Brain Tumor Stem Cells | Pediatric Research
Brain Tumor Stem Cells | Pediatric Research"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cancers are composed of heterogeneous cell populations ranging from highly proliferative immature cells to more differentiated cells of various cell lineages. Recent advances in
stem cell research have allowed for the demonstration of the existence of cancer stem cells in acute myeloid leukemia, breast cancer, and, most recently, in brain tumors. Each of these has
some similarities with the normal stem cells in the corresponding organs. In brain tumors, putative cancer stem cells have been identified from glioblastoma multiforme, medulloblastoma and
ependymoma. These tumor-derived cells self-renew under clonal conditions, and differentiate into neuron- and glia-like cells as well as into abnormal cells with mixed phenotypes. The tumor
stem cells, but not the rest of tumor cells form secondary tumors by transplantation into immunodeficient mouse brain. In this review, we discuss the cellular and molecular relationships
between brain tumor stem cells and normal neural stem cells, and also the possible clinical implications of brain tumor stem cells. SIMILAR CONTENT BEING VIEWED BY OTHERS BRAIN CANCER STEM
CELLS: RESILIENCE THROUGH ADAPTIVE PLASTICITY AND HIERARCHICAL HETEROGENEITY Article 16 June 2022 CANCER STEM CELLS: ADVANCES IN KNOWLEDGE AND IMPLICATIONS FOR CANCER THERAPY Article Open
access 05 July 2024 CELL-LINEAGE CONTROLLED EPIGENETIC REGULATION IN GLIOBLASTOMA STEM CELLS DETERMINES FUNCTIONALLY DISTINCT SUBGROUPS AND PREDICTS PATIENT SURVIVAL Article Open access 25
April 2022 MAIN Cancers, rather than consisting of single cell types, contain a variety of different cells with different phenotypic and genetic characteristics. There are numerous tumor
types that can be found within the CNS. Metastatic tumors arise from tissue outside the CNS, and spread hematogenously to the brain. Primary CNS tumors are thought to arise directly from CNS
tissue. Generally, these exclude such intracranial tumors as meningioma and acoustic neurinoma that, while existing only within the skull, they are not thought to arise from brain tissue.
The most common form of brain tumors in pediatric patients is medulloblastoma, and in adults is glioblastoma multiforme (GBM). Both tumor types have aggressive characteristics, and cause
high mortality and morbidity. Medulloblastomas generally occur in the posterior fossa, although metastases can spread throughout the CNS, including so-called “drop mets” in the spinal cord.
GBM are the most aggressive form of gliomas, a heterogeneous group of tumors. While medulloblastoma is often treatable, there is still a high rate of morbidity and mortality. To date, GBM
remains one of the most difficult cancers to treat, with less than 5% of 5-y survival rate, despite of multiple treatments such as direct surgery, radiation therapy, and chemotherapy.
Traditional thinking holds that any (or many) cells within a tumor will be capable of giving rise to another tumor (1). The cancer stem cell hypothesis dictates that tumors arise from a
single, self-renewing cell type, which then gives rise to the rest of the tumor, including a variety of “more differentiated” cell types. These cancer stem cells would be a minority
population within the tumor. The practical implications of this hypothesis is that any curative therapy will need to kill or stop the cancer stem cell from proliferating, whereas therapies
targeted at derivatives of the cancer stem cell will not be curative. Studies have provided strong evidence for the cancer stem cell hypothesis in leukemia and in breast cancer (2,3). The
first cancer which was found to contain stem cells was acute myeloid leukemia (AML) (2) (Table 1). A subfraction of cells in AML resembled normal hematopoietic stem cells based upon their
morphologic and immunohistochimal characteristics. It was found that this subset of cells, but not the rest of the tumor cells, could form AML when xenotransplanted into immnodeficient mice.
