenuating effects of gastrin-releasing peptide on stem/progenitor cells"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Aberrant neuroendocrine signaling is frequent yet poorly understood feature of prostate cancers. Membrane metalloendopeptidase (MME) is responsible for the catalytic inactivation of
neuropeptide substrates, and is downregulated in nearly 50% of prostate cancers. However its role in prostate carcinogenesis, including formation of castration-resistant prostate
carcinomas, remains uncertain. Here we report that MME cooperates with PTEN in suppression of carcinogenesis by controlling activities of prostate stem/progenitor cells. Lack of MME and PTEN
results in development of adenocarcinomas characterized by propensity for vascular invasion and formation of proliferative neuroendocrine clusters after castration. Effects of MME on
prostate stem/progenitor cells depend on its catalytic activity and can be recapitulated by addition of the MME substrate, gastrin-releasing peptide (GRP). Knockdown or inhibition of GRP
receptor (GRPR) abrogate effects of MME deficiency and delay growth of human prostate cancer xenografts by reducing the number of cancer-propagating cells. In sum, our study provides a
definitive proof of tumor-suppressive role of MME, links GRP/GRPR signaling to the control of prostate stem/progenitor cells, and shows how dysregulation of such signaling may promote
formation of castration-resistant prostate carcinomas. It also identifies GRPR as a valuable target for therapies aimed at eradication of cancer-propagating cells in prostate cancers with
MME downregulation. SIMILAR CONTENT BEING VIEWED BY OTHERS SECRETED SPERMIDINE SYNTHASE REVEALS A PARACRINE ROLE FOR PGC1Α-INDUCED GROWTH SUPPRESSION IN PROSTATE CANCER Article Open access
23 April 2025 PRL-MEDIATED STAT5B/ARRB2 PATHWAY PROMOTES THE PROGRESSION OF PROSTATE CANCER THROUGH THE ACTIVATION OF MAPK SIGNALING Article Open access 10 February 2024 NEUROPILIN-2
PROMOTES LINEAGE PLASTICITY AND PROGRESSION TO NEUROENDOCRINE PROSTATE CANCER Article 19 August 2022 INTRODUCTION Prostate cancer is the most frequently diagnosed cancer and is the second
leading cause of cancer-related death in men in the United States1. While most prostate cancers are adenocarcinomas, a significant percentage also have dysregulation of neuroendocrine
signaling, such as excessive accumulation of cells with neuroendocrine differentiation and/or overproduction of neuropeptides2,3,4. A large amount of data demonstrate neuropeptides, such as
gastrin-releasing peptide (GRP), are associated with accelerated prostate cancer progression and inferior prognosis5,6,7. GRP can promote cell proliferation and accelerate migration and
invasion of prostate cancer cells5,8,9,10. Targeting of the GRP receptor suppresses growth in cell culture and xenograft models11. However, specific mechanisms by which neuropeptide
dysregulation contributes to the pathogenesis of prostate cancer remain insufficiently elucidated. Local concentration of neuropeptides is regulated in part by membrane metalloendopeptidase
(MME, aka Neutral endopeptidase). MME is a cell-surface peptidase member of the M13 family of zinc peptidases, which also includes endothelin converting enzymes (ECE-1 and ECE-2), KELL and
PEX. MME cleaves peptide bonds on the amino side of hydrophobic amino acids and is the key enzyme in processing of a variety of physiologically active peptides, such as GRP, neurotensin
(NT), and vasoactive intestinal peptide (VIP)10,12. MME is downregulated in nearly 50% of primary and metastatic prostate cancers, independently predicting an inferior prognosis13,14,15. In
addition to its downregulation by androgen withdrawal16,17, MME expression is also downregulated by methylation, suggesting its tumor-suppressive effects15,18. Indeed, MME expression reduces
growth, motility9, and survival19,20 of prostate cancer cells in cell culture. Consistent with these observations, replacements of MME inhibit tumorigenicity of prostate cancer cells in
xenograft experiments21,22. Nevertheless, mice lacking _Mme_ show no prostate cancer-related phenotype23, and the role of MME in prostate cancer progression remains uncertain. At least part
of MME effects are mediated by the PI3K/AKT pathway that plays a key role in multiple cellular processes, including cell survival, proliferation, and cell migration reviewed in ref. 24. MME
associates with and stabilizes the PTEN tumor suppressor protein, resulting in increased PTEN phosphatase activity, thereby inhibiting AKT activating phosphorylation25. MME may also have
PTEN-independent mechanisms of AKT inhibition by processing neuropeptides, such as GRP, which are known to activate AKT20. Consistent with a possibility of potential cooperation between MME
and PTEN in suppression of carcinogenesis, downregulation of MME is observed in 42% and 63% of PTEN-deficient cases of human primary and metastatic prostate cancers, respectively26. However,
it remains unknown if catalytically dependent neuropeptide-based mechanisms of MME tumor suppression play a role in prostate cancer progression. The mouse prostate is composed of a series
of branching ducts, each containing distal and proximal regions relative to the urethra27. Proliferating, transit-amplifying cells are preferentially located in the distal region of the
prostatic ducts, whereas cells with stem cell-like properties, such as low cycling rate, self-renewal ability, high ex vivo proliferative potential, and androgen withdrawal resistance,
