Transient receptor potential vanilloid 1-based gene therapy alleviates orthodontic pain in rats
Transient receptor potential vanilloid 1-based gene therapy alleviates orthodontic pain in rats"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Orthodontic pain that is induced by tooth movement is an important sequela of orthodontic treatment and has a significant effect on patient quality of life. Studies have shown that
the high expression of transient receptor potential vanilloid 1 (TRPV1) in trigeminal ganglions plays a vital role in the transmission and modulation of orofacial pain. However, little is
known about the role of TRPV1 in orthodontic pain. In this study, male Sprague–Dawley rats were randomly assigned to six groups to study the role of TRPV1 in the modulation of tooth-movement
pain. The expression levels of TRPV1 mRNA and protein were determined by real-time PCR and western blot, respectively. Moreover, pain levels were assessed using the rat grimace scale (RGS).
The role of TRPV1 in modulating tooth-movement pain was examined by injecting a TRPV1 antagonist into the trigeminal ganglia of rats. A lentivirus containing a TRPV1 shRNA sequence was
constructed and transduced into the rats’ trigeminal ganglia. The results showed that the expression levels of TRPV1 protein and mRNA were elevated following tooth-movement pain. Pain levels
increased rapidly on the 1st day, peaked on the 3rd day and returned to baseline on the 14th day. The TRPV1 antagonist significantly reduced tooth-movement pain. The lentivirus containing a
TRPV1 shRNA sequence was able to inhibit the expression of TRPV1 and relieved tooth-movement pain. In conclusion, TRPV1-based gene therapy may be a treatment strategy for the relief of
orthodontic pain. SIMILAR CONTENT BEING VIEWED BY OTHERS RETROGRADE NERVE GROWTH FACTOR SIGNALING MODULATES TOOTH MECHANICAL HYPERALGESIA INDUCED BY ORTHODONTIC TOOTH MOVEMENT VIA
ACID-SENSING ION CHANNEL 3 Article Open access 04 June 2021 TRPA1 TRIGGERS HYPERALGESIA AND INFLAMMATION AFTER TOOTH BLEACHING Article Open access 31 August 2021 THE ROLE OF TRPV2 AS A
REGULATOR ON THE OSTEOCLAST DIFFERENTIATION DURING ORTHODONTIC TOOTH MOVEMENT IN RATS Article Open access 22 August 2023 INTRODUCTION Pain caused by tooth movement can be perceived during
the entire duration of an orthodontic treatment, e.g., separator placement, initial archwire engagement, banding, wearing elastics, rapid maxillary expansion, and debonding, and it can
especially be perceived during the initial phases of treatment1. It has been shown that 85% of orthodontic patients experience mild to moderate pain, while 9% of them endured severe pain on
the first day of treatment2,3. This unpleasant experience is one of the main factors contributing to poor compliance and treatment termination4,5. Therefore, the successful management of
orthodontic pain is critical in clinical practice. To date, several approaches have been used to alleviate orthodontic pain, such as nonsteroidal anti-inflammatory drugs (NSAIDs), mechanical
vibration, laser therapy, and behavioural therapy6,7,8,9,10,11,12. Unfortunately, none of these studies have shown any of these methods to be truly effective or clinically validated. Gene
therapy, which is the delivery of genes to target cells to alter gene expression, is a viable and promising modality in the treatment of orthodontic pain11. The expression of genes to
inhibit pain was upregulated in the primary afferent nerve and the dorsal horn of the spinal cord when an analgesic gene, a pain related receptor gene, or a cytokine gene were inserted into
the specific transport carrier or when the recombinant vector was imported into the nervous system13. RNA interference (RNAi) is a powerful tool to silence gene expression or translation by
neutralising targeted mRNA molecules in mammalian cells and is widely used clinically14. Particularly, small interfering RNA (siRNA) is more efficient at interference than traditional
antisense RNA, and it has become more popular in the application of the targeted interference of gene expression and related gene functions that are involved in pain regulation15,16. Studies
have shown in a rat neuropathic pain model that the intrathecal injection of siRNA containing the transient receptor potential vanilloid 1 (TRPV1) gene can downregulate TRPV1 expression and
thereby ease pain. Successful anti-nociceptive gene therapy can be achieved by the downregulation of pro-nociceptive genes and/or upregulation of anti-nociceptive genes17,18. TRPV1 has been
widely recognised as a key component of both inflammatory and neuropathic pain in the sensory system19. It is a non-selective cation channel that is mainly distributed in the primary and
secondary sensory neurons such as the trigeminal ganglia (TG)19. Since it is a multi-modal sensory receptor, TRPV1 can be activated by capsaicin, nociceptive thermal stimulation ( ≥ 43 °C),
extracellular acidic pH, mechanical signals, and exogenous chemical stimuli20,21. When TRPV1 is activated by such stimuli, the resulting physiological response is pain. Pain transmitters,
