Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa
Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Neuroendocrine abnormalities in anorexia nervosa (AN) include hypercortisolemia, hypogonadism, and hypoleptinemia, and neuroendocrine predictors of menstrual recovery are unclear.
Preliminary data suggest that increases in fat mass may better predict menstrual recovery than leptin. High doses of cortisol decrease luteinizing hormone (LH) pulse frequency, and cortisol
predicts regional fat distribution. We hypothesized that an increase in fat mass and decrease in cortisol would predict menstrual recovery in adolescents with AN. Thirty-three AN girls 12–18
y old and 33 controls were studied prospectively for 1 y. Body composition [dual energy x-ray absorptiometry (DXA)], leptin, and urinary cortisol (UFC) were measured at 0, 6, and 12 mo.
Serum cortisol was measured overnight (every 30 min) in 18 AN subjects and 17 controls. AN subjects had higher UFC/cr·m2 and cortisol area under curve (AUC), and lower leptin levels than
controls. Leptin increased significantly with recovery. When menses-recovered AN subjects were compared with AN subjects not recovering menses and controls, menses-recovered AN subjects had
higher baseline cortisol levels and greater increases in leptin than controls and greater increases in fat mass than AN subjects not recovering menses and controls (adjusted for multiple
comparisons). In a logistic regression model, increasing fat mass, but not leptin, predicted menstrual recovery. Baseline cortisol level strongly predicted increases in the percentage of
body fat. We demonstrate that 1) high baseline cortisol level predicts increases in body fat and 2) increases in body fat predict menses recovery in AN. SIMILAR CONTENT BEING VIEWED BY
OTHERS EFFECT OF TIME RESTRICTED EATING VERSUS DAILY CALORIE RESTRICTION ON SEX HORMONES IN MALES AND FEMALES WITH OBESITY Article 12 June 2024 OMEGA-3 FATTY ACIDS AND AUTONOMIC FUNCTION IN
ADOLESCENTS WITH ANOREXIA: A RANDOMIZED TRIAL Article 28 July 2022 OLDER ADULT WOMEN WITH CHRONIC ANOREXIA NERVOSA: HETEROGENEOUS ADAPTATION TO UNDERNUTRITION OVER TIME Article 30 July 2024
MAIN AN, a model of severe undernutrition, is associated with hypogonadotropic hypogonadism resulting in primary or secondary amenorrhea or delayed menarche. Weight recovery occurs in up to
50% of adolescents with AN and should result in recovery of the hypothalamo-pituitary-gonadal (H-P-G) axis (1). However, a temporal association between weight gain and menstrual recovery is
not always observed (2,3). Not all adolescents with AN who resume menses are weight recovered, and not all weight-recovered adolescents with AN resume menstrual function. In addition,
neuroendocrine predictors of menstrual recovery are unclear. We have demonstrated higher cortisol (4) and lower leptin levels (3,5) in AN girls compared with healthy adolescents. Leptin is
an adipocytokine, and leptin-deficient or -resistant mice (6,7) and humans with leptin and leptin receptor mutations (8,9) are hypogonadal. In a recent study, leptin administration was
associated with resumption of menses in five of eight women with hypothalamic amenorrhea (10). These data suggest that leptin is an important regulator of the H-P-G axis and that an increase
in leptin along with an increase in fat mass may predict recovery of the H-P-G axis in AN. Conversely, Golden _et al._ (11) observed no differences in fat mass between AN girls who
recovered menses and those who did not. In addition, possible effects of cortisol on the H-P-G axis have been demonstrated. Cortisol in high doses decreases gonadotropin-releasing hormone
(GnRH) secretion and LH pulse frequency in healthy women (12,13), and inverse associations have been noted between cortisol and LH pulse frequency (14) as well as between cortisol and
menstrual frequency (15). Samuels _et al._ (16), conversely, did not observe a decrease in gonadotropin pulsatility following hydrocortisone administration. Girls with AN have
hypercortisolemia (4), and it is unclear whether baseline cortisol levels or a reduction in cortisol may predict menstrual recovery. In addition, in a study of adult amenorrheic and
eumenorrheic women of comparable low body mass index (BMI), our group reported greater fat mass, particularly trunk fat, in the eumenorrheic group, suggestive of effects of body composition
on reproductive function (17). Our group has also demonstrated that cortisol is an important predictor of body composition in adults, with higher baseline cortisol levels predicting greater
increases in trunk fat in adults with AN (18). It has not been determined, however, whether a higher baseline cortisol level, predicting greater increases in fat mass, also predicts greater
chances of recovering menses. Relative contributions of hypercortisolemia, hypoleptinemia, and decreased fat mass to hypogonadism in adolescent AN are unclear, and it is uncertain whether
recovery of any one of these factors better predicts menstrual recovery than others. We hypothesized that, in AN girls, an increase in leptin associated with increases in fat mass
contributes to and predicts menstrual recovery. In addition, we hypothesized that alterations in cortisol may predict menses recovery, either as a consequence of recovery of the H-P-G axis
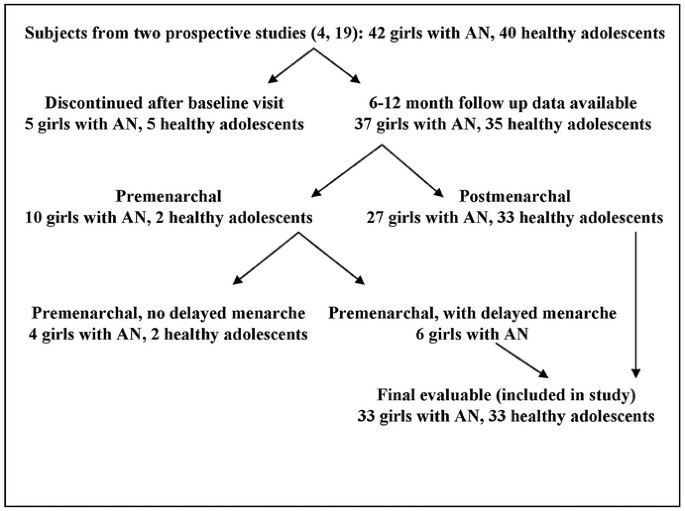
from decreasing cortisol levels or from greater increases in fat mass and leptin in AN girls with higher cortisol levels at baseline. SUBJECTS AND METHODS SUBJECT SELECTION. Data from two
previously completed studies by Soyka _et al._ (19) and Misra _et al._ (3,4,20) were pooled to determine predictors of menstrual recovery in girls with AN. Of the 42 adolescent girls with AN
(meeting the DSM-IV criteria) and 40 controls 12–18 y old enrolled in these studies, follow-up data at 6 or 12 mo was available for 37 AN subjects and 35 controls. Of these adolescents, 10
AN subjects and two controls were premenarchal. Among the premenarchal group, girls with delayed menarche [age older than 15.3 y (mean age at menarche + 2 SDs for American girls) (21)] were
included in our study because delayed menarche was likely subsequent to low weight 3 and onset of menses expected following weight gain. Girls younger than 15.3 y old were not included
because they were still in the normal age range for attaining menarche. Thus, four AN subjects and two controls were excluded from the final analysis, and 33 AN subjects and 33 healthy
adolescents were evaluable for predictors of menstrual recovery (Fig. 1). Baseline characteristics and methods of recruitment for 16 controls and 15 AN subjects have been reported in a
previous article by Soyka _et al._ (19) and for 17 controls and 18 AN subjects in articles by Misra _et al._ (3,4,20). Characteristics of girls with AN did not differ in the two studies nor
did characteristics for controls. The overall mean age was 16.1 ± 1.5 y in AN subjects and 15.4 ± 1.6 y in controls (_p_ = not significant). Bone age was 15.7 ± 1.5 y in AN subjects and 15.8
± 1.6 in healthy adolescents (_p_ = not significant). Mean duration since diagnosis was 5.0 ± 9.4 mo, and mean duration of amenorrhea was 10.6 ± 10.2 mo. Mean age at menarche among
postmenarchal girls was 12.8 ± 1.5 y in AN subjects _versus_ 12.4 ± 1.0 y in controls (_p_ = not significant). Postmenarchal AN subjects had been amenorrheic at least 3 mo at study
initiation. Nineteen of 33 AN subjects (57.6%) recovered menstrual function during the 1-y follow-up. Recovery of menstrual function was defined as three or more menstrual periods over the
preceding 6 mo with at least one menstrual period in the preceding 3 mo. Healthy controls did not have a history of eating disorders. Our institutional review board approved the study, and
informed assent and consent was obtained from all. EXPERIMENTAL PROTOCOL. Subjects were screened to rule out thyroid dysfunction, hypergonadotropic hypogonadism, and hyperprolactinemia and
were evaluated at a baseline visit and at 6 and 12 mo. The baseline visit included a history and physical examination and fasting blood sample for leptin. Subjects from the study by Misra
_et al._ (3,4,20) (17 controls and 18 AN subjects) had frequent sampling for cortisol level performed every 30 min overnight (2000 h to 0800 h) at the General Clinical Research Center (GCRC)
of Massachusetts General Hospital. Total AUC for cortisol was calculated using the computerized algorithm Cluster (1 × 2) (22). For all subjects, body composition was assessed by DXA. A
24-h urine collection was completed UFC and creatinine (cr), and baseline UFC/cr·m2 was available in 29 AN subjects and 29 controls. Subjects were followed over a year and leptin and body
composition measurements repeated at 6 and 12 mo. Twelve-month data were available for 29 AN subjects and 29 controls, and 6-mo data in an additional four AN subjects and four controls.
Among the four AN girls for whom only 6-mo data were available, two resumed menses by 6 mo and continued to maintain menstrual function, whereas the two AN girls who had not resumed menses
at the 6-mo follow-up continued to be amenorrheic and of low weight at 12 mo (information from primary care provider and parent). These girls were included in the study given that their
clinical and menstrual status at 12 mo was not different from that at 6 mo. Frequent sampling for cortisol was repeated at 10% increase in BMI in 11 of the 18 AN girls from the study by
Misra _et al._ (4). Eight of these 11 had resumed menses. UFC/cr·m2 data at follow-up were available in 16 AN and 13 controls [all from the study by Misra _et al._ (4) because the study by
Soyka _et al._ (19) did not include urinary cortisol measurements after the baseline visit]. ANTHROPOMETRIC MEASUREMENTS. Height was measured at the GCRC in triplicate on a single
stadiometer and averaged. Weight was measured on an electronic scale in a hospital gown. BMI was calculated as the ratio of weight (kg) to height (m2). Standards of Greulich and Pyle (23)
were used to determine bone age. Body composition was determined using whole-body DXA (QDR 4500, Hologic Inc., Waltham, MA) (24,25). The percentage of trunk fat was calculated as follows:
[trunk fat/total fat]*100 (26). BIOCHEMICAL ASSESSMENT. We used radioimmunoassay (RIA) to measure serum cortisol (Diagnostic Products Corp., Los Angeles, CA) [limit of detection, 1 μg/dL;
sensitivity, 0.21 μg/dL; coefficient of variation (CV), 2.5–4.1%] and leptin (Linco Diagnostics, St. Louis, MO) (sensitivity, 0.5 ng/mL; CV, 3.4–8.3%). Samples were stored at –80°C until
analysis and were run in duplicate. UFC was measured by the hospital laboratory using the GammaCoat 125I RIA (Diasorin Inc., Stillwater, MN; detection limit 1 μg/dL; CV 7%). STATISTICAL
METHODS. All data are described as mean ± SD. Data were analyzed using the JMP program (version 4, Cary, NC). A _t_ test was used to calculate differences between means. When comparisons
involved more than two groups, we used analysis of variance (ANOVA) followed by the Tukey-Kramer test for intergroup comparisons. A paired _t_ test was used to compare endpoints at follow-up
_versus_ baseline. To compare proportions, we used Fisher's exact test. Correlational analysis was used to determine associations between continuous variables such as baseline serum or
urinary cortisol, baseline body fat, and changes in body fat. Logistic regression was used to determine predictors of menstrual recovery and odds ratios calculated from parameter estimates
of covariates. For the four subjects in whom only baseline and 6-mo data were available, 6-mo data were carried forward for analysis. For these girls, 12-mo values were imputed based on
within-group average change between 6 and 12 mo, and we found similar differences between the groups as with carry forward analysis. RESULTS COMPARISON OF AN GIRLS WHO RECOVERED MENSES
_VERSUS_ THOSE WHO DID NOT RECOVER MENSES _VERSUS_ HEALTHY ADOLESCENTS. Girls with AN recovering menses did not differ from AN girls not recovering menses for baseline BMI, fat mass,
percentage of body fat, and leptin (Table 1). Both AN groups had lower BMI, fat mass, percentage of body fat, and leptin than controls. Baseline cortisol AUC [performed only in subjects from
the study by Misra _et al._ (4)] was higher in AN recovering menses compared with the other groups (_p_ < 0.0001, ANOVA) (Fig. 2). For girls who underwent frequent sampling for cortisol
(4), when AN girls were dichotomized based on median cortisol AUC, eight of the nine girls with a cortisol level greater than the median resumed menses _versus_ four of nine girls with a
cortisol level below the median (_p_ = 0.07). Baseline UFC/cr·m2 trended higher in AN girls _versus_ controls. Correlation between cortisol AUC and UFC/cr·m2 was fair (_r_ = 0.52, _p_ =
0.002 for all subjects; _r_ = 0.56, _p_ = 0.01 for AN girls). Girls with AN recovering menses had greater increases in BMI, fat mass, percentage of body fat, percentage of trunk fat, and
leptin than healthy adolescents, and greater increases in BMI, fat mass, and percentage of body fat than AN not recovering menses (Table 1). Change in fat mass in the three groups is
illustrated in Figure 3. When AN girls were divided into two groups based on median change in BMI (Δ BMI) over follow-up, 12 of the 16 girls with Δ BMI greater than the median resumed menses
_versus_ seven of the 17 girls with Δ BMI less than the median (_p_ = 0.05). Similarly, when AN girls were dichotomized based on median change in fat mass (Δ fat mass) over follow-up, 13 of
16 AN girls with Δ fat mass greater than the median resumed menses _versus_ five of the 16 AN girls with Δ fat mass less than the median (_p_ = 0.006). When AN girls were dichotomized based
on median change in leptin (Δ leptin), 11 of 16 girls with Δ leptin greater than the median resumed menses _versus_ seven of 16 girls with Δ leptin less than the median (_p_ = not
significant). Final measurements of BMI, fat mass, percentage of body fat, percentage of trunk fat, and leptin and cortisol levels were compared (Table 1). Final BMI, fat mass, and
percentage of body fat were higher in AN girls resuming menses _versus_ those who did not but continued to be lower than in controls. All AN girls with a final percentage of body fat
>24.4% regained menstrual function, whereas no AN girl with a final percentage of body fat <18.1% regained menstrual function (Fig. 4). Final percentage of trunk fat in AN girls
resuming menses approached that in controls and was higher than in AN girls not resuming menses. Paired _t_ tests of baseline and 12-mo data in AN who resumed menses (Table 2) showed that
girls recovering menses had significant increases in BMI, fat mass, percentage of body fat, percentage of trunk fat, and leptin, but not in UFC/cr·m2. Frequent sampling for cortisol was
repeated in 11 AN girls who gained >10% of their BMI during follow-up. Eight of these girls also resumed menses. In this subset who underwent repeat frequent sampling, paired _t_ tests in
girls resuming menstrual function demonstrated significant increases in BMI (16.9 _versus_ 19.4 kg/m2, _p_ = 0.0008), fat mass (9.3 _versus_ 13.4 kg, _p_ = 0.0002), percentage of body fat
(18.8 _versus_ 24.1%, _p_ = 0.0008), and leptin (4.6 _versus_ 9.0 ng/mL, _p_ = 0.007) similar to the group as a whole who resumed menses. Cortisol AUC decreased minimally from 6683 to 5940
μg/dL·12 h in this subset (_p_ = not significant). Cortisol as a predictor of changes in body composition over time in AN. Baseline cortisol AUC predicted Δ BMI (_r_ = 0.54, _p_ = 0.02) and
was a strong predictor of Δ fat mass and percentage of body fat (_r_ = 0.70, _p_ = 0.002 and _r_ = 0.75, _p_ = 0.0006) (Fig. 5). Baseline fat mass and leptin did not predict Δ fat mass in
AN. However, baseline percentage of body fat did predict Δ percentage of body fat (_r_ = −0.49, _p_ = 0.005). When baseline percentage of body fat, cortisol AUC, and leptin were entered into
a regression model, the sole significant predictor of Δ percentage of body fat was baseline cortisol AUC, which contributed to 55.9% of the variability. UFC/cr·m2 also predicted Δ
percentage of body fat (_r_ = 0.40, _p_ = 0.04) (Fig. 5) and remained an independent predictor after adjusting for baseline percentage of body fat (8.3% of the variability). In the group as
a whole, cortisol was again a significant and independent predictor of changes in percentage of body fat over time (data not reported). Baseline characteristics in AN with baseline cortisol
AUC above the median _versus_ those with AUC below the median did not differ [BMI (16.7 ± 1.5 kg/m2 _versus_ 16.7 ± 1.1 kg/m2), fat mass (9.2 ± 3.5 kg _versus_ 8.8 ± 2.7 kg), percentage of
body fat (18.7 ± 4.5% _versus_ 18.2 ± 4.5%), duration of illness (8.4 ± 2.5 mo _versus_ 3.9 ± 2.5 mo), or duration of amenorrhea (3.6 ± 1.9 mo _versus_ 5.3 ± 1.9 mo)]. PREDICTORS OF
MENSTRUAL RECOVERY (LOGISTIC REGRESSION). When logistic regression was performed using Δ BMI, Δ body fat, and Δ leptin to determine which of these parameters predicted menstrual recovery, Δ
body fat, but not Δ leptin or Δ BMI, was a significant predictor of menstrual recovery (_p_ = 0.01). For every quartile increase in Δ fat mass, the odds ratio (OR) for recovering menses was
3.0 (_p_ = 0.009), and for an increase in Δ fat mass from below the median to above the median, the OR of recovering menses was 9.5 (_p_ = 0.007). Similarly, when final percentage of body
fat, final BMI, and final leptin were entered into a logistic regression model, final percentage of body fat was a significant predictor of menstrual recovery (_p_ = 0.03). The OR of
resuming menses for final percentage of body fat above the median compared with below the median was 15.5 (_p_ = 0.003). Trunk fat was not an independent predictor of menstrual recovery.
DISCUSSION We demonstrate that a higher baseline cortisol level in AN predicts greater subsequent increases in body fat, and increase in body fat predicts menstrual recovery. Thus, AN girls
with higher cortisol levels at baseline may gain more fat mass with weight gain, predicting greater chances of menstrual recovery. The role of cortisol in predicting menstrual recovery in AN
may stem not from its effect on the H-P-G axis, but from the effect of prerecovery cortisol concentrations on subsequent changes in body composition. In healthy women, high-dose cortisol
causes either a decrease (12,13) or no change (16) in LH pulsatility, and Rickenlund _et al._ (15) have reported an inverse correlation between cortisol and menstrual frequency. AN is
associated with hypercortisolemia in adults (27) and adolescents (4), and it is tempting to postulate that hypercortisolemia may contribute to hypogonadotropic hypogonadism associated with
AN. However, in this study, baseline serum cortisol was higher in AN girls who recovered menses compared with the group that did not recover menses and controls, and UFC/cr·m2 was higher in
AN recovering menses _versus_ controls. No change in overnight serum cortisol occurred with weight gain in eight AN girls who also recovered menses. Given that marked increases in BMI, fat
mass, and leptin did occur in this subset who underwent repeat frequent sampling following weight gain suggests that small sample size cannot explain the lack of change in cortisol with
menses recovery. AN girls may have residual elevations in cortisol despite increased weight, and, indeed, adult AN studies do report that weight gain is not associated with changes in
cortisol (18). Baseline characteristics including BMI, fat mass, and duration of illness or of amenorrhea did not differ in AN subjects with higher _versus_ lower cortisol values (based on
median AUC). Thus, higher baseline cortisol was not indicative of severity of illness. Baseline cortisol was a strong and independent predictor of increase in fat mass after adjusting for
baseline fat and leptin. Similarly, UFC/cr·m2 independently predicted increase in body fat. AN girls with higher baseline cortisol are thus more likely to have greater increases in fat mass
with weight gain, particularly because cortisol does not decrease with weight gain. Increase in fat mass, in turn, independently predicted menstrual recovery on logistic regression. This is
in contrast to data reported by Golden _et al._ (11) in which fat mass did not differ in AN girls who recovered menses _versus_ those who did not. In our analyses, serum cortisol was a
stronger predictor of changes in fat mass than was urinary cortisol. In a previous study (4), we reported that higher serum cortisol concentrations observed in girls with AN are a
consequence of increased secretory frequency and half-life. We postulate that UFC/cr·m2 is a less sensitive measure than serum cortisol of hypercortisolemia in AN due to increased cortisol
half-life and reduced urinary clearance in AN. In addition, although detailed instructions were provided to all subjects regarding 24-h urine collection, we suspect lack of completeness of
this collection in some adolescents noted to have very low 24-h urinary volumes. Although correction for creatinine would take into account some inaccuracies in collection, diurnal variation
limits the accuracy of this correction method. Therefore, our serum frequent sampling cortisol data are likely more accurate than our urinary cortisol data. Studies have demonstrated that
cortisol binding globulin (CBG) levels are not elevated in AN (28). Therefore, we do not believe that lack of a stronger correlation between serum and urinary cortisol is a consequence of
high CBG levels in AN. In addition, baseline BMI and body composition did not differ between girls who did (4) and did not (19) have frequent sampling for serum cortisol performed (data not
reported). Thus, it is not likely that innate differences between subjects from the two studies may have accounted for the stronger correlations of serum cortisol with body fat _versus_ that
of UFC/cr·m2 with body fat. These data would, of course, be stronger if overnight serum cortisol estimations had been performed for all subjects. Leptin is an adipocytokine that is low in
AN (3,29–31), and leptin deficiency or resistance causes hypogonadism (6–9). Administration of Rh leptin to women with hypothalamic amenorrhea caused resumption of menstrual function in five
of eight women (10). Given these data, we expected an association between change in leptin and resumption of menses. Indeed, AN girls who resumed menses had greater increases in leptin than
controls. When compared with AN girls not recovering menses, change in leptin in the menses-recovered group was higher but did not reach statistical significance. A significant difference
may have been observed with more subjects. However, with this sample size, we did observe significantly greater increases in fat mass in the group recovering menses _versus_ the group not
recovering menses. In addition, on regression modeling, increase in body fat (but not in leptin) predicted menstrual recovery. These data suggest that increase in fat mass may result in
signals to the H-P-G axis that cause an awakening of this axis separate from those associated with increases in leptin. However, mediators of effects of increased fat mass on the
reproductive axis are unclear, and more studies are necessary to confirm these findings. In this study of adolescents with AN, we demonstrate that increases in fat mass are the most
important predictor of menstrual recovery and that baseline cortisol most strongly predicts subsequent increases in fat mass. Although increases in leptin were greater in AN girls who
recovered menses _versus_ those not recovering menses, leptin did not independently predict menstrual recovery in this study. The mechanism whereby increases in fat mass cause recovery of
the H-P-G axis independent of increases in leptin is unclear, but maybe related to signaling by fat-related factors yet to be characterized. ABBREVIATIONS * AN: anorexia nervosa; * AUC: area
under the curve; * BMI: body mass index; * DXA: dual energy x-ray absorptiometry; * GnRH: gonadotropin-releasing hormone; * H-P-G axis: hypothalamo-pituitary-gonadal axis; * UFC/cr·m2:
urinary free cortisol normalized for creatinine and surface area REFERENCES * Swenne I 2004 Weight requirements for return of menstruations in teenage girls with eating disorders, weight
loss and secondary amenorrhoea. _Acta Paediatr_ 93: 1449–1455 Article CAS Google Scholar * Brambilla F, Monteleone P, Bortolotti F, Dalle Grave R, Todisco P, Favaro A, Santonastaso P,
Ramacciotti C, Paoli R, Maj M 2003 Persistent amenorrhoea in weight-recovered anorexics: psychological and biological aspects. _Psychiatry Res_ 118: 249–257 Article Google Scholar * Misra
M, Miller KK, Almazan C, Ramaswamy K, Aggarwal A, Herzog DB, Neubauer G, Breu J, Klibanski A 2004 Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin
index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. _J Clin Endocrinol Metab_ 89: 3486–3495 Article CAS Google Scholar * Misra M, Miller KK,
Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A 2004 Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects
on bone metabolism. _J Clin Endocrinol Metab_ 89: 4972–4980 Article CAS Google Scholar * Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A 2005 Secretory
dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. _Am J Physiol Endocrinol Metab_ 289: E373–E381 Article CAS Google Scholar * Zhang Y, Proenca R,
Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. _Nature_ 372: 425–432 Article CAS Google Scholar * Chen H, Charlat O,
Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP 1996 Evidence that the diabetes gene encodes the leptin receptor:
identification of a mutation in the leptin receptor gene in db/db mice. _Cell_ 84: 491–495 Article CAS Google Scholar * Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D,
Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary
dysfunction. _Nature_ 392: 398–401 Article CAS Google Scholar * Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH,
Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. _Nature_ 387: 903–908 Article CAS Google
Scholar * Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. _N Engl J Med_ 351: 987–997
Article CAS Google Scholar * Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR 1997 Resumption of menses in anorexia nervosa. _Arch Pediatr Adolesc Med_ 151: 16–21
Article CAS Google Scholar * Saketos M, Sharma N, Santoro N 1993 Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. _Biol Reprod_ 49: 1270–1276
Article CAS Google Scholar * Barbarino A, De Marinis L, Folli G, Tofani A, Della Casa S, D'Amico C, Mancini A, Corsello SM, Sambo P, Barini A 1989 Corticotropin-releasing hormone
inhibition of gonadotropin secretion during the menstrual cycle. _Metabolism_ 38: 504–506 Article CAS Google Scholar * Laughlin GA, Dominguez CE, Yen SS 1998 Nutritional and
endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. _J Clin Endocrinol Metab_ 83: 25–32 CAS PubMed Google Scholar * Rickenlund A, Thoren M, Carlstrom K, von
Schoultz B, Hirschberg AL 2004 Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. _J Clin Endocrinol
Metab_ 89: 702–707 Article CAS Google Scholar * Samuels MH, Luther M, Henry P, Ridgway EC 1994 Effects of hydrocortisone on pulsatile pituitary glycoprotein secretion. _J Clin Endocrinol
Metab_ 78: 211–215 CAS PubMed Google Scholar * Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, Herzog DB, Klibanski A 2004 Preservation of neuroendocrine control of
reproductive function despite severe undernutrition. _J Clin Endocrinol Metab_ 89: 4434–4438 Article CAS Google Scholar * Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A
2001 Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. _Am J Clin Nutr_ 73: 865–869 Article CAS Google
Scholar * Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A 2002 Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. _J Clin Endocrinol
Metab_ 87: 4177–4185 Article CAS Google Scholar * Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A 2003 Alterations in growth
hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. _J Clin Endocrinol Metab_ 88: 5615–5623 Article CAS Google Scholar * Herman-Giddens
ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM 1997 Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric
Research in Office Settings Network. _Pediatrics_ 99: 505–512 Article CAS Google Scholar * Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for
endocrine pulse detection. _Am J Physiol_ 250: E486–E493 Article CAS Google Scholar * Greulich WW, Pyle SJ 1959 _Radiographic Atlas of Skeletal Development of Hand and Wrist, 2nd Ed_.
Stanford University Press, Stanford, Google Scholar * Kelly TL, Berger N, Richardson TL 1998 DXA body composition: theory and practice. _Appl Radiat Isot_ 49: 511–513 Article CAS Google
Scholar * Visser M, Fuerst T, Lang T, Salamone L, Harris TB 1999 Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and
Body Composition Study- Dual Energy X-ray Absorptiometry and Body Composition Working Group. _J Appl Physiol_ 87: 1513–1520 Article CAS Google Scholar * Misra M, Soyka LA, Miller KK,
Grinspoon S, Levitsky LL, Klibanski A 2003 Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. _Am J Clin Nutr_ 77: 1361–137 Article CAS Google
Scholar * Biller BM, Federoff HJ, Koenig JL, Klibanski A 1990 Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. _J Clin
Endocrinol Metab_ 70: 311–317 Article CAS Google Scholar * Casper RC, Chatterton RT Jr, Davis JM 1979 Alterations in serum cortisol and its binding characteristics in anorexia nervosa. _J
Clin Endocrinol Metab_ 49: 406–411 Article CAS Google Scholar * Kopp W, Blum WF, Ziegler A, Mathiak K, Lubbert H, Herpertz S, Deter HC, Hebebrand J 1998 Serum leptin and body weight in
females with anorexia and bulimia nervosa. _Horm Metab Res_ 30: 272–275 Article CAS Google Scholar * Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C,
Zizzari P, Lang F, Epelbaum J, Estour B 2003 Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. _J Clin Endocrinol Metab_ 88: 109–116
Article CAS Google Scholar * Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A 1996 Serum leptin levels in women with anorexia
nervosa. _J Clin Endocrinol Metab_ 81: 3861–3863 CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroendocrine Unit, Massachusetts General
Hospital and Harvard Medical School, Boston, 02114, MA Madhusmita Misra, Rajani Prabhakaran, Karen K Miller, Patrika Tsai, Alvin Lin, Noel Lee & Anne Klibanski * Pediatric Endocrine
Unit, Massachusetts General Hospital and Harvard Medical School, Boston, 02114, MA Madhusmita Misra & Rajani Prabhakaran * Eating Disorders Unit, Massachusetts General Hospital and
Harvard Medical School, Boston, 02114, MA David B Herzog Authors * Madhusmita Misra View author publications You can also search for this author inPubMed Google Scholar * Rajani Prabhakaran
View author publications You can also search for this author inPubMed Google Scholar * Karen K Miller View author publications You can also search for this author inPubMed Google Scholar *
Patrika Tsai View author publications You can also search for this author inPubMed Google Scholar * Alvin Lin View author publications You can also search for this author inPubMed Google
Scholar * Noel Lee View author publications You can also search for this author inPubMed Google Scholar * David B Herzog View author publications You can also search for this author inPubMed
Google Scholar * Anne Klibanski View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Madhusmita Misra. ADDITIONAL
INFORMATION This work was supported in part by NIH grants M01-RR-01066, DK 062249 and K23 RR018851. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Misra, M., Prabhakaran, R., Miller, K. _et al._ Role of Cortisol in Menstrual Recovery in Adolescent Girls with Anorexia Nervosa. _Pediatr Res_ 59, 598–603 (2006).
https://doi.org/10.1203/01.pdr.0000203097.64918.63 Download citation * Received: 21 September 2005 * Accepted: 29 November 2005 * Issue Date: April 2006 * DOI:
https://doi.org/10.1203/01.pdr.0000203097.64918.63 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Record heat in california could interfere with your fourth of july guacamoleAn avocado hangs from a tree at a farm in Pauma Valley on March 5, 2014 near Valley Center, California. David McNew | Ge...
Trump reported making more than $1. 6 billion while president | common dreamsDonald Trump reported making more than $1.6 billion in outside revenue and income during his four years as President of ...
Spread yourself thin | Nature Reviews ImmunologyAccess through your institution Buy or subscribe In a similar manner to the intimate interaction between T cells and ant...
Vha transitions to covid-19 operational plan implementing health protection levels to enhance safety of veterans, visitors and employees | va syracuseSyracuse , NY — Syracuse VA Medical Center is implementing COVID-19 Health Protection Levels as part VA’s consistent, na...
Perinatal characteristics and parents' perspective of health status of nicu graduates born at termABSTRACT OBJECTIVE: Long-term outcomes of preterm infants have been extensively studied, but few studies have examined l...
Latests News
Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosaABSTRACT Neuroendocrine abnormalities in anorexia nervosa (AN) include hypercortisolemia, hypogonadism, and hypoleptinem...
Parents of autistic children are stressed. Here’s what they want you to knowIf you’re a parent or carer of a child who’s autistic, the odds are you’re spinning more plates than the average person....
Who is john galt? Ayn rand, libertarians and the gopAyn Rand (1904-82) has arisen from the dead. Over the last decade the pop philosopher and propaganda fictionist extraord...
Apple iPhone 6 (128GB) - Price in India, Specifications, Comparison (5th June 2025) | Gadgets 360English Edition हिंदी বাংলা தமிழ் తెలుగు മലയാളം ગુજરાતી मराठी Deutsch Française HomeMobilesPhone FinderApple PhonesApple...
Daily infographic: dot awards funding to community colleges to prepare veterans for jobs in truckingHome/Media/Infographics/FreightWaves Infographics/Daily Infographic: DOT awards funding to community colleges to prepare...