mainly reside in the proximal region of the prostatic ducts28,29,30,31,32. Thus, approaches based on the isolation of cells according to their displayed stem cell-specific markers can be
complemented by careful evaluation of stem cell compartments in situ. In the current study, we used autochthonous mouse model of prostate neoplasia associated with deficiency of _Pten_ tumor
suppressor gene. In this model, prostate carcinogenesis is initiated by the prostate epithelium-specific inactivation of _Pten_ driven by PB-_Cre4_ transgene (_Pten_PE−/−
mice33,34,35,36,37,38). The majority of mice show early stages of prostate cancer, such as high-grade prostatic intraepithelial neoplasms (HG-PINs) and few animals show early adenocarcinomas
characterized by stromal invasion. Thus, it is well suitable for testing if additional genetic alterations, such as _Mme_ inactivation, may accelerate cancer progression. We report that
lack of both MME and PTEN leads to aggressive prostate cancers manifesting frequent vascular invasion and increased neuroendocrine differentiation after castration. Formation of such cancers
is preceded by morphologically detectable neoplastic lesions at the prostate stem/progenitor cell compartment. The effect of MME deficiency on stem/progenitor cells can be recapitulated by
its substrate GRP and is abrogated by either GRP receptor (GRPR) antagonist or _GRPR_ siRNA knockdown. Knockdown or inhibition of GRP receptor (GRPR) delay growth of human prostate cancer
xenografts by reducing the pool of cancer-propagating cells. RESULTS MME COOPERATES WITH PTEN IN SUPPRESSION OF PROSTATE CANCER IN AUTOCHTHONOUS MOUSE MODEL To test the cooperation of _Mme_
and _Pten_ genes in suppression of prostate cancer in vivo we first evaluated MME expression in HG-PINs and early invasive adenocarcinomas typical for _Pten_PE−/− mice. While irregular MME
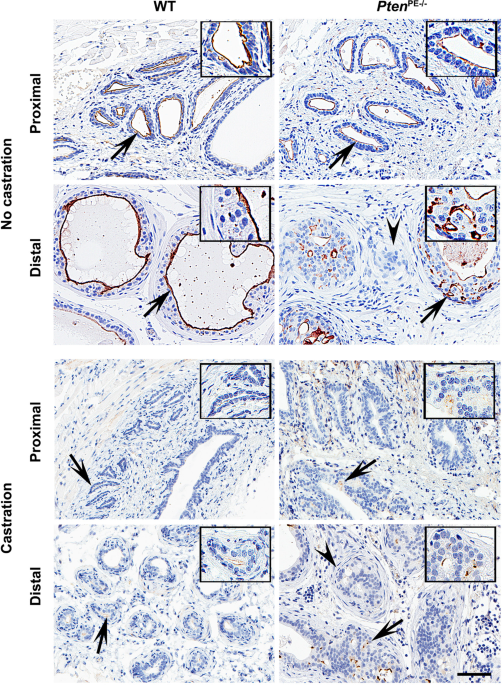
expression was observed in the majority of neoplastic lesions, MME was absent in the areas of stromal invasion (Fig. 1, Supplementary Fig. 1). No significant alterations in MME expression
were detected in the proximal regions of prostatic ducts, consistent with the lack of neoplastic lesions in that part of the prostate in _Pten_PE−/− mice (Fig. 2a, Supplementary Fig. 2a,
Supplementary Table 1). Consistent with the reported regulation of MME by the androgen receptor (AR)16,17,39, castration of both WT and _Pten_PE−/− mice resulted in downregulation but not
complete abrogation of MME expression (Fig. 1). Next, we tested if MME deficiency can accelerate prostate carcinogenesis in _Pten_PE−/− mouse model. We crossed _Mme_−/− and _Pten_PE−/− mice,
and evaluated prostates of age-matched wild-type (WT), _Mme_−/−, _Pten_PE−/−, and _Mme_−/−_Pten_PE−/− strains (Fig. 2, Supplementary Fig. 2, Supplementary Table 1). Consistent with previous
observations23, _Mme_−/− mice did not develop any neoplastic lesions by 16 months of age, while _Pten_PE−/− mice showed low- and high-grade PINs at 3 months. At 16 months 29% of _Pten_PE−/−
mice developed early invasive adenocarcinomas, characterized by separate nests of neoplastic cells in desmoplastic stroma (Fig. 2, Supplementary Table 1). All neoplastic lesions were in the
distal regions of prostatic ducts. Eighty-six percent of _Mme__−/−__Pten_PE−/− mice developed adenocarcinomas in the same location (Fisher’s exact test _P_ = 0.0025). Furthermore, contrary
to _Pten_PE−/− mice, _Mme_−/−_Pten_PE−/− mice developed dysplastic lesions followed by adenocarcinomas in the proximal regions of the prostatic ducts (Fig. 2a, Supplementary Fig. 1,
Supplementary Table 1). Some adenocarcinomas of _Mme__−/−__Pten_PE−/− mice showed distinct vascular invasion, a feature not characteristic for prostatic lesions in _Pten_PE−/− mice (Fig. 2a,
Supplementary Figs. 2 and 3). Consistent with these histological observations, prostatic epithelium of _Mme__−/−__Pten_PE−/− mice was characterized by significantly higher proliferative
rate according to Ki67 staining (Fig. 2, Supplementary Fig. 2), expressed higher amounts of epigenetic reprogramming factor EZH2 (Fig. 2, Supplementary Fig. 4), and showed increased number
of CK5 and p63 positive cells (Supplementary Fig. 2) as compared to prostatic lesions in _Pten_PE−/− mice. Prostatic epithelium lesions of _Mme__−/−__Pten_PE−/− mice also showed higher
levels of pAKT (Fig. 2, Supplementary Fig. 4), confirming additional MME-dependent mechanisms of regulation of this downstream target of PTEN20,25. Consistent with our previous
observation35, prostatic neoplastic lesions of _Pten_PE−/− mice had increased number of synaptophysin-positive neuroendocrine cells in the distal regions of prostatic ducts (Fig. 2,
Supplementary Fig. 2). However, this number was not additionally elevated in _Mme__−/−__Pten_PE−/− mice. In sum, lack of both MME and PTEN not only promoted lesions typically observed in the
prostates of _Pten_PE−/− mice but also resulted in a distinct new neoplasms located in the proximal regions of prostatic ducts. MME LOSS LEADS TO MORE AGGRESSIVE TUMOR PHENOTYPE WITH
INCREASED PROLIFERATION OF NEUROENDOCRINE CLUSTERS AFTER CASTRATION As compared to _Pten_PE−/− mice, recurrent tumors in all castrated _Mme__−/−__Pten_PE−/− mice (_n_ = 8) had increased
levels of phosphorylated MET, and reduced expression of E-cadherin, signatures of more aggressive phenotype (Fig. 3). As previously reported35, recurrent tumors in castrated _Pten_PE−/− mice
show a decreased expression of AR and an increased number of neuroendocrine cells. MME deficiency in castrated _Pten_PE−/− mice did not further affect AR expression levels (Fig. 3a).