inflammatory mediators, and injuries can regulate the activity of TRPV1 and cause a cascade of signals to be produced in the downstream cells. It is well-documented that orthodontic tooth
movement can induce an acidic extracellular micro-environment, initiate mechanical signals, and generate inflammatory mediators6. These nociceptive signals can activate TRPV1 and induce
painful sensations22,23. TRPV1 expression in TG and periodontal tissues was increased following orthodontic tooth movement24,25,26. Moreover, blocking TRPV1 in periodontal tissues with its
antagonist alleviated orthodontic pain26. All of these findings suggest that TRPV1 plays an essential role in the modulation of orthodontic pain. However, whether TRPV1-based gene therapy
could alleviate tooth-movement-induced orofacial pain is poorly understood. Therefore, this study was aimed at examining whether TRPV1-based gene therapy can alleviate tooth-movement-induced
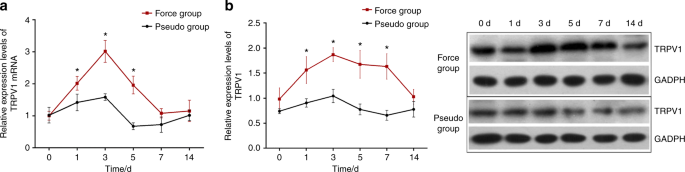
orofacial pain in rats. RESULTS THE EFFECTS OF ORTHODONTIC FORCE ON TRPV1 EXPRESSION IN THE TG OF RATS As displayed in Fig. 1, TRPV1 mRNA and protein expression were increased following
orofacial pain that was induced by tooth movement. A two-way ANOVA with repeated measures revealed that the TRPV1 mRNA and protein expression levels were significantly influenced by group
(_P_ value of 0.001 for mRNA expression and 0.004 for protein expression), time (both _P_ _<_ 0.001), and interactions (_P_ < 0.001). Further analyses revealed that the TRPV1 protein
expression levels were significantly higher in the force group than those in the pseudo-force group on the 1st day (_P_ < 0.001), the 3rd day (_P_ < 0.001), the 5th day (_P_ <
0.001), and the 7th day (_P_ < 0.001), while no differences were observed between the two groups at baseline (_P_ = 0.479) or on the 14th day (_P_ = 0.455). Likewise, TRPV1 mRNA
expression levels were significantly higher in the force group than in the pseudo group on the 1st day (_P_ = 0.023), the 3rd day (_P_ < 0.001), and the 5th day (_P_ < 0.001), while no
differences were seen between the two groups at baseline (_P_ = 0.930), on the 7th day (_P_ = 0.356) or on the 14th day (_P_ = 0.978). The details are also shown in Table 1. THE EFFECTS OF
THE TRPV1 ANTAGONIST ON TRPV1 EXPRESSION IN THE TG OF RATS The effects of the TRPV1 antagonist (SB366791) on TRPV1 protein and mRNA expression is shown in Fig. 2. A two-way repeated measures
ANOVA revealed that TRPV1 mRNA and protein expression were significantly influenced by group (_P_ value of 0.001 for mRNA expression and 0.001 for protein expression), time (both _P_ <
0.001), and interaction (both _P_ < 0.05). Further analyses revealed that TRPV1 protein expression levels were higher in the force + TRPV1 antagonist (SB366791) group than those in the
force + normal saline group on day 1 (_P_ < 0.001), day 3 (_P_ < 0.001), day 5 (_P_ < 0.001), day 7 (_P_ = 0.002), and day 14 (_P_ = 0.002). The baseline levels were similar between
the two groups (_P_ = 0.714), as presented in Table 2. Likewise, TRPV1 mRNA expression levels were higher in the force + TRPV1 antagonist group than in the force + saline group on day 1
(_P_ <0.001), day 3 (_P_ < 0.001), and day 5 (_P_ < 0.001), while no statistically significant differences between the two groups were present at baseline (_P_ = 0.994), on day 7
(_P_ = 0.254), or on day 14 (_P_ = 0.982). LENTIVIRUS VECTOR TRANSDUCTION As depicted in Fig. 3a, both in vivo fluorescence imaging and immunostaining showed successful lentiviral
transduction of the TG. The in vivo fluorescence images also confirmed that the 100% accuracy of the TG-injection. THE EFFECT OF THE LENTIVIRAL VECTOR ON TRPV1 EXPRESSION IN THE TG OF RATS
As shown in Fig. 3b, the TRPV1 mRNA and protein expression that was induced by the lentiviral vector displayed a unique pattern. TRPV1 expression decreased following orofacial pain. A
two-way repeated measures ANOVA found that both the TRPV1 protein and mRNA expression levels were significantly influenced by group (_P_ value of 0.001 for mRNA expression and 0.001 for
protein expression), time (both _P_ < 0.001), and the interactions (both _P_ < 0.001). As shown in Table 3, the TRPV1 protein expression levels in the force + TRPV1 shRNA lentivirus
group were significantly lower than those of the force + blank lentivirus group on the 1st day (_P_ < 0.001), the 3rd day (_P_ < 0.001), the 5th day (_P_ < 0.001), the 7th day (_P_
< 0.001), and the 14th day (_P_ < 0.001), except for the baseline (_P_ = 0.064). The TRPV1 mRNA expression levels were also lower in the force + TRPV1 shRNA lentivirus group compared
with those of the force + blank lentivirus group on the 1st day (_P_ < 0.001), the 3rd day (_P_ < 0.001), the 5th day (_P_ = 0.016), the 7th day (_P_ = 0.009), and the 14th day (_P_ =
0.033), but no differences were seen between the two groups at baseline (_P_ = 0.999). ASSESSMENT OF OROFACIAL PAIN The RGS scores, which are regarded as the surrogates for the pain levels
