The changing landscape of phase i trials in oncology
The changing landscape of phase i trials in oncology"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
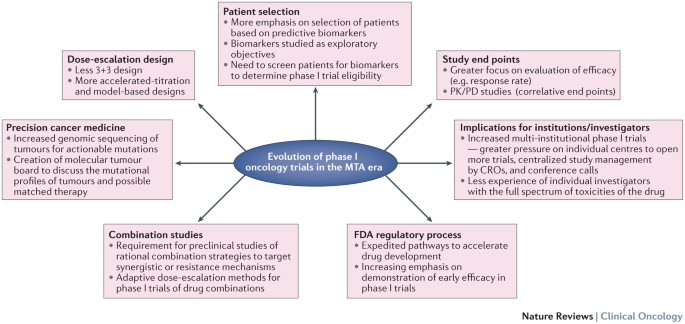
KEY POINTS * Several aspects of the design of phase I trials have evolved in the era of molecular targeted agents to enable better assessment of these novel therapies and maximize the
efficiency of drug development * Current phase I trial designs increasingly use new dose-escalation approaches and biomarker-driven patient selection, while expanding study objectives to
include efficacy evaluation and pharmacokinetics/ pharmacodynamics (PK/PD), in addition to safety * Preclinical evidence supporting a biological or pharmacological rationale and exploration
of PK/PD interactions between drug partners are necessary for phase I trials of combination therapies that include targeted agents * Changes to the regulatory approval process help to
expedite drug development, particularly for novel agents with a well-established biological mechanism, a predictive biomarker, and clear evidence of efficacy in early trials * Changes in the
goals and conduct of phase I trials have resulted in a shift towards multi-institutional studies and centralized management, with a significant impact on the structure of phase I programmes
* Both the efficiency and rate of drug approval need to improve despite the limited acceptance of novel trial designs and difficulties associated with early phase biomarker integration
ABSTRACT Advances in our knowledge of the molecular pathogenesis of cancer have led to increased interest in molecularly targeted agents (MTAs), which target specific oncogenic drivers and
are now a major focus of cancer drug development. MTAs differ from traditional cytotoxic agents in various aspects, including their toxicity profiles and the potential availability of
predictive biomarkers of response. The landscape of phase I oncology trials is evolving to adapt to these novel therapies and to improve the efficiency of drug development. In this Review,
we discuss new strategies used in phase I trial design, such as novel dose-escalation schemes to circumvent limitations of the classic 3 + 3 design and enable faster dose escalation and/or
more-precise dose determinations using statistical modelling; improved selection of patients based on genetic or molecular biomarkers; pharmacokinetic and pharmacodynamic analyses; and the
early evaluation of efficacy — in addition to safety. Indeed, new expedited approval pathways that can accelerate drug development require demonstration of efficacy in early phase trials.
The application of molecular tumour profiling for matched therapy and the testing of drug combinations based on a strong biological rationale are also increasingly seen in phase I studies.
Finally, the shift towards multi-institutional trials and centralized study management results in consequent implications for institutions and investigators. These issues are also
highlighted herein. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MOLECULAR TUMOUR BOARDS — CURRENT AND FUTURE CONSIDERATIONS FOR PRECISION ONCOLOGY Article 16 October 2023 SYSTEMATIC REVIEW
AND META-ANALYSIS OF MOLECULAR TUMOR BOARD DATA ON CLINICAL EFFECTIVENESS AND EVALUATION GAPS Article Open access 02 April 2025 A STANDING PLATFORM FOR CANCER DRUG DEVELOPMENT USING
CTDNA-BASED EVIDENCE OF RECURRENCE Article 30 September 2024 REFERENCES * American Cancer Society. _Cancer Facts and Figures 2015_ [online], (2015). * Euhus, D., Di Carlo, P. A. &
Khouri, N. F. Breast cancer screening. _Surg. Clin. North Am._ 95, 991–1011 (2015). Article PubMed Google Scholar * National Lung Screening Trial Research Team. Reduced lung-cancer
mortality with low-dose computed tomographic screening. _N. Engl. J. Med._ 365, 395–409 (2011). * Rajput, A. & Bullard Dunn, K. Surgical management of rectal cancer. _Semin. Oncol._ 34,
241–249 (2007). Article PubMed Google Scholar * van Gijn, W. _ et al_. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of
the multicentre, randomised controlled TME trial. _Lancet Oncol._ 12, 575–582 (2011). Article PubMed Google Scholar * Pharmaceutical Research and Manufacturers of America. _Medicines in
development: Cancer_ [online], (2014). * Hay, M., Thomas, D. W., Craighead, J. L., Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. _Nat.