The corresponding secondary AML in mice possessed similar histopathological characteristics to the primary tumor. The second cancer type in which caner stem cells were identified was breast
cancer (3). In this study, the authors used markers associated with normal ductal stem cells, to positively (CD44) and negatively (CD24) sorted cells. When small numbers of these cells were
injected into immunodeficient mice, they formed tumors at very high frequency, while the stem cell negative fraction did not form tumors. These secondary tumors were histologically similar
to the primary tumors and also contained a subpopulation of CD44+, CD24− cancer stem cells which could, in turn form tumors in other mice. In addition to providing the first documentations
of cancer stem cells, both of these studies raised the strong possibility that the origin of the cancer stem cells, themselves, lay in the tissue-specific or adult stem cells resident within
the tissue. Thus, although the cancer stem cell hypothesis does not require cancer stem cells to be derived from mutations of normal stem cells, there is evidence to suggest that, at least
in these two cancers, such is the case. NEURAL STEM CELLS AND BRAIN TUMOR STEM CELLS Neural stem cells are a form of tissue-specific stem cell. They are self-renewing and capable of forming
the major cell types intrinsic to the brain, neurons, astrocytes and oligodendrocytes. Neural stem cells were originally isolated in culture by exposing dissociated CNS cells to epidermal
growth factor (EGF) in a relatively minimal medium (4). As a result, large spheres of cells formed from single cells which could then be re-dissociated and seeded at extremely low density to
produce new spheres, indicating a capacity for self-renewal. In the proliferative and undifferentiated state, these cells express the intermediate filament, nestin. However, when EGF is
removed, the cells begin to express markers typical of differentiated neurons and glia. Thus, these cells meet the criteria of being stem cells in that they are both self-renewing and
multipotent. Neural stem cells can be isolated from the CNS at virtually any stage of development after neural tube closure, and possibly before, through adulthood (4,5). _In vivo_, neural
stem cells are generally reside within specialized germinal zones situated along the surface of the neural tube—the developing and mature ventricular system. However, there is some
heterogeneity of cells that share the critical features of neural stem cells; self-renewal and multipotency (6). During development, neural stem cells divide rapidly and extensively
self-renew. Some investigators call these cells multipotent progenitors, since, in general, stem cells are thought as relatively slowly dividing. Radial glia, the bipolar cells extending
from the ventricular to the pial surface of the developing neural tube have been long perceived as “simple” guides for migrating cortical neurons. Several recent studies, however, have
demonstrated that radial glia, themselves function as neural stem cells during prenatal rodent development. Later, in postnatal stages and adulthood, a type of neural stem cell exists that
divides relatively slowly and produces more rapidly dividing self-renewing progenitors which, in turn, produces more committed progenitors. Recent evidence has indicated that these putative,
slowly dividing cells express glial fibrillary acidic protein (GFAP), a classic marker for astrocytes (7,8). Likewise, in the adult human brain, neural stem cells are also GFAP-positive
cells (9). Neural stem cells have been isolated and enriched from fetal and adult human brain and grown as neurospheres and adherent cultures. Human neural stem cells are CD133-positive and
CD24-negative (10). Flow sorting for these markers, greatly enriches cultures for neurosphere-forming cells, although this set of markers does not completely purify neural stem cells.
Following the isolation of neural stem cells, there was wide speculation that mutations of neural stem cells were the origins of many brain tumors. There are several lines of evidence
supporting the hypothesis that brain tumors arise from aberrant neural stem cell proliferation (11) including the fact that many brain tumors contain neuronal and glial elements and express
the intermediate filament nestin (12). Direct evidence for a neural stem cell origin of human brain tumors is difficult to obtain. However, an elegant series of studies using transgenic
models has demonstrated that nestin-expressing progenitors overexpressing the oncogene, c-Myc, can give rise to gliomas and medulloblastomas (13,14). Transformation of GFAP positive cells
_in vivo_ can give rise to gliomas, as well (15). In the past, this finding would have been taken as evidence of astrocytes being the origin of these experimental tumors, but now, given that
postnatal neural stem cells are known to be GFAP positive (8), these results are also consistent with a neural stem cell origin. Despite evidence that neural stem cells can be the source of
brain tumors under some experimental conditions, it is unlikely that they are the source of all brain tumors. Medulloblastomas are tumors of the cerebellum. Recent microarray and animal
model studies strongly indicate that the source of many, if not all medulloblastomas is the external granule cell (16). This cell type, like neural stem cells, is self-renewing, but it is
not multipotent under most conditions. Additionally, many tumors appear to have origins away from the ventricular surface, the site of neural stem cells _in vivo_, making it more likely that
they arise from transformation of other progenitors or more fully differentiated glia. One potential cell of origin could be the recently identified glial-like cells with a capacity to
self-renew and produce neurons, astrocytes and oligodendrocytes that are dispersed throughout the cortex and cerebellum, at sites distant from the ventricular surface (17). Despite the fact
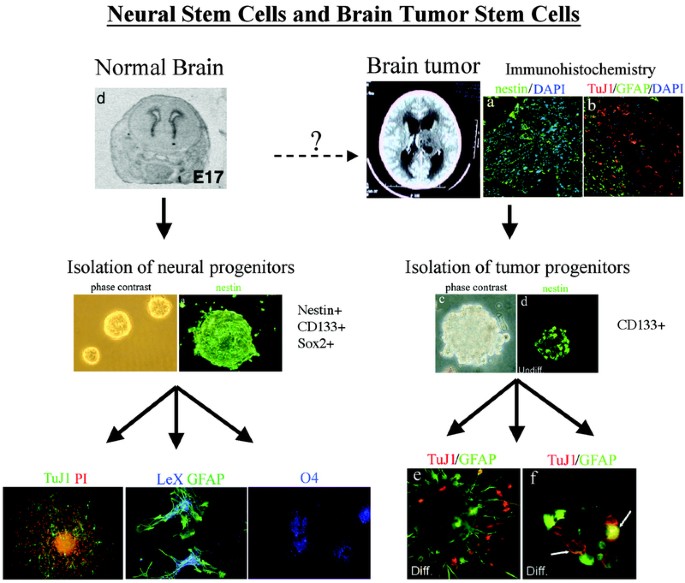
that not all brain tumors are likely to be derived from neural stem cells, many brain tumors do contain stem cells within them that bear many similarities to neural stem cells. We found that
several brain tumors, including astrocytomas (low and high grade), ependymoma, glioblastoma multiforme, and medulloblastomas contained cells that proliferated in the presence of EGF, basic
fibroblast growth factor (bFGF) and leukemia inhibitory factor (LIF) under conditions identical to those reported for embryonic human neural stem cells (18). Others have also obtained
similar data with gliomas (19,20) medulloblastomas (20) and ependymomas (21). These cells readily formed neurospheres as shown in Fig. 1. Immunocytochemical analysis of these tumor-derived
spheres demonstrated that most expressed nestin (Fig. 1). Following growth factor withdrawal, most tumor-derived spheres gave rise to cells with glial and neuronal morphology and antigenic
characteristics (Fig. 1). Tumor-derived spheres could be passaged clonally, and these clones could again be demonstrated to be multipotent. The numbers of neuron-like and glial-like cells in
each clone within a tumor were remarkably similar although the relative proportions varied greatly from tumor to tumor (18). The data described above demonstrate the presence of
self-renewing, multipotent progenitors within brain tumors, but do not prove that they are cancer stem cells. Two studies from different groups, however, do demonstrate this. Galli _et al._
(22) demonstrated that neurosphere cultures generated from GBM cells (in a manner very similar to Hemmati et al. (18)) could give rise to GBMs in immunodeficient mice. Tumors could result
from cultures that had been expanded from single cells and bore the same cytogenetic abnormalities as the parent tumors. Singh _et al._ (23) used a slightly different strategy. They sorted
glioblastomas and medulloblastomas for CD133 and found that the CD133- positive cells transplanted at low density readily formed tumors in immunodeficient mice. These tumors could be
re-sorted for CD133 positive cells, which could then form new tumors in recipient mice. CD133 negative cells, even at much higher concentrations, did not form tumors. More recently,
tumorigenic stem cells were isolated from ependymomas from various brain and spinal cord regions. Interestingly, these cells had the properties of radial glia. Taken together, the studies of
Singh _et al._ (20,23) Galli _et al._ (22) and our own study (18) strongly support the hypothesis that at least some brain tumors contain cancer stem cells with at least some of the
characteristics of neural stem cells. CRITICAL REGULATOR CANDIDATES OF SELF-RENEWAL OF BRAIN TUMOR STEM CELLS The isolation of cancer stem cells from brain tumors is important because of the
implications that only through attacking these cells, will we be able to eradicate the tumors that contain them. As stated above, cancer stem cells and adult stem cells share the properties
of self-renewal and multipotentiality. Several studies have demonstrated that genes known to play roles in the self-renewal of somatic stem cells also are likely to play similar roles in
cancer stem cells. Some of these pathways, which are not mutually exclusive, are described here. BMI-1. Bmi-1 is a polycomb transcription factor and is known to regulate chrmomatin
remodeling. Bmi-1 has been previously shown to regulate the proliferation of hematopoetic stem cells as well as leukemic stem cells (24). Analysis of Bmi-1 mutant mice have shown that it is