in the rats, were coded by the images that were captured from the videotapes. Compared to the baseline of 0, the RGS scores increased in all the groups that were tested. The signs of pain in
response to orthodontic treatment included a decreased width of the palpebral fissures, ears that tended to curl and angle outwards or forwards, flattened and elongated noses, and whiskers
that tended to bunch and move forward (shown in Fig. 4). All of these features were observed in both the orthodontic force and pseudo-force groups but were much subtler and were seen for a
shorter duration in the latter group. A two-way repeated measures ANOVA showed that the orofacial pain assessments of all groups were significantly influenced by group (_P_ value of 0.001
for force group, 0.001 for antagonist group and 0.027 for lentivirus group, respectively), time (all _P_ < 0.001), and the interactions (all _P_ < 0.001). The study showed that the
groups with similar RGS scores shared similar TRPV1 expression trends. As displayed in Fig. 5a and Table 4, the RGS scores in the force group were significantly higher than those in the
pseudo-force group on the 1st day (_P_ < 0.001), the 3rd day (_P_ < 0.001), the 5th day (_P_ = 0.001), and the 7th day (_P_ = 0.087), while similarities between the two groups were
seen at baseline and on the 14th day (_P_ = 0.970). As for the effects of the TRPV1 antagonist SB366791 on the response of rats to the orthodontic treatments, although SB366791 upregulated
TRPV1 protein and mRNA expression in the TG in this study, the RGS scores in antagonist group C were significantly higher than those in the control group D on days 1 (_P_ = 0.005), 3 (_P_
<0.001), and 5 (_P_ = 0.016), but no differences were seen between the two groups on day 7 (_P_ = 0.698) or day 14 (_P_ = 0.111) (Fig. 5b). In terms of the comparisons of the RGS score
between the force + TRPV1 shRNA lentivirus group and the force + blank lentivirus group, the scores of the former group were significantly lower than those of the latter group on days 1 (_P_
< 0.001) and 3 (_P_ < 0.001), while no statistically significant differences were found at baseline or on day 5 (_P_ = 0.999), day 7(_P_ = 0.849), or day 14 (_P_ = 0.999) (Fig. 5c).
DISCUSSION Our study shows that TRPV1 was expressed and functional in the TG of healthy rats. The expression of TRPV1 was upregulated by orthodontic forces that coincided with tooth-movement
pain in rats, indicating that TRPV1 was involved in orthodontic pain. These results were in accordance with those reported previously24,27. Notably, TRPV1 activation by orthodontic tooth
movement directly affects TG function, which in turn modulates orthodontic pain. This hypothesis arises from: (a) the TRPV1 mRNA expression in the TG was validated by a lack of expression in
rats from the negative control, the pseudo-force group, (b) the high levels of TRPV1 protein expression in the TG of rats in the force group, as shown in the western blot assays, (c) the
RGS changes upon TRPV1 activation in the force group compared with either the baseline or the pseudo-force groups, and (d) this variation in pain level was consistent with the TRPV1 protein
and mRNA expression in the TG, which further illustrates that changes in TRPV1 expression play an important role in the transmission and regulation of pain signals during tooth movement.
Importantly, TRPV1-mediated increases in both TRPV1 mRNA and protein expression were also significantly inhibited by both the TRPV1 antagonist and the inoculation of the TRPV1 shRNA
lentivirus into the TG of the rats. Numerous novel and potent TRPV1 modulators have been identified to date, especially antagonists that can be used in pain treatment. These compounds
include capsazepine, SB366791 (a cinnamide analogue), AMG-9810 (a cinnamide analogue), A-425619 (a urea analogue), BCTC (a urea analogue), and JNJ-17203212 (a urea analogue)28,29,30,31,32.
All of these antagonists are small molecule compounds. Among them, SB366791 is an authorised neotype and is both a powerful and selective TRPV1 antagonist. It can competitively bind to the
TRPV1 receptor and thereby effectively inhibit the TRPV1 activation process that is mediated by capsaicin, acid stimulation, pain, and burns28. Additionally, different viral vector types,
including adenovirus, lentivirus, herpes simplex virus, and adeno-associated viruses, have been developed to transduce genes of interest into their target cells33,34,35,36. In particular,
lentiviruses are characterised by their effective integration of RNA interference sequences against proinflammatory genes into TG and their stable gene expression, with an easier
transduction of DNA into neurons and neuroglial cells37. Therefore, a lentivirus was used in the present study to deliver RNA interference sequences against proinflammatory genes into the TG
of rats. We demonstrated that the lentivirus vector, representing a form of gene therapy, was much better than the chemical antagonist SB366791 at relieving the pain of tooth movement.
Hence, owing to its high therapeutic efficacy, the application of lentivirus vectors in clinical practice will become a viable treatment strategy for orthodontic pain relief once state
administrative drug authorities can regulate the quality and safety of these products and supervise how they are clinically used. In comparison with the control group, TG inoculation with
SB366791 upregulates TRPV1 mRNA and protein expression on days 1, 3, and 5 following tooth movements and alleviates the pain levels at these time intervals. These findings were inconsistent
with our expectations, which could be attributed to the fact that TRPV1 synthesis occurs in a primary sensory afferent neuron, such as the dorsal root and the TG and is followed by
bidirectional axonal transport to the central and peripheral axon terminals. However, the TRPV1 receptor is distributed in the primary afferent terminals that are switched off under normal
conditions, and no glutamate neurotransmitters are released by TRPV1-dependent activation. The inflammatory factors that are locally generated facilitate the synthesis of TRPV1, which
activates the receptor ion channels during peripheral inflammation. Conversely, protein phosphorylation is catalysed by either PKA or PKC, which are activated by the voltage-gated Ca2+
channel-dependent mechanism that causes the receptors to open in response to an elevation in TRPV138,39. SB366791 can inhibit glutamatergic release and transmission in a subset of neurons
via a presynaptic mechanism following peripheral inflammation40. These results provide evidence for a mechanism by which TRPV1 contributes to inflammatory pain. The levels of TRPV1 mRNA and
protein expression in the TG of the TRPV1 shRNA lentivirus group were significantly lower than those in the blank lentivirus control group at all time intervals. Moreover, the pain levels,
expressed as RGS, were significantly lower in the lentivirus group on days 1 and 3, but no significant differences after day 5 were observed compared to the control group, which likely
occurs because siRNA is the key RNAi molecule that affects pain relief. This gene inhibition is transient, which is quite different from gene knockout models, in which the inhibition lasts
longer41. Lentivirus vectors that target transduction mechanisms reduced TRPV1 expression only in the TG. The body was still able to stabilise its internal environment through the nervous,
immune and endocrine systems, activating other related signalling pathways or signalling molecules that can regulate neuronal excitement and affect pain transmission, ultimately changing
pain perception42. All of the reasons mentioned above could explain the differences we observed in this experiment at the molecular or the behavioural level. In conclusion, this study showed
that TRPV1 mRNA and protein expression levels in the TG of rats, together with the RGS scores, were significantly upregulated following the orthodontic treatments, and they rapidly
increased on day 1, peaked on day 3, maintained their high levels on days 5 and 7, and declined to baseline on day 14. The increases in TRPV1 mRNA and protein expression, however, were
significantly inhibited by the inoculation of the TRPV1 antagonist SB366791 and the lentivirus vector that transfects RNA interference sequences against proinflammatory genes into the TG of
rats, which both led to the alleviation of orthodontic pain. The TRPV1 shRNA lentivirus group showed a much higher therapeutic efficacy in the relief of orthodontic pain compared with that
of the SB366791 group. Our findings suggest that the use of a TRPV1 shRNA lentivirus in clinical practice will become a viable treatment strategy for orthodontic pain relief. Future quality
and safety evaluations of these vectors should be performed for clinical approval by the drug administrative authorities. MATERIALS AND METHODS ANIMALS Two hundred and sixteen male
Sprague–Dawley rats, weighing between 200 g and 300 g, were obtained from the Animal Experimental Center at Sichuan University. They were maintained in the animal facility and kept in an
air-conditioned room at 21 °C with a 12 h light-dark cycle. Standard rat chow and water were provided ad libitum. Animal experiments were performed in accordance with protocols that were
approved by the ethical committee of the State Key Laboratory of Oral Diseases, Sichuan University (protocol No.WCCSIRB-D-2014-084). The rats were randomly assigned to 6 groups: force group
(group A; _n_ = 36), pseudo-force group (group B; _n_ = 36), force + TRPV1 antagonists (SB366791, positive control) group (group C; _n_ = 36), force + normal saline group (group D; _n_ =
36), force + TRPV1 shRNA lentivirus group (group E; _n_ = 36), and force + blank lentivirus group (group F; _n_ = 36). Rats were anaesthetised with an intraperitoneal injection of 7% chloral
hydrate in normal saline (NS) solution at the dose of 0.06 mL·g−1 body weight, and then fixed Ni-Ti alloy closed-coil springs were ligated to the rats with ligation wires (0.2 mm in
diameter) between the left maxillary first molar and the upper incisor of the rats to simulate orthodontic forces. In all force groups, the springs delivered a 40 g force measured, as by a
force meter (Tiantian, Changsha, China), while a 0 g force was used for the pseudo-force group. Rats were euthanized by decapitation after being anaesthetised with pentobarbital sodium (50
mg·kg−1·bw) on days 0, 1, 3, 5, 7, and 14 (_n_ = 6 for each group per day). The rats in all groups that were euthanized on day 14 were used in the orofacial pain assessments on days 0, 1, 3,
5, 7, and 14. Moreover, rats that were euthanized on day 0 did not receive any interventions and were chosen as the baseline control for each group. The details of animal use in different
groups are depicted in Supplementary Fig. 1. All sections of this report adhere to the ARRIVE Guidelines for reporting animal research43. LENTIVIRUS VECTOR PREPARATION A lentivirus vector
encoding an enhanced red fluorescence protein was recombined with the rat TRPV1 RNA interference sequence (sense: CAGATAACACAGTTGACAA). The recombined sequence was amplified with a
polymerase chain reaction (PCR) assay. Viral vectors were packaged and harvested by transfection into 293 T cells, and this was followed by visualisation under a fluorescent microscope. The
viral titre was determined using TaqMan PCR and expressed as transducing unit (TU) per mL. The viral vector titre was 1.0 × 109 TU·mL−1. In addition, blank lentivirus vectors that did not
contain the RNAi sequence were simultaneously prepared as a control. TG INOCULATION A 15 μL aliquot of the TRPV1 antagonist solution (SB366791, Sigma-Aldrich, USA), 15 μL of a normal saline
solution, 10 μL of a TRPV1 shRNA lentivirus solution (virus 3.3 × 105 TU·μL−1, polybrene 6 × 10−3 TU·μL−1, Qiagen, USA), and 10 μL of a blank lentivirus vector (virus 3.3 × 105 TU·μL−1,
polybrene 6 × 10−3 TU·μL−1) were inoculated into the TG of animals in groups C, D, E, and F, respectively. The TRPV1 antagonist was inoculated into the TG of group C rats 30 min before
spring mounting, which was followed by TG inoculations on days 1, 3, 5, 7, and 14. The group D rats were treated similarly but received normal saline TG inoculations. On each inoculation
day, all of the animals in these two groups were inoculated, and six rats were sacrificed four hours after the inoculations. The TG were rapidly harvested and placed in liquid nitrogen for
PCR and western blot analyses. The animals in groups E and F were inoculated with either the TRPV1 shRNA lentivirus or the blank lentivirus seven days before the orthodontic springs were
applied. Six rats in each group were euthanized on days 0, 1, 3, 5, 7, and 14, and the TG were harvested. IMMUNOFLUORESCENCE STAINING Twelve rats that received the same inoculations as the
group E rats but did not have springs inserted were euthanized, and the TG were harvested and subjected to immunofluorescence staining analyses on days 1, 3, 5, and 7 after the lentiviral
inoculation. The TG specimens were embedded in a cutting compound (OCT) at an optimal temperature and cut at a 14 μm thickness along the TG macroaxis in a freezing microtome. The prepared
sections were deparaffinized, rinsed with phosphate-buffered saline (PBS) three times, blocked with a 3% bovine serum albumin (BSA) solution, and incubated for 30 min after fixation in
acetone for 15 min. Thereafter, the sections were stained with a primary rabbit anti-TRPV1 monoclonal antibody (1:500, Abcam) and incubated for another 45 minutes at 37 °C followed by
rinsing with PBS three times for 5 min. The sections were further stained with an immunofluorescent tetramethylrhodamine (TRITC)-labelled secondary antibody (1:100), incubated for 1 h at 37
°C, rinsed with PBS and finally mounted onto slides for observation under a fluorescence microscope (DM4000B, Leica, Germany). VERIFICATION OF TG TRANSDUCTION IN VIVO Twelve rats were
euthanized to verify the transduction of the viral vectors into the TG using an in vivo fluorescence imaging system (In-Vivo Xtreme, Bruker, Germany) on days 1, 3, 5, and 7 after TRPV1 shRNA
lentivirus inoculation and prior to the application of the orthodontic force springs. All rats were routinely imaged on days 1, 3, 5, and 7 after lentivirus inoculation, and three rats were
imaged on each day. Cherry was selected as the fluorescence reporter gene, and it has a fluorescence detector with excitation/emission wavelengths of 587/610 nm. OROFACIAL PAIN ASSESSMENT
For the animals in groups C, D, E, and F that were sacrificed on day 14, orofacial pain levels were assessed using the RGS between 7:00 pm and 9:00 pm on days 0, 1, 3, 5, and 7 in a room
with a level of background noise less than 45 dB following the protocols described previously44. Briefly, the tested rats were placed individually into transparent cubicles with a 20.0 cm ×
10.5 cm × 9.0 cm volume. After acclimation to the environment for 15 min, the rats were continuously videotaped for 30 min. For each rat on each assessment day, ten facial expression images
were extracted every 3 min to be used for the RGS scoring. The RGS was scored independently by two analysts by examining the facial expression changes in the orbits, nose, ears, and whiskers
of the rats. The two analysts’ scores of the pain levels were averaged. For each rat, the RGS scores that were determined before the interventions were applied were used as the baseline
RGS. Differences in the RGS scores between the baseline and experimental tooth-movement groups were regarded as the surrogate pain levels for each rat at each assessment time point. THE
REAL-TIME RT-PCR ASSAY Total RNA was extracted from the TG using the Takara MiniBEST Universal RNA Extraction Kit (Takara, Shiga, Japan) according to the manufacturer’s protocols. Then, cDNA
was reverse transcribed using the M-MLV test kit (M1705, Promega) following the manufacturer’s recommendations. Rat TRPV1 (NM_031983) mRNA expression was quantified in the TG samples using
triplex RT-PCR performed in a LightCycler480 (Roche, Switzerland) RT-PCR platform with the SYBR Premix Ex Taq (Perfect Real-time, TAKARA, Dalian, China) according to the manufacturer’s
protocol. To quantify the amount of specific mRNA expression in the samples, a standard curve was generated for normal rat TG samples. GAPDH served as an internal standard. PCR was performed
using specific primers for rat GAPDH (forward primer: TTCAACGGCACAGTCAAGG, reverse primer: CTCAGCACCAGCATCACC, expected size: 114 bp) and TRPV1 (forward primer: AAGGATGGAACAACGGGCTAG,
reverse primer: TCCTGGTAGTGAAGATGTGGG, expected size: 127 bp). The final reaction volume of 12 µL consisted of 6 µL of the SYBR Premix Ex Taq, 0.3 μL of the 5 µmol·L−1 primer mix, 0.6 μL of
the reverse transcription nucleic acid template, and 5.1 μL of RNase-free H2O. The thermal profile was set at 95 °C for 2 min and 94 °C for 10 s, followed by 40 cycles at 60 °C for 30 s and
40 cycles at 60 °C for 10 s. Relative mRNA transcript level calculations were performed using the comparative CT method (ΔΔCT). The average CT values of the three complex holes for each
sample were calculated and marked as CTTRPV1 and CTGAPDH, ΔΔCT = N(CTTRPV1 − CTGAPDH) − (CTTRPV1 − CTGAPDH). WESTERN BLOT ANALYSIS The TG tissues were cut into pieces and homogenised with
the RIPA lysis buffer plus phenylmethanesulfonyl fluoride (PMSF) at a ratio of 20 mg to 150–250 μL, resulting in a final concentration of 1 mm. The homogenate was incubated on ice for 30 min
followed by centrifugation at 14 000 × _g_ for 5 min. The supernatant was stored at −80 °C before analysis. The lysates were run on SDS-PAGE and separated by electrophoresis (Tanon,
Shanghai, China). Proteins were transferred onto polyvinylidene fluoride (PVDF) membranes and blocked with 5% skim milk in a Tris-buffered saline with Tween 20 (TBST) solution for 1 h at
room temperature. The sealed PVDF membranes were incubated in the primary TRPV1 goat polyclonal antibody (1:200, Santa Cruz, USA) for 2 h at room temperature, washed with TBST four times and
incubated with the second HRP-conjugated goat anti-rabbit antibody (Beyotime Biotechnology, China) for 1 h. The protein blot densities were analysed using ImageJ Software (National
Institutes of Health, Bethesda), which marked the target intensity band as ATRPV1 and the internal reference intensity band (GAPDH) as AGAPDH; protein expression at the corresponding time
point = ATRPV1/AGAPDH. STATISTICAL ANALYSES Statistical analyses were performed using SPSS 16.0 (SPSS, Chicago, Illinois, USA) and GraphPad Prism 6.0 software (GraphPad Software, San Diego,
USA). The results are depicted as the means ± the standard deviation (SD). A one-way ANOVA (Bonferroni post hoc test) was employed to analyse the differences between TRPV1 expression and
orofacial pain among different time intervals in each group. A two-way ANOVA with repeated measures was used to examine the effects of time (0, 1, 3, 5, 7, and 14 d), groups (the force group
vs. the pseudo-force group, the force + TRPV1 antagonist group vs. the force + normal saline group, the force + TRPV1 shRNA lentivirus group vs. the force + blank lentivirus group), and the
interactions with both TRPV1 expression and orofacial pain. For the two-group comparisons at each time interval, the Bonferroni post hoc test was used if the pretest for normality was not
rejected at the 0.05 significance level. _P_ values less than 0.05 were considered statistically significant. REFERENCES * Banerjee, S., Banerjee, R., Shenoy, U., Agarkar, S. &
Bhattacharya, S. Effect of orthodontic pain on quality of life of patients undergoing orthodontic treatment. _Indian J. Dent. Res._ 29, 4–9 (2018). Article Google Scholar * Campos, M. J.,
Fraga, M. R., Raposo, N. R., Ferreira, A. P. & Vitral, R. W. Assessment of pain experience in adults and children after bracket bonding and initial archwire insertion. _Dent. Press J.
Orthod._ 18, 32–37 (2013). Article Google Scholar * Rakhshan, H. & Rakhshan, V. Pain and discomfort perceived during the initial stage of active fixed orthodontic treatment. _Saudi
Dent. J._ 27, 81–87 (2015). Article Google Scholar * Krishnan, V. Orthodontic pain: from causes to management--a review. _Eur. J. Orthod._ 29, 170–179 (2007). Article Google Scholar *
Bergius, M., Berggren, U. & Kiliaridis, S. Experience of pain during an orthodontic procedure. _Eur. J. Oral. Sci._ 110, 92–98 (2002). Article Google Scholar * Long, H. et al. Current
advances in orthodontic pain. _Int. J. Oral. Sci._ 8, 67–75 (2016). Article Google Scholar * Gupta, M. et al. Controlling pain during orthodontic fixed appliance therapy with non-steroidal
anti-inflammatory drugs (NSAID): a randomized, double-blinded, placebo-controlled study. _J. Orofac. Orthop._ 75, 471–476 (2014). Article Google Scholar * Yadav, S. et al. Effect of
low-frequency mechanical vibration on orthodontic tooth movement. _Am. J. Orthod. Dentofac. Orthop._ 148, 440–449 (2015). Article Google Scholar * Deana, N. F., Zaror, C., Sandoval, P.
& Alves, N. Effectiveness of low-level laser therapy in reducing orthodontic pain: a systematic review and meta-analysis. _Pain. Res. Manag._ 2017, 8560652 (2017). Article Google
Scholar * Sawada, A., Usui, N., Shimazaki, K., Taira, M. & Ono, T. The effects of cognitive behavioral therapy on experimental orthodontic pain. _Orthod. Waves_ 74, 10–14 (2015).
Article Google Scholar * Prabhakar, A. R., Paul, J. M. & Basappa, N. Gene therapy and its implications in dentistry. _Int. J. Clin. Pediatr. Dent._ 4, 85–92 (2011). Article Google
Scholar * Hussain, A. S., Al Toubity, M. J. & Elias, W. Y. Methodologies in orthodontic pain management: a review. _Open Dent. J._ 11, 492–497 (2017). Article Google Scholar *
Glorioso, J. C. & Fink, D. J. Gene therapy for pain: introduction to the special issue. _Gene Ther._ 16, 453–454 (2009). Article Google Scholar * Simonato, M. et al. Progress in gene
therapy for neurological disorders. _Nat. Rev. Neurol._ 9, 277–291 (2013). Article Google Scholar * Leung, R. K. & Whittaker, P. A. RNA interference: from gene silencing to
gene-specific therapeutics. _Pharmacol. Ther._ 107, 222–239 (2005). Article Google Scholar * Rohl, T. & Kurreck, J. RNA interference in pain research. _J. Neurochem._ 99, 371–380
(2006). Article Google Scholar * Zychowska, M. et al. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain.
_Eur. J. Pharmacol._ 791, 377–388 (2016). Article Google Scholar * Singh, A. K. & Vinayak, M. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential
regulation of pro-inflammatory mediators. _Phytother. Res._ 30, 1164–1171 (2016). Article Google Scholar * Gunthorpe, M. J. & Chizh, B. A. Clinical development of TRPV1 antagonists:
targeting a pivotal point in the pain pathway. _Drug Discov. Today_ 14, 56–67 (2009). Article Google Scholar * Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel
in the pain pathway. _Nature_ 389, 816–824 (1997). Article Google Scholar * Zheng, J. Molecular mechanism of TRPchannels. _Compr. Physiol._ 3, 221–242 (2013). PubMed PubMed Central
Google Scholar * Ghilardi, J. R. et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. _J. Neurosci._ 25, 3126–3131 (2005). Article Google Scholar *
Honore, P. et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain
associated with inflammation and tissue injury in rats. _J. Pharmacol. Exp. Ther._ 314, 410–421 (2005). Article Google Scholar * Qiao, H., Gao, Y., Zhang, C. & Zhou, H. Increased
expression of TRPV1 in the trigeminal ganglion is involved in orofacial pain during experimental tooth movement in rats. _Eur. J. Oral. Sci._ 123, 17–23 (2015). Article Google Scholar *
Ohkura, M. et al. Orthodontic force application upregulated pain-associated prostaglandin-I2/PGI2-receptor/TRPV1 pathway-related gene expression in rat molars. _Odontology_ 106, 2–10 (2018).
Article Google Scholar * Gao, Y. et al. Blocking of TRPV-1 in the parodontium relieves orthodontic pain by inhibiting the expression of TRPV-1 in the trigeminal ganglion during
experimental tooth movement in rats. _Neurosci. Lett._ 628, 67–72 (2016). Article Google Scholar * Zhang, C. D. et al. Expression of TRPV1 and CGRP in rat trigeminal ganglion during
orthodontic tooth movement. _Shanghai Kou. Qiang. Yi. Xue._ 24, 6–12 (2015). PubMed Google Scholar * Gunthorpe, M. J. et al. Identification and characterisation of SB-366791, a potent and
selective vanilloid receptor (VR1/TRPV1) antagonist. _Neuropharmacology_ 46, 133–149 (2004). Article Google Scholar * Gavva, N. R. et al. AMG 9810
[(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. _J. Pharmacol. Exp. Ther._ 313,
474–484 (2005). Article Google Scholar * El Kouhen, R. et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1
receptor antagonist, blocks channel activation by vanilloids, heat, and acid. _J. Pharmacol. Exp. Ther._ 314, 400–409 (2005). Article Google Scholar * Behrendt, H. J., Germann, T., Gillen,
C., Hatt, H. & Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. _Br. J.
Pharmacol._ 141, 737–745 (2004). Article Google Scholar * Wiskur, B. J. et al. A novel Trpv1 receptor antagonist Jnj-17203212 attenuates colonic hypersensitivity in rats. _Methods Find.
Exp. Clin. Pharmacol._ 32, 557–564 (2010). Article Google Scholar * Teramoto, S., Ishii, T., Matsuse, T. & Fukuchi, Y. Recombinant adeno-associated virus vectors efficiently transduce
foreign gene into bovine aortic endothelial cells: comparison with adenovirus vectors. _Jpn. J. Pharmacol._ 84, 206–212 (2000). Article Google Scholar * Delenda, C. Lentiviral vectors:
optimization of packaging, transduction and gene expression. _J. Gene Med._ 6, S125–S138 (2004). Article Google Scholar * Benavides, O. J., Satlin, L., Burrow, C., Wilson, P. & Herold,
B. Herpes simplex virus (HSV) as a model vector for gene therapy for renal disease. _J. Am. Soc. Nephrol._ 13, 125a (2002). Google Scholar * Daya, S. & Berns, K. I. Gene therapy using
adeno-associated virus vectors. _Clin. Microbiol. Rev._ 21, 583–593 (2008). Article Google Scholar * Ogawa, N. et al. Gene therapy for neuropathic pain by silencing of TNF-alpha expression
with lentiviral vectors targeting the dorsal root ganglion in mice. _PLoS ONE_ 9, e92073 (2014). Article Google Scholar * Wang, W., Cao, X. H., Liu, C. J. & Liu, L. J. Cannabinoid WIN
55,212-2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways. _Neurol. Sci._ 33, 79–85 (2012). Article Google Scholar * Sanz-Salvador, L., Andres-Borderia, A.,
Ferrer-Montiel, A. & Planells-Cases, R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. _J. Biol. Chem._ 287, 19462–19471
(2012). Article Google Scholar * Lappin, S. C., Randall, A. D., Gunthorpe, M. J. & Morisset, V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal
dorsal horn following peripheral inflammation. _Eur. J. Pharmacol._ 540, 73–81 (2006). Article Google Scholar * Christoph, T. et al. Investigation of TRPV1 loss-of-function phenotypes in
transgenic shRNA expressing and knockout mice. _Mol. Cell. Neurosci._ 37, 579–589 (2008). Article Google Scholar * Nakamura, A. et al. G protein-gated inwardly rectifying potassium (KIR3)
channels play a primary role in the antinociceptive effect of oxycodone, but not morphine, at supraspinal sites. _Br. J. Pharmacol._ 171, 253–264 (2014). Article Google Scholar * Kilkenny,
C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. _J. Pharmacol.
Pharmacother._ 1, 94–99 (2010). Article Google Scholar * Liao, L. et al. Evaluation of pain in rats through facial expression following experimental tooth movement. _Eur. J. Oral. Sci._
122, 121–124 (2014). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by both National Natural Science Foundation of China (Contract No. 81571004, No.
81500884) and Applied and Fundamental Research Program funded by Department of Science and Technology of Sichuan Province (Contract No. 2018JY0558). All authors listed in the manuscript
contributed to the conception, acquisition, analysis and interpretation of data, design of the manuscript, critically revised the manuscript, and approved the final submitted version. AUTHOR
INFORMATION Author notes * These authors contributed equally: Rui Guo, Yang Zhou AUTHORS AND AFFILIATIONS * State Key Laboratory of Oral Diseases & National Clinical Research Center for
Oral Diseases & Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, China Rui Guo, Yang Zhou, Hu Long, Jing Wen, Huimin Hu, Hong Yang, Zhouqiang
Wu & Wenli Lai * Jiangsu Key Laboratory of Oral Diseases, Department of Orthodontics, Stomatology Hospital Affiliated with Nanjing Medical University, Nanjing, China Di Shan Authors *
Rui Guo View author publications You can also search for this author inPubMed Google Scholar * Yang Zhou View author publications You can also search for this author inPubMed Google Scholar
* Hu Long View author publications You can also search for this author inPubMed Google Scholar * Di Shan View author publications You can also search for this author inPubMed Google Scholar
* Jing Wen View author publications You can also search for this author inPubMed Google Scholar * Huimin Hu View author publications You can also search for this author inPubMed Google
Scholar * Hong Yang View author publications You can also search for this author inPubMed Google Scholar * Zhouqiang Wu View author publications You can also search for this author inPubMed
Google Scholar * Wenli Lai View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Hu Long or Wenli Lai. ETHICS
DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Guo, R., Zhou, Y., Long, H. _et al._ Transient receptor potential
Vanilloid 1-based gene therapy alleviates orthodontic pain in rats. _Int J Oral Sci_ 11, 11 (2019). https://doi.org/10.1038/s41368-019-0044-3 Download citation * Received: 12 July 2018 *
Revised: 06 December 2018 * Accepted: 13 December 2018 * Published: 11 March 2019 * DOI: https://doi.org/10.1038/s41368-019-0044-3 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Peripheral blood regulatory T cells in patients with diffuse systemic sclerosis (SSc) before and after autologous hematopoietic SCT: a pilot studyThe present pilot study aims to evaluate the frequency and the function of regulatory T (Treg) cells in patients with di...
Ex-civil service chief warns next 20 years will be 'dominated' by conflictSIR SIMON CASE, WHO SERVED AS THE HEAD OF THE CIVIL SERVICE FOR FOUR DIFFERENT PRIME MINISTERS, SAID THE GOVERNMENT NEED...
An account of the alcyonarians collected by the royal indian marine survey ship “investigator” in the indian oceanABSTRACT THE first part of the memoir of the Alcyonarians of the Indian Ocean was published in 1906, and reviewed in NAT...
Irb infra q4 profit rises 14% to ₹215 cr, annual net jumps to ₹6,481 cr on strong toll revenueNEW DELHI: IRB Infrastructure Developers Ltd on Monday posted 14 per cent growth in net profit at Rs 214.7 crore for Mar...
How to watch the snp national conference 2024 — scottish national partySNP Conference kicks off Friday 30th August. Over the weekend, members will debate policy, elect members to new position...
Latests News
Transient receptor potential vanilloid 1-based gene therapy alleviates orthodontic pain in ratsABSTRACT Orthodontic pain that is induced by tooth movement is an important sequela of orthodontic treatment and has a s...
The centennial of the planetariumThe first planetarium projector was completed 100 years ago, providing the public with an unparalleled view of the night...
Former president jimmy carter dies at 100Jimmy Carter never wanted to be a celebrity. A politician, yes: He wanted to effect change, mostly in compassionate huma...
Gaffe-prone blowhard biden is no churchill: rarely have so few had to clarify so muchMORE FROM RICH LOWRY The president of the United States is a blowhard — again. If the country thought that it was gettin...
Large-scale protein level comparison of deltaproteobacteria reveals cohesive metabolic groupsABSTRACT Deltaproteobacteria, now proposed to be the phyla Desulfobacterota, Myxococcota, and SAR324, are ubiquitous in ...