Corticotropin-releasing factor receptor 1 (crf1) antagonism in patients with alcohol use disorder and high anxiety levels: effect on neural response during trier social stress test video feedback
Corticotropin-releasing factor receptor 1 (crf1) antagonism in patients with alcohol use disorder and high anxiety levels: effect on neural response during trier social stress test video feedback"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In preclinical models of alcohol use disorder, the corticotropin-releasing factor (CRF) receptor is upregulated, particularly in the extended amygdala. This upregulation is thought
to play a role in stress-induced relapse to drinking by a mechanism that is independent of the hypothalamic-pituitary-adrenal axis. As part of a double-blind, placebo-controlled clinical
study with pexacerfont, a selective, orally available, and brain-penetrant CRF1 receptor antagonist which has anti-anxiety effects in preclinical studies, we examined the effect of
pexacerfont on the neural response to a social stress task adapted to fMRI. Subjects were 39 individuals (4 women) with high trait anxiety and moderate to severe alcohol use disorder
randomized to receive pexacerfont or placebo. The task involved feedback of videoclips of an individual performing the Trier Social Stress Test. Pexacerfont had no effect on the neural
response to self-observation under stress. The neural response to viewing oneself under stress vs an unknown other under stress activated prefrontal brain regions including insula, inferior
frontal gyrus as well as medial, superior frontal gyri. These regions of activation overlap with those found in studies using similar paradigms. Potential applications of this task to probe
neurocircuitry that is disrupted in addiction is discussed. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS CHEMOGENETIC
INHIBITION OF CENTRAL AMYGDALA CRF-EXPRESSING NEURONS DECREASES ALCOHOL INTAKE BUT NOT TRAUMA-RELATED BEHAVIORS IN A RAT MODEL OF POST-TRAUMATIC STRESS AND ALCOHOL USE DISORDER Article Open
access 21 March 2024 PEOPLE WHO BINGE DRINK SHOW NEUROENDOCRINE TOLERANCE TO ALCOHOL CUES THAT IS ASSOCIATED WITH IMMEDIATE AND FUTURE DRINKING- RESULTS FROM A RANDOMIZED CLINICAL EXPERIMENT
Article 16 September 2023 INSULAR CORTEX CORTICOTROPIN-RELEASING FACTOR INTEGRATES STRESS SIGNALING WITH SOCIAL AFFECTIVE BEHAVIOR Article Open access 26 February 2022 INTRODUCTION In
preclinical models of alcohol use disorder (AUD) [1], the corticotropin-releasing factor-1 (CRF1) receptor is upregulated, particularly in the extended amygdala which contains
extrahypothalamic CRF neurons located in the bed nucleus of the stria terminalis and central nucleus of the amygdala [2]. This upregulation is thought to play a role in stress-induced
relapse to drinking by a mechanism that is independent of the hypothalamic–pituitary–adrenal (HPA) axis [3, 4]. This effect is most pronounced in animals with high levels of anxiety-like
behavior [3]. CRF1 receptor antagonists have been evaluated in clinical populations with anxiety and depression with largely negative results [5, 6]. Central administration of a CRF1
receptor antagonist blocked alcohol withdrawal-induced anxiety in rodents [7]. Peripheral administration of a CRF1 receptor antagonist reduced alcohol self-administration in a rodent model
of alcohol dependence and in rats genetically bred to prefer alcohol [8]. It also blocked reinstatement of stress-induced alcohol seeking in these two animal models related to AUD [8]. The
effect of CRH1 antagonists to reduce these stress-induced behaviors is independent of the HPA axis [9, 10]. In patients with moderate to severe AUD [1] and high trait anxiety, Kwako et al.
