A new approach to quantify visceral fat via bioelectrical impedance analysis and ultrasound compared to mri
A new approach to quantify visceral fat via bioelectrical impedance analysis and ultrasound compared to mri"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Visceral adipose tissue (VAT) has been linked to systemic proinflammatory characteristics, and measuring it accurately usually requires sophisticated instruments. This
study aimed to estimate VAT applying a simpler method that uses total subcutaneous fat and total body fat (BF) measurements. METHOD As part of our experimental approach, the subcutaneous fat
mass (SFT) was measured via US (SFTtotal), and VAT was quantified by assessing MRI data. Both parameters were added to obtain total body fat (BFcalc). Those results were then compared to
values obtained from a bioelectrical impedance analysis (BFBIA). Multiple regression analyses were employed to develop a simplified sex-specific equation for SFT, which was subsequently used
in conjunction with BFBIA to determine VAT (VATEq). RESULT We observed excellent reliability between BFBIA and BFcalc, with no significant difference in body fat values (20.98 ± 8.36 kg vs.
21.08 ± 8.81 kg, _p_ = 0.798, ICC 0.948). VATEq_female/male revealed excellent reliability when compared to VATMRI, and no significant difference appeared (women: 0.03 ± 0.66 kg with a 95%
CI ranging from −1.26 kg to 1.32 kg, _p_ = 0.815, ICC: 0.955.; men: −0.01 ± 0.85 kg with a 95% CI ranging from −1.69 kg to 1.66 kg, _p_ = 0.925, ICC: 0.952). CONCLUSION Taking an
experimental approach, VAT can be determined without MRI. SIMILAR CONTENT BEING VIEWED BY OTHERS DEVELOPMENT OF A SEX-SPECIFIC VISCERAL FAT AREA ESTIMATION USING DISCRETE MULTI-WAVELENGTH
NEAR-INFRARED SPECTROSCOPY MEASUREMENTS IN KOREAN INDIVIDUALS Article 18 November 2024 ESTIMATED VISCERAL ADIPOSITY IS ASSOCIATED WITH RISK OF CARDIOMETABOLIC CONDITIONS IN A POPULATION
BASED STUDY Article Open access 27 April 2021 AGE- AND SEX-SPECIFIC VISCERAL FAT REFERENCE CUTOFFS AND THEIR ASSOCIATION WITH CARDIO-METABOLIC RISK Article 20 January 2021 INTRODUCTION
Overweight and obesity are associated with a range of chronic illnesses including cardiovascular disease, diabetes mellitus and cancer [1]; they are also strong predictors of increased
mortality [2]. In recent decades, body fat distribution has become a major focus of research, as there is evidence that it is more important than total body fat mass (BF) in predicting
obesity-related diseases [3]. The proportion of abdominal fat tissue comprising visceral (VAT) and subcutaneous adipose tissue (SAT) is a critical correlate for all health complications
related to overweight and obesity [4, 5]. These two fat compartments are structurally and metabolically distinct [6, 7]. VAT is of particular interest due to its association with
proinflammatory and angiogenic activation, including cytokine secretion such as IL-6 and other immune response regulators [7, 8]. It is also well-established that men tend to accumulate more
VAT than SAT, while women tend to store more of the latter [9, 10]. Several studies have shown that weight loss through diet and exercise interventions triggers a significant reduction in
VAT mass in obese individuals, partly due to catecholamine-induced lipolysis caused by greater β1- β3-adrenoreceptor density in visceral fat [7, 8, 11,12,13,14,15]. A reduction in VAT
coincides with a drop in glucose, insulin, and leptin levels, contributing to a protective effect in relation to cardiovascular and metabolic conditions [8]. Given that VAT is known to
contribute significantly to cluster of health conditions that augment the likelihood of developing heart disease, stroke, and diabetes, quantifying VAT precisely is extremely important. It
is a parameter that could serve as a potential parameter for classifying an individual’s cardiovascular risk profile. Although reference methods for measuring VAT such as MRI, CT, and DXA
exist, they are costly and time-consuming. Some studies have suggested applying single-slice MRI to determine VAT, but this method may not always accurately yield the total VAT mass
[16,17,18]. Non-ionizing and cost-effective techniques such as abdominal bioelectrical impedance analysis (BIA) may be valuable for measuring total abdominal adipose tissue, but they are
incapable of specifically quantifying visceral fat in comparison to MRI [19]. Moreover, while simpler methods like the waist-hip ratio (WHR) or waist circumference (WC) are frequently used,
they do not specifically quantify VAT mass, as they provide information only on fat distribution [18, 20, 21]. The caliper is capable of assessing subcutaneous fat folds but is error-prone,
particularly in the abdominal area [22]; it thus systematically underestimates the thickness of subcutaneous fat. Ultrasound (US) is a promising alternative thanks to its ability to quantify
subcutaneous fat tissue and visualize visceral fat depth [23]. However, quantifying the total VAT mass is a complex task, and alternative practical and feasible methods suitable to a
clinical setting have yet to be established. In writing this paper we relied on the fundamental concept that the combined mass of subcutaneous fat (SFT) and VAT corresponds to total body fat
when applying the “addition method”. By rearranging this formula, it becomes theoretically feasible to calculate VAT solely based on SFT and total BF measurements. BF is easily obtained
through BIA in this context. Determining total SFT involves measuring the entirety of subcutaneous fat across the body via US and a previously implemented systematic mapping technique [22].