However, recurrent tumors in _Mme__−/−__Pten_PE−/− mice had a higher frequency of neuroendocrine clusters (≥ 3 cells) and such clusters were more proliferative according to Ki67 staining
(Fig. 3b, c). Thus, MME deficiency promotes the increase in neuroendocrine differentiation of neoplastic cells after castration. MME LOSS PROMOTES ACTIVITIES OF PTEN-DEFICIENT MOUSE PROSTATE
STEM/PROGENITOR CELLS The proximal regions of prostatic ducts are particularly enriched in prostate epithelium stem/progenitor cells28,29,30,31,32. Thus we evaluated potential effects of
MME and PTEN deficiency on prostate stem/progenitor cells, isolated as the CD49fhi/Sca-1+ fraction by fluorescence-activated cell sorting (FACS). _Mme_−/− mice had the same number of
stem/progenitor cells as age-matched WT mice (5.7% vs 5.6%). In contrast, _Pten_PE−/− mice showed a significant increase of the stem cell pool (8.4%) consistent with previous reports
suggesting PTEN’s involvement in regulation of prostate stem/progenitor cells40,41,42. The pool of CD49fhi/Sca-1+ cells deficient for both PTEN and MME, constituted 12.1% of the prostate
epithelium, representing an additional 44% increase as compared to PTEN-deficient CD49fhi/Sca-1+ cells (Fig. 4a, _P_ < 0.0001). Formation of prostaspheres is used as a functional cell
culture test for presence, growth, and self-renewal potential of prostate stem/progenitor cells. Consistent with FACS results, CD49fhi/Sca-1+ cells isolated from prostates of
_Mme__−/−__Pten_PE−/− mice showed the highest frequency of prostaspheres in multiple consecutive sphere dissociation and regeneration passages (Fig. 4b). Furthermore, prostaspheres deficient
for both genes had larger size, as compared to WT, MME, or PTEN-deficient stem/progenitor cells (Fig. 4c). In all groups CD49flo/Sca-1− luminal cells formed very few spheres after the first
plating and no spheres were observed after the first passage (Fig. 4d). Thus, it is unlikely that the increase in prostaspheres in PTEN and MME deficient cells resulted from a reprograming
of differentiated cells towards a stem cell state. To directly test if the observed results represent direct effects of MME and/or PTEN on prostate stem/progenitor cells, we isolated
CD49fhi/Sca-1+ stem/progenitor cells and CD49flo/Sca-1− luminal cells from prostates of WT, _Mme_−/−, _Pten__loxP/loxP_, and _Mme_−/−_Pten__loxP/loxP_ mice and infected them with adenovirus
expressing Cre recombinase (Ad-_Cre_). Consistent with our previous experiments, lack of both _Pten_ and _Mme_ had the most pronounced effect on frequency of CD49fhi/Sca-1+ stem/progenitor
cells in consecutive passages (Fig. 4e). Luminal cells formed only few spheres with the same frequency in all groups (Fig. 4f). Taken together, these results showed that MME cooperates with
PTEN in regulation of prostate stem/progenitor cell functions. GRP PROMOTES ACTIVITIES OF PTEN-DEFICIENT MOUSE PROSTATE STEM/PROGENITOR CELLS To identify mechanisms by which MME may affect
regulation of prostate stem/progenitor cells, we next examined the expression of MMEs main substrates, GRP, NT, and VIP in the prostates of WT, _Mme__−/−_, and _Pten_PE−/− and
_Mme__−/−__Pten_PE−/− strains. Strong GRP expression was detected only in prostates of _Mme__−/−__Pten_PE−/− mice, while NT and VIP were not detected in all cases (Supplementary Fig. 5). To
test if GRP could recapitulate the effects of MME deficiency, we isolated prostate cells from _Mme__−/−__Pten_PE−/− mice, and either performed knockdown of GRP receptor (GRPR, Fig. 5a) or
administered GRPR antagonist [Tyr4, d-Phe12]-Bombesin. Both approaches reversed effects of MME deficiency on size and formation frequency of prostaspheres (Fig. 5b, c). Consistent with the
observation of frequent vascular invasion by prostate adenocarcinomas in _Mme__−/−__Pten_PE−/− mice, we detected increased cell motility and invasion of prostate cells isolated from
_Mme__−/−__Pten_PE−/− mice, as compared to those prepared from _Pten_PE−/− mice (Fig. 5d, e). This effect was reversed by either GRPR knockdown or treatment with [Tyr4, d-Phe12]-Bombesin. We
next isolated prostate cells form _Pten_PE−/− mice, and treated them with the MME enzyme inhibitor Thiorphan. Sphere size (Fig. 5f), sphere-forming capacity (Fig. 5g), cell motility (Fig.
5h), and invasion (Fig. 5l) were each increased by MME catalytic inhibition; and more importantly _Grpr_ siRNA knockdown or a GRPR antagonist abrogated the stimulated functions of MME enzyme
inhibition on the prostate cells form _Pten_PE−/− mice. Finally, to test if GRP directly affects the function of PTEN-deficient prostate stem/progenitor cells, CD49fhi/Sca-1+ prostate stem
cells isolated from _Pten__loxP/loxP_ were infected with Ad-_Cre_ followed by treatment with GRP and/or [Tyr4, d-Phe12]-Bombesin. GRP addition reproduced the effects of MME deficiency on
formation frequency and size of prostasphere, while these effects were abrogated by [Tyr4, d-Phe12]-Bombesin (Fig. 5j, k). Taken together, these data suggest that the MME substrate GRP
mediates in part the growth and invasive effect of PTEN-deficient prostate cells observed in association with loss or catalytic inhibition of MME, and GRP is a key MME target responsible for
stimulating prostate stem/progenitor cells. GRP PROMOTES EXPANSION OF HUMAN PROSTATE CANCER-PROPAGATING CELLS To further assess the relevance of our observations to human disease we tested
the effects of GRP and [Tyr4, d-Phe12]-Bombesin on PTEN-deficient androgen-refractory human prostate cancer cells DU145 and PC3. Consistent with previous reports5,7,9, GRP promoted cell
proliferation, migration, and invasion, and these effects were negated by addition of [Tyr4, d-Phe12]-Bombesin in both cell lines (Supplementary Fig. 6). Similar effects cell migration and
invasion were also observed in androgen-sensitive human prostate adenocarcinoma cells LNCaP (Supplementary Fig. 7). In agreement with our observations on mouse prostate epithelium cells,
GRPR knockdown rescued the stimulating effects of GRP and inhibitory effects of [Tyr4, d-Phe12]-Bombesin, suggesting the crucial role of GRP/GRPR in control of the above parameters in both
species (Supplementary Figs. 6 and 7). GRP treatment also increased expression of synaptophysin in DU145, PC3 cells, and LNCaP cells, thereby suggesting that GRP/GRPR signaling may influence
neuroendocrine commitment (Supplementary Fig. 8). Next we tested if GRP/GRPR signaling has an immediate effect on prostate cancer cells with stem cell-like properties. Such cells, called