conducted a double-blind, placebo-controlled clinical study with pexacerfont [11]. The aim was to investigate whether pexacerfont, reduced stress-induced craving for alcohol. Stress was
induced with two laboratory procedures: the Trier Social Stress Test (TSST) [12] and with personalized stress scripts [13]. Pexacerfont did not reduce subjective stress or craving reported
as a consequence of these two laboratory stress provocations [11]. The drug also had no effect on blood-oxygen level dependent (BOLD) responses to alcohol cues or affective [fearful vs
neutral stimuli from the International Affective Picture System (IAPS) [14]]. Pexacerfont, consistent with preclinical studies, did not reduce HPA-related biomarkers which are reliably
activated in the TSST such as plasma cortisol or adrenocorticotropic hormone (ACTH), and did not affect these markers in the dexamethasone/CRF stimulation test. Notwithstanding the absence
of a drug effect on these aforementioned outcomes, the TSST itself (regardless of drug condition: pexacerfont or placebo), produced a robust stress response with significant plasma cortisol
and ACTH concentration elevations over baseline 20 min after the TSST. Accordingly, there was a significant elevation in subjective stress response as measured by the Subjective Units of
Distress Scale (SUDS; [15]) at 20 min post-TSST and in alcohol craving as measured by the Alcohol Urge Questionnaire (AUQ; [16]) at 40 min post-TSST. These results are consistent with the
large body of literature reporting that the TSST is a reliable laboratory paradigm for stress induction as measured by these objective and self-report measures. We previously developed an
fMRI task that involved video feedback of oneself performing the TSST during fMRI scanning [17]. This task combines self-referential processing under conditions of stress compared to viewing
an unknown other under the same stress conditions (TSST). Task-based connectivity on similar tasks with the amygdala as a seed was sensitive to treatment for anxiety [18] and for predicting
levels of clinical anxiety [19]. We examined whether there was an effect of pexacerfont on BOLD response to viewing SELF vs OTHER during the TSST among individuals with moderate to severe
AUD and high trait anxiety. Since the putative mechanism of the CRH1 receptor antagonist, pexacerfont, dampens CRF-induced upregulation in the extended amygdala in AUD, we investigated
whether pexacerfont modulated the neural response during this self-referential processing task using a seed-based analysis with right/left amygdala as a region of interest (ROI). Further, we
explored, as regions of interest, other brain areas known to be activated in self-referential processing [20], anterior cingulate cortex (ACC), left, right inferior frontal gyrus
(IFG)/insula. Of note, these were the regions we found to be robustly activated in the SELF vs OTHER contrast in our previous study of this task [17]. We also investigated whether neural
modulation was related to the magnitude of the stress response while performing the actual TSST as measured by cortisol, ACTH, and subjective report of stress and of alcohol craving. METHODS
PARTICIPANTS Methods have been reported previously [11]. We report here the results from the subset of subjects from the parent study [11] who completed the TSST and fMRI sessions with the
TSST video feedback task (_N_ = 39; 4 female). For subject characteristics, see Table 1. Trait anxiety was assessed with the Spielberger State Trait Anxiety Inventory-Trait version (STAI-T;
[21]; the study inclusion criterion score for this instrument was a STAI-T score >39. There were no significant group differences in age or scores on the Addiction Severity Index (ASI)
[22], Childhood Trauma Questionnaire (CTQ) [23], Neuroticism factor of the NEO Personality Inventory-Revised (NEO; [24] or Spielberger State-Trait Anxiety Inventory-Trait Version (STAI-T)
[21] between the pexacerfont and placebo groups(Table 1). Subjects were right-handed and diagnosed with alcohol dependence (AD) (DSM-IV) [25] which is equivalent to moderate to severe AUD
[1]. Participants were excluded if they had other significant psychiatric or medical disorders. Informed consent was obtained as approved by the NIH Institutional Review Board. Details of
eligibility criteria are provided at http://www.clinicaltrials.gov/ct2/show/NCT01227980. STUDY DRUG ADMINISTRATION Subjects were randomized to pexacerfont or matched placebo, which was
administered for 30 days. They received a loading dose of 300 mg of pexacerfont given once daily for the first 7 days, followed by 100 mg once daily for 23 days, or placebo. A separate