also important for self-renewal of normal neural stem cells (25). Bmi-1 binds to p14INKa and p16ARF, and inhibits the activation of their signaling (26). These two downstream genes are
highly expressed in primary GBM as well as oligodendrocytoma, another type of glioma. The high level of bmi-1 expression in our tumor-derived progenitors in spheres (18) suggests that this
gene, as well as its signaling pathway, plays an important role in the proliferation of not only GBM but also stem cells in GBM. Recently bmi-1 and its associated genes were found as
critical indicators of the prognosis of various cancers including glioma, according to the cDNA microarray data set (27). This also suggests that bmi-1 and the genes that it regulates have a
strong influence on proliferation of tumor cells. PTEN AND THE PI3 KINASE-AKT PATHWAY. Activation of PI3 kinase leads to phosphoryolation of Akt which, in turn, leads to promotion of
proliferation and cell survival, among other things (28). A part of this cascade includes TOR (target of rapamycin). Many tumors frequently have enhanced activity of this pathway. Mutations
of the EGF receptor, for example, are frequently found in GBM and confer a worse prognosis with a more aggressive tumor. Another mechanism by which this pathway is regulated is via the
phosphatase PTEN (phosphatase and tenascin homolog deleted on chromosome 10). PTEN suppresses Akt phosphorylation by reversing PI3 kinase-driven phosphorylation. PTEN is a tumor suppressor
gene, and is frequently deleted in GBM. We have previously demonstrated that PTEN is a critical regulator of neural stem cell self-renewal, as its loss induces greater proliferation as well
as enhanced recruitment of neural stem cells from a quiescent to an active cycling state (29,30). Drugs that interact with this pathway are potential candidates for treatment of brain
tumors. Inhibitors of EGFR activation have been recently shown to be effective in treating patients with the EGFR VIII mutation (31), an active form of the receptor. Rapamycin is a drug that
inhibits TOR and is under investigation for a variety of cancers, including GBM. It is unknown, however, whether these agents selectively inhibit cancer stem cell proliferation. MATERNAL
EMBRYONIC LEUCINE-ZIPPER KINASE (MELK). MELK encodes a serine/threonine kinase with a leucine-zipper domain, and is a poorly characterized member of snf1/AMPK family. Several members of this
family are known as tumor survival factors under conditions of nutrient starvation. Our previous studies demonstrated that normal neural stem cells highly express MELK. Functional analysis
demonstrated that MELK regulates the self-renewal of murine (32) and human (unpublished) neural stem cells. MELK was recently identified by microarray analysis to be highly correlated with
malignancy of a variety of tumors and to be an important regulator of cell cycle progression in these cancer cells (33). We have found that MELK is highly expressed in brain tumors as well
as in brain tumor stem cell containing cultures (18). Functional studies in these cultures indicate that MELK regulates the proliferation of both putative cancer stem cells as well as non
stem cell components (unpublished). The mechanism by which MELK regulates proliferation in cancer stem cells or other cells is still unknown. Previous studies have suggested that one
mechanism of MELK action is by regulated the transcription factor B-Myb, a known cell cycle regulator (32). However, our own preliminary data suggest that this is not the only function of
MELK. MELK has both kinase and RNA processing functions, both of which could contribute to its net effects. WNT AND HEDGEHOG SIGNALING IN MEDULLOBLASTOMAS. As described above, cancer stem
cells have been reported for medulloblastomas, although there is some disagreement in the literature about whether these cells represent true stem cells. The Wnt and sonic hedgehog signaling
cascades promote the proliferation of numerous cells, including granule cell progenitors of the cerebellum, the presumed cells of origin of medulloblastoms. Both have also been directly
shown to regulate the growth of murine and human medulloblastomas (16). Mutations of the sonic hedgehog receptor patched (which functions to inhibit the pathway) results in the formation of
medulloblastomas in both mice and humans. It remains to be seen whether these effects are on a cancer stem cell, _per se_. CLINICAL SIGNIFICANCE OF THE CANCER STEM CELL THEORY The isolation
of cancer stem cells is an important step in the understanding of tumor biology. However, there are also practical implications in the diagnosis and treatment of brain for the clinician.
SIGNIFICANCE FOR BRAIN TUMOR STRATIFICATION. Traditional anatomic/pathologic categorization of tumors have very limited ability to completely stratify patients into meaningful subgroups for
prognosis and intervention. Protein expression by immunohistochemistry has greatly enhanced the potential link between pathologic diagnosis and prognosis. However, relative lack of useful
antibodies shows the limitation of this strategy. Recently, large scale gene expression study by microarray and analyses of specific pathways have been more successful at regrouping patients
within broad histologic categories (16). The existence of caner stem cells raises the possibility that expression profiling and molecular pathway analysis of these cells will provide
further useful stratification of tumors. For example, one might isolate cancer stem cells from individual patients and then analyze gene or protein expression profiles as well as the
activity of the specific pathways described above (Fig. 2). SIGNIFICANCE FOR BRAIN TREATMENT. The isolation and demonstration of brain tumor stem cells, suggests that, for those tumors that
contain such cells, treatment can only be gauged as successful if the stem cell component is successfully eradicated (Fig. 2). Since, by their nature, stem cells are heartier and more
resistant to insult, this may prove to be a daunting task. There are several potential avenues to address this issue, however. First, since cancer stem cells may use specific molecular
pathways to drive their self-renewal, one might use known and as-yet-to-be-discovered selective inhibitors of these pathways to attack them. In fact, as intimated above, two groups of
agents, EGF receptor inhibitors and rapamycin-related compounds, that are already in clinical trial and showing some promise are predicted to inhibit cancer stem cell self-renewal (31).
Another way to attack cancer stem cells would be to use them as targets for small molecule screens, an approach that we are taking. Finally, if specific antigens are expressed by cancer stem
cells, then these antigens could be used in immunotherapies, which have already shown some promise in the treatment of glioma. ARE THERE IMPLICATIONS FOR NEURAL REPAIR. Neural stem and
progenitor cells are potential sources of therapy for a wide variety of disorders, ranging from enzymatic deficiency to neurodegenerative disease. It is well known that transplantation of
pluripotent embryonic stem cells into the brain or other tissue can result in teratoma formation. The possibility that neural stem and progenitor cells can give rise to tumors theoretically
raises the possibility that transplantation of the cells could result in tumor formation. It is important to state, however, that this simply has not been shown to be the case, at least in
the short to moderate timespans used in animal studies. To our knowledge, despite more than one thousand reports of neural stem and progenitor cell transplantation, none have reported
malignant transformation. CONCLUSIONS The isolation and initial characterization of brain tumor stem cells provides hope that new avenues of therapy will be opened up. However, many
questions remain before our fully utilizing these discoveries. Among these are the following: Do all brain tumors contain cancer stem cells? What are the differences and similarities between
cancer stem cells from different tumors? Does malignant progression of low grade to high grade gliomas directly involve brain tumor stem cells? Is CD-133 the best marker for the prospective
isolation of cancer stem cells? Can we attack brain tumor stem cells without attacking normal neural stem cells? Would this matter? These questions and many others highlight the early state
of this field and the great amount of work needed before putting the discovery of cancer stem cells to productive use. ABBREVIATIONS * bFGF: basic fibroblast growth factor * GBM:
glioblastoma multiforme * GFAP: glial fibrillary acidic protein * LIF: leukemia inhibitory factor * PTEN: phosphatase and tenascin homolog * TOR: target of rapamycin REFERENCES * Reya T,
Morrison SJ, Clarke MF, Weissman IL 2001 Stem cells, cancer, and cancer stem cells. _Nature_ 414: 105–111 Article CAS Google Scholar * Bonnet D, Dick JE 1997 Human acute myeloid leukemia
is organized as a hierarchy that originates from a primitive hematopoietic cell. _Nat Med_ 3: 730–737 Article CAS Google Scholar * Al-Hajj M, Wicha MS, Benito-Hernandez Morrison SJ,
Clarke MF 2003 Prospective identification of tumorigenic breast cancer cells. _Proc Natl Acad Sci U S A_ 100: 3983–3988 Article CAS Google Scholar * Reynolds BA, Weiss S 1992 Generation
of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. _Science_ 255: 1707–1710 Article CAS Google Scholar * Weiss S, Dunne C, Hewson J, Wohl C,
Wheatley M, Peterson AC, Reynolds BA 1996 Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. _J Neurosci_ 16: 7599–7609 Article CAS Google
Scholar * Hitoshi S, Tropepe V, Ekker M, van der Kooy D 2002 Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain.
_Development_ 129: 233–244 CAS PubMed Google Scholar * Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A 1999 Subventricular zone astrocytes are neural stem cells in the
adult mammalian brain. _Cell_ 97: 703–716 Article CAS Google Scholar * Imura T, Kornblum HI, Sofroniew MV 2003 The predominant neural stem cell isolated from postnatal and adult forebrain
but not early embryonic forebrain expresses GFAP. _J Neurosci_ 23: 2824–2832 Article CAS Google Scholar * Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S,
Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A 2004 Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain
migration. _Nature_ 427: 740–744 Article CAS Google Scholar * Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL 2000 Direct isolation of human
central nervous system stem cells. _Proc Natl Acad Sci U S A_ 97: 14720–14725 Article CAS Google Scholar * Oliver TG, Wechsler-Reya RJ 2004 Getting at the root and stem of brain tumors.
_Neuron_ 42: 885–888 Article CAS Google Scholar * Rorke LB 1997 Pathologic diagnosis as the gold standard. _Cancer_ 79: 665–667 Article CAS Google Scholar * Rao G, Pedone CA, Coffin
CM, Holland EC, Fults DW 2003 c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. _Neoplasia_ 5: 198–204 Article CAS Google
Scholar * Rao G, Pedone CA, Valle LD, Reiss K, Holland EC, Fults DW 2004 Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from
nestin-expressing neural progenitors in mice. _Oncogene_ 23: 6156–6162 Article CAS Google Scholar * Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC 2002 Ink4a-Arf loss
cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. _Cancer Res_ 62: 5551–5558 CAS PubMed
Google Scholar * Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel
JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR 2002 Prediction of central nervous system embryonal tumour outcome based on gene
expression. _Nature_ 415: 436–442 Article CAS Google Scholar * Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA 2003 Identification and
isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. _Nat Med_ 9: 439–447 Article CAS Google Scholar * Hemmati HD, Nakano I,
Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI 2003 Cancerous stem cells can arise from pediatric brain tumors. _Proc Natl Acad Sci U S A_ 100: 15178–15183
Article CAS Google Scholar * Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA 2002 Human cortical glial tumors contain neural stem-like cells expressing astroglial
and neuronal markers in vitro. _Glia_ 39: 193–206 Article Google Scholar * Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB 2003 Identification of a cancer stem
cell in human brain tumors. _Cancer Res_ 63: 5821–5828 CAS PubMed Google Scholar * Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J,
Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ 2005 Radial glia cells are candidate stem cells of ependymoma. _Cancer Cell_ 8: 323–335 Article CAS Google Scholar * Galli
R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A 2004 Isolation and characterization of tumorigenic, stem-like neural precursors from
human glioblastoma. _Cancer Res_ 64: 7011–7021 Article CAS Google Scholar * Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB 2004
Identification of human brain tumour initiating cells. _Nature_ 432: 396–401 Article CAS Google Scholar * Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke
MF 2003 Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. _Nature_ 423: 302–305 Article CAS Google Scholar * Molofsky AV, Pardal R, Iwashita T, Park IK,
Clarke MF, Morrison SJ 2003 Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. _Nature_ 425: 962–967 Article CAS Google Scholar * Molofsky AV, He
S, Bydon M, Morrison SJ, Pardal R 2005 Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence
pathways. _Genes Dev_ 19: 1432–1437 Article CAS Google Scholar * Glinsky GV, Berezovska O, Glinskii AB 2005 Microarray analysis identifies a death-from-cancer signature predicting therapy
failure in patients with multiple types of cancer. _J Clin Invest_ 115: 1503–1521 Article CAS Google Scholar * Sinor AD, Lillien L 2004 Akt-1 expression level regulates CNS precursors.
_J Neurosci_ 24: 8531–8541 Article CAS Google Scholar * Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H 2001 Negative regulation of neural
stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. _Science_ 294: 2186–2189 Article CAS Google Scholar * Groszer M, Erickson R, Scripture-Adams DD, Dougherty
JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H 2005 PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. _Proc Natl Acad Sci U S A_
Dec 22; [Epub ahead of print] * Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN,
Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS 2005 Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. _N
Engl J Med_ 353: 2012–2024 Article CAS Google Scholar * Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, Kim KJ, Ou J, Groszer M, Imura T, Freije WA, Nelson SF, Sofroniew
MV, Wu H, Liu X, Terskikh AV, Geschwind DH, Kornblum HI 2005 Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. _J Cell Biol_ 170: 413–427
Article CAS Google Scholar * Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, Yi S, Bai W, Frantz G, Zhang Z, Koeppen H, de Sauvage FJ, Davis DP 2005 Maternal embryonic leucine zipper
kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. _Cancer Res_ 65: 9751–9761 Article CAS Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Departments of Psychiatry, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, 90095, CA Harley I Kornblum *
Departments of Pharmacology, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, 90095, CA Harley I Kornblum * Departments of Neurosurgery, David Geffen
School of Medicine at University of California, Los Angeles, Los Angeles, 90095, CA Ichiro Nakano * Departments of Pediatrics, David Geffen School of Medicine at University of California,
Los Angeles, Los Angeles, 90095, CA Ichiro Nakano & Harley I Kornblum Authors * Ichiro Nakano View author publications You can also search for this author inPubMed Google Scholar *
Harley I Kornblum View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Harley I Kornblum. ADDITIONAL INFORMATION Some
of the work described here was supported by a Jonsson Comprehensive Cancer Center Interdisciplinary Grant. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Nakano, I., Kornblum, H. Brain Tumor Stem Cells. _Pediatr Res_ 59 (Suppl 4), 54–58 (2006). https://doi.org/10.1203/01.pdr.0000203568.63482.f9 Download citation * Received: 07
December 2005 * Accepted: 21 December 2005 * Issue Date: 01 April 2006 * DOI: https://doi.org/10.1203/01.pdr.0000203568.63482.f9 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Dwp change for people on benefits born within three-year period rolled outThe Department for Work and Pensions is launching a new initiative to support benefits claimants born in specific years....
Ryanair passengers praise 'perfect' cabin bag with 'lots of pockets' - YorkshireLiveWhat's OnRyanair passengers praise 'perfect' cabin bag with 'lots of pockets'The VANKEV Backpack is the ideal travel com...
Moyes in tribute to victims and emergency services after liverpool parade crashTHE EVERTON BOSS BECAME THE LATEST FIGUREHEAD TO EXPRESS SOLIDARITY WITH THOSE AFFECTED BY THE CITY CENTRE INCIDENT 11:3...
Foreign office issues fresh warnings to brits heading to turkeyThe UK Foreign Office has today issued a fresh caution to Brits planning to visit Turkey, advising extra care when using...
Karine jean-pierre roasted over ‘orwellian’ tweet touting ‘0% inflation’EXPLORE MORE President Biden’s truth-averse top spokesperson was ridiculed after she touted “0% inflation in July” follo...
Latests News
Brain Tumor Stem Cells | Pediatric ResearchABSTRACT Cancers are composed of heterogeneous cell populations ranging from highly proliferative immature cells to more...
Opposition politicians urge scots to move into financial nightmare — scottish national partyIt’s not just the financial nightmare of Brexit that Westminster obsessed politicians want the people of Scotland to res...
Liquid biopsy of sclc chemosensitivityAccess through your institution Buy or subscribe Small-cell lung cancer (SCLC) is arguably the most-aggressive form of l...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Why does cbo prepare cost estimates?Memorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...