Mechanical overload-induced release of extracellular mitochondrial particles from tendon cells leads to inflammation in tendinopathy
Mechanical overload-induced release of extracellular mitochondrial particles from tendon cells leads to inflammation in tendinopathy"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Tendinopathy is one of the most common musculoskeletal diseases, and mechanical overload is considered its primary cause. However, the underlying mechanism through which mechanical
overload induces tendinopathy has not been determined. In this study, we identified for the first time that tendon cells can release extracellular mitochondria (ExtraMito) particles, a
subtype of medium extracellular particles (mEPs), into the environment through a process regulated by mechanical loading. RNA sequencing systematically revealed that oxygen-related
reactions, extracellular particles, and inflammation were present in diseased human tendons, suggesting that these factors play a role in the pathogenesis of tendinopathy. We simulated the
disease condition by imposing a 9% strain overload on three-dimensional mouse tendon constructs in our cyclic uniaxial stretching bioreactor. The three-dimensional mouse tendon constructs
under normal loading with 6% strain exhibited an extended mitochondrial network, as observed through live-cell confocal laser scanning microscopy. In contrast, mechanical overload led to a
fragmented mitochondrial network. Our microscopic and immunoblot results demonstrated that mechanical loading induced tendon cells to release ExtraMito particles. Furthermore, we showed that
mEPs released from tendon cells overloaded with a 9% strain (mEP9%) induced macrophage chemotaxis and increased the production of proinflammatory cytokines, including IL-6, CXCL1, and
IL-18, from macrophages compared to mEP0%, mEP3%, and mEP6%. Partial depletion of the ExtraMito particles from mEP9% by magnetic-activated cell sorting significantly reduced macrophage
chemotaxis. N-acetyl-L-cysteine treatment preserved the mitochondrial network in overloaded tendon cells, diminishing overload-induced macrophage chemotaxis toward mEP9%. These findings
revealed a novel mechanism of tendinopathy; in an overloaded environment, ExtraMito particles convey mechanical response signals from tendon cells to the immune microenvironment, culminating
in tendinopathy. SIMILAR CONTENT BEING VIEWED BY OTHERS AUTOPHAGY GUARDS TENDON HOMEOSTASIS Article Open access 23 April 2022 MULTI-OMICS ANALYSIS OF HUMAN TENDON ADHESION REVEALS THAT
ACKR1-REGULATED MACROPHAGE MIGRATION IS INVOLVED IN REGENERATION Article Open access 07 May 2024 MULTI-OMIC SINGLE CELL ANALYSIS RESOLVES NOVEL STROMAL CELL POPULATIONS IN HEALTHY AND
DISEASED HUMAN TENDON Article Open access 03 September 2020 INTRODUCTION The tendon primarily consists of type I collagen, which is stretched into parallel elastic fiber bundles, with tendon
cells embedded within the matrix1. The main function of the tendon is to transmit the force generated from the muscles to the bones at the entheses, thus driving joint movements. Because of
the uniqueness of the tendon’s physiological function and structural features, tendons undergo numerous repetitive stretches daily. This regular stress often leads to tendon degeneration,
which gives rise to tendinopathy2. Tendinopathy is one of the most common musculoskeletal diseases, with a 10.52 per 1000 person-years incidence of tendinopathy in the lower limb2 and a 2%
to 3.8% incidence of tendinopathy in the upper limb for adults in the general population3,4. Tendinopathy seriously affects the quality of life of patients. Up to 5% of patients with upper
limb tendinopathy have taken approximately 29 sick days from work5. Tendinopathy also poses a substantial economic burden. Absenteeism due to lateral epicondylitis was estimated to cost
approximately £27 million annually in the United Kingdom alone6. Moreover, tendinopathy may further develop into tendon rupture, which leads to limb dysfunction and ultimately surgical
intervention7. While tendinopathy can be managed well with analgesics and physical therapy in most patients, effective treatments that directly address the underlying pathogenesis are still
lacking8. One main reason is that the underlying pathological mechanism remains unclear. Multiple factors contribute to the pathogenesis of tendinopathy. Some studies have reported that
tendinopathy is a process of extracellular matrix (ECM) degeneration and dysregulation9,10. Moreover, over the past decade, studies have reported that inflammation plays a role in the
development of tendinopathy11. Tendon cells, as the residential stromal component, interact with immune cells from the infiltrating compartment and immune-sensing compartment, resulting in a
complex cascade of inflammatory responses and subsequent matrix degradation11. Although the intrinsic mechanisms of tendinopathy remain a matter of debate, some theories propose that these
pathological changes might work together in the development of tendinopathy12,13. Subcellular responses, such as lysosomal quality control, endoplasmic reticulum stress, and mitochondrial
dynamics, have been found to affect both inflammation and tissue regeneration14,15,16. However, the subcellular responses in tendinopathy are largely unknown. In this study, we performed RNA
sequencing (RNA-seq) on human tendons with tendinopathy to systematically explore the cellular responses in tendinopathy at the molecular level. We used an in vitro three-dimensional (3D)
uniaxial stretching bioreactor platform to closely mimic the in vivo mechanical stimulation of tendons. Based on data obtained from transcriptomic profiling, the 3D uniaxial stretching
bioreactor platform, live-cell confocal laser scanning microscopy (CLSM), and magnetic-activated cell sorting (MACS) technology, we identified for the first time that extracellular
mitochondria (ExtraMito), a novel subpopulation of tendon-derived medium extracellular particles (mEPs), play an essential role in passing mechanical information from tendon cells into the
immune microenvironment. In this study, we explored new pathological mechanisms at the subcellular level that can potentially be used to address tendinopathy. MATERIALS AND METHODS HUMAN
TENDON SAMPLE COLLECTION The collection of human tendon samples for sequencing analysis was performed according to the protocols reviewed and approved by the Ethics Committee of Guangdong
Provincial People’s Hospital (ethics approval number KY-Z-2020-586-02). The study was conducted based on the guidelines of the Declaration of Helsinki. Informed consent was obtained from the
donors prior to tissue collection. The patient information remains confidential. Limited patient data, including sex and tendinopathy-related medical descriptions, were obtained. Unhealthy
tendon tissues were surgically collected from 2 patients diagnosed with rotator cuff tendinopathy by magnetic resonance imaging. Healthy tendon tissues were collected from the hamstring
tendons of 3 patients who underwent anterior cruciate ligament reconstruction. RNA SEQUENCING, MAPPING, AND ANALYSIS Total RNA was extracted from human tendon samples by TRIzol reagent
(Invitrogen, Waltham, United States). The RNA quality was determined by an Agilent 2200 instrument, and the samples were stored at −80 °C. RNA with an RNA integrity number (RIN) > 7.0 was
acceptable for cDNA library construction. cDNA libraries were established for each RNA sample using the TruSeq Stranded mRNA Library Prep Kit (Illumina, Inc., San Diego, United States)
according to the manufacturer’s instructions. Briefly, the protocol consisted of the following steps. Using oligo (dT) magnetic beads, poly(A)-containing mRNA was purified from 1 µg of total
RNA and fragmented into 200–600 bp fragments using divalent cations at 85 °C for 6 min. The cleaved RNA fragments were subsequently subjected to first- and second-strand complementary DNA
(cDNA) synthesis. The dUTP mixture was used for second-strand cDNA synthesis, which allowed for the removal of the second strand. The cDNA fragments were end-repaired, A-tailed and ligated
with indexed adapters. To remove the second-strand cDNA, the ligated cDNA products were purified and treated with uracil DNA glycosylase. Purified first-strand cDNA was enriched by PCR to
create cDNA libraries. Quality control of the libraries was conducted with an Agilent 2200 platform, and the libraries were sequenced with a NovaSeq 6000 platform on a 150 bp paired-end run.
By removing the adaptor sequences and low-quality reads, clean reads were obtained. The clean reads were subsequently aligned to the human genome (GRCh38) using HISAT217. For quality
control, the raw sequencing data, including the quality distribution of nucleotides, GC content, proportion of PCR duplication, position-specific sequencing quality, and kmer frequency, were
evaluated via FAST-QC. HTseq was applied to obtain gene counts, and the RPKM method was used to determine gene expression18. The data were analyzed by the DESeq2 algorithm19. The genes that
were differentially expressed between healthy and unhealthy tendons were considered to have a _P_ value < 0.05. The computed _Z_ score was used to plot a heatmap of the differentially
expressed genes. R (version 4.2.2; Boston, United States) software was used for the data analysis. HALLMARK GENE SET ANALYSIS AND GENE ONTOLOGY (GO) ANALYSIS OF THE RNA SEQUENCING DATA The
top 100 upregulated differentially expressed genes (DEGs) (ranked by fold change) were subjected to enrichment analysis. To evaluate the associations of the various biological events
activated in unhealthy tendons, the h.all.v7.4.symbols.gmt sets from the Molecular Signatures Database20 were downloaded for the hallmark gene set analysis. For the GO functional analysis,
annotations from the R package “org.Hs.eg.db (version 3.1.0)” were retrieved. The R package “clusterProfiler (version 3.14.3)” was used for the enrichment analysis. A _P_ value < 0.05 was
considered to indicate statistical significance. BIOINFORMATIC ANALYSIS OF THE MICROARRAY DATASETS Microarray datasets were obtained from the Gene Expression Omnibus (GEO) database
(ncbi.nlm.nih.gov/geo/) under the ID GSE26051. The data were analyzed using the limma algorithm21. Differentially expressed genes between healthy and unhealthy tendons were identified with a
_P_ value threshold of <0.05. The top 500 upregulated DEGs, ranked by fold change, were subjected to enrichment analysis. GO enrichment analysis was carried out in the same manner as
that for the RNA sequencing data. We used GSEA software (version 3.0), which was obtained from the GSEA website22 (software.broadinstitute.org/gsea/index.jsp). We downloaded the
h.all.v7.4.symbols.gmt subset for hallmark analysis and the c5.go.bp.v7.4.symbols.gmt subset for BP analysis from the Molecular Signatures Database20
(http://www.gsea-msigdb.org/gsea/downloads.jsp). The minimum set size was set at 5, and the maximum was set at 5000, with the analysis involving 1000 permutations. A _P_ value < 0.05 was
considered to indicate statistical significance. PORTRAYING THE MACROPHAGE LANDSCAPE WITH XCELL To explore macrophage infiltration in tendon tissues via transcriptomic analysis, the xCell
webtool (https://xcell.ucsf.edu/) was used to digitally portray the cellular landscape23. Standard RNA-Seq expression was normalized to gene length as transcripts per kilobase million (TPM)
and submitted for analysis with the “RNA-seq = True” option. A _P_ value of <0.2 was considered to indicate statistical significance according to the instructions of xCell23. ISOLATION OF
MOUSE TENDON CELLS The use of the mice was approved, and the mice were housed and maintained in accordance with the Institutional Animal Care and Use Committee guidelines of the University
of Western Australia Animal Ethics Committee (RA/3/100/1526; RA/3/100/1731). Six- to eight-week-old C57BL/6 mice were euthanized by cervical dislocation. The middle portions of the Achilles
tendons and patellar tendons were isolated and rinsed with phosphate-buffered saline (PBS) containing 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco™, Waltham, United States).
Isolated tendon tissue was digested with 3 mg/mL type II collagenase in α-Minimal Essential Medium (MEM Alpha, Gibco™) for 3 h. After filtering through a 70 μm cell strainer, the cells were
centrifuged and cultured in Alpha MEM supplemented with 10% fetal bovine serum (FBS, Gibco™), 100 U/mL penicillin, and 100 μg/mL streptomycin. The incubation conditions were 37 °C in a
humidified atmosphere containing 5% CO2. Passage 3 cells were used in the experiments. 3D TENDON CONSTRUCT FORMATION AND MECHANICAL STIMULATION 3D tendon construct formation and mechanical
stimulation were adapted from published methods24,25. Mouse tendon cells were seeded into a T-75 flask (106 cells) and cultured to complete confluence. The cells were then stimulated with 25
ng/mL connective tissue growth factor (CTGF; PeproTech, Waltham, United States) and 4.4 μg/mL ascorbic acid (Sigma‒Aldrich, Burlington, United States) for 6 days to generate an
extracellular matrix and to form monolayer cell sheets. Monolayer cell sheets were then detached from the flask and subsequently attached to custom-made tissue hooks in a bioreactor to form
3D tendon constructs26. These 3D tendon constructs were cultured in a bioreactor containing Alpha MEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Extracellular vesicles were depleted from the FBS beforehand27. For uniaxial 3D stretching, the programmable bioreactor was set up for 8 h/day, followed by 16 h of rest, with 0%, 3%, 6%, or
9% strain, at 37 °C and 5% CO2 for 6 days. The strain (in percentage) was calculated as follows: Strain (%) = ((final length – initial length)/initial length) × 100. For example, a 0% strain
indicates static culture, whereas a 100% strain implies stretching the tissue to double its original length. The middle thirds of the 3D tendon constructs were obtained for downstream
analysis after mechanical stimulation. CHEMICAL TREATMENTS, TRANSFECTION, AND STAINING OF TENDON CONSTRUCTS To protect the intracellular mitochondrial network in the overloaded tendon
constructs, they were incubated with 2.5 mM NAC (Sigma, Burlington, United States) during mechanical stimulation by 9% or 0% strain. To label mitochondria with an RFP in the 3D tendon
construct, cell sheets were incubated with Mitochondria-RFP (C10601; CellLight, Thermo Fisher Scientific, Waltham, United States), a baculovirus-packaged DNA construct, for 16 h following
the manufacturer’s protocol and then assembled into the bioreactor to form tendon constructs. To label the mitochondria with MTR and cytoplasm with CFSE for particle characterization, 3D
tendon constructs were stained with 1 μM CFSE for 15 min and 20 nM MTR for 30 min before mechanical stimulation. For live-cell imaging to observe mitochondrial morphology in tendon
constructs, 3D tendon constructs were stained with 20 nM MTR for 30 min after mechanical stimulation, washed three times, and observed in live-cell imaging solution (A14291DJ, Invitrogen,
Waltham, United States). REVERSE TRANSCRIPTION QUANTITATIVE POLYMERASE CHAIN REACTION (RT–QPCR) Tendon constructs were pulverized into powder using a mortar and pestle under liquid nitrogen
conditions. Total RNA was extracted using TRIzol reagent (15596-026, Invitrogen) and a PureLink™ RNA Mini Kit (Invitrogen) according to the manufacturer’s instructions. The RNA concentration
was evaluated spectrophotometrically by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized from RNA using Moloney murine leukemia virus
(M-MLV) reverse transcriptase (Promega, Madison, United States). The cDNA was subsequently amplified and quantified via real-time PCR using the iQTM SYBR® Green Supermix (Bio-Rad
Laboratories, Hercules, CA, USA). RT‒PCR was repeated using three individual samples from each group, and each sample was analyzed in triplicate. The cycle threshold (Ct) was calculated for
each qPCR reaction. The relative expression levels of the target genes were calculated using the comparative 2-ΔΔCt method. The sequences of primers used for the genes are listed in
Supplementary Table 2. IMMUNOBLOT ANALYSIS The tendon constructs were frozen using liquid nitrogen and ground into powder using a mortar and pestle before protein extraction. Tendon
constructs or mEP pellets were incubated with radioimmunoprecipitation assay lysis buffer supplemented with protease inhibitor (Roche, Basel, Switzerland) and a phosphatase inhibitor
cocktail (Sigma‒Aldrich) for 30 min at 4 °C. After centrifugation, the debris was pelleted, and the supernatants were transferred into 1 mL tubes, which were subsequently diluted with 4 ×
SDS sampling buffer and boiled for 5 min. The protein concentration was evaluated via a Bio-Rad protein assay (Bio-Rad, Hercules, United States). Unless otherwise stated, equal amounts of
protein were separated via SDS‒polyacrylamide gel electrophoresis and subsequently transferred to nitrocellulose membranes (Millipore). The membranes were incubated at 4 °C overnight with
primary antibodies against β-actin JLA20 (1:5000; Developmental Studies Hybridoma Bank, Iowa City, United States), VDAC (1:1000; ab15895, Abcam, Cambridge, United Kingdom), TOM20 (1:1000;
ab186735, Abcam), CD63 (1:1000; ab217345, Abcam), Annexin A2 (1:1000; #8235, Cell Signaling, Danvers, United States), Lamin A/C (1:2000; #4777, Cell Signaling), and Albumin (1:1000;
ab175934, Abcam) and then incubated with the corresponding secondary antibodies conjugated with horseradish peroxidase (Sigma‒Aldrich) for 1 h at room temperature. Immunoreactivity was
evaluated by the Western Lightning Ultra Detection Kit (PerkinElmer, Waltham, Massachusetts, United States) according to the manufacturer’s instructions. Detection of chemiluminescence was
performed with a ChemiDoc™ MP Imaging System (Bio-Rad). Uncropped images are provided in Supplementary Fig. 9. Quantification was performed with ImageJ (National Institutes of Health,
Bethesda, United States). FINITE ELEMENT ANALYSIS (FEA) To illustrate the uneven strain distribution in the tendon construct, a two-dimensional plane stress finite element model of the
sample was generated via simulation via the ANSYS software package (ANSYS, Canonsburg, USA; v2021R1). The construct was modeled in a concave shape to match the geometry of the experimental
sample, which was assumed to be isotropic, linearly elastic, and incompressible in the simulation. A Young’s modulus of 10 GPa (a typical value obtained from the stress‒strain curve of
tendon28) was used as input in the simulation. The 2-D model was generated by dividing the geometry into mesh elements with a mesh size of 0.2 mm, in which the approximate solutions to the
partial differential equations describing the mechanical response of the construct subjected to a known applied strain of 3% were computed, with a well-defined (fixed) boundary condition
assigned to one end of the construct. TENDON-DERIVED MEP COLLECTION mEPs were isolated from the conditioned media of tendon constructs according to published methods29. Briefly, tendon
constructs were cultured in a bioreactor containing 30 mL of Alpha MEM for each tendon construct supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Extracellular
vesicles in the FBS were depleted beforehand27. After mechanical stimulation for 6 days, conditioned media were obtained and centrifuged at 400 × g to pellet nonadherent cells for 5 min. The
supernatant was subsequently centrifuged to pellet cellular debris at 1500 × g for 10 min. The remaining supernatant was subsequently centrifuged to pellet mEPs at 18000 × g for 30 min. To
separate the mEP fractions through a high-resolution iodixanol density step-gradient, the pellet containing mEPs was resuspended in a 40% (v/v) iodixanol (OptiPrep, Burlington, United
States) solution. A density step gradient was set up by carefully layering decreasing concentrations of iodixanol (20, 15, 13, 11, 9, 7, and 5%) on top of the 40% mEPs containing iodixanol
solution. The column was then centrifuged for 16 hs at 4 °C at 200,000 × g. Afterward, 1.25 mL of the gradient from the top (fraction 1) was collected, and 1.5 mL of the fractions (fractions
2–8) were subsequently collected. All the fractions were mixed with 4 mL of ice-cold PBS, except for Fraction 8, which was mixed with 8 mL of ice-cold PBS. The fractions were subsequently
centrifuged at 18000 × g for 30 min to collect the pellets of each fraction. CHEMOTAXIS ASSAY AND CYTOKINE PRODUCTION ANALYSIS The RAW 264.7 mouse macrophage line was used for the subsequent
experiments. The chemotaxis of RAW 264.7 cells was assessed with a colorimetric cell migration assay kit (ECM508, MILLIPORE QCMTM, Sigma) based on the Boyden chamber principle, with inserts
utilizing polycarbonate membranes with 8 μm pores. Briefly, RAW 264.7 cells were starved by incubating them for 24 h prior to the assay in serum-free Dulbecco’s modified Eagle’s medium
(DMEM, ATC302002, ATCC, Manassas, United States). Then, 300 μL of 1 × 106 RAW 264.7 cells was added to the insert. Then, 500 μl of serum-free DMEM was added to the lower chamber, with either
PBS as a negative control or mEPs in PBS as a chemoattractant. To evaluate the biological function of the mEPs, mEPs from 500 μm tendon constructs in 500 μL medium were applied to each
insert; for a tendon construct with a length of 1 cm, one-twentieth of the mEPs were used. The mass of the 500 μm tendon construct was found to be 1.1 ± 0.1 mg, with the protein amount shown
as mEP0% (5.85 ± 0.18 µg), mEP3% (5.87 ± 0.34 µg), mEP6% (6.94 ± 0.21 µg), and mEP9% (8.88 ± 0.30 µg). After incubating for 24 h at 37 °C in 5% CO2, the remaining cell suspension from the
top chamber of the insert was removed. The migration insert was placed into a well containing the cell stain from the kit and left for 20 min at room temperature. The nonmigratory cell layer
from the interior of the moist insert was then removed with cotton-tipped swabs. The inserts were then air-dried and observed by an Eclipse TE2000-S microscope (Nikon, Konan, Japan). For
quantification, the stained insert was moved to a well containing extraction buffer for 15 min at room temperature, after which the mixture was gently tilted back and forth during incubation
to extract the stain from the underside. The optical density (OD) of the extracted stain was measured at 560 nm by a Cell Imaging MultiMode Reader (Cytation 5, BioTek, Winooski, U.S.) with
Gen5 software (BioTek). To evaluate the ability of tendon-derived mEPs to induce the production of inflammatory cytokines, RAW 264.7 cells were cultured with tendon-derived mEPs, PBS (as the
negative control), or 500 ng/mL lipopolysaccharide (LPS) (as the positive control) for 8 h in 6-well plates. The concentration of mEPs was the same as that used in the chemotaxis assay.
However, to examine whether mEPs from various conditions mediate the release of inflammatory cytokines in a dose-dependent manner, we used a 10-fold mEP concentration gradient. These
included mEPs from tendon constructs of 50 μm, 500 μm, and 5 mm in 500 μL medium. FBS-free media supplemented with 200 μg/mL bovine serum albumin (BSA) were used for culture. Conditioned
media were then collected for cytokine measurements using a bead-based multiplex assay panel, LEGENDplexTM Mouse Macrophage Panel (13-plex) (740845; BioLegend, San Diego, United States), per
the manufacturer’s instructions. Briefly, the samples were incubated with the capture beads for 2 h at room temperature on a plate shaker. Then, the beads were spun down and washed.
Detection antibodies were added to the beads, which were incubated for 1 h at room temperature, followed by incubation with SA-PE at room temperature on a plate shaker. The beads were then
spun down, washed and resuspended in wash buffer. As the beads are differentiated by size and internal fluorescence intensity, analyte-specific populations can be identified and quantified
by flow cytometry (FACS Canto II Flow Cytometry, BD, Franklin Lakes, United States). The instrument was set up according to the manufacturer’s protocol. The concentration of each cytokine
was analyzed by a standard curve generated in the same assay using the standard samples provided in the assay panel. The data were analyzed with LEGENDplex™ software (BioLegend). MEP
CHARACTERIZATION BY FLOW CYTOMETRY AND DYNAMIC LIGHT SCATTERING For mEP characterization, nanoparticles with 4 different diameters (220 nm, 450 nm, 880 nm, and 1340 nm) were applied for the
calibration of size with an LSR Fortessa flow cytometer (BD) using SSC measurements (voltage: 412; gain: 1.0). For lipophilic membrane identification of mEPs, mEPs were stained with PKH26
(MINI26, Sigma‒Aldrich) after collection following the manufacturer’s protocol. Briefly, mEPs were suspended in Diluent C (CGLDIL, Sigma). Then, the mEPs were stained with 2 × 10–6 M PKH26
for 5 min. mEPs containing only the diluent were used as the control. The mEPs were then analyzed by an LSR Fortessa flow cytometer (BD) (voltage: 342; gain: 1.0). The data were processed
and analyzed with FlowJo software (V10, BD Life Sciences, Franklin Lakes, United States). Dynamic light scattering is a commonly used method for determining the size of small particles. To
characterize the size of the mEPs by dynamic light scattering, the mEPs were suspended in cuvettes and measured by a Zetasizer Nano Series (Malvern, United Kingdom) with Zetasizer Software
(version 7.13, Malvern). The refractive index value “n” was set as 1.40, and the absorption value “k” was set as 0.01. DEPLETION OF THE MITOCHONDRIA FROM TENDON-DERIVED MEPS Free
mitochondria were depleted from mEPs by MACS. Tendon-derived mEPs were incubated with anti-rabbit IgG magnetic beads coupled with anti-TOM20 antibodies (Miltenyi Biotec, Bergisch Gladbach,
Germany). Then, suspensions of magnetically labeled mEPs or sham-labeled mEPs were applied to an LD column (130-042-901; Miltenyi Biotec) in the magnetic field of a MidiMACS Separator
(130-042-302; Miltenyi Biotec). The flow through the LD column included TOM20- mEPs. The magnetically labeled TOM20+ mEPs were retained in the column and then eluted after the column was
removed from the separator. CONFOCAL LASER SCANNING MICROSCOPY (CLSM) IMAGING AND PROCESSING For live-cell imaging of mitochondrial morphology, pieces of the middle portion of the tendon
constructs were cut out and transferred from the bioreactor to 35-mm glass-bottom Petri dishes (P35G-0.17-14-C; MatTek, Ashland, United States). The mEP imaging procedure was modified from a
published method30. The mEPs in SlowFadeTM Glass Antifade Mountant (S36917, Thermo Fisher Scientific) were applied to 35-mm glass-bottom Petri dishes (P35G-0.17-14-C, MatTek) and flattened
against the surface tension before imaging. To observe the morphology of RAW 264.7 cells after treatment with or without ExtraMito-containing mEPs, 3D tendon constructs were stained with 20
nM MTR for 30 min before mechanical stimulation. Then, MTR-labeled mEP9% was obtained from MTR-labeled tendon constructs after 6 days of mechanical stimulation with 9% strain. RAW 264.7
cells were prelabeled with 1 μM CFSE for 15 min. CFSE-labeled RAW 264.7 cells were then cultured with or without MTR-labeled mEP9% on 13 mm microscope coverslips (ProSciTech, Kirwan,
Australia) placed in 24-well plates. The concentration of mEPs was the same as that used in the chemotaxis and cytokine production assays. After 24 h of incubation, the RAW 264.7 cells were
washed with PBS and fixed in 4% paraformaldehyde (PFA). Hoechst 33342 (1:5000; 62249; Thermo Fisher Scientific) was then applied for nuclear staining. The coverslips were mounted with
ProLong Diamond Antifade Medium (P36970, Invitrogen). Images were acquired on a Nikon A1Si confocal microscope with a 60× water immersion objective or a Nikon A1R confocal microscope with a
100x oil immersion objective. The CLSM instrument was equipped with a perfect focus system (PFS) and a Tokai Hit incubation chamber for live-cell imaging, set at 37 °C and 5% CO2.
Three-dimensional images were acquired by _Z_-stacks with 0.1–0.15 μm interstack intervals. Denoising, deconvolution, 3D rendering, and quantification of images were performed using
NIS-Elements Advanced Research (Nikon), Imaris (Bitplane, Zurich, Switzerland), and ImageJ (National Institutes of Health, Bethesda, United States). TRANSMISSION ELECTRON MICROSCOPY (TEM)
The mEPs were fixed in a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) (CEMS15960-01, ProSciTech). Carbon-coated copper 200 mesh grids
(GSCU200CC, ProSciTech) were glow discharged in a PELCO easiGlow (Ted Pella, Inc., Redding, United States). Fixed mEPs were then added to the glow-discharged grids held by self-clamping
tweezers for 1 min. The solution was then removed with Whatman filter paper, and the grids were negatively stained with 1% (w/v) uranyl acetate (Electron Microscopy Sciences, Hatfield,
Pennsylvania) for 30 s. An F200 transmission electron microscope (JEOL, Tokyo, Japan) with a Camera OneView 4k (Gatan, Pleasanton, United States) and DigitalMicrograph (Gatan) was used for
imaging at 200 kV. STATISTICAL ANALYSIS GraphPad Prism software (version 8, GraphPad Software, Inc., San Diego, United States) was used for statistical calculations. A two-tailed Student’s
_t_ test was performed for means comparisons between two groups. For comparisons of multiple group data, one-way analysis of variance (ANOVA) with Tukey’s post hoc test was used. At least
three biological replicates were performed for each experiment. In each imaging experiment, at least five different areas were scanned for each biological replicate. The data are presented
as the means ± SEMs. A _P_ value < 0.05 was considered to indicate statistical significance and is marked by asterisks (ns, no significance; *_P_ < 0.05; **_P_ < 0.01; and ***_P_
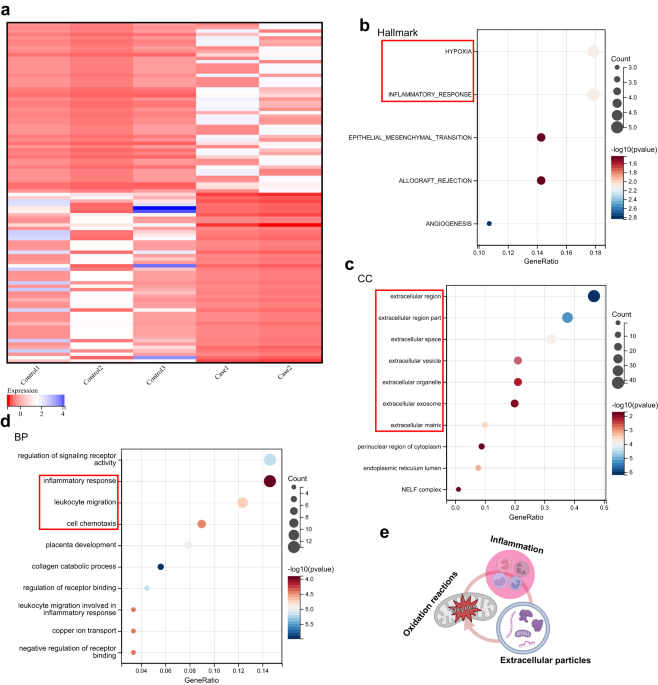
< 0.001). RESULTS TRANSCRIPTOMIC PROFILING REVEALS OXYGEN-RELATED REACTIONS, EXTRACELLULAR PARTICLES, AND INFLAMMATION IN HUMAN TENDINOPATHIC TISSUES To investigate the subcellular
responses in tendinopathy, we performed RNA-seq to obtain transcriptomic profiles from healthy human tendons and tendinopathic tissues. Healthy tendon tissues were obtained from 3 male
donors. Diseased tendons were obtained from a male patient and a female patient with chronic tendinopathy. Transcriptomic profiling identified 1083 upregulated genes and 1261 downregulated
genes (_P_ value < 0.05, |logFoldChange | > 2). The expression of the top 50 upregulated genes and the top 50 downregulated genes is shown in a heatmap (Fig. 1a, Supplementary Table
1). To evaluate the biological events activated in tendons with tendinopathy, we performed enrichment analysis of the hallmark gene sets of the top 100 upregulated genes in tendons with
tendinopathy. The results revealed that “hypoxia” and “inflammatory response” were the top-ranked biological events (Fig. 1b). For functional analysis, we used the Gene Ontology (GO)
cellular component (CC) and biological process (BP) terms. GO CC analysis of the top 100 upregulated genes revealed that the DEGs were enriched in extracellular components (Fig. 1c). We also
noted that the highest ranked cellular parts were “extracellular vesicle” and “extracellular organelle” according to the GO CC analysis. The GO BP analysis of these genes indicated that the
highly ranked functional activities were related to the inflammatory response, including “leukocyte migration” and “cell chemotaxis” (Fig. 1d). We then accessed archived microarray data
(GSE26051) from the Gene Expression Omnibus (GEO) database. These microarray data included 23 diseased tendons with tendinopathy and 23 normal tendons as controls. The enrichment analysis of
the microarray data supported our RNA sequencing findings. Gene Set Enrichment Analysis (GSEA) of the microarray data revealed that several inflammatory responses, such as “positive
regulation of leukocyte adhesion of vascular endothelial cell” and “positive regulation of leukocyte tethering or rolling”, were significantly activated in tendinopathy (Supplementary Fig.
1a). Furthermore, GSEA of hallmark gene sets showed that enrichment in “hypoxia” was positively associated with tendinopathy (Supplementary Fig. 1b). GO CC analysis of the top 500 genes
upregulated in the tendons from patients with tendinopathy compared to healthy individuals revealed that most of the genes activated in patients with tendinopathy were significantly related
to extracellular regions and extracellular particles, including different extracellular components (Supplementary Fig. 1c). Taken together, these data indicated that oxygen-related
reactions, extracellular particles, and the inflammatory response may play essential roles in the pathology of tendinopathy (Fig. 1e). A BIOREACTOR PLATFORM MIMICS THE MECHANICAL ENVIRONMENT
OF THE TENDON To further explore the relationships among oxygen-related reactions, inflammation, and extracellular particles, we established an in vitro model to closely mimic the in vivo
mechanical environment and to clearly observe tendon cell function in tendinopathy. This model enabled us to apply 3D force to tendon constructs in a bioreactor (Fig. 2a)25,31. We applied
four cyclic stretching regimens of 0% (static culture), 3%, 6%, or 9% strain (0.25 Hz; 8 h/day; 6 days) to the tendon constructs. Consistent with our previous study on an ex vivo rabbit
tendon model26, examination of tenogenic-related genes for evaluating cell function, including Scleraxis (_Scx_), Mohawk (_Mkx_), Tenomodulin (_Tnmd_), and Collagen 1 (_Col1a1_), by reverse
transcription–quantitative polymerase chain reaction (RT–qPCR) showed that, compared with static culture with 0% strain, 6% strain increased the expression level of _Scx_ (2.51-fold change,
FC), _Mkx_ (3.39 FC), _Tnmd_ (1.58 FC), and _Col1a1_ (2.45 FC) in tendon constructs (Fig. 2b), indicating that optimal loading with 6% strain was beneficial for cell function. However,
compared with the 6% strain, the 9% strain decreased the expression levels of _Scx_ (0.52 FC), _Mkx_ (0.60 FC), _Tnmd_ (0.76 FC), and _Col1a1_ (0.66 FC) in the tendon constructs, revealing
that 9% strain was overload that impaired cell function. The expression levels of _Scx_, _Mkx_, _Tnmd_, and _Col1a1_ did not significantly change in the tendon constructs receiving 3% strain
compared to those in the tendon constructs receiving 0% strain. Together with the findings of previous work26, we conclude that 0% strain and 3% strain underload mouse tendon constructs, 6%
strain is a normal loading percentage, and that 9% strain overloads and simulates the mechanical overload environment in tendinopathy. 3D CYCLIC STRAIN DOSE-DEPENDENTLY AFFECTS THE
MITOCHONDRIAL NETWORK IN TENDON CONSTRUCTS We then investigated the impact of underload, normal load, and overload on oxygen-related reactions in the tendon constructs. Mitochondria, as the
powerhouses of cells, control the response to oxygen32. To study the effects of cyclic strains on oxygen-related reactions, we used live-cell CLSM to observe the morphology of MitoTracker
Red (MTR)-labeled mitochondria in 3D mouse tendon constructs (Fig. 3a). Volume-rendered 3D reconstruction of confocal images showed that in an underloaded environment with 0% strain or 3%
strain, the mitochondria tended to be rod-like and puncta-like. A normal load of 6% strain could induce the formation of elongated mitochondria and activate the mitochondrial network. The
mitochondrial network has been reported to increase energy production, maintain mitochondrial homeostasis, and serve as a signal of active mitochondrial dynamics33,34,35. In tendon
constructs overloaded with 9% strain, the mitochondria were fragmented and appeared to be rounded and punctate. Quantitative analysis revealed the largest mitochondrial footprint, highest
mitochondrial aspect ratio, and greatest number of branch junctions per mitochondrion in the tendon constructs receiving 6% strain, compared to those receiving 0%, 3%, and 9% strain, which
indicated the highest mitochondrial content, longest mitochondria, and most mitochondrial networks, respectively (Fig. 3b, c). However, although there was no significant difference in the
tenogenesis of the tendon constructs of the 0% strain and 3% strain groups (Fig. 2b), we found that 3% strain still increased the mitochondrial footprint in the tendon constructs compared to
that in the 0% strain group according to quantitative analysis of mitochondrial morphology (Fig. 3c). Further assays for mitochondrial homeostasis were conducted by immunoblotting for two
mitochondrial markers, voltage-dependent anion channel (VDAC) and translocase of outer mitochondrial membrane 20 (TOM20) (Fig. 3d, e). Consistent with the findings for the tenogenic-related
genes, the immunoblot results revealed the highest expression of VDAC and TOM20 in the 6% strain group, confirming that the greatest amount of mitochondria was present in the tendon
constructs receiving a normal load. Increased mitochondrial content in tendon constructs receiving 3% strain compared to 0% strain was not reproducibly detected by immunoblotting. This
discrepancy might be because the size of the tendon constructs we used for immunoblotting was greater than the size we scanned in the microscopy experiments, resulting in less mean strain in
the sample section, which was below a certain threshold required for mitochondrial reaction (Supplementary Fig. 2). Taken together, these results show that cyclic strain has an impact on
mitochondria in tendon cells. Compared to underloading, normal loading increases the mitochondrial content and activates the mitochondrial network in tendon cells, while overloading
eliminates this effect. CYCLIC STRAIN INDUCES THE RELEASE OF EXTRACELLULAR MITOCHONDRIAL (EXTRAMITO) PARTICLES FROM TENDON CELLS Previous studies have reported that ExtraMito particles are
intercellular communicators that can affect inflammatory responses29,36. As the RNA sequencing data indicated the potential relationships between extracellular particles, inflammation and
tendinopathy, we hypothesized that mitochondria affected by different levels of cyclic strains could be further released into the extracellular environment as particles and affect the immune
microenvironment. To date, little is known about the capacity of tendon cells to release mitochondria into the extracellular environment. We then explored the existence and regulation of
tendon-derived ExtraMito particles. As ExtraMito particles are a subtype of mEP29, we first collected mEPs from the conditioned media of tendon constructs stimulated by different levels of
cyclic strain, including 0% strain, 3% strain, 6% strain and 9% strain, in the bioreactors. The mEPs were obtained by differential centrifugation (Fig. 4a). Flow cytometry showed that the
sizes of these mEPs were similar among the different degrees of strain and were mainly between 220 nm and 880 nm (Supplementary Fig. 3). The size of the particles was further characterized
by dynamic light scattering, a technique commonly used for nanoparticle characterization. The results confirmed that the particles obtained were mainly between 100 nm and 1000 nm in size
(Fig. 4b). Poststaining of mEPs revealed that most of the mEPs could be stained with PKH26, a lipophilic membrane dye, revealing the presence of a membrane in most of these mEPs (more than
75%) (Fig. 4c). The mEPs were also characterized by transmission electron microscopy (Fig. 4d). We further assessed proteins from different cellular compartments and found that CD63 (cell
membrane marker), Annexin A2 and β-actin (cytoplasmic marker) were in high concentrations in the tendon-derived mEPs, while LaminA/C (nuclear marker) and Albumin (protein from culture
medium) were in low abundance (Supplementary Fig. 4). To detect the ExtraMito particles in mEPs, we then prestained mitochondria with MitoTracker Red (MTR) and cytoplasm with
carboxyfluorescein succinimidyl ester (CFSE) in tendon constructs before applying mechanical stimulation. After 6 days of cyclic stretching, CLSM revealed that ExtraMito particles were
released by the tendon constructs, regardless of the degree of strain (Fig. 4e). There were two forms of ExtraMito particles, namely, mitochondria-encapsulated mEPs and free extracellular
mitochondria (Fig. 4f). To exclude the possibility of MTR leakage, we transfected tendon constructs with red fluorescent protein (RFP) fused to mitochondria instead of MTR. After 6 days of
cyclic stretching in the bioreactor system, fluorescence confocal imaging confirmed the presence of the two forms of ExtraMito particles from the conditioned media (Supplementary Fig. 5). To
quantify the amount of ExtraMito particles released from tendon constructs in different mechanical environments, we examined the mitochondrial markers VDAC and TOM20 at the protein level
(Fig. 4g, h). Immunoblotting revealed that the highest level of ExtraMito particles was produced by tendon constructs that received 9% strain (ExtraMito9%). Collectively, these data suggest
that tendon cells can release ExtraMito particles, which is enhanced by mechanical overload. EXTRACELLULAR PARTICLES FROM TENDON CELLS INDUCE MACROPHAGE INFILTRATION AND CYTOKINE RELEASE We
further explored the relationship between inflammation and mEPs, including ExtraMito particles. Macrophage infiltration and increased proinflammatory cytokine production have been reported
as two major pathological features of tendinopathy37,38. Our RNA sequencing data also showed that “leukocyte migration” and “cell chemotaxis” were activated in tendinopathy (Fig. 1d). Here,
the effects of mEPs, including ExtraMito particles, on macrophage infiltration were first tested. We performed a chemotaxis cell migration assay with the RAW 264.7 macrophage line for mEPs
released from 3D tendon cell constructs in different loading environments in a migration chamber system based on the Boyden chamber principle (Fig. 5a, b). RAW 264.7 cells exhibited
significantly greater chemotaxis toward mEPs excreted by tendon constructs receiving 9% strain (mEP9%) than those excreted by tendon constructs receiving mEP0%, mEP3% or mEP6%, which was
consistent with previous reports showing that overload could induce significant macrophage infiltration and tendinopathy2,39. To validate the macrophage infiltration results, we analyzed the
transcriptomic profiles of healthy human tendons and tendons with tendinopathy via xCell, a gene signature-based method for inferring the content of infiltrated macrophages in tendon
tissues23. The results revealed an increase in the number of macrophages in the tendons with tendinopathy, which also indicated that overload induced macrophage infiltration into the tendon
microenvironment (Fig. 5c). To evaluate the ability of mEPs from tendon cells to induce the secretion of inflammatory cytokines from macrophages, 13 key cytokines from the conditioned media
of RAW 264.7 cells were evaluated after culturing RAW 264.7 cells with mEP0%, mEP3%, mEP6%, or mEP9%. Cytokines including multifunctional cytokine Interleukin 6 (IL-6), proinflammatory
cytokines C-X-C Motif Chemokine Ligand 1 (CXCL1), IL-18, IL-23, IL-12p70, Tumor Necrosis Factor α (TNF-α), IL-12p40, and IL-1β, and anti-inflammatory cytokines Free Active Transforming
Growth Factor β1 (TGF-β1), C-C Motif Chemokine Ligand 22 (CCL22), IL-10, Granulocyte Colony Stimulating Factor (G-CSF), and C-C Motif Chemokine Ligand 17 (CCL17), were tested by a bead-based
multiplex assay panel with fluorescence-encoded beads and flow cytometry (Fig. 5d). Our results showed that RAW 264.7 cells released increasing amounts of IL-6, CCL22, GCSF, CCL17, CXCL1,
IL-23, IL-18, TNF-α and IL-12p40 when treated with mEPs from mouse tendon cells, compared to PBS-treated RAW 264.7 cells (Fig. 5e). Among these cytokines, the secretion levels of IL-6, CXCL1
and IL-18 varied when mEPs from tendons subjected to different loading environments were applied. Interestingly, it has been reported that IL-6, CXCL1 and IL-18 can induce inflammatory
reactions40,41, indicating that the mechanical messages carried by mEPs can be passed to the immune microenvironment and mainly affect proinflammation. Moreover, compared to mEP0%, mEP3% and
mEP6%, mEP9% induced the highest secretion of IL-6 and CXCL1 from RAW 264.7 cells. As tendon cells have been reported to synthesize cytokines13, we further directly examined the cytokines
inside mEPs. Our data showed that the levels of IL-6, CXCL1 and IL-18 within mEPs were lower than the detectable levels (Supplementary Fig. 6), indicating that these cytokines were mainly
secreted from RAW 264.7 cells rather than from mouse tendon mEPs. To examine whether mEPs from various conditions mediate the release of inflammatory cytokines in a dose-dependent manner, we
cultured RAW 264.7 cells with varying doses of mEP0%, mEP3%, mEP6% and mEP9%. We used a 10-fold mEP concentration gradient. The analysis of the conditioned media using the bead-based
multiplex assay indicated that, considering the general concentration effect, mEPs mediated the release IL-6, CCL22, G-CSF, CCL17, CXCL1, IL-23, IL-18, TNF-α, and IL-12p40 by RAW 264.7 cells
in a dose-dependent manner (Supplementary Fig. 7). The ability of mEP0%, mEP3%, mEP6% and mEP9% to induce the secretion of most cytokines exhibited similar trends at different doses.
Notably, mEP9% significantly induced the highest expression levels of IL-6 and IL-18 compared to those induced by mEP0%, mEP3%, and mEP6% at different doses (Fig. 5f). However, at a low
dose, mEP9% did not induce higher CXCL1 secretion than mEP0%, mEP3%, or mEP6%. The simple effect analysis of cells treated with different mEPs revealed a significant increase in CXCL1
expression levels when treating with mEP9% at higher doses. This trend was not observed with the other mEP treatments, suggesting that mEP9% has specific effects on inducing CXCL1 expression
that cannot be compensated for by increasing the quantity of mEP0%, mEP3%, or mEP6%. These data suggested that mEPs released from tendon cells stimulated by different degrees of strain
could induce RAW 264.7 macrophages to secrete proinflammatory cytokines. Taken together, these results suggest that mEPs secreted from overloaded tendon cells induce macrophage chemotaxis
and increase the secretion of the proinflammatory cytokines IL-6 and CXCL1 from macrophages. TENDON-DERIVED EXTRAMITO PARTICLES ARE ESSENTIAL FOR THE INDUCTION OF MACROPHAGE CHEMOTAXIS To
investigate whether the functional mEPs were directly absorbed by RAW 264.7 cells, we cultured RAW 264.7 cells with MTR-prelabeled tendon-derived ExtraMito particles within the mEP9%
population. After 24 h of incubation, we observed that the mitochondria from the tendon constructs could be taken up by RAW 264.7 cells (Fig. 6a). Interestingly, we noticed that the RAW
264.7 cells that absorbed the tendon-derived ExtraMito particles had a more elongated morphology than the cells not treated with mEP9%, and even compared to their counterparts treated with
mEP9% but without observable tendon-derived ExtraMito9% uptake (Fig. 6b). Untreated RAW 264.7 cells displayed a rounded and plump morphology and lacked cytoplasmic extensions. In contrast,
the RAW 264.7 cells treated with mEP9% generally presented an activated phenotype with flattened and elongated shapes, which was more obvious for RAW 264.7 cells that absorbed the
tendon-derived ExtraMito particles. These results suggested that the tendon-derived ExtraMito particles might exert specific effects on macrophages. To the best of our knowledge, few studies
have addressed the specific function of ExtraMito particles as a component of mEPs42,43. To determine the contribution of the tendon-derived ExtraMito particles within mEP9%, which act as a
chemoattractant to RAW 264.7 cells, we first depleted mitochondria from mEP9% using the TOM20 antibody via MACS technology (Fig. 6c). Depletion of mitochondria was evaluated by the
mitochondrial markers VDAC and TOM20 at the protein level (Fig. 6d, e). Immunoblotting revealed diminished levels of VDAC and TOM20 in TOM20-depleted mEP9% compared to sham-depleted mEP9%.
These results confirmed that post-MACS depletion, the mitochondrial component in mEPs, was strongly reduced. Immunoblotting also revealed residual ExtraMito particles in mEPs, corroborating
the presence of mitochondria within particles resistant to MACS-mediated depletion. Next, TOM20-depleted mEP9% or sham-depleted mEP9% was added to a migration chamber system to serve as a
chemoattractant. RAW 264.7 cells displayed a significantly reduced chemotaxis toward TOM20-depleted mEP9% (Fig. 6f, g), compared to sham-depleted mEP9%, indicating the indispensability of
ExtraMito particles within mEPs for macrophage chemotaxis. We then directly applied TOM20+ particles sorted from mEP9% to RAW 264.7 cells as chemoattractants in the chemotaxis assay.
Surprisingly, compared to those in response to PBS, TOM20+ particles did not significantly change RAW 264.7 cell chemotaxis (Fig. 6h, i), which implied that the ExtraMito particles of mEP9%
were necessary but not sufficient alone to induce macrophage chemotaxis. To further confirm the capacity of mEP9% ExtraMito particles to induce RAW 264.7 cell chemotaxis, we separated the
mEP9% fractions through a high-resolution iodixanol density step gradient (Supplementary Fig. 8). Immunoblotting of different mEP9% fractions for the mitochondrial markers TOM20 and VDAC
demonstrated enrichment of ExtraMito particles in Fractions 4–8 (Fig. 6j). We therefore defined Fractions 4–8 as purified ExtraMito9% and Fractions 1–3 as mEP9% with less ExtraMito
particles. We subsequently introduced purified ExtraMito9%, mEP9% with less ExtraMito particles, unseparated mEP9%, or PBS into the migration chamber system to serve as chemoattractants.
Notably, purified ExtraMito9% exhibited chemoattraction to RAW 264.7 cells that was comparable to that of unseparated mEP9% (Fig. 6k, l). In contrast, the mEP9% with less ExtraMito particles
had negligible chemoattractive effects on RAW 264.7 cells, akin to the outcome of the PBS control group. These results highlight the important role of ExtraMito particles in inducing
macrophage chemotaxis in a mechanical overload environment. Next, to further explore the role of ExtraMito particles, a component of mEP9% that induces inflammatory cytokine release from
macrophages, RAW 264.7 cells were incubated with TOM20-depleted mEP9% or sham-depleted mEP9%. The bead-based multiplex assay revealed that there were no significant changes in the release of
IL-6, CXCL1, or IL-18 after the depletion of ExtraMito particles from mEPs (Fig. 6m), implying that the rest components of mEP9%, except for mitochondria, mainly induced inflammation. Taken
together, these data suggest that ExtraMito particles, as a component of mEPs, mainly contributes to macrophage chemotaxis. PROTECTION OF THE INTRACELLULAR MITOCHONDRIAL NETWORK IN
OVERLOADED PARENTAL TENDON CELLS PREVENTS MACROPHAGE CHEMOTAXIS INDUCED BY EXTRAMITO PARTICLES Given that overload impairs the intracellular mitochondrial network, we hypothesized that the
damaged intracellular mitochondrial network causes the release of ExtraMito particles, which can induce macrophage chemotaxis. We subsequently applied N-acetylcysteine (NAC), an antioxidant
normally supplied as a mitochondrial protector and stressed mitochondria rescuer44,45, to regulate mitochondrial homeostasis in tendon constructs in a mechanical overload environment with 9%
strain. Live-cell CLSM showed that NAC treatment could reverse the impairment of the mitochondrial network labelled by MTR in tendon constructs receiving 9% strain (Fig. 7a). Quantitative
analysis revealed a larger mitochondrial footprint, a greater mitochondrial aspect ratio, and more branch junctions per mitochondrion in the tendon constructs after NAC treatment in the 9%
strain environment (Fig. 7b). Then, the mEPs released by the tendon constructs subjected to 9% strain and treated with NAC (termed NAC-treated mEP9%) were collected and applied to a
migration chamber system as a chemoattractant for RAW 264.7 cells. As expected, the chemotaxis assay demonstrated that, compared to mEP9%, NAC-treated mEP9% significantly lost the capacity
to induce macrophage chemotaxis (Fig. 7c, d), indicating that the impaired intracellular mitochondrial network was responsible for macrophage chemotaxis induced by mEP9%. DISCUSSION In the
present study, we showed that the release of ExtraMito particles from tendon cells during mechanical overload is a novel pathological mechanism of tendinopathy. We showed that these newly
identified tendon-derived ExtraMito particles serve as a cell‒cell communicators to transmit mechanical signals from mechanically stimulated tendon cells to macrophages (Fig. 8). Our data
indicate that overload damages the intracellular mitochondrial network and triggers the release of ExtraMito particles and mEPs by tendon cells. Then, the ExtraMito particles and mEPs
promote the chemotaxis of macrophages and the release of inflammatory cytokines, ultimately causing inflammatory reactions. By protecting the intracellular mitochondrial network in tendon
cells from breakdown, we can reduce overload-induced macrophage chemotaxis. Inflammation is considered a contributing factor to tendinopathy. Immune cell infiltration and further cytokine
production are essential for inflammatory reactions. Our work indicated that both of these processes were dependent on the degree of mechanical stimulation, supporting the theory that
overload initiates the pathological progression of tendinopathy2,8. Macrophage infiltration has been characterized in human tendinopathy46. The present study revealed that the ExtraMito
particles within mEPs are fundamental for inducing macrophage infiltration. Several proinflammatory cytokines, such as IL-1β, TNF-α, CXCL1 and IL-6, have been reported to initiate catabolic
processes, especially in the early phase of tendinopathy13,47,48. We found that the mechanism underlying the loading-induced release of IL-6, CXCL1, and IL-18 was dependent on the degree of
mechanical loading. Among them, IL-6 and CXCL1 secretion was associated with mEPs released from tendon cells in an overload environment. These data suggest that the newly identified
tendon-derived mEPs and ExtraMito particles affect the immune microenvironment from several perspectives, including both macrophage infiltration and cytokine production. The bioreactor
platform we applied in the present study was a 3D uniaxial model. Compared to a 2D loading model, this 3D loading model allows tendon cells to receive mechanical stimuli not only through
cell‒cell interactions but also via cell–ECM interactions across the entire cell body. Previous work has suggested that this platform can more closely mimic the in vivo tendon mechanical
environment for tendon cells from the perspective of inducing differentiation25. The present study further proved that it could induce tendon cells to secrete extracellular particles. These
extracellular factors can ultimately affect macrophages to create an immune microenvironment similar to that in vivo. Using live-cell confocal microscopy, we observed that mitochondria were
responsive to mechanical loading. Mitochondria, the “energy power stations” of cells, can transform between elongated or fragmented states and into interconnected networks to maintain their
fitness, which is required for normal cell metabolism49,50. An interconnected mitochondrial network is a combination of diverse mitochondrial morphologies indicating active mitochondrial
dynamics, which increases the controllability, robustness, and efficiency of the network in response to perturbations, as well as enhancing oxidative capacity34,35. These networks appeared
more frequently when the cells were in a normal loading environment in the present study. Consistent with the findings of previous studies26,51,52, the present study confirmed through the
analysis of mitochondrial geometric features that only moderate mechanical loading is beneficial for cell metabolism. Mitochondria-targeted drugs have been applied in animal studies and
clinical trials for several diseases, such as Parkinson’s disease, cardiovascular disease, and cancer53,54. Although oxidative stress has been reported to be involved in tendinopathy55, to
the best of our knowledge, no mitochondria-targeted drug has been reported to be clinically used for treating tendinopathy. The present study showed that protection of mitochondria from
overload not only rescues tendon cells themselves but also improves the immune microenvironment, which highlights the potential of mitochondria treatment for tendinopathy. The nomenclature
used in the field of extracellular particles/extracellular vesicles is rapidly evolving. Microvesicles are heterogeneous extracellular vesicles that typically range in size from 150 nm to
1000 nm. However, in some cases, they can be as large as 10 μm56,57. Some studies have termed mitochondria-derived extracellular vesicles “mitovesicles“43,58. Some studies have referred to
these vesicles as “mitochondria extracellular vesicles” or “extracellular mitochondria“29,59. The term “microvesicle” has been assigned to an extracellular vesicle in a plasma
membrane-derived biogenesis pathway60, which was not discussed in the present study. Thus, in this study, we opted for a more general term, “mEPs,” to describe these medium-sized
particles/vesicles, and we used “ExtraMito” to denote the mitochondrial portion. Previous studies have shown that these particles, which can be released from activated monocytes or
platelets, are related to inflammatory reactions29,36,61. The limitation of some previous studies is that their extracellular particles were heterogeneous, meaning that the function of the
mitochondrial portion was not distinguished from that of the nonmitochondrial part42,43. Using the Migration Chamber system and MACS technology, we showed for the first time that the
ExtraMito portion of mEPs from overloaded tendon constructs is indispensable for macrophage chemotaxis. Interestingly, our data showed that the mitochondrial portion could not work alone as
a chemoattractant. However, the lack of these mitochondria particles significantly reduced macrophage chemotaxis. As chemotaxis requires three independent steps—chemosensing, polarization
and locomotion62—we hypothesized that ExtraMito particles participate in only a part of these steps. Since we observed morphological changes in the polarization of RAW 264.7 cells after the
uptake of tendon-derived ExtraMito particles, even when compared to their counterparts treated with mEPs but without ExtraMito particles uptake, we assumed that ExtraMito particles mainly
worked through the polarization step. As we did not obtain the mitochondrial portion encapsulated in mEPs, synergy between the free mitochondrial portion and the mitochondrial portion
encapsulated in mEPs could not be inferred. Tools to extract the specific particle portions are lacking and need to be developed. The field of mechanobiology is interdisciplinary and aims to
study how force propagates in cells and affects cells and how cells, in turn, remodel the microenvironment to adapt to physical and mechanical stimuli. While studies in this field have
traditionally focused on individual cell types, cell–cell communication is a critical process for maintaining biological homeostasis. This work represents the first demonstration of
ExtraMito particles functioning as an intercellular crosstalk signal in mechanobiology. While our conclusions were drawn based on tendinopathy, these findings may extend to other diseases
induced by mechanical overload, such as pressure overload-induced cardiac fibrosis and heart failure63 and ocular hypertension-caused glaucoma64, as inflammation has been found to be an
essential associated event in these overload-induced diseases65,66. In conclusion, we provide evidence that tendon cells can release mEPs into the extracellular environment, which contain
mechanical response messages that regulate the immune microenvironment. Furthermore, the ExtraMito portion of mEPs is essential for inducing macrophage chemotaxis. Our data reveal a novel
pathological mechanism of tendinopathy and provide new insights for the treatment of tendinopathy and related inflammatory reactions. REFERENCES * Riley, G. Tendinopathy–from basic science
to treatment. _Nat. Clin. Pract. Rheumatol._ 4, 82–89 (2008). Article PubMed Google Scholar * Millar, N. L. et al. Tendinopathy. _Nat. Rev. Dis. Prim._ 7, 1 (2021). Article PubMed
Google Scholar * Miranda, H., Viikari-Juntura, E., Heistaro, S., Heliövaara, M. & Riihimäki, H. A population study on differences in the determinants of a specific shoulder disorder
versus nonspecific shoulder pain without clinical findings. _Am. J. Epidemiol._ 161, 847–855 (2005). Article PubMed Google Scholar * Shiri, R., Varonen, H., Heliövaara, M. &
Viikari-Juntura, E. Hand dominance in upper extremity musculoskeletal disorders. _J. Rheumatol._ 34, 1076–1082 (2007). PubMed Google Scholar * Walker-Bone, K., Palmer, K. T., Reading, I.,
Coggon, D. & Cooper, C. Occupation and epicondylitis: a population-based study. _Rheumatol. (Oxf., Engl.)_ 51, 305–310 (2012). Article Google Scholar * Hopkins, C. et al. Critical
review on the socio-economic impact of tendinopathy. _Asia-Pac. J. Sports Med., Arthrosc., Rehab. Technol._ 4, 9–20 (2016). Google Scholar * Pennisi, E. Tending tender tendons. _Sci. (N.
Y., N. Y.)_ 295, 1011 (2002). Article CAS Google Scholar * Chen, Z. et al. Challenges and perspectives of tendon-derived cell therapy for tendinopathy: from bench to bedside. _Stem Cell
Res. Ther._ 13, 444 (2022). Article PubMed PubMed Central Google Scholar * Guerquin, M. J. et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. _J.
Clin. Investig._ 123, 3564–3576 (2013). Article CAS PubMed PubMed Central Google Scholar * Langberg, H. et al. Eccentric rehabilitation exercise increases peritendinous Type I collagen
synthesis in humans with Achilles tendinosis. _Scand. J. Med. Sci. Sports_ 17, 61–66 (2007). Article CAS PubMed Google Scholar * Millar, N. L., Murrell, G. A. & McInnes, I. B.
Inflammatory mechanisms in tendinopathy - towards translation. _Nat. Rev. Rheumatol._ 13, 110–122 (2017). Article CAS PubMed Google Scholar * Abate, M. et al. Pathogenesis of
tendinopathies: inflammation or degeneration? _Arthritis Res. Ther._ 11, 235 (2009). Article PubMed PubMed Central Google Scholar * D’Addona, A., Maffulli, N., Formisano, S. & Rosa,
D. Inflammation in tendinopathy. _Surg. : J. R. Coll. Surg. Edinb. Irel._ 15, 297–302 (2017). Google Scholar * Zhu, S. Y. et al. Lysosomal quality control of cell fate: a novel therapeutic
target for human diseases. _Cell Death Dis._ 11, 817 (2020). Article CAS PubMed PubMed Central Google Scholar * Xu, W. N. et al. Sesn2 serves as a regulator between mitochondrial
unfolded protein response and mitophagy in intervertebral disc degeneration. _Int. J. Biol. Sci._ 19, 571–592 (2023). Article CAS PubMed PubMed Central Google Scholar * Zhao, C., Liao,
Y., Rahaman, A. & Kumar, V. Towards understanding the relationship between ER stress and unfolded protein response in amyotrophic lateral sclerosis. _Front. Aging Neurosci._ 14, 892518,
https://doi.org/10.3389/fnagi.2022.892518 (2022). Article CAS PubMed PubMed Central Google Scholar * Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low
memory requirements. _Nat. Methods_ 12, 357–360 (2015). Article CAS PubMed PubMed Central Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with
high-throughput sequencing data. _Bioinforma. (Oxf., Engl.)_ 31, 166–169 (2015). CAS Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and
dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article PubMed PubMed Central Google Scholar * Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0.
_Bioinforma. (Oxf., Engl.)_ 27, 1739–1740 (2011). CAS Google Scholar * Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies.
_Nucleic Acids Res._ 43, e47 (2015). Article PubMed PubMed Central Google Scholar * Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting
genome-wide expression profiles. _Proc. Natl. Acad. Sci. USA_ 102, 15545–15550 (2005). Article CAS PubMed PubMed Central Google Scholar * Aran, D., Hu, Z. & Butte, A. J. xCell:
digitally portraying the tissue cellular heterogeneity landscape. _Genome Biol._ 18, 220 (2017). Article PubMed PubMed Central Google Scholar * Chen, Z. et al. Applying a
Three-dimensional Uniaxial Mechanical Stimulation Bioreactor System to Induce Tenogenic Differentiation of Tendon-Derived Stem Cells. _Journal of visualized experiments : JoVE_,
https://doi.org/10.3791/61278 (2020). * Wang, T. et al. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling
pathway. _FASEB J. : Off. Publ. Feder. Am. Soc. Exp. Biol._ 32, 4804–4814 (2018). Article CAS Google Scholar * Wang, T. et al. Programmable mechanical stimulation influences tendon
homeostasis in a bioreactor system. _Biotechnol. Bioeng._ 110, 1495–1507 (2013). Article CAS PubMed Google Scholar * Mohammadi, M. R. et al. Isolation and characterization of
microvesicles from mesenchymal stem cells. _Methods (San. Diego, Calif.)_ 177, 50–57 (2020). Article CAS PubMed Google Scholar * Özer Kaya, D. in _Comparative Kinesiology of the Human
Body_ (eds S. Angin & I. E. Şimşek) 115–147 (Academic Press, 2020). * Puhm, F. et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce Type
I IFN and TNF responses in endothelial cells. _Circ. Res._ 125, 43–52 (2019). Article CAS PubMed Google Scholar * Kajimoto, T. & Nakamura, S. I. Quantitative analysis of cargo
density in single-extracellular vesicles by imaging. _Bio-Protoc._ 8, e3111 (2018). Article CAS PubMed PubMed Central Google Scholar * Chen, Z., Chen, P., Ruan, R. & Zheng, M. In
vitro 3D mechanical stimulation to tendon-derived stem cells by bioreactor. _Methods Mol. Biol. (Clifton, N. J.)_ 2436, 135–144 (2022). Article CAS Google Scholar * McElroy, G. S. &
Chandel, N. S. Mitochondria control acute and chronic responses to hypoxia. _Exp. Cell Res._ 356, 217–222 (2017). Article CAS PubMed PubMed Central Google Scholar * Hood, D. A., Memme,
J. M., Oliveira, A. N. & Triolo, M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. _Annu. Rev. Physiol._ 81, 19–41 (2019). Article CAS PubMed Google
Scholar * Hoitzing, H., Johnston, I. G. & Jones, N. S. What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research.
_BioEssays : N. Rev. Mol., Cell. Dev. Biol._ 37, 687–700 (2015). Article Google Scholar * Pickles, S., Vigié, P. & Youle, R. J. Mitophagy and quality control mechanisms in
mitochondrial maintenance. _Curr. Biol.: CB_ 28, R170–r185 (2018). Article CAS PubMed Google Scholar * Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for
bactericidal group IIA-secreted phospholipase A2 to promote inflammation. _Blood_ 124, 2173–2183 (2014). Article CAS PubMed PubMed Central Google Scholar * Schubert, T. E., Weidler, C.,
Lerch, K., Hofstädter, F. & Straub, R. H. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. _Ann. Rheum. Dis._ 64, 1083–1086 (2005). Article CAS
PubMed PubMed Central Google Scholar * Tang, C. et al. The roles of inflammatory mediators and immunocytes in tendinopathy. _J. Orthop. Transl._ 14, 23–33 (2018). Google Scholar *
Sunwoo, J. Y., Eliasberg, C. D., Carballo, C. B. & Rodeo, S. A. The role of the macrophage in tendinopathy and tendon healing. _J. Orthop. Res. : Off. Publ. Orthop. Res. Soc._ 38,
1666–1675 (2020). Article Google Scholar * Kim, Y. K., Na, K. S., Myint, A. M. & Leonard, B. E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the
neuroendocrine system in major depression. _Prog. Neuro-Psychopharmacol. Biol. psychiatry_ 64, 277–284 (2016). Article CAS Google Scholar * Song, A. Q. et al. NLRP1 inflammasome
contributes to chronic stress-induced depressive-like behaviors in mice. _J. Neuroinflam._ 17, 178 (2020). Article CAS Google Scholar * Rosina, M. et al. Ejection of damaged mitochondria
and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. _Cell Metab._ 34, 533–e512 (2022). Article CAS PubMed PubMed Central Google Scholar * D’Acunzo,
P. et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. _Sci. Adv_. 7, https://doi.org/10.1126/sciadv.abe5085 (2021). *
Zalewska, A., Szarmach, I., Żendzian-Piotrowska, M. & Maciejczyk, M. The effect of N-Acetylcysteine on respiratory enzymes, ADP/ATP ratio, glutathione metabolism, and nitrosative stress
in the salivary gland mitochondria of insulin resistant rats. _Nutrients_ 12, https://doi.org/10.3390/nu12020458 (2020). * Shafie, B., Pourahmad, J. & Rezaei, M. N-acetylcysteine is more
effective than ellagic acid in preventing acrolein induced dysfunction in mitochondria isolated from rat liver. _J. Food Biochem._ 45, e13775 (2021). Article CAS PubMed Google Scholar *
Millar, N. L. et al. Inflammation is present in early human tendinopathy. _Am. J. Sports Med._ 38, 2085–2091 (2010). Article PubMed Google Scholar * Kendal, A. R. et al. Multi-omic
single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. _Sci. Rep._ 10, 13939 (2020). Article CAS PubMed PubMed Central Google Scholar *
Herman, J. et al. Pro-inflammatory effects of human apatite crystals extracted from patients suffering from calcific tendinopathy. _Arthritis Res. Ther._ 23, 131 (2021). Article CAS PubMed
PubMed Central Google Scholar * Malla, B. et al. Teriflunomide Preserves Neuronal Activity and Protects Mitochondria in Brain Slices Exposed to Oxidative Stress. _Int. J. Mol. Sci._ 23,
https://doi.org/10.3390/ijms23031538 (2022). * Mahmoodzadeh, S. et al. Age-related decline in murine heart and skeletal muscle performance is attenuated by reduced Ahnak1 expression. _J.
Cachexia, Sarcopenia Muscle_ 12, 1249–1265 (2021). Article PubMed Google Scholar * Liao, H. et al. Mechanotranduction pathways in the regulation of mitochondrial homeostasis in
Cardiomyocytes. _Front. Cell Dev. Biol._ 8, 625089 (2020). Article PubMed Google Scholar * Flockhart, M. et al. Excessive exercise training causes mitochondrial functional impairment and
decreases glucose tolerance in healthy volunteers. _Cell Metab._ 33, 957-.e956, (2021). * Jiang, Q. et al. Mitochondria-targeted antioxidants: a step towards disease treatment. _Oxid. Med.
Cell. Longev._ 2020, 8837893 (2020). Article PubMed PubMed Central Google Scholar * Zinovkin, R. A. & Zamyatnin, A. A. Mitochondria-targeted drugs. _Curr. Mol. Pharmacol._ 12,
202–214 (2019). Article CAS PubMed PubMed Central Google Scholar * Murrell, G. A. Oxygen free radicals and tendon healing. _J. shoulder Elb. Surg._ 16, S208–214, (2007). Article Google
Scholar * van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. _Nat. Rev. Mol. Cell Biol._ 19, 213–228 (2018). Article PubMed Google
Scholar * Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. _Annu. Rev. Cell Dev. Biol._ 30,
255–289 (2014). Article CAS PubMed Google Scholar * D’Acunzo, P. et al. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain
tissues. _Nat. Protoc._ https://doi.org/10.1038/s41596-022-00719-1 (2022). * Amari, L. & Germain, M. Mitochondrial extracellular vesicles - origins and roles. _Front. Mol. Neurosci._ 14,
767219 (2021). Article CAS PubMed PubMed Central Google Scholar * Tricarico, C., Clancy, J. & D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. _Small GTPases_ 8,
220–232 (2017). Article CAS PubMed Google Scholar * Miliotis, S., Nicolalde, B., Ortega, M., Yepez, J. & Caicedo, A. Forms of extracellular mitochondria and their impact in health.
_Mitochondrion_ 48, 16–30 (2019). Article CAS PubMed Google Scholar * Iglesias, P. A. & Devreotes, P. N. Navigating through models of chemotaxis. _Curr. Opin. Cell Biol._ 20, 35–40
(2008). Article CAS PubMed Google Scholar * Tang, C. et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent
exosomal communication. _FASEB J.: Off. Publ. Feder. Am. Soc. Exp. Biol._ 37, e22699 (2023). Article CAS Google Scholar * Lauwers, A., Breda, J. B. & Stalmans, I. The natural history
of untreated ocular hypertension and glaucoma patients. _Survey Ophthalmol._ 68, 388–424 (2023). * Liu, X., Shi, G. P. & Guo, J. Innate immune cells in pressure overload-induced cardiac
hypertrophy and remodeling. _Front. Cell Dev. Biol._ 9, 659666 (2021). Article PubMed PubMed Central Google Scholar * Vargas, J. L. & Di Polo, A. Neuroinflammation in glaucoma:
soluble tumor necrosis factor alpha and the connection with excitotoxic damage. _Neural Regen. Res._ 11, 424–6, (2016). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS
We acknowledge the support of the Australian Research Council Industrial Transformation Training Centre for Personalised Therapeutics Technologies (IC170100016), the Western Australia
Innovation Seed Fund (2022/GR000685), the Program of Science and Technology of Guangzhou (202102020427), and the Guang Dong Basic and Applied Basic Research Foundation (2021A1515011008;
2020A1515110511). Several figure infographics were prepared with Biorender. We acknowledge Catherine Rinaldi, Peta Clode, Crystal Cooper, Alysia Hubbard and Liisa Hirvonen for the facilities
and the scientific and technical assistance of Microscopy Australia at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by
the University, State and Commonwealth Governments. FUNDING Australian Research Council Industrial Transformation Training Centre for Personalised Therapeutics Technologies (IC170100016),
Western Australia Innovation Seed Fund (2022/GR000685), the Program of Science and Technology of Guangzhou (202102020427), and Guang Dong Basic and Applied Basic Research Foundation
(2021A1515011008; 2020A1515110511). AUTHOR INFORMATION Author notes * These authors contributed equally: Ziming Chen, Mengyuan Li. AUTHORS AND AFFILIATIONS * Centre for Orthopaedic Research,
School of Surgery, The University of Western Australia, Nedlands, WA, 6009, Australia Ziming Chen, Peilin Chen, Euphemie Landao Bassonga, Junjie Gao, Delin Liu, David Wood & Ming H.
Zheng * Department of Joint Osteopathy and Traumatology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangdong, China
Mengyuan Li & Qiujian Zheng * School of Medicine, Monash University, Clayton, VIC, VIC 3800, Australia Peilin Chen * Perron Institute for Neurological and Translational Science,
Nedlands, WA, 6009, Australia Andrew Tai, Euphemie Landao Bassonga, Junjie Gao, Delin Liu & Ming H. Zheng * BRITElab, Harry Perkins Institute of Medical Research, QEII Medical Centre,
Nedlands, and Centre for Medical Research, The University of Western Australia, Perth, WA, 6009, Australia Jiayue Li & Brendan F. Kennedy * Department of Electrical, Electronic and
Computer Engineering, School of Engineering, The University of Western Australia, Nedlands, WA, 6009, Australia Jiayue Li & Brendan F. Kennedy * Australian Research Council Centre for
Personalised Therapeutics Technologies, Melbourne, VIC, Australia Jiayue Li & Brendan F. Kennedy * Department of Orthopaedics, Shanghai Jiao Tong University Affiliated Shanghai Sixth
People’s Hospital, Shanghai, 200233, China Junjie Gao * Institute of Physics, Faculty of Physics, Astronomy and Informatics, Nicolaus Copernicus University in Toruń, Grudziadzka 5, 87-100,
Torun, Poland Brendan F. Kennedy Authors * Ziming Chen View author publications You can also search for this author inPubMed Google Scholar * Mengyuan Li View author publications You can
also search for this author inPubMed Google Scholar * Peilin Chen View author publications You can also search for this author inPubMed Google Scholar * Andrew Tai View author publications
You can also search for this author inPubMed Google Scholar * Jiayue Li View author publications You can also search for this author inPubMed Google Scholar * Euphemie Landao Bassonga View
author publications You can also search for this author inPubMed Google Scholar * Junjie Gao View author publications You can also search for this author inPubMed Google Scholar * Delin Liu
View author publications You can also search for this author inPubMed Google Scholar * David Wood View author publications You can also search for this author inPubMed Google Scholar *
Brendan F. Kennedy View author publications You can also search for this author inPubMed Google Scholar * Qiujian Zheng View author publications You can also search for this author inPubMed
Google Scholar * Ming H. Zheng View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Qiujian Zheng or Ming H. Zheng.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, Z., Li, M., Chen, P. _et al._ Mechanical overload-induced release of
extracellular mitochondrial particles from tendon cells leads to inflammation in tendinopathy. _Exp Mol Med_ 56, 583–599 (2024). https://doi.org/10.1038/s12276-024-01183-5 Download citation
* Received: 10 March 2023 * Revised: 17 December 2023 * Accepted: 17 December 2023 * Published: 01 March 2024 * Issue Date: March 2024 * DOI: https://doi.org/10.1038/s12276-024-01183-5 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Record heat in california could interfere with your fourth of july guacamoleAn avocado hangs from a tree at a farm in Pauma Valley on March 5, 2014 near Valley Center, California. David McNew | Ge...
Trump reported making more than $1. 6 billion while president | common dreamsDonald Trump reported making more than $1.6 billion in outside revenue and income during his four years as President of ...
Sturgeon warned: forget independence and sort out our schoolsA spokesman for the SNP said: “The Tories are desperately trying to distract from the complete shambles that is their ha...
Vic news - 9news - latest updates and breaking headlines victoriaVICTORIAN PRINCIPALS GRANTED SWEEPING NEW EXPULSION POWERS Principals in Victoria are being given extraordinary new powe...
The capsule | designboom.comDESIGNER'S OWN WORDS: A new product characteristics: - easy to assemble (joined supplies) - short-term completion o...
Latests News
Mechanical overload-induced release of extracellular mitochondrial particles from tendon cells leads to inflammation in tendinopathyABSTRACT Tendinopathy is one of the most common musculoskeletal diseases, and mechanical overload is considered its prim...
Access to this page has been deniedYour browser appears to have Javascript disabled.For instructions on how to enable Javascript please click here.If you h...
Channelnews : samsung s20 leak reveals snapdragon, 12gb ramLeak after leak – Samsung’s upcoming Galaxy S20 range has been victim to many over the past few days. Now, the latest in...
Itchy ears could be 'red-flag' sign of incurable health conditionA GP wants to remind people not to itch or clean their ears with cotton buds. The expert also explained that ear itching...
Reply to: hypertension and severe covid-19We thank Kow et al. for their thoughtful comments [1] on our review article titled “COVID-19 pandemic and hypertension: ...