To enhance this procedure’s practicality, it would be additionally beneficial to utilize a simplified equation. Three key points arise from this objective: * (1) Will adding SFT and VAT
align with to total BF determined by BIA? * (2) Is it feasible to derive a simplified equation (SFTEq) applying only three to four measuring points to accurately represent total SFT
(SFTtotal)? * (3) Can VAT be accurately determined by utilizing BFBIA and SFTEq according to key point two? SUBJECTS AND METHODS ETHICAL ASPECTS The study was approved by the Ethics
Committee of the Medical Faculty, University of Leipzig (097/17-ek, 089/18-ek) and followed the latest revision of the Declaration of Helsinki. All participants signed written informed
consent forms and received an information letter. PARTICIPANTS We enrolled 49 subjects aged a mean 49.98 ± 20.86 years in this study (Table 1). Based on BMI and BF, we included participants
of normal weight and overweight to examine a wide range of body types. Study participants were excluded in case of pregnancy, any metal in the body, or any type of cardiac devices or leg
edema (ie, due to heart failure). SAMPLE SIZE Our study is based on an experimental design. A total sample size of 41 was calculated using G-Power (Version 3.1.9.2) to achieve a power of 0.8
at a significance level of α < 0.05, with the ability to detect a difference of 10% (±22.5%) between measurements. To account for potential variations in body types, eight additional
participants were included in the study taking an oversampling approach. STUDY DESIGN Body fat measurements were taken within a single day. Prior to the examination, a medical history
assessment was conducted and the participants’ weight, height and waist-to hip ratio (WHR) were assessed. SFT was then measured via US, total body fat was determined using bioelectrical
impedance analysis (BIA), and we quantified visceral fat via magnetic resonance imaging (MRI). The measurements were taken in the morning, and participants were asked to fast overnight
before measurements were taken. They were also instructed not to engage in any exercise or make any dietary changes the day before the measurements. WAIST-TO-HIP RATIO As an additional
anthropometric parameter, waist circumference (WC) was measured using a tape measure at the end of a normal expiration, specifically at the level of the lower floating rib. For hip
circumference (HC), the tape was positioned around the hips at the level of the trochanter major and greatest gluteal protuberance. WHR was calculated by dividing WC by HC. SUBCUTANEOUS FAT
TISSUE MEASUREMENT BY ULTRASOUND To determine the SFT, the right side of the body was systematically divided into 56 rectangles, excluding head, hand, foot, and genital area. A previous
study demonstrated the reliability of this mapping method [22]. The center of each rectangle was measured via US, and the length, width, depth, and fat density (0.949 g/cm3) [24] were
multiplied to obtain the subcutaneous fat thickness. All width and length measurements were taken with an accuracy of 0.1 cm using a tape measure. The total subcutaneous fat mass on the
right side of the body was obtained by adding together measurements from all 56 fields and doubling the measured SFT in the overall subcutaneous fat mass (SFTtotal). Detailed instructions
were provided in a previous study [22]. After mapping, measurements were taken with the subject in supine position. US images were acquired using a B-Mode device (GE Healthcare GmbH, LOGIQ
e, Vivid series). Depending on the approximate tissue depth, a 12 MHz linear transducer was used to measure SFT in longitudinal position. An optimum of brightness, gain and dynamic range was
adjusted to improve tissue delineation. US gel was applied in the center of the field, with the probe placed longitudinally and perpendicularly to the tissue being examined, which revealed
optimum echogenicity. When the boundaries were clearly visible, the US probe was lifted slowly until the ultrasound began to extinguish, applying the least amount of pressure possible. The
area of interest was then frozen and measured from the beginning of the cutis to the muscle fascia. In the abdominal area, the image was taken when the subject stopped breathing at mid-tidal
expiration. SFT was measured by an experienced scientific sonographer with five years of training. BIOELECTRICAL IMPEDANCE ANALYSIS (BIA) As human body tissues possess capacitive and
resistive characteristics [25], this method relies on measuring resistance, which is then converted into total body water (TBW) predictions via an algorithm utilizing the relationship
between volume, length, and resistivity. A resistivity value is assumed and included in the regression analysis to determine body composition [26]. Two skin electrodes are attached on each
hand and foot for analysis, and the potential difference is measured. A constant electrical alternating current of 0.4 mA at 50 kHz is applied using a single frequency bioelectrical
impedance device (AKERN BIA 101 Anniversary, AKERN-Srl, Florence, Italy). Free fat mass (FFM) and physiological fluids are good electrical conductors, while fat mass (FM) reacts with strong
resistance. FM is obtained by subtracting FFM from weight. Although concerns have been raised about BIA’s accuracy, Ward et al. clarified that they are of comparable magnitude to the gold
standard when performed under standardized conditions [26]. To ensure sufficient variability in VAT across the sample, a wide range of BMIs and body fat types was included in our study. The
examination was conducted in the morning, and the bioelectrical impedance analysis (BIA) done immediately after the participants had spent two hours in recumbent position during the US
measurement. We obtained BF parameters using BodyComposition V. 8.5 Professional Software (www.medi-cal.de). This software applies statistical analysis to determine the FFM hydration
fraction instead of using the specified hydration level of 73.2% for a healthy population. MRI A Philips Achieva 1.5 T MRI scanner was used in our study to acquire images of the abdomen. A
whole-body coil was used to obtain sufficient imaging coverage in the peripheral regions of the homogeneous magnetic field. The system’s gradient strength was 33 mT/m with a maximum gradient
slew rate of 122 T/m/s. Participants were examined in supine position and MRI imaging was done from the diaphragm to symphysis. Our MRI protocol is in Table 2. Imaging visceral adipose
tissue (VAT) is challenging; motion artefacts caused by respiration, peristalsis and vascular pulsation, as well as ghost artefacts, must be minimized to ensure accurate delineation of
adipose tissue from organs [27]. The abdomen and pelvis were measured during respiratory arrest. Compared to inspiration, the expiratory maneuver provides a well-defined respiratory position
enhancing reproducibility [28]. Due to the longer echo time (TE), T2-weighted measurements produce lower flow artifacts (dark vessel lumen) [29]. This contributes to fewer ghost artifacts
in phase direction (anterior-posterior). In addition, adipose tissue exhibits high signal intensity due to the long T1 and T2 values of fatty compounds, making fast T2-weighted sequences
well-suited to define borders to the intestine and abdominal wall [29]. The short measurement time of under one second per slice reduces the impact of intestinal motility on image quality.
Furthermore, motion artefacts are reduced on 2D slice acquisitions compared to a similar 3D sequence. Hence, we measured abdomen and pelvis with a cross-sectional 2D slice selective single
shot turbo spin-echo sequence (SSH-TSE). To ensure full coverage, acquisition was performed twice, for the lower and upper abdomen, respectively. Interleaved staircase acquisition was done
within such a slab with 8 mm slices and 2 mm gaps. The reconstructed slices are taken correspondingly to cover 10 mm in slice direction in subsequent volume estimations. To cover the full
slab, the acquisition was again subdivided into 2 runs. The interleaved staircase configuration ensured 10 mm slice and 10 mm gap alternations before using a 10 mm offset in the second run
to yield the remaining gap. This setup minimizes cross-talk and magnetization transfer effects by prolonging the duration between the saturation of adjacent slices. The complete study
resulted in 4 packages of 15 slices each, thus, 60 MRI slices were used to quantify VAT. We evaluated the images using the PACS JiveX software from Visus Health IT GmbH (www.visus.com). Each
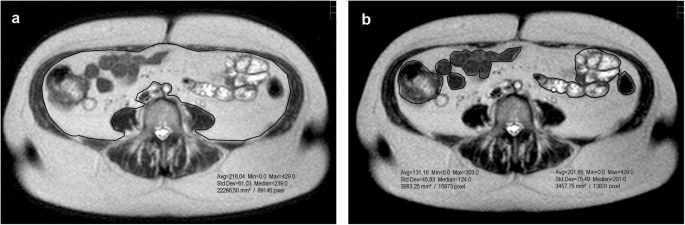
slice image was individually processed using the polygonal traction measurement software tool to determine total intra-abdominal area (Fig. 1a) and the boundaries between bowel structures
(Fig. 1b) of SSH-TSE images. Both results were subtracted (cm2) and multiplied by 10 mm slice thickness and a density of 0.949 g/cm3 to obtain VAT mass. Our measurements showed excellent
intra-rater (ICC 0.993, _p_ = 0.778) and inter-rater (ICC 0.997, _p_ = 0.263) reliability. T1 sequences were only used to help identify adipose tissue. STATISTICS The initial stage of our
data analysis involved comparing two techniques for measuring the overall amount of body fat: bioelectrical impedance analysis (BFBIA) and the “addition method” (BFcalc). The addition method
combines measurements of VAT acquired through MRI and the total subcutaneous fat measured using US (SFTtotal) across all fields (1–56). In the next step, we simplified the procedure by
developing a concise equation specifically for men and women (SFTEq_female/male) to quantify total subcutaneous fat. This enabled us to identify representative locations for subcutaneous fat
mass. This preliminary process laid the foundation for our subsequent quantification of VAT. The third step comprised calculating VAT (VATEq_female/male) by employing the representative SFT
locations obtained from SFTEq_female/male and the BFBIA values in a multiple regression analysis. Finally, we compared VAT measured by MRI (VATMRI) with VATEq_female/male to assess the
reliability and accuracy of these methods. Statistical analysis was performed using GraphPad Prism 9.1.1 (GraphPad Software Inc., California, USA, www.graphpad.com) to calculate the mean,
standard deviation (SD), Pearson’s correlation coefficient (r), and Radj (adjusted R2). A two-sided paired t-test with a _p_-value (α < 0.05) and 95% confidence interval (CI), and a
Bland-Altman plot [30] were used when differences between methods passed the normality test. Reliability was determined using the Intraclass coefficient (ICC) in SPSS 27 (SPSS Inc.,
Illinois, USA), with two-way mixed, single measures, absolute agreement. The ICC classifications were as follows: poor reliability (≤0.5), moderate reliability (>0.5–0.75), good
reliability (>0.75–0.9), and excellent reliability (values greater than 0.90) [31]. To generate a simplified subcutaneous fat equation for men and women through regression analysis, the
subcutaneous fat fields (1–56) were narrowed down using principal component analysis (PCA). Only 25 fields were integrated, as they revealed the highest intra- und interrater reliability
[22] of each body part and were easily detectable via anatomical landmarks. Requirements for PCA use were tested in advance (e.g., normality, linearity, multicollinearity). This statistical
analysis can only be used to reduce dimensions when variables correlate, therefore Bartlett’s sphericity test gives an indication of the correlation’s strength with significant differences
(_p_ < 0.05) indicating that PCA can be used for the data [32, 33]. The F-test in PCA compares the variance between components to the variance within components [34]. A statistically
significant F-value (i.e., a high value) indicates that at least one principal component differs significantly from the others, and can explain a significant proportion of the variance in
the data set [34]. We then ran the Kayser-Meyer-Olkin test (KMO) to test our sample’s adequacy, measuring the proportion of variance between variables attributable to common variance [35]. A
KMO value of at least 0.6 is required to ensure an adequate sample [33]. To generate the number of factors/components (i.e. arm, leg, abdomen), we relied on the number above the eigenvalue
of one [36]. The eigenvalue describes how much variance can be explained by the factor [37]. The resulting component matrix illustrates the factors’ loadings, which should be at least
>0.5, indicating to degree to which a given factor contributes to explaining the variable across all individuals [38]. A rotation matrix is then generated via VariMax Rotation to
analytically ensure that certain variables per factor load high and others load low [39]. Finally, two values exhibiting high loadings for each component were selected for multiple
regression analysis. We took a stepwise approach using the SFTEq to identify suitable measurement locations. The inclusion variant was employed in the VATEq to integrate fixed parameters,
such as SFT and BFBIA parameters. In the case of males, waist circumference (WC) was further incorporated into the equation, leading to enhanced outcomes. Conversely, for women, hip
circumference (HC) was included in the VAT equation to offer a more comprehensive explanation of the proportionate variance and a lower 95% CI. RESULTS Our comparison of total body fat
methods and VAT results are shown in Table 3 with their corresponding ICC and _p_-value. Sex-specific data are also integrated. TOTAL BODY FAT: BIA VS. ADDITION METHOD (BFCALC = SFTTOTAL +
VATMRI) A strong correlation is evident between BFBIA and BFcalc (_r_ = 0.948) (Fig. 2a) for our total sample. Furthermore, a paired _t_-test (α < 0.05) reveals no significant difference
between instruments, and excellent reliability is observed for BFcalc compared to BFBIA (_p_ = 0.798, ICC = 0.948). Figure 2b visualizes the associated Bland-Altman plot of BFBIA and BFcalc
displaying a mean of difference of −0.10 ± 2.82 kg with the 95% confidence interval (CI) values of −5.63 to 5.42 kg. PRINCIPAL COMPONENT ANALYSIS (PCA) To select the most accurate fields for
an SFT equation, PCA was performed as described. Our female sample’s PCA showed significant and sufficient sphericity according to Bartlett’s test (χ2 = 452.70) and a KMO measure of sample
adequacy (0.708). Three components were identified which explained 78.73% of the total variance of the variables. Two variables with the highest loading of each component were used for
stepwise multiple linear regression analysis, after confirming the requirements for this analysis (e.g., normality, linearity etc.). Three fields of the female sample exhibited significant
influence on the subcutaneous fat mass, _F_(3,21) = 137.11, _p_ < 0.001, _n_ = 25. This is statistically significant, and suggests that the three principal components can explain a part
of variance in the data set. Similarly, our male sample’s PCA revealed significant and sufficient sphericity (Bartlett’s test: χ2 = 333.05) and a KMO measure of sample adequacy (0.71). We
identified four components that explained 83.86% of the total variance of all variables. We demonstrate that four fields of the male sample revealed significant influence on the subcutaneous
fat mass, _F_(3,20) = 59.54, _p_ < 0.001, _n_ = 24. SUBCUTANOUS FAT: SFTTOTAL VS. SFTEQ In our male participants, we found that three independent variables (fields 14, 18, and 48 in cm)
included in the SFT Eq. (1) accounted for 94.4% of the total variance, demonstrating a strong effect size (Cohen’s f2 = 15.67). Similarly, four independent variables (fields 17, 37, 32, and
26 in cm) presented in the SFT Eq. (2) in women explained 94.5% of the total variance, exhibiting a strong effect size (Cohen’s f2 = 17.18) compared to SFTtotal. We detected no significant
difference between SFTEq_female/male and SFTtotal, indicating excellent correlation and reliability (refer to Table 3). Figure 3 illustrates all landmarks visually for SFTEq.
$$\begin{array}{ll}{SF}{T}_{{Eq}{{\_}}{female}}\left({kg}\right)=0.561+F18\,\left({lateral}\,{abdomen}\right)*
4.819\\\qquad\qquad\qquad\qquad\quad+\,F48\left({mid}\,{lateral}\,{posterior}\,{thigh}\right)* 4.462\\\qquad\qquad\qquad\qquad\quad+\,F14\left({umbilical}\right)* 1.589\end{array}$$ (1)
$$\begin{array}{ll}{SF}{T}_{{Eq}{{\_}}{male}}\left({kg}\right)=2.638+2.153* F17\left({lateral}\,{breast}\right)\\\qquad\qquad\qquad\qquad+\,6.132*
F37\,\left({lower}\,{lateral}\,{thigh}\right)\\\qquad\qquad\qquad\qquad+\,1.311* F32\,\left({posterior}\,{neck}\right)\\\qquad\qquad\qquad\qquad+\,2.040* F26\,({lower}\,{back})\end{array}$$
(2) VISCERAL FAT: VATMRI VS. VATEQ We included variables from BIA, the SFTEq fields, and WC and HC in a linear multiple regression model to arrive at an equation to estimate VAT in both men
and women (refer to Eqs. (3) and (4)). Statistical analysis revealed that WC demonstrated significant benefits only for men, and HC for women. The inclusion variant multiple regression
analysis revealed a VATEq_female (3) explaining 87.1% of variance for men (Cohen’s f2 = 7.20) and 87.8% (4) for women (Cohen’s f2 = 7.20). Comparing VATEq values with MRI showed a strong
positive correlation, as depicted in Fig. 4a and Table 3 for both men and women. Our findings also demonstrate excellent reliability. The average difference was 0.03 ± 0.66 kg with a 95%
confidence interval (CI) ranging from −1.26 kg to 1.32 kg for women, and −0.01 ± 0.85 kg with a 95% CI ranging from −1.69 kg to 1.66 kg for men, as illustrated in Fig. 4b.
$$\begin{array}{l}{{\boldsymbol{VAT}}}_{{\boldsymbol{Eq}}{{\_}}_{{\boldsymbol{female}}}}({kg})={{\rm{BF}}}_{{\rm{BIA}}}({kg})* 0.139\\\qquad\qquad\qquad\qquad\quad+\,F14\,\left({cm}\right)*
0.587-F18\left({cm}\right)* 0.983\\\qquad\qquad\qquad\qquad\quad-\,F48\left({cm}\right)* 1.802+{HC}({cm})* 0.109-7.758\end{array}$$ (3)
$$\begin{array}{ll}{{\boldsymbol{VAT}}}_{{\boldsymbol{Eq}}{{\_}}{\boldsymbol{male}}}\left({kg}\right)=B{F}_{{BIA}}\left({kg}\right)*
0.144\\\qquad\qquad\qquad\qquad\quad+\,F17\left({cm}\right)* 0.302+F26\left({cm}\right)* 0.073\\\qquad\qquad\qquad\qquad\quad-\,F32\left({cm}\right)* 0.730-F37({cm})*
1.763\\\qquad\qquad\qquad\qquad\quad+\,{WC}({cm})* 0.151-11.502\end{array}$$ (4) DISCUSSION Our study demonstrates that total BF measurements obtained through BIA and the addition method
delivered highly reliable results between the two methods. We also succeeded in developing simplified sex-specific equations which accurately estimated the total SFT yielded by the mapping
method (SFTtotal). These excellent reliability results indicate that VAT can be determined without requiring MRI. TOTAL BODY FAT: BFBIA VS. ADDITION METHOD (BFCALC = SFTTOTAL + VATMRI) As we
describe the addition procedure here for the first time, there is no other (published) data for comparison. Considering the Bland-Altman plot of total body fat measurement methods, both BIA
and the addition method revealed a favorable mean difference. The lower and upper 95% confidence interval of −5.63 to 5.42 kg is a relatively large range. However, the absolute deviation of
total body fat between methods remains consistent across our sample, resulting in a decrease in relative error with increasing total body fat. Bland-Altman plots help visualize differences,
but they do not show whether the observed limits are clinically acceptable, as they depend on clinical need [32]. Johnsen et al. demonstrated that BIA and US correlate equivalently with
total BF, suggesting that a combination of methods is generally feasible thanks to their equal sensitivity [40]. Our study confirms excellent reliability and correlation between BFBIA and
BFcalc. Froelich et al. reported similar fat-mass values when determined by BIA and MRI (23.3 ± 10.9 kg and 22.7 ± 9.9 kg), but did not test for absolute agreement [18]. Our results acquired
through BIA, US, and MRI measurements confirm their findings. Regarding our sex-biased results, the relative mean of the difference between male BFBIA and BFcalc was 8.13% (±23.79%) and
female −3.58% (±9.85%). Although BIA’s fat mass was slightly higher in men and lower in women compared to BFcalc, that difference was not significant, and the two methods demonstrated
excellent reliability. This is evidence that sex is no limitation when applying these methods. Alicandro et al. reported excellent reliability in FFM for men and women when comparing BIA to
DXA (ICC: male 0.95; female 0.89) [41], since DXA is considered the gold standard for assessing total fat mass. In conclusion: when compared to BIA, the addition method for assessing total
body fat utilizing SFTtotal and VATMR fulfills the prerequisite for simplifying the SFT measuring process. SUBCUTANEOUS FAT: SFTTOTAL VS. SFTEQ Two distinct equations for estimating SFT have
been derived using PCA and multiple regression analysis. The reliability of these equations is remarkably high, and there are no noteworthy inconsistencies between SFTtotal and the SFT
estimates for female and male individuals (SFTEq_female/male). It is therefore justifiable to utilize these equations to determine subcutaneous fat, and the precise locations the formula
incorporates can be employed to calculate VAT. There is no alternative study of which we are aware that assesses SFT applying the same methodology. The equations we describe account for
different landmarks based on sex, with the lateral abdomen, mid lateral posterior thigh and umbilical area included for women and lateral breast, lower lateral thigh, lower back and
posterior neck for men. Agrawal et al. confirmed significant sex-dimorphism in body fat distribution [42], highlighting the need for sex-specific equations when assessing SFT. Compared to
other body fat equations, our calculations differ in the output variable. While most calculate body density (BD) over the subcutaneous skinfold thickness, our formula specifically calculates
the SFT mass. However, there is some similarity with the 3-factor formula of Jackson & Pollock in the location of such measurements [43, 44]. The known landmarks for Jackson &
Pollock’s formula include the abdomen (near the navel), mid-anterior thigh, and lateral chest. While our locations may differ slightly, they cover a similar area. Nevill et al. [45] adapted
Jackson & Pollock’s formula again, but their positions remained the same. In contrast, Durning and Womersely [46] included biceps, triceps, scapula and iliac crest in their body fat
equation, with only the iliac crest bearing a slight resemblance to our landmark positions. Goran et al. [47] and Leahy et al. [48] added the calf to their formula. The development of these
formulas and their correlation with total fat highlight the sensitivity of the sites. However, note that they employ a double-indirect approach: Although those particular sites presumably
represent total SFT, which in turn correlates with total fat, they have not been specifically validated for total subcutaneous fat as an initial step. To reduce this gap, we take into
account the measurement of total SFT. Most equations rely on caliper measurements which require caution as the caliper can vary from the reference depending on the body fat mass. Our
equation is only applicable to our population and cannot yet be generalized without further verification studies. VISCERAL FAT: VATMRI VS. VATEQ Regarding VATEq for men and women, the 95%
confidence intervals (CIs) are significantly lower than those of the addition method, indicating their applicability within an acceptable mean range of ±1.4 kg. Furthermore, the excellent
reliability supports this conclusion. In addition to SFTtotal and BIA parameters, anthropometric parameters (WC and HC) were included in the respective VATEq to improve its validity. Given
that VAT correlates positively with a higher WHR, it is unsurprising that our VATEq_male model incorporates WC. Our investigation has also provided evidence supporting the notion that men
exhibit higher VAT levels than women (3.85 kg vs. 2.96 kg, _p_ < 0.016). Onat et al. revealed higher VAT in men as well [49]. Rantalainen et al. suggested that VAT may be influenced by
sex-specific mRNA and miRNA expression in abdominal and gluteal adipose tissues [50]. Including either WHR or WC for men in the VAT equation contributed similarly to the proportion of the
variance. Including these parameters resulted in an approximate 5% increase in adjusted R2 compared to the equation without WC or WHR and a lower 95% CI. Considering that WHR incorporates
multiple measurements, we opted for WC as an additional parameter in our formula. Some studies have also indicated that WC is a better VAT indicator than WHR [49, 51,52,53]. We were able to
confirm those findings while also observing that HC and VAT exhibited the weakest correlation in men (_r_ = 0.689). Conversely, women showed a 6% higher adjusted R2 and a lower 95% CI
between methods when HC was included, compared to WHR or WC alone, or without additional parameters. HC exhibited a highly positive correlation with SFT (_r_ = 0.801) and VAT (_r_ = 0.887)
in women. When considering VATEq parameters, HC and WC are more heavily weighted within the formula than are subcutaneous fat depths and BIA because of their high values (e.g., 100 cm). This
means that more technically demanding methods such as BIA and US do not contribute as strongly to the formula as do simple circumference measurement. The Bland-Altman plot, similar to the
comparison with total body fat methods, demonstrates consistent absolute deviation between methods across our total sample. This leads to a reduction in relative error as VAT increases.
These findings suggest that the VAT formula is indeed applicable for overweight male and female adults. CONCLUSION Our approach employing BIA and US may function as an initial step towards
determining total VAT without MRI, DXA or CT. The following steps describe its application for practical use: * 1. Assessing WC in males and HC in females. * 2. Measuring subcutaneous fat
depth at sex-specific landmarks via ultrasound. * 3. Applying single-frequency BIA with two electrodes on each hand and foot. * 4. Insert the obtained results into the sex-specific VAT
formula. OUTLOOK With the rising global prevalence of obesity and related health conditions, there is growing awareness of the detrimental effects of excess visceral fat. Numerous innovative
devices have been implemented to precisely measure visceral fat; but they are often time-consuming and expensive. As an experimental approach, our method can be employed to simplify the
quantification of VAT and incorporate it as an additional parameter when assessing cardiovascular risk profiles. Further investigations, including the implementation of multifrequency BIA
and its practical use in clinical setting, are necessary to refine this methodology. LIMITATION Certain areas, such as the hands, feet, head, and genitals, were neglected, even though they
may contain a small amount of subcutaneous fat. Our study cohort consisted only of young, normal-weight and older, overweight individuals. As young, normal-weight individuals have minimal
visceral adipose tissue, the VATEq primarily applies to overweight individuals. Further studies with larger samples and more diverse body types are necessary to validate these results. Our
findings only apply to this study population and rely on fasting adults with diverse body types measured by a single frequency bioelectrical impedance analysis, thus they cannot be
extrapolated to multifrequency BIA. DATA AVAILABILITY The authors confirm that the data supporting the findings of this study are available within the article; further inquiries can be
directed to the corresponding authors. REFERENCES * Crudele L, Piccinin E, Moschetta A. Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients. 2021;13.
https://doi.org/10.3390/nu13062101. * Ladeiras-Lopes R, Sampaio F, Bettencourt N, Fontes-Carvalho R, Ferreira N, Leite-Moreira A, et al. The ratio between visceral and subcutaneous abdominal
fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Revista Espanola de Cardiologia. 2017;70:331–7. https://doi.org/10.1016/j.rec.2016.09.010
Article PubMed Google Scholar * Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. https://doi.org/10.1152/physrev.00033.2011
Article CAS PubMed Google Scholar * Després J-P, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global
cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49. https://doi.org/10.1161/ATVBAHA.107.159228 Article CAS PubMed Google Scholar * Kuk JL, Church TS, Blair SN, Ross R.
Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–84.
https://doi.org/10.2337/diacare.29.03.06.dc05-1500 Article PubMed Google Scholar * Dadson P, Landini L, Helmiö M, Hannukainen JC, Immonen H, Honka M-J, et al. Effect of bariatric surgery
on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292–9. https://doi.org/10.2337/dc15-1447 Article CAS PubMed
Google Scholar * Villaret A, Galitzky J, Decaunes P, Estève D, Marques M-A, Sengenès C, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in
angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–63. https://doi.org/10.2337/db10-0398 Article CAS PubMed PubMed Central Google
Scholar * Kloting N, Stumvoll M, Bluher M. Biologie des viszeralen Fetts. Der Internist. 2007;48:126–33. https://doi.org/10.1007/s00108-006-1781-x Article CAS PubMed Google Scholar *
Nauli AM, Matin S. Why do men accumulate abdominal visceral fat. Front Physiol. 2019;10:1486. https://doi.org/10.3389/fphys.2019.01486 Article PubMed PubMed Central Google Scholar *
Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. https://doi.org/10.1186/2042-6410-3-13
Article PubMed PubMed Central Google Scholar * Arner P, Hellström L, Wahrenberg H, Brönnegård M. Beta-adrenoceptor expression in human fat cells from different regions. J Clin Invest.
1990;86:1595–600. https://doi.org/10.1172/JCI114880 Article CAS PubMed PubMed Central Google Scholar * Lönnqvist F, Thöme A, Nilsell K, Hoffstedt J, Arner P. A pathogenic role of
visceral fat beta 3-adrenoceptors in obesity. J Clin Invest. 1995;95:1109–16. https://doi.org/10.1172/JCI117758 Article PubMed PubMed Central Google Scholar * Lee S, Kuk JL, Davidson LE,
Hudson R, Kilpatrick K, Graham TE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol.
2005;99:1220–5. https://doi.org/10.1152/japplphysiol.00053.2005 Article PubMed Google Scholar * Okura T, Nakata Y, Lee DJ, Ohkawara K, Tanaka K. Effects of aerobic exercise and obesity
phenotype on abdominal fat reduction in response to weight loss. Int J Obes. 2005;29:1259–66. https://doi.org/10.1038/sj.ijo.0803013 Article CAS Google Scholar * Merlotti C, Ceriani V,
Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and
meta-analysis. Int J Obes. 2005;41:672–82. https://doi.org/10.1038/ijo.2017.31. * Xu Z, Liu Y, Yan C, Yang R, Xu L, Guo Z, et al. Measurement of visceral fat and abdominal obesity by
single-frequency bioelectrical impedance and CT: a cross-sectional study. BMJ Open. 2021;11:e048221. https://doi.org/10.1136/bmjopen-2020-048221 Article PubMed PubMed Central Google
Scholar * Linder N, Schaudinn A, Garnov N, Bluher M, Dietrich A, Schutz T, et al. Age and gender specific estimation of visceral adipose tissue amounts from radiological images in morbidly
obese patients. Sci Rep. 2016;6:22261. https://doi.org/10.1038/srep22261 Article CAS PubMed PubMed Central Google Scholar * Froelich MF, Fugmann M, Daldrup CL, Hetterich H, Coppenrath
E, Saam T, et al. Measurement of total and visceral fat mass in young adult women: a comparison of MRI with anthropometric measurements with and without bioelectrical impedance analysis.
Brit J Radiol. 2020;93:20190874. https://doi.org/10.1259/bjr.20190874 Article PubMed Google Scholar * Browning LM, Mugridge O, Dixon AK, Aitken SW, Prentice AM, Jebb SA. Measuring
abdominal adipose tissue: comparison of simpler methods with MRI. Obes Facts. 2011;4:9–15. https://doi.org/10.1159/000324546 Article PubMed PubMed Central Google Scholar * Shuster A,
Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Brit J Radiol. 2012;85:1–10.
https://doi.org/10.1259/bjr/38447238 Article CAS PubMed PubMed Central Google Scholar * Bi X, Seabolt L, Shibao C, Buchowski M, Kang H, Keil CD, et al. DXA-measured visceral adipose
tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. Eur J Clin Nutr. 2015;69:329–36. https://doi.org/10.1038/ejcn.2014.227
Article CAS PubMed Google Scholar * Hoffmann J, Thiele J, Kwast S, Borger MA, Schröter T, Falz R, et al. Measurement of subcutaneous fat tissue: reliability and comparison of caliper and
ultrasound via systematic body mapping. Sci Rep. 2022;12. https://doi.org/10.1038/s41598-022-19937-4 * Woldemariam MM, Evans KD, Butwin AN, Pargeon RL, Volz KR, Spees C. Measuring abdominal
visceral fat thickness with sonography: a methodologic approach. J Diagn Med Sonography. 2018;34:91–6. https://doi.org/10.1177/8756479317747210 Article Google Scholar * Lochner S,
Moghaddam N, Graw A & Graw M. Physikalische Eigenschaften von Fettgewebe - Vergleichende Untersuchungen an Erwachsenen und Kindern als Grundlage für die Entwicklung virtueller
Menschmodelle; Available from: https://www.rechtsmedizin.med.uni-muenchen.de/service/downloads/sdt2010_berlin_fett.pdf * Foster KR, Lukaski HC. Whole-body impedance–what does it measure? Am
J Clin Nutr. 1996;64:388S–96S. https://doi.org/10.1093/ajcn/64.3.388S Article CAS PubMed Google Scholar * Ward LC. Bioelectrical impedance analysis for body composition assessment:
reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019;73:194–9. https://doi.org/10.1038/s41430-018-0335-3 Article PubMed Google Scholar * Scott B, Reeder
Ergin, Atalar Bradley D, Bolster Elliot R, McVeigh. Quantification and reduction of ghosting artifacts in interleaved echo-planar imaging. Magn Reson Med. 1997;38:429–39.
https://doi.org/10.1002/mrm.1910380312 * Vu K-N, Haldipur AG, Roh AT-H, Lindholm P, Loening AM. Comparison of end-expiration versus end-inspiration breath-holds with respect to respiratory
motion artifacts on T1-weighted abdominal MRI. AJR. Am J Roentgenol. 2019:1–6. https://doi.org/10.2214/AJR.18.20239 * Yang RK, Roth CG, Ward RJ, deJesus JO, Mitchell DG. Optimizing abdominal
MR imaging: approaches to common problems. Radiographics. 2010;30:185–99. https://doi.org/10.1148/rg.301095076 Article PubMed Google Scholar * Bland JM, Altman DG. Measuring agreement in
method comparison studies. Stat Methods Med Res. 1999;8:135–60. https://doi.org/10.1191/096228099673819272 Article CAS PubMed Google Scholar * Koo TK, Li MY. A guideline of selecting
and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. https://doi.org/10.1016/j.jcm.2016.02.012 Article PubMed PubMed Central Google
Scholar * Bloomer C, Rehm G, (eds.). Using principal component analysis to find correlations and patterns at diamond light source. Geneva, Switzerland: JACoW; 2014. Google Scholar *
Tabachnick BG, Fidell LS. Using multivariate statistics. New York, NY: Pearson; 2019. Google Scholar * Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments.
Philos Trans Ser A Math Phys Eng Sci. 2016;374:20150202. https://doi.org/10.1098/rsta.2015.0202 Article Google Scholar * Cureton EE. Factor analysis: an applied approach. Hillsdale, New
Jersey: L. Erlbaum Associates; 1983. * Hayton JC, Allen DG, Scarpello V. Factor retention decisions in exploratory factor analysis: a tutorial on parallel analysis. Organ Res Methods.
2004;7:191–205. https://doi.org/10.1177/1094428104263675 Article Google Scholar * Grimm LG, Yarnold PR, (eds.). Reading and understanding multivariate statistics. 13th ed. Washington, DC:
American Psychological Assoc; 2009. Google Scholar * Tinsley HEA, Brown SD, (eds.). Handbook of applied multivariate statistics and mathematical modeling. San Diego: Academic Press; 2010.
Google Scholar * Osborne J. Best practices in quantitative methods. Los Angeles, Calif.: Sage Publ; 2008. Book Google Scholar * Johnson, K. E., Miller, B., Mclain, T. A., Gibson, A. L.,
Otterstetter, R. Bioelectrical impedance and ultrasound to assess body composition in college-aged adults. J Adv Nutr Hum Metab. 2016. https://doi.org/10.14800/janhm.1176 * Alicandro G,
Battezzati A, Bianchi ML, Loi S, Speziali C, Bisogno A, et al. Estimating body composition from skinfold thicknesses and bioelectrical impedance analysis in cystic fibrosis patients. J Cyst
Fibros. 2015;14:784–91. https://doi.org/10.1016/j.jcf.2015.07.011 Article PubMed Google Scholar * Agrawal S, Wang M, Klarqvist MDR, Smith K, Shin J, Dashti H, et al. Inherited basis of
visceral, abdominal subcutaneous and gluteofemoral fat depots. Nat Commun. 2022;13:3771. https://doi.org/10.1038/s41467-022-30931-2 Article CAS PubMed PubMed Central Google Scholar *
Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Brit J Nutr. 1978;40:497–504. https://doi.org/10.1079/bjn19780152 Article CAS PubMed Google Scholar *
Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–81. Article CAS PubMed Google Scholar * Nevill AM, Metsios
GS, Jackson AS, Wang J, Thornton J, Gallagher D. Can we use the Jackson and Pollock equations to predict body density/fat of obese individuals in the 21st century? Int J Body Compos Res.
2008;6:114–21. CAS PubMed PubMed Central Google Scholar * Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on
481 men and women aged from 16 to 72 years. Brit J Nutr. 1974;32:77–97. https://doi.org/10.1079/bjn19740060 Article CAS PubMed Google Scholar * Goran MI, Toth MJ, Poehlman ET.
Cross-validation of anthropometric and bioelectrical resistance prediction equations for body composition in older people using the 4-compartment model as a criterion method. J Am Geriatrics
Soc. 1997;45:837–43. https://doi.org/10.1111/j.1532-5415.1997.tb01511.x Article CAS Google Scholar * Leahy S, Toomey C, McCreesh K, O’Neill C, Jakeman P. Ultrasound measurement of
subcutaneous adipose tissue thickness accurately predicts total and segmental body fat of young adults. Ultrasound Med Biol. 2012;38:28–34. https://doi.org/10.1016/j.ultrasmedbio.2011.10.011
Article PubMed Google Scholar * Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk.
Int J Obes Relat Metab Disord. 2004;28:1018–25. https://doi.org/10.1038/sj.ijo.0802695 Article CAS PubMed Google Scholar * Rantalainen M, Herrera BM, Nicholson G, Bowden R, Wills QF, Min
JL, et al. MicroRNA expression in abdominal and gluteal adipose tissue is associated with mRNA expression levels and partly genetically driven. PloS One. 2011;6:e27338.
https://doi.org/10.1371/journal.pone.0027338 Article CAS PubMed PubMed Central Google Scholar * Lundblad MW, Jacobsen BK, Johansson J, Grimsgaard S, Andersen LF, Hopstock LA.
Anthropometric measures are satisfactory substitutes for the DXA-derived visceral adipose tissue in the association with cardiometabolic risk-The Tromsø Study 2015-2016. Obes Sci Practice.
2021;7:525–34. https://doi.org/10.1002/osp4.517 Article Google Scholar * Burton RF. The waist-hip ratio: a flawed index. Ann Hum Biol. 2020;47:629–31.
https://doi.org/10.1080/03014460.2020.1820079 Article PubMed Google Scholar * Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference,
waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Rel Metab Disord. 2001;25:652–61.
https://doi.org/10.1038/sj.ijo.0801582 Article CAS Google Scholar Download references FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Outpatient Clinic of Sports Medicine, University of Leipzig, Rosa-Luxemburg-Str. 20-30, 04103, Leipzig, Germany Jana Hoffmann & Martin Busse * Department of Radiology,
Helios Klinik, 04435, Schkeuditz, Germany Jens Thiele * Helios Health Institute, 13125, Berlin, Germany Stefan Kwast * University Department of Cardiac Surgery, Heart Center, 04289, Leipzig,
Germany Michael Andrew Borger & Thomas Schröter * Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, 04103, Leipzig, Germany Jochen Schmidt Authors
* Jana Hoffmann View author publications You can also search for this author inPubMed Google Scholar * Jens Thiele View author publications You can also search for this author inPubMed
Google Scholar * Stefan Kwast View author publications You can also search for this author inPubMed Google Scholar * Michael Andrew Borger View author publications You can also search for
this author inPubMed Google Scholar * Thomas Schröter View author publications You can also search for this author inPubMed Google Scholar * Jochen Schmidt View author publications You can
also search for this author inPubMed Google Scholar * Martin Busse View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Idea, concept and
research design were conducted by JH and MB. Writing by JH. Data collection by JH and JT. Data analysis by JH and JS. Data interpretation by JH and SK. All authors contributed to the
critical review and approved the submitted manuscript. CORRESPONDING AUTHOR Correspondence to Jana Hoffmann. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hoffmann, J.,
Thiele, J., Kwast, S. _et al._ A new approach to quantify visceral fat via bioelectrical impedance analysis and ultrasound compared to MRI. _Int J Obes_ 48, 209–217 (2024).
https://doi.org/10.1038/s41366-023-01400-7 Download citation * Received: 09 June 2023 * Revised: 19 September 2023 * Accepted: 10 October 2023 * Published: 27 October 2023 * Issue Date:
February 2024 * DOI: https://doi.org/10.1038/s41366-023-01400-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Understanding Stalin - The AtlanticHow did Stalin become Stalin? Or, to put it more precisely: How did Iosif Vissarionovich Djugashvili—the grandson of ser...
I really look up to nayanthara: director vignesh shivn's tweet is winning the internetNayanthara went through an ugly phase when her relationships fell out. Her photographs with actor Silambarasan were leak...
Folgers is trying to be cool — but it has a problem with its reputationRACHEL MARTIN, HOST: Folgers is trying to be cool. As the biggest seller of ground coffee in U.S. stores, the brand has ...
Week in politics: pelosi steps down; trump announces 2024 presidential runSCOTT SIMON, HOST: Most of the midterm elections have been called, and we do know congressional leadership will be diffe...
Sunday brunch's tim lovejoy forced to step in as show guest swears live on airSUNDAY BRUNCH HOST TIM LOVEJOY WAS FORCED TO APOLOGISE AS A SHOW GUEST SWORE LIVE ON AIR DURING SUNDAY'S EPISODE MO...
Latests News
A new approach to quantify visceral fat via bioelectrical impedance analysis and ultrasound compared to mriABSTRACT BACKGROUND Visceral adipose tissue (VAT) has been linked to systemic proinflammatory characteristics, and measu...
Attention Required! | CloudflareАшер Кричевский – главный раввин Омской области. Он учился в Израиле и в Нью-Йорке, проходил практику в Аргентине, дипло...
Civil Rights Pioneer Thrives With Granddaughter’s Help2:16 Videos de AARP Civil Rights Pioneer Thrives With Granddaughter’s Help Facebook Twitter LinkedIn In 1956 Rob Grier b...
Time is of the essence: micrornas and age-associated neurodegenerationAging is a key risk factor in neurodegenerative disease; however, little is known about cellular pathways that mediate a...
Watch Live Sports TV - News & Live Sports Channels on SkyWatch Sky Sports On now Back Live EFL SF: Stockport v Leyton Orient Live Live 1st ODI: ENG v WI Live PL Retro: Birmingha...