cancer propagating or cancer stem cells29,43, are characterized by their ability for long-term self-renewal, high potential for proliferation, high tumorigenicity, capacity to generate the
whole spectrum of heterogeneity of their original tumors, and resistance to androgen withdrawal. Prostate cancer-propagating cells can be isolated by either high enzymatic activity of ALDH
(ALDEFLUOR assay)44 or by detection of CD44 expression45. In both approaches cancer-propagating cells isolated from DU145, PC3, and LNCaP cells were increased in number after GRP treatment
(Fig. 6a–d, Supplementary Fig. 7e, f). Cancer-propagating cells also formed spheres in greater number and size. These changes were diminished by [Tyr4, d-Phe12]-Bombesin and/or GRPR siRNA
(Fig. 6a–g). To study the effect of GRP on human prostate cancer xenografts in vivo, immunodeficient NSG mice were subcutaneously injected with PC3 cells and treated with [Tyr4,
d-Phe12]-Bombesin when the tumor size reached 10 mm3 (Fig. 6h–j). Daily intraperitoneal injections of [Tyr4, d-Phe12]-Bombesin reduced both tumor volume and weight by 70% by the time of
animal euthanasia at 26 days after the beginning of treatment. Importantly, according to the ALDEFLUOR assay, a fraction of prostate cancer-propagating cells was significantly reduced in
tumors treated with [Tyr4, d-Phe12]-Bombesin as compared to controls treated with vehicle alone. The same outcomes have been observed after transplantation of PC3 cells infected with
lentivirus-expressing GRPR shRNA (Supplementary Fig. 9). Thus, the effects of GRP/GRPR signaling abrogation in inhibiting prostate cancer cell tumorigenicity result from diminishing the
population of cancer-propagating cells. DISCUSSION Our study provides direct genetic support to previous reports proposing _Mme_ role as a tumor suppressor gene. Our findings show that _Mme_
cooperates with _Pten_ in suppression of prostate carcinogenesis. Lack of _Mme_ leads to formation of advanced adenocarcinomas characterized by intravascular invasion, a feature not
observed adenocarcinomas of _Pten_PE−/− mice. In agreement with their more aggressive behavior adenocarcinomas of composite mice also have higher proliferative rate, increased number of CK5
and p63 positive cells, and elevated levels of EZH2 expression. It has been reported previously that _Pten_ conditional deletion in the prostate epithelium leads to basal cell proliferation
with concomitant expansion of the prostate stem/progenitor-cell-like Sca-1+ and BCL-2+ subpopulation42. CD49fhi/Sca-1+ _Pten_-deficient neoplastic cells exhibits cancer-propagating cell
properties, such as high sphere-forming capacity, sustained self-renewal, increased proliferation, and tumorigenic potential, as compared with other isogenic subpopulations40,41. According
to our studies, the combined _Mme_ and _Pten_ deficiency leads to further increase of number and growth potential of prostate stem/progenitor cells. These observations suggest that _Mme_ and
_Pten_ cooperate in controlling stem/progenitor cells. However, since lack of _Pten_ may also stimulate a basal to luminal transdifferentiation in conjunction with proliferation46,47,48,
further studies are needed to evaluate the role of cell plasticity as an additional factor influencing effects of combined _Mme_ and _Pten_ deficiency. Our findings also show that the lack
of both _Mme_ and _Pten_ leads to the formation of dysplastic lesions and adenocarcinomas in the proximal regions of prostatic ducts, the structures particularly enriched in stem/progenitor
cells. Thus, _Mme_ may playing a particularly critical role at the site of prostate stem/progenitor cell niche. Our findings also show that effects of MME on prostate stem/progenitor cells
depend on the presence of its downstream effector, GRP, Furthermore, we have observed that abrogation of GRP/GRPR signaling may diminish the pool of cancer-propagating cells, thereby
highlighting an important role of neuroendocrine signaling in the regulation of cell stemness. Previously, prostate cancer-propagating cells have been shown to be regulated by the
PTEN/PI3K/AKT pathway40. Our study suggests that this effect is induced by neuropeptide signaling regulated by MME and is potentiated by MME loss. This provides a rationale for using the
MME/GRP pathway for targeting of cancer-propagating cells, perhaps as a part of combinatorial therapy approaches. Our study shows that accelerated progression of prostate carcinomas
associated with MME deficiency is not associated with increase in the number of neuroendocrine cells. Neuroendocrine cells are important for prostate development49. The majority of human
prostate adenocarcinomas and mouse models associated with _Pten_ deficiency contain neuroendocrine cells35,50. However, with the exception of highly aggressive neuroendocrine/small cell
carcinomas, it remains debatable if increased number of neuroendocrine cells, as defined by their expression of neuroendocrine markers, such as synaptophysin and calcitonin gene-related
peptide, are essential for the accelerated progression of pre-castrate prostate adenocarcinoma6,51,52. Our study suggests that accumulation of un-cleaved neuropeptides may mitigate the
requirement for neuroendocrine cell expansion or lead to a feedback inhibition of neuroendocrine cell differentiation in MME-deficient prostate cancers. The recent introduction of therapies
that better target the androgen axis has led to a significant increase in frequency of castrate-resistant prostate cancer with neuroendocrine differentiation2. It has been shown that
inactivation of the _RB1_ tumor suppressor gene increases cell plasticity along with the promotion of an aggressive neuroendocrine phenotype3,4,53. In castrated mice with combined _Pten_ and
_Trp53_ deficiency some prostate tumors arising from luminal cells had regions of highly proliferative cells with overt neuroendocrine differentiation. However, other tumors showed only
limited foci of neuroendocrine differentiation without any detectable proliferation50. In this report _Pten_ inactivation alone resulted in modest increase in neuroendocrine foci, consistent
with our findings. In our model associated with _Pten_ and _Mme_ deficiency we observed increased number of neuroendocrine neoplastic clusters and increased proliferation in such clusters
after castration. Furthermore, as compared to _Pten_PE−/− mice, recurrent tumors in castrated _Mme__−/−__Pten_PE−/− mice had more aggressive phenotype based on increased levels of
phosphorylated MET and reduced expression of E-cadherin. The mechanisms by which MME deficiency facilitates neuroendocrine transdifferentiation of the prostate epithelium or leads to
expansion neuroendocrine cell lineage after castration remain to be investigated. It also remains to be investigated if MME deficiency represents an alternative to _RB1_ loss for progression
of some castrate-resistant prostate cancer. Our mouse model based on inactivation of _Mme_ and _Pten_ should offer an important tool for answering these important questions of prostate
cancer pathogenesis and testing new therapeutic approaches. MATERIALS AND METHODS MICE _ARR__2__PB-Cre_ transgenic male mice on FVB/N background (_PB-Cre4_)37 were crossed with
_Pten__loxP/loxP_54 female mice on the 129/BALB/c background. Resulting _PB-Cre4Pten__loxP/loxP_ male mice were designated as _Pten_PE−/− mice. _Pten_PE−/− male mice were crossed with _Mme_
null female mice on C57BL6 background (_Mme__−/−_)23. Offspring with _PB-Cre4 Mme__−/−_ _Pten__loxP/loxP_ genotype were designated as _Mme__−/−__Pten__PE−/−_ mice. To minimize the
confounding effects of genetic background _Pten_PE−/− and _Mme__−/−__Pten_PE−/− mice were backcrossed to FVB/N for at least 10 crosses and all control experiments were performed on age and
sex-matched randomized mice of the same background. All animal experiments were carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The protocol was approved by the Institutional Laboratory Animal Use and Care Committee at Cornell University. All efforts were made to minimize
animal suffering. IMMUNOHISTOCHEMISTRY AND QUANTITATIVE IMAGE ANALYSIS Immunoperoxidase staining of paraffin sections of paraformaldehyde-fixed tissue was performed by a modified Elite
avidin-biotin-peroxidase (ABC) technique28. Antigen retrieval was done by boiling the slides in 10 mM citric buffer (pH 6.0) for 10 min. The primary antibodies to MME (Santa Cruz; Dallas,
TX; #sc-80021, 1:100), Ki67 (Leica Microsystems; Bannockburn, IL; #NCLKi67p, 1:1000), EZH2 (Cell Signaling Technology; Danvers, MA; #5246, 1:200), keratin 5 (CK5, Covance; Dallas, TX,
#PRB-160P, 1:2500), keratin 8 (CK8, Developmental Studies Hybridoma Bank; Iowa City, IA; #TROMA-I, 1:10), p63 (Santa Cruz, #sc-8431, 1:1000), PTEN (Cell Signaling Technology, #9559 S,
1:800), pAKT (Cell Signaling Technology, # 3787 S, 1:50), SYP (BD Biosciences; #611880, 1:500), GRP (Santa Cruz, #sc-7788, 1:100), NT (Santa Cruz, #sc-20806, 1:1000), and VIP (Abcam;
Cambridge, MA; #ab8556, 1:200), E-cadherin (Cell Signaling Technology #3195 S, 1:200), phospho-MET (Cell Signaling Technology, #3077, 1:50) were incubated with deparaffinized sections at 4
°C overnight, followed by incubation with secondary biotinylated antibody (1 h, room temperature) and modified theh avidin-biotin-peroxidase (ABC) technique. Methyl green was used as the
counterstain in immunoperoxidase stainings. Slides were scanned by a ScanScope CS (Leica Biosystems, Vista, CA) with ×40 objective followed by lossless compression. For double fluorescence
antibodies against SYP (BD Biosciences, #611880) and Ki67 (Abcam, #16667) were used at a concentration of 1:40 and 1:50, respectively, for overnight incubation at 4 °C, followed by the
incubation with secondary antibodies (Alexa Fluor 568 donkey-anti-mouse #A10037, Alexa Fluor 488 donkey-anti-rabbit #21206, Life Technologies, 1:200 each, 2 h, room temperature) and
counterstaining with 4′6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich #D9542). Immunofluorescent sections were observed and imaged using a Leica TCS SP2 confocal laser scanning microscope
system (Leica Microsystems). Quantitative analysis of immunohistochemistry (IHC) was performed with the ImageJ software (W. Rasband, National Institutes of Health, Bethesda, MD).
TUMORIGENICITY EXPERIMENTS Human prostate cancer PC3 cells (5 × 106 cells) were suspended in the mixture of 200 µl phosphate-bufferd saline (PBS) and 200 µl Matrigel (BD Biosciences,
#356237) and injected subcutaneously into 5-week-old NSG (NOD.Cg-_Prkdc_scid_Il2rg_tm1Wjl/SzJ4; The Jackson Laboratory, stock number #005557) male mice. Intraperitoneal injection of GRPR
antagonist (0.8 μg/g body weight/day in PBS) was started when the tumor size reached 10 mm3. After 26 days of treatment with GRPR antagonist, mice were euthanized and subjected to necropsy.
Tumor xenografts were collected pathological evaluation and ALDEFLUOR assay followed by FACS analysis. CASTRATION EXPERIMENTS Male mice were weighed and anesthetized using isoflurane.
Pressure was applied to the abdomen of each mouse to push both testes down into the scrotal sac. An approximately 1 cm incision was made across the midline of the scrotal sac to expose the
testicular membrane. The midline between the left and right testes sacs were located and a small incision is made on the left side of the membrane midline. The testes, attached fatty tissue,
the vas deferens, and associated blood vessels are carefully pulled out through the incision. The blood vessels were cauterized and the testes was removed by severing below the
cauterization. Same procedure was repeated to remove testes from the right side of the midline. Remaining tissue was pushed back into incision which was then closed with wound clips. Post
procedure, the mouse was placed on a heating pad till it recovered from the effects of the anesthetic followed by placement in a clean cage with fresh chow and water. PATHOLOGIC ASSESSMENT
Moribund mice, as well as those sacrificed according to schedule, were anesthetized with avertin and, if necessary, subjected to cardiac perfusion at 90 mm Hg with PBS. After macroscopic
evaluation during necropsy, lung, liver, prostate, and lymph nodes were fixed in phosphate-buffered 4% paraformaldehyde tissues, embedded in paraffin, and 4-μm-thick sections were stained
with hematoxylin (Mayer’s haemalum) and eosin. Striated muscle layer was used for identification of proximal (periurethral) and distal regions of prostatic ducts in transverse sections, as
previously described32. Mouse prostatic intraepithelial neoplasia (PIN) and adenocarcinoma were defined according to earlier publications28,34,38,55. Briefly, distal PIN1 has 1 or 2 layers
of atypical cells; PIN2 has 3 or more layers of atypical cells, PIN3 occupies the near entire glandular lumen; and PIN 4 fills and distorts the glandular profile, and is frequently marked by
pronounced desmoplastic reaction. In agreement with the recent consensus report34, PIN1 and PIN2 represent low-grade PIN (LG-PIN) and PIN3 and 4 represent high-grade PIN (HG-PIN). Due to
different architecture of the proximal regions of prostatic ducts, current PIN classification cannot be carefully applied to atypical proliferative lesions found in those structures. Thus we
named those lesions as proximal duct dysplasia to stress their dissimilarity to PIN of distal regions of prostatic ducts. Given the complexity and controversial nature of the interpretation
of stromal microinvasion we used term early adenocarcinoma only for neoplasms with invasive stromal growth confirmed by serial sections followed by 3D reconstruction. We used term advanced
adenocarcinoma for neoplasms invading blood and lymphatic vessels. All pathological evaluations were performed in blinded fashion. CELL CULTURE DU145, PC3, and LNCaP cell lines were obtained
from the American Type Culture Collection (ATCC) and cultured in minimum essential medium (Cellgrow, #10-010-CV), F-12K (Cellgrow, #10-025-CV) and RPMI 1640 (VWR, cat # 4500-396),
respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS, GIBCO, #16141-079) and penicillin–streptomycin (Cellgrow, #30-002-Cl). The cultures were maintained at 37 °C in
a 5% CO2 incubator. All cell lines were confirmed to be free of mycoplasma. Primary mouse prostate cells were isolated following described procedures28,56. For GRP experiments, 5 nM GRP
(Sigma, #G8022) and 4 μM GRPR antagonist ([Tyr4, d-Phe12]-Bombesin, Sigma, #B0650) were used for cell culture experiments. Media was changed every 3 days. Lipofectamin 2000 reagent
(Invitrogen; Carlsbad, CA, #11668-030) was used for the transfection following the manufacturer’s recommendations. Control siRNA (Santa Cruz, #sc-37007) and two independent human _GRPR_
siRNAs (Santa Cruz, # sc-106924 and Life Technologies, #145216), human GRPR shRNA in Lentiviral Vector (Genomics Online; Limerick, PA, # ABIN3479889) and mouse _Grpr_ siRNAs (Life
Technologies, #157912 and #157914) were used for all knockdown experiments. MME inhibitor dl-thiorphan, (1 μM, Sigma, #T6031)57 was used to treat cells at 37 °C for 20 min, followed by
subsequent experiments. 5-Bromo-2′deoxyuridine (BrdU) staining, cell motility, and invasion assays were performed as previously described28,58. ALDEFLUOR ASSAY AND FACS For detection of
aldehyde dehydrogenase (ALDH) enzymatic activity, 106 cells were placed in ALDEFLUOR buffer and processed for staining with the ALDEFLUOR Kit (Stem Cell, #01700) according to the
manufacturer’s protocol. Unstained and ALDH inhibitor (diethylaminobenzaldehyde, DEAB)-treated cells served as controls. For detection of CD44-expressing cancer cells, prostate cancer cells
were stained for CD44 (BD Bioscience, #553134) to sort out CD44-positive and CD44-negative cells. Cell sorting and data analysis were performed on a FACS Aria II sorter equipped with the
FACS DiVa software (BD Bioscience). PROSTASPHERE ASSAY The preparation of prostate epithelial cell suspensions, stem/basal cells, and luminal cells from male mice were performed based on
previously described prostrate sphere assays28,56. Briefly, 104 mouse prostate stem/basal cells, mouse prostate luminal cells, human prostate cancer cells, and human prostate
cancer-propagating cells were resuspended in 120 µl of a 1:1 mixture of Matrigel (BD Biosciences, #354234) and PrEGM (Lonza, #CC-3166), and plated around the rim of a well of a 12-well
tissue culture plate. Matrigel mix was alloµwed to solidify at 37 °C for 15 min, and 1 ml of PrEGM was added per well. Media was changed every 3 days. To recover the spheres, each well was
treated with enzyme mixture: 750 µl collegenase/dispase 4 mg/ml (Roche, #10269638001), 30 mg BSA (Sigma, #A3311), and 1 µl DNase1 10 mg/ml (Sigma, D4513), followed by Trypsin 0.25% EDTA
(Cellgrow, 25-052-Cl) to make cell suspensions, which were ready for passage. The sphere-forming efficiency was calculated as previously described28. A constant number of cells was used for
each passaging. QUANTITATIVE REAL-TIME PCR RNA was extracted using TRIzol reagent according to the manufacturer’s instructions (Thermo Fisher). cDNA was produced using the SuperScript III
First-Strand Synthesis kit (Thermo Fisher). Real-time PCR was performed using PerfeCTa SYBR Green Super Mix Reagent (Quanta bio) on C1000 Touch Thermal Cycler PCR machine (Bio-Rad). _SYP_
expression was assessed using forward primer 5′-TGCGCTAGAGCATTCTGGG-3′ and reverse primer 5′-CTTAAAGCCCTGGCCCCTTCT-3′. WESTERN BLOT For western blot cell lysates were prepared using RIPA
buffer (50 mM Tris-HCl, (pH 7.4), 1% Nonidet P-40, 0.25% Na-deoxicholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, Aprotinin, leupeptin, pepstatin: 1 μg/ml each, 1 mM Na3VO4, 1 mM NaF), followed
by sonication for 10 s five times on ice. Lysates were then separated by 12% SDS-PAGE and transferred to PVDF membrane (Millipore #IPVH00010). The membrane was incubated overnight at 4 °C
with antibodies to detect GRPR (Santa Cruz, #sc-32903, 1:1000) and GAPDH (Advanced Immunohistochemical Inc.; Long Beach, CA; #2-RGM2,1:5000), followed by incubation for 1 h at room
temperature with corresponding horseradish peroxidase-conjugated anti-rabbit secondary antibodies (Santa Cruz, #sc-2004, 1:2000) or anti-mouse secondary antibodies (Santa Cruz, #sc-2005,
1:2000) and developed using chemiluminescent substrate (Thermo Scientific, Rockford, IL, #34077). STATISTICS Statistical analyses were performed with InStat 3.10 and Prism 7 software.
(GraphPad, Inc., San Diego, CA). Two-tailed unpaired _t_-test, direct Fisher’s tests, and log-rank Mantel–Haenszel test were used as appropriate. To ensure adequate power to detect a
pre-specified effect size all sample sizes were chosen based on initial pilot experiments, including animal studies. No samples or animals were excluded from the analysis. The variance was
similar between the groups that were statistically compared. REFERENCES * Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. _CA Cancer J. Clin._ 70, 7–30 (2020). Article
PubMed Google Scholar * Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. _Nat. Rev. Urol._ 15, 271–286 (2018).
Article CAS PubMed Google Scholar * Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. _Science_ 355, 78–83
(2017). Article CAS PubMed PubMed Central Google Scholar * Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer.
_Science_ 355, 84–88 (2017). Article CAS PubMed PubMed Central Google Scholar * Qiao, J. et al. Activation of GRP/GRP-R signaling contributes to castration-resistant prostate cancer
progression. _Oncotarget_ 7, 61955–61969 (2016). PubMed PubMed Central Google Scholar * Sun, Y., Niu, J. & Huang, J. Neuroendocrine differentiation in prostate cancer. _Am. J. Transl.
Res._ 1, 148–162 (2009). CAS PubMed PubMed Central Google Scholar * Uchida, K. et al. Murine androgen-independent neuroendocrine carcinoma promotes metastasis of human prostate cancer
cell line LNCaP. _Prostate_ 66, 536–545 (2006). Article CAS PubMed Google Scholar * Ischia, J., Patel, O., Bolton, D., Shulkes, A. & Baldwin, G. S. Expression and function of
gastrin-releasing peptide (GRP) in normal and cancerous urological tissues. _BJU Int._ 113(Suppl 2), 40–47 (2014). Article CAS PubMed Google Scholar * Sumitomo, M. et al. Neutral
endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. _J. Clin. Invest_. 106, 1399–1407 (2000). Article CAS PubMed PubMed Central Google
Scholar * Zheng, R. et al. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. _Oncogene_ 25,
5942–5952 (2006). Article CAS PubMed Google Scholar * Mansi, R., Fleischmann, A., Macke, H. R. & Reubi, J. C. Targeting GRPR in urological cancers–from basic research to clinical
application. _Nat. Rev. Urol._ 10, 235–244 (2013). Article CAS PubMed Google Scholar * Erdos, E. G. & Skidgel, R. A. Neutral endopeptidase 24.11 (enkephalinase) and related
regulators of peptide hormones. _FASEB J._ 3, 145–151 (1989). Article CAS PubMed Google Scholar * Freedland, S. J. et al. Loss of CD10 (neutral endopeptidase) is a frequent and early
event in human prostate cancer. _Prostate_ 55, 71–80 (2003). Article PubMed Google Scholar * Kalin, M. et al. Novel prognostic markers in the serum of patients with castration-resistant
prostate cancer derived from quantitative analysis of the pten conditional knockout mouse proteome. _Eur. Urol._ 60, 1235–1243 (2011). Article PubMed CAS Google Scholar * Osman, I. et
al. Neutral endopeptidase protein expression and prognosis in localized prostate cancer. _Clin. Cancer Res._ 10, 4096–4100 (2004). Article CAS PubMed Google Scholar * Shen, R. et al.
Androgen-induced growth inhibition of androgen receptor expressing androgen-independent prostate cancer cells is mediated by increased levels of neutral endopeptidase. _Endocrinology_ 141,
1699–1704 (2000). Article CAS PubMed Google Scholar * Zheng, R., Shen, R., Goodman, O. B. Jr. & Nanus, D. M. Multiple androgen response elements cooperate in androgen regulated
activity of the type 1 neutral endopeptidase promoter. _Mol. Cell Endocrinol._ 259, 10–21 (2006). Article CAS PubMed Google Scholar * Usmani, B. A. et al. Methylation of the neutral
endopeptidase gene promoter in human prostate cancers. _Clin. Cancer Res._ 6, 1664–1670 (2000). CAS PubMed Google Scholar * Sumitomo, M. et al. Neutral endopeptidase promotes phorbol
ester-induced apoptosis in prostate cancer cells by inhibiting neuropeptide-induced protein kinase C delta degradation. _Cancer Res._ 60, 6590–6596 (2000). CAS PubMed Google Scholar *
Sumitomo, M. et al. Neutral endopeptidase inhibits neuropeptide-mediated transactivation of the insulin-like growth factor receptor-Akt cell survival pathway. _Cancer Res._ 61, 3294–3298
(2001). CAS PubMed Google Scholar * Dai, J. et al. Tumor-suppressive effects of neutral endopeptidase in androgen-independent prostate cancer cells. _Clin. Cancer Res._ 7, 1370–1377
(2001). CAS PubMed Google Scholar * Iida, K., Zheng, R., Shen, R. & Nanus, D. M. Adenoviral neutral endopeptidase gene delivery in combination with paclitaxel for the treatment of
prostate cancer. _Int. J. Oncol._ 41, 1192–1198 (2012). CAS PubMed PubMed Central Google Scholar * Lu, B. et al. Neutral endopeptidase modulation of septic shock. _J. Exp. Med._ 181,
2271–2275 (1995). Article CAS PubMed Google Scholar * Sumitomo, M., Shen, R. & Nanus, D. M. Involvement of neutral endopeptidase in neoplastic progression. _Biochim. Biophys. Acta_
1751, 52–59 (2005). Article CAS PubMed Google Scholar * Sumitomo, M. et al. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. _Cancer Cell_ 5, 67–78
(2004). Article CAS PubMed Google Scholar * Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. _Cancer Cell_ 18, 11–22 (2010). Article CAS PubMed PubMed
Central Google Scholar * Sugimura, Y., Cunha, G. R. & Donjacour, A. A. Morphogenesis of ductal networks in the mouse prostate. _Biol. Reprod._ 34, 961–971 (1986). Article CAS PubMed
Google Scholar * Cheng, C. Y. et al. miR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment. _Cell Rep._ 6, 1000–1007 (2014). Article
CAS PubMed PubMed Central Google Scholar * Fu, D. J. et al. Stem cell pathology. _Annu Rev. Pathol._ 13, 71–92 (2018). Article CAS PubMed Google Scholar * Salm, S. N. et al.
TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. _J. Cell Biol._ 170, 81–90 (2005). Article CAS PubMed PubMed Central Google Scholar * Tsujimura,
A. et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. _J. Cell Biol._ 157, 1257–1265 (2002). Article CAS PubMed PubMed Central Google
Scholar * Zhou, Z., Flesken-Nikitin, A. & Nikitin, A. Y. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic
ducts. _Cancer Res._ 67, 5683–5690 (2007). Article CAS PubMed Google Scholar * Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient
tumorigenesis. _Nature_ 436, 725–730 (2005). Article CAS PubMed PubMed Central Google Scholar * Ittmann, M. et al. Animal models of human prostate cancer: the consensus report of the
New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. _Cancer Res._ 73, 2718–2736 (2013). Article CAS PubMed PubMed Central Google Scholar *
Liao, C. P. et al. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. _Cancer Res._ 67, 7525–7533 (2007).
Article CAS PubMed Google Scholar * Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. _Cancer Cell_ 4, 209–221
(2003). Article CAS PubMed Google Scholar * Wu, X. et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. _Mech. Dev._
101, 61–69 (2001). Article CAS PubMed Google Scholar * Zhou, Z. et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. _Cancer Res._ 66,
7889–7898 (2006). Article CAS PubMed Google Scholar * Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. _Cancer Cell_ 19,
792–804 (2011). Article CAS PubMed PubMed Central Google Scholar * Dubrovska, A. et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like
cell populations. _Proc. Natl. Acad. Sci. USA_ 106, 268–273 (2009). Article CAS PubMed Google Scholar * Mulholland, D. J. et al. Lin-Sca-1+CD49fhigh stem/progenitors are
tumor-initiating cells in the Pten-null prostate cancer model. _Cancer Res._ 69, 8555–8562 (2009). Article CAS PubMed PubMed Central Google Scholar * Wang, S. et al. Pten deletion leads
to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. _Proc. Natl. Acad. Sci. USA_ 103, 1480–1485 (2006). Article CAS PubMed PubMed Central Google
Scholar * Kreso, A. & Dick, J. E. Evolution of the cancer stem cell model. _Cell Stem Cell_ 14, 275–291 (2014). Article CAS PubMed Google Scholar * Li, T. et al. ALDH1A1 is a marker
for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. _Lab Invest._ 90, 234–244 (2010). Article CAS PubMed Google Scholar * Patrawala, L. et al. Highly
purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. _Oncogene_ 25, 1696–1708 (2006). Article CAS PubMed Google
Scholar * Choi, N., Zhang, B., Zhang, L., Ittmann, M. & Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate
cancer initiation. _Cancer Cell_ 21, 253–265 (2012). Article CAS PubMed PubMed Central Google Scholar * Lawson, D. A. et al. Basal epithelial stem cells are efficient targets for
prostate cancer initiation. _Proc. Natl. Acad. Sci. USA_ 107, 2610–2615 (2010). Article CAS PubMed PubMed Central Google Scholar * Wang, Z. A. et al. Lineage analysis of basal
epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. _Nat. Cell Biol._ 15, 274–283 (2013). Article CAS PubMed PubMed
Central Google Scholar * Cheng, C. Y., Zhou, Z. & Nikitin, A. Y. Detection and organ-specific ablation of neuroendocrine cells by synaptophysin locus-based BAC cassette in transgenic
mice. _PLoS ONE_ 8, e60905 (2013). Article CAS PubMed PubMed Central Google Scholar * Zou, M. et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of
castration-resistant prostate cancer. _Cancer Discov._ 7, 736–749 (2017). Article CAS PubMed PubMed Central Google Scholar * Aggarwal, R., Zhang, T., Small, E. J. & Armstrong, A. J.
Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. _J. Natl. Compr. Canc. Netw._ 12, 719–726 (2014). Article PubMed Google Scholar * Bonkhoff, H. & Berges, R.
From pathogenesis to prevention of castration resistant prostate cancer. _Prostate_ 70, 100–112 (2010). Article CAS PubMed Google Scholar * Park, J. W. et al. Reprogramming normal human
epithelial tissues to a common, lethal neuroendocrine cancer lineage. _Science_ 362, 91–95 (2018). Article CAS PubMed PubMed Central Google Scholar * Lesche, R. et al. Cre/loxP-mediated
inactivation of the murine Pten tumor suppressor gene. _Genesis_ 32, 148–149 (2002). Article CAS PubMed Google Scholar * Park, J. H. et al. Prostatic intraepithelial neoplasia in
genetically engineered mice. _Am. J. Pathol._ 161, 727–735 (2002). Article PubMed PubMed Central Google Scholar * Lukacs, R. U., Goldstein, A. S., Lawson, D. A., Cheng, D. & Witte,
O. N. Isolation, cultivation and characterization of adult murine prostate stem cells. _Nat. Protoc._ 5, 702–713 (2010). Article CAS PubMed PubMed Central Google Scholar * Roques, B. P.
et al. The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. _Nature_ 288, 286–288 (1980). Article CAS PubMed Google Scholar * Flesken-Nikitin, A. et al. Ovarian
surface epithelium at the junction area contains a cancer-prone stem cell niche. _Nature_ 495, 241–245 (2013). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We would like to thank David Dupee, Aditi Iyengar, and Elaina Wang for expert technical assistance; Lavanya Sayam (NYSTEM supported FACS Core) for her help with
fluorescence-activated cell sorting; and all members of the Nikitin Lab for their advice and support. This work has been supported by NIH (CA096823 and CA197160) and NYSTEM (C023050 and
C028125) grants to A.Y.N., NIH (CA72717) and the Genitourinary Oncology Research fund (Weill Cornell) to D.M.N., and fellowship funding from the Cornell Comparative Cancer Biology Training
Program to C.-Y.C. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Sciences, and Cornell Stem Cell Program, Cornell University, Ithaca, NY, 14850, USA Chieh-Yang
Cheng, Zongxiang Zhou, Meredith Stone, Andrea Flesken-Nikitin & Alexander Yu. Nikitin * Harvard Medical School, Children’s Hospital, Boston, MA, 02115, USA Bao Lu * Department of
Medicine, Weill Cornell Medicine and Meyer Cancer Center, New York, NY, 10021, USA David M. Nanus Authors * Chieh-Yang Cheng View author publications You can also search for this author
inPubMed Google Scholar * Zongxiang Zhou View author publications You can also search for this author inPubMed Google Scholar * Meredith Stone View author publications You can also search
for this author inPubMed Google Scholar * Bao Lu View author publications You can also search for this author inPubMed Google Scholar * Andrea Flesken-Nikitin View author publications You
can also search for this author inPubMed Google Scholar * David M. Nanus View author publications You can also search for this author inPubMed Google Scholar * Alexander Yu. Nikitin View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Alexander Yu. Nikitin. ETHICS DECLARATIONS CONFLICT OF INTEREST The
authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cheng, CY., Zhou, Z., Stone, M. _et al._ Membrane metalloendopeptidase suppresses
prostate carcinogenesis by attenuating effects of gastrin-releasing peptide on stem/progenitor cells. _Oncogenesis_ 9, 38 (2020). https://doi.org/10.1038/s41389-020-0222-3 Download citation
* Received: 29 January 2020 * Revised: 02 March 2020 * Accepted: 05 March 2020 * Published: 23 March 2020 * DOI: https://doi.org/10.1038/s41389-020-0222-3 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
Trending News
Farms can claim £20,000 each for flood repairs, defra says - farmers weekly© FLPA/REX Shutterstock Flood-hit farmers in north-west England will be able to claim up to £20,000 to help restore dama...
Va puget sound health care system annual report | va puget sound health care | veterans affairsPRESS RELEASE May 27, 2021 Seattle , WA — The Annual Report is a look back at the accomplishments of the previous year a...
Channelnews : microsoft takes on apple, google with mobile games app storeMicrosoft is planning to launch a new app store for games on both iPhones and Android smartphones – as long as its A$100...
Qandeel baloch’s brother confesses to killing her for ‘honour’, says he has no regrets - scoopwhoopIn the latest update regarding the case of Pakistani Internet sensation Qandeel Baloch’s honour killing, the victim’s br...
Surge in imported dengue fever cases to france from overseasTHERE ARE CONCERNS THAT TIGER MOSQUITOES WILL SPREAD THE DISEASE WITHIN THE COUNTRY There have been 500 cases of dengue ...
Latests News
Membrane metalloendopeptidase suppresses prostate carcinogenesis by attenuating effects of gastrin-releasing peptide on stem/progenitor cellsABSTRACT Aberrant neuroendocrine signaling is frequent yet poorly understood feature of prostate cancers. Membrane metal...
Scam, fraud alerts - protect your digital identityLeaving AARP.org Website Cancel You are leaving AARP.org and going to the website of our trusted provider. The provider’...
Do not be fooled by twitter’s excellent elon musk impersonatorsPhoto: Twitter Elon Musk’s Twitter is verified. You can find him and his little blue check mark tweeting about SpaceX, T...
Why britain needs new tv news channels | thearticleTwo new news stations could soon be coming to British televisions. First there is GB News, the work of a company called ...
Front Matter | Science NewsScience News was founded in 1921 as an independent, nonprofit source of accurate information on the latest news of scien...