Biotechnol._ 32, 40–51 (2014). Article CAS PubMed Google Scholar * Pharmaceutical Research and Manufacturers of America. _Researching Cancer Medicines: Setbacks and Stepping Stones_
[online], (2014). * DiMasi, J. A. & Grabowski, H. G. Economics of new oncology drug development. _J. Clin. Oncol._ 25, 209–216 (2007). Article PubMed Google Scholar * Le Tourneau, C.,
Stathis, A., Vidal, L., Moore, M. J. & Siu, L. L. Choice of starting dose for molecularly targeted agents evaluated in first-in-human phase I cancer clinical trials. _J. Clin. Oncol._
28, 1401–1407 (2010). Article CAS PubMed Google Scholar * Ivy, S. P., Siu, L. L., Garrett-Mayer, E. & Rubinstein, L. Approaches to phase 1 clinical trial design focused on safety,
efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committee. _Clin. Cancer Res._
16, 1726–1736 (2010). Article PubMed PubMed Central Google Scholar * Le Tourneau, C., Lee, J. J. & Siu, L. L. Dose escalation methods in phase I cancer clinical trials. _J. Natl
Cancer Inst._ 101, 708–720 (2009). Article CAS PubMed PubMed Central Google Scholar * LoRusso, P. M., Boerner, S. A. & Seymour, L. An overview of the optimal planning, design, and
conduct of phase I studies of new therapeutics. _Clin. Cancer Res._ 16, 1710–1718 (2010). Article CAS PubMed Google Scholar * Mick, R. & Ratain, M. J. Model-guided determination of
maximum tolerated dose in phase I clinical trials: evidence for increased precision. _J. Natl Cancer Inst._ 85, 217–223 (1993). Article CAS PubMed Google Scholar * Dowlati, A. _ et al_.
Multi-institutional phase I trials of anticancer agents. _J. Clin. Oncol._ 26, 1926–1931 (2008). Article PubMed Google Scholar * Postel-Vinay, S. _ et al_. Towards new methods for the
determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents — dose-Limiting Toxicity and Toxicity Assessment
Recommendation Group for Early Trials of Targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. _Eur. J. Cancer_ 50, 2040–2049 (2014). Article PubMed
Google Scholar * Le Tourneau, C. _ et al_. Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the
literature. _Eur. J. Cancer_ 47, 1468–1475 (2011). Article CAS PubMed Google Scholar * Paoletti, X. _ et al_. Defining dose-limiting toxicity for phase 1 trials of molecularly targeted
agents: results of a DLT-TARGETT international survey. _Eur. J. Cancer_ 50, 2050–2056 (2014). Article CAS PubMed Google Scholar * Adamina, M. & Joerger, M. Dose-toxicity models in
oncology. _Expert Opin. Drug Metab. Toxicol._ 7, 201–211 (2011). Article PubMed Google Scholar * Simon, R. _ et al_. Accelerated titration designs for phase I clinical trials in oncology.
_J. Natl Cancer Inst._ 89, 1138–1147 (1997). Article CAS PubMed Google Scholar * Penel, N. _ et al_. “Classical 3 + 3 design” versus “accelerated titration designs”: analysis of 270
phase 1 trials investigating anti-cancer agents. _Invest. New Drugs_ 27, 552–556 (2009). Article PubMed Google Scholar * Dancey, J., Freidlin, B. & Rubinstein, L. in _Statistical
methods for dose-finding experiments_ (ed. Chevret, S.) 91 (Wiley Press, 2006). Book Google Scholar * Skolnik, J. M., Barrett, J. S., Jayaraman, B., Patel, D. & Adamson, P. C.
Shortening the timeline of pediatric phase I trials: the rolling six design. _J. Clin. Oncol._ 26, 190–195 (2008). Article PubMed Google Scholar * Onar-Thomas, A. & Xiong, Z. A
simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric
phase I oncology trials. _Contemp. Clin. Trials_ 31, 259–270 (2010). Article PubMed PubMed Central Google Scholar * Doussau, A. _ et al_. Dose-finding designs in pediatric phase I
clinical trials: comparison by simulations in a realistic timeline framework. _Contemp. Clin. Trials_ 33, 657–665 (2012). Article CAS PubMed Google Scholar * Sposto, R. & Groshen, S.