pharmacokinetic (PK) study was conducted using this dosing regimen in which cerebrospinal fluid was sampled in steady state in a group of healthy volunteers; data were presented in the
parent study. PK modeling based on this study provided support for >90% central CRF1 receptor occupancy. All participants remained hospitalized throughout the study and were
simultaneously in standard inpatient treatment for AUD. TSST AND CUE REACTIVITY SESSION (TSST/CR) Stress induction was achieved in the laboratory using the TSST. This procedure took place on
Day 18. The TSST was immediately followed by an alcohol cue reactivity (CR) session. The TSST consisted of the subject delivering a 5-min oral presentation in front of a panel of unfamiliar
individuals. Then subjects were instructed to carry out mental arithmetic (serial subtraction) for 5 min. The TSST was videotaped for the fMRI task. Throughout the TSST/CR session,
subjective anxiety was rated with the SUDS and alcohol craving was rated with the AUQ. The endocrine response (ACTH and cortisol) was measured with serial blood draws every 10 min from −20
to 90 min. approximately. FMRI TASK The video feedback task for the TSST was similar to that described previously [17]. Audio-visual recordings were reviewed and edited into 30 s video
clips, numbering 7. These video clips were chosen during periods when participants appeared uncomfortable or were making errors. Seven similar clips were obtained from a volunteer “other”
who was unknown to the participant and who was the same gender and race as the participant. Clips were chosen from “other” during periods where the subject’s performance was unremarkable to
avoid the confound of an empathetic or envious response to another’s performance. The fMRI task consisted of random alternating presentation in fixed order of SELF and OTHER videoclips, each
of 30 s duration, totaling 7-SELF and 7-OTHER video clips for a total imaging time of 900 s (15 min). FMRI SCANNING SESSION Scanning session took place on Day 23 of the study. Three other
tasks were conducted in this session involving stimuli from the International Affective Picture System, alcohol cues and emotional faces and are reported elsewhere [11]. The order of
presentation of the four tasks was randomized across participants. Subjects underwent an fMRI scan on a 3 Tesla General Electric MRI Scanner using a standard quadrature head coil. The
functional MRI (fMRI) scans consisted of 450 temporal (with TR, repetition, or sampling time, of 2 s or as previously mentioned a total time of 900 s) volumes (64 × 64 × 36) consisting of
3.8 mm thick slices with in-plane sampling of 3.75 × 3.75 mm using a T2* weighted echo planar sequence. Structural scans were acquired using a T1-weighted MP-RAGE sequence with 256 × 256 ×
144 voxels with 0.9375 × 0.9375 mm in plane sampling and slice thickness of 1 mm. ANALYSIS All preprocessing and statistical tests on functional images were performed on an Apple Mac Pro
3.33 GHz 6-Core Intel Xeon computer using the Analysis of Functional NeuroImaging (AFNI) software package [26]. The functional images were blurred to a 6 mm full-width at half-maximum
resolution (composed of intrinsic or acquired smoothing plus the application of a Gaussian smoothing filter of 4 mm) and slice-time corrected to account for difference in acquisition times
between slices in each volume. Motion correction of the fMRI was done to the third temporal image (and 12 motion correction regressors from this correction were used in later statistical
analyses). Also note that voxels showing spatial aligned temporal corrections greater than 0.3 mm where censored in later analyses. The fMRI volumetric sequence was then aligned to the
MP-RAGE image and ultimately transformed to Talairach space. For each subject, a voxel-wise generalized least squares time series fit was constructed using AFNI 3dREMLfit that included
estimation of the temporal auto-correlation structure. A boxcar design corresponding to the 30 s trier stimulus intervals (AFNI BLOCK duration 30 and magnitude 1 option) convoluted with a
standard hemodynamic transfer function (HTF) was used as the primary regressors as well as the previously mentioned motion regressors. For Group analysis, 3dMVM [27], a group analysis
program from AFNI that performs traditional ANOVA and ANCOVA style computations, was used. The resultant activation maps were presented in standard (Talairach) space and displayed as 3.5 ×
3.5 × 3.5 mm voxels. The SELF versus OTHER contrast (SELF > OTHER) was tested at a per voxel _p_ value of 0.0001 (all subjects combined regardless of drug group: pexacerfont or placebo).
Then _t_ tests were conducted comparing the SELF vs OTHER contrast for the pexacerfont group compared to the placebo group. To further help control for multiple voxel tests only voxel
cluster sizes greater than 20 at cluster threshold of 0.05 were deemed significant. In an exploratory analysis, we examined further the effect of Pexacerfont on the SELF > OTHER contrast
by adding psychological characteristics to the model and examining whether there was an interaction between each of these measures and DRUG (pexacerfont vs Placebo) on SELF vs OTHER. The
measures were addiction severity (ASI), history of childhood trauma (CTQ), Neuroticism (NEO-PR), trait anxiety (STAI-T), post-traumatic stress disorder symptom severity (PTSD Symptom
Severity Interview—PSSI) [28]. Objective measures of stress (cortisol and ACTH), as well as subjective measures of alcohol craving (AUQ) and distress (SUDS) (all entered as peak change from
baseline post-TSST), were entered as covariates in the SELF > OTHER analysis to determine whether the experienced stress during the actual TSST affected the neural response to viewing the
SELF under stress. For the ROI analysis, bilateral insula, left IFG, right IFG, ACC, left amygdala, right amygdala were ROIs. The p-values were determined for difference in average fMRI
response in each ROI between drug conditions (pexacerfont vs placebo). ROIs were based on AFNI segmentation map re-sampled to fMRI grid for each subject. In addition, for each drug condition
(pexacerfont or placebo), the correlation between BOLD activation in each ROI and psychometric/stress variable listed above were compared. RESULTS There was no significant effect of DRUG on
BOLD response for the SELF > OTHER contrast. There was no significant DRUG x Psychological/Stress response interaction on SELF vs OTHER in the whole brain or ROI analyses. SELF >
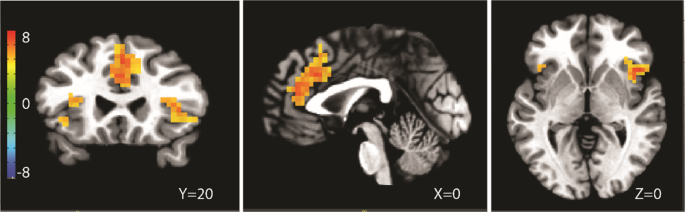
OTHER for the entire group, regardless of DRUG condition yielded significant activations in bilateral insula, superior, medial, and inferior frontal gyri (Table 2, Fig. 1). Covarying for
psychological/stress response measures did not alter neural activation of the SELF vs OTHER contrast in the whole brain or ROI analyses (see Table 3 and Fig. 2 for cortisol covariate as an
example). DISCUSSION We report here the neural response to viewing the self under stress in individuals with moderate-severe AUD with high trait anxiety. We found no effect of pexacerfont, a
CRF1 receptor antagonist on this response. In addition, baseline psychological factors (addiction severity, trait anxiety, PTSD symptoms, childhood trauma, neuroticism) did not interact
with the drug condition to affect the neural response to the observation of oneself under stress compared to an unknown other. The subjects experienced an objective and subjective stress
response during the actual TSST [11] as exemplified by the significant elevation in plasma cortisol and ACTH as well as distress ratings compared to baseline at the 20-min timepoint
(post-TSST). As we know from the original study [11], there was no drug effect on these TSST outcomes and during the dexamethasone/CRF stress test, there was also no effect of pexacerfont on
either cortisol or ACTH plasma concentrations. This was not unexpected as CRF1 antagonists exert their anxiolytic, anti-stress effect in a manner independent of the HPA axis, perhaps by
modulating neurocircuitry related to the extended amygdala. Therefore, we wanted to explore whether pexacerfont may modulate the neural response to viewing oneself undergoing the TSST even
though it did not modulate the peripheral HPA axis response provoked by the TSST in real time. We found that it did not, neither in whole brain nor in ROI analyses that included the
amygdala. Similarly, in the parent study [11], there was no effect of pexacerfont on amygdala BOLD activation in response to fearful vs neutral faces. This latter brain region is where the
CRF system is upregulated in AUD and where, theoretically, CRF1 antagonists exert their effect to suppress alcohol self-administration [reviewed in ref. [3]] in preclinical studies.
Notwithstanding preclinical studies of alcohol self-administration in animal models of AUD, pexacerfont did not modulate biological markers of stress during the actual TSST [11] or neural
activation during viewing oneself under stress as reported here. A limitation of the present study is that we did not have subjective ratings of affect or stress during the fMRI video
feedback task. We do know from our pilot study of this task in healthy controls [17] that the task did engender subjective report of stress where stress ratings viewing the SELF were
significantly greater than viewing OTHER. Further, we previously reported gender differences in neural activation to SELF vs OTHER where males activated right insula/IFG as well as superior,
middle frontal gyrus, cingulate gyrus to a greater degree than women while reporting less stress during the actual TSST. We were unable to examine gender differences in the SELF vs OTHER
contrast in the present study due to the small number of women (_n_ = 4), however, when we examined these outcomes in males only, the results were unchanged. Another limitation is that the
sample size (_N_ = 50) for the parent study which was calculated based on the effect size of naltrexone to reduce cue induced alcohol craving in a laboratory session. This may not be
adequate to detect a pexacerfont effect on the neural response to the self under stress and further, the sample size for imaging from the parent study [11] was smaller, i.e., _N_ = 39. Three
other tasks were conducted with the task reported here, however, the order of the tasks was randomized across participants so as to minimize an order effect on task outcome. Lastly, we
considered that since both the SELF and OTHER conditions included viewing a stress induction task (TSST), the neural response to the stress component of the task may have been diminished.
Examining the effect of pexacerfont on the response to SELF vs baseline or OTHER vs baseline yielded no significant results. Importantly, in this group of individuals with moderate-severe
AUD with high trait anxiety, the brain regions activated while viewing the SELF versus an unknown OTHER under stress, overlap with those previously reported with this same task in healthy
control subjects [17], namely prefrontal cortical and limbic regions, including the superior, medial, inferior frontal gyri as well as insula. These regions have been shown to be activated
in a meta-analysis of self-face recognition apart from stress conditions [20]. In addition to the frontal lobes other brain regions are involved in self-recognition including regions of the
parietal, temporal and occipital lobes [29]. While the regions found in the results of the present study overlap partially with those found in self-recognition/observation tasks that do not
involve stress, the stress condition added to this study makes comparison difficult. The effect of observing oneself vs another in pain vs no pain [30] significantly activated in similar
brain regions reported in the present study: inferior and middle frontal gyri and insula. Self-observation in patients with social anxiety disorder (compared to controls) was associated with
greater connectivity between insula and amygdala [18]. Activation in the insula and middle frontal gyrus during self-observation was also sensitive to social anxiety disorder treatment
(cognitive behavioral therapy or acceptance commitment therapy), where the activation in these regions during self-observation decreased with treatment. Self-observation is related to
self-monitoring which is impaired in addiction [31]; the salience network, which mediates self-monitoring, is structurally [32, 33] and functionally [34,35,36] impaired in AUD. Targeting
nodes in the salience network such as insula or ACC with noninvasive brain stimulation is a potential therapeutic approach for addiction [37]. This fMRI task robustly engages nodes in the
salience network. Therefore, this kind of task could be used in conjunction with noninvasive brain stimulation approaches to activate targeted salience network nodes. Further, combining this
task with alcohol/drug cue exposure could help to elucidate how drugs of addiction can shift salience and alter salience network functioning. REFERENCES * American Psychiatric Association.
Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013. * Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. A central
amygdala CRF circuit facilitates learning about weak threats. Neuron. 2017;93:164–78. Article CAS PubMed Google Scholar * Heilig M, Koob GF. A key role for corticotropin-releasing factor
in alcohol dependence. Trends Neurosci. 2007;30:399–406. Article CAS PubMed PubMed Central Google Scholar * Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and
chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–86. Article CAS PubMed PubMed Central Google Scholar * Binneman B,
Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH 1 antagonist) in the treatment of major depression. Am J
Psychiatry. 2008;165:617–20. Article PubMed Google Scholar * Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. Multicenter, randomized, double-blind, active comparator
and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–25. Article CAS PubMed Google Scholar
* Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of
ethanol withdrawal. Brain Res. 1993;605:25–32. Article CAS PubMed Google Scholar * Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, et al.
3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2, 6-dimethyl-imidazo [1, 2-b] pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1
antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–26. Article CAS PubMed PubMed Central Google Scholar * Lê AD, Harding S, Juzytsch W, Watchus J, Shalev
U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–24. Article PubMed Google Scholar
* Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration
and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195:345–55. Article CAS PubMed Google Scholar * Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan
R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology.
2015;40:1053–63. Article CAS PubMed Google Scholar * Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a
laboratory setting. Neuropsychobiology. 1993;28:76–81. Article CAS PubMed Google Scholar * Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in
humans. Ann NY Acad Sci. 2004;1032:254–7. Article PubMed Google Scholar * Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective
Ratings. The Center for Research in Psychophysiology, University of Florida; 1999. * Wolpe J. Subjective units of distress scale. the practice of behavior therapy; 1969. * Bohn MJ, Krahn
DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol: Clin Exp Res. 1995;19:600–6. Article CAS PubMed Google Scholar * Lee
MR, Cacic K, Demers CH, Haroon M, Heishman S, Hommer DW, et al. Gender differences in neural-behavioral response to self-observation during a novel fMRI social stress task. Neuropsychologia.
2014;53:257–63. Article PubMed Google Scholar * Brown LA, Young KS, Goldin PR, Torre JB, Burklund LJ, Davies CD, et al. Self-referential processing during observation of a speech
performance task in social anxiety disorder from pre-to post-treatment: evidence of disrupted neural activation. Psychiatry Res: Neuroimaging. 2019;284:13–20. Article PubMed Google Scholar
* Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW, et al. Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to
psychosocial stress. Behav Neurosci. 2019;133:203–11. Article PubMed PubMed Central Google Scholar * Hu C, Di X, Eickhoff SB, Zhang M, Peng K, Guo H, et al. Distinct and common aspects
of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci Biobehav Rev. 2016;61:197–207. Article
PubMed Google Scholar * Spielberger CD. Manual for the state and trait anxiety inventory. Consulting Psychologist Press, Palo Alto, CA; 1983. * Alterman AI, Cacciola JS, Habing B, Lynch
KG. Addiction Severity Index Recent and Lifetime summary indexes based on nonparametric item response theory methods. Psychol Assess. 2007;19:119–32. Article PubMed Google Scholar *
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl.
2003;27:169–90. Article Google Scholar * Costa PT Jr, McCrae RR. Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. J Pers Assess.
1997;68:86–94. Article PubMed Google Scholar * First MB. User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. American Psychiatric
Publication; 1997. * Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. Article CAS PubMed Google
Scholar * Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear
model. Neuroimage. 2014;99:571–88. Article PubMed Google Scholar * Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post‐traumatic
stress disorder. J Trauma Stress. 1993;6:459–73. Article Google Scholar * Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, et al. Neural substrates for functionally
discriminating self‐face from personally familiar faces. Hum Brain Mapp. 2006;27:91–98. Article PubMed Google Scholar * Benuzzi F, Lui F, Ardizzi M, Ambrosecchia M, Ballotta D, Righi S,
et al. Pain mirrors: neural correlates of observing self or others’ facial expressions of pain. Front Psychol. 2018;9:1825. Article PubMed PubMed Central Google Scholar * Zilverstand A,
Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. Article CAS
PubMed PubMed Central Google Scholar * Galandra C, Basso G, Manera M, Crespi C, Giorgi I, Vittadini G, et al. Salience network structural integrity predicts executive impairment in
alcohol use disorders. Sci Rep. 2018;8:14481. Article PubMed PubMed Central Google Scholar * Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, et al. Cortical thickness,
surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. Article PubMed PubMed
Central Google Scholar * Halcomb ME, Chumin EJ, Goñi J, Dzemidzic M, Yoder KK. Aberrations of anterior insular cortex functional connectivity in nontreatment-seeking alcoholics. Psychiatry
Res: Neuroimaging. 2019;284:21–28. Article PubMed Google Scholar * Sullivan EV, Müller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, et al. A selective insular
perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol psychiatry. 2013;74:547–55. Article PubMed PubMed Central Google Scholar *
Butcher TJ, Chumin EJ, West JD, Dzemidzic M, Yoder KK. Cerebral blood flow in the salience network of individuals with alcohol use disorder. Alcohol Alcohol. 2021;57:445–51. Article PubMed
Central Google Scholar * Padula CB, Tenekedjieva LT, McCalley DM, Al-Dasouqi H, Hanlon CA, Williams LM, et al. Targeting the salience network: a mini-review on a novel neuromodulation
approach for treating alcohol use disorder. Front Psychiatry. 2022;13:893833. Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the clinical and
research staff involved in patient care, data collection, and technical support ICBR (the NIAAA Office of the Clinical Director and the NIAAA Clinical Core Laboratory), at the NIH Clinical
Center (Departments of Nursing, Nutrition, and Pharmacy). The authors would also like to express their gratitude to the participants who took part in these studies. The content of this
article is solely the responsibility of the authors. FUNDING This study was supported by the NIAAA Division of Intramural Clinical and Biological Research (DICBR) and carried out under a
Cooperative Research and Development Agreement (CRADA) between the NIAAA and Bristol Meyers Squibb. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Veterans Affairs Medical Center, Washington,
DC, USA Mary R. Lee * Clinical Neuroimaging Research Core, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA Daniel Rio & Reza Momenan
* Division of Treatment and Recovery, Health Services, and Recovery Branch (THSRB), National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA
Laura Kwako * Office of the Clinical Director, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA David T. George * Center for Social and
Affective Neuroscience, Department of Biomedical and Clinical Sciences, Linköping University Hospital, Linköping, Sweden Markus Heilig Authors * Mary R. Lee View author publications You can
also search for this author inPubMed Google Scholar * Daniel Rio View author publications You can also search for this author inPubMed Google Scholar * Laura Kwako View author publications
You can also search for this author inPubMed Google Scholar * David T. George View author publications You can also search for this author inPubMed Google Scholar * Markus Heilig View author
publications You can also search for this author inPubMed Google Scholar * Reza Momenan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
MH, RM, LK, MRL designed experiments; LK, DTG performed experiments. DR, MRL analyzed data. MH, RM, LK, MRL, DTG, MRL wrote and edited the manuscript. CORRESPONDING AUTHOR Correspondence to
Mary R. Lee. ETHICS DECLARATIONS COMPETING INTERESTS MH is an Associate Editor for Neuropsychopharmacology. The other authors declare that they have no competing conflicts of interest.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, M.R., Rio, D., Kwako, L. _et al._ Corticotropin-Releasing Factor receptor 1 (CRF1) antagonism in patients with alcohol use disorder
and high anxiety levels: effect on neural response during Trier Social Stress Test video feedback. _Neuropsychopharmacol._ 48, 816–820 (2023). https://doi.org/10.1038/s41386-022-01521-z
Download citation * Received: 09 September 2022 * Revised: 29 November 2022 * Accepted: 02 December 2022 * Published: 23 December 2022 * Issue Date: April 2023 * DOI:
https://doi.org/10.1038/s41386-022-01521-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Submerged valleys and barrier reefsABSTRACT As I have never visited the Pacific Islands, I do not attempt to bring their valleys under the same category as...
What's at stake for automakers in fight over emissions standardsSCOTT SIMON, HOST: Some of the world's largest automakers want to renegotiate a White House proposal to roll back f...
North korea and us war threats reignite - kim refuses to denucleariseKim Jong-un’s totalitarian regime has handed a heavy blow to Trump by vowing to hold on to its nuclear weapons. The herm...
Essel group chairman subash chandra put out this really weird tweet & got trolled for it - scoopwhoopOn October 12, Essel group chairman and Zee owner Dr Subhash Chandra put out this strange tweet: In his tweet, he says, ...
Lipid storage disorders block lysosomal trafficking by inhibiting a trp channel and lysosomal calcium releaseABSTRACT Lysosomal lipid accumulation, defects in membrane trafficking and altered Ca2+ homoeostasis are common features...
Latests News
Corticotropin-releasing factor receptor 1 (crf1) antagonism in patients with alcohol use disorder and high anxiety levels: effect on neural response dABSTRACT In preclinical models of alcohol use disorder, the corticotropin-releasing factor (CRF) receptor is upregulated...
Madras hc issues notice to sii, icmr & india’s drug regulator on a petition against covishieldThe Madras High Court on Friday issued notices to Pune-based Serum Institute of India (SII), Indian Council of Medical R...
CONSUMER NEWS - Los Angeles TimesFord Recalling 72,600 Trucks: Ford Motor Co. said it is recalling the trucks to correct a problem that may cause emissio...
US Judge blocks immigration raids in certain places of worshipNewsletters ePaper Sign in HomeIndiaKarnatakaOpinionWorldBusinessSportsVideoEntertainmentDH SpecialsOperation SindoorNew...
El camino real's evan wardlow pulls out all the stops on defenseAny high school basketball player who claps his hands while playing defense, daring the player he is guarding to try to ...