A wide-spectrum paired comparison of the properties of the Rolling 6 and 3+3 Phase I study designs. _Contemp. Clin. Trials_ 32, 694–703 (2011). Article PubMed Google Scholar *
O'Quigley, J., Pepe, M. & Fisher, L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. _Biometrics_ 46, 33–48 (1990). Article CAS PubMed
Google Scholar * Iasonos, A., Zohar, S. & O'Quigley, J. Incorporating lower grade toxicity information into dose finding designs. _Clin. Trials_ 8, 370–379 (2011). Article PubMed
PubMed Central Google Scholar * Yuan, Z., Chappell, R. & Bailey, H. The continual reassessment method for multiple toxicity grades: a Bayesian quasi-likelihood approach. _Biometrics_
63, 173–179 (2007). Article CAS PubMed Google Scholar * Ezzalfani, M., Zohar, S., Qin, R., Mandrekar, S. J. & Deley, M. C. Dose-finding designs using a novel quasi-continuous
endpoint for multiple toxicities. _Stat Med._ 32, 2728–2746 (2013). Article PubMed PubMed Central Google Scholar * Van Meter, E. M., Garrett-Mayer, E. & Bandyopadhyay, D.
Proportional odds model for dose-finding clinical trial designs with ordinal toxicity grading. _Stat. Med._ 30, 2070–2080 (2011). Article PubMed PubMed Central Google Scholar * Van
Meter, E. M., Garrett-Mayer, E. & Bandyopadhyay, D. Dose-finding clinical trial design for ordinal toxicity grades using the continuation ratio model: an extension of the continual
reassessment method. _Clin. Trials_ 9, 303–313 (2012). Article PubMed PubMed Central Google Scholar * Goodman, S. N., Zahurak, M. L. & Piantadosi, S. Some practical improvements in
the continual reassessment method for phase I studies. _Stat. Med._ 14, 1149–1161 (1995). Article CAS PubMed Google Scholar * Piantadosi, S., Fisher, J. D. & Grossman, S. Practical
implementation of a modified continual reassessment method for dose-finding trials. _Cancer Chemother. Pharmacol._ 41, 429–436 (1998). Article CAS PubMed Google Scholar * Rogatko, A.,
Babb, J. S., Tighiouart, M., Khuri, F. R. & Hudes, G. New paradigm in dose-finding trials: patient-specific dosing and beyond phase I. _Clin. Cancer Res._ 11, 5342–5346 (2005). Article
CAS PubMed Google Scholar * O'Quigley, J. & Shen, L. Z. Continual reassessment method: a likelihood approach. _Biometrics_ 52, 673–684 (1996). Article CAS PubMed Google
Scholar * Cheung, Y. K. & Chappell, R. Sequential designs for phase I clinical trials with late-onset toxicities. _Biometrics_ 56, 1177–1182 (2000). Article CAS PubMed Google Scholar
* O'Quigley, J. & Conaway, M. Extended model-based designs for more complex dose-finding studies. _Stat. Med._ 30, 2062–2069 (2011). Article PubMed PubMed Central Google
Scholar * Zhang, W., Sargent, D. J. & Mandrekar, S. An adaptive dose-finding design incorporating both toxicity and efficacy. _Stat. Med._ 25, 2365–2383 (2006). Article PubMed Google
Scholar * Thall, P. F. & Cook, J. D. Dose-finding based on efficacy-toxicity trade-offs. _Biometrics_ 60, 684–693 (2004). Article PubMed Google Scholar * Thall, P. F., Cook, J. D
& Estey, E. H. Adaptive dose selection using efficacy-toxicity trade-offs: illustrations and practical considerations. _J. Biopharm. Stat._ 16, 623–638 (2006). Article PubMed Google
Scholar * Mandrekar, S. J., Qin, R. & Sargent, D. J. Model-based phase I designs incorporating toxicity and efficacy for single and dual agent drug combinations: methods and challenges.
_Stat. Med._ 29, 1077–1083 (2010). Article PubMed PubMed Central Google Scholar * Le Tourneau, C., Gan, H. K., Razak, A. R. & Paoletti, X. Efficiency of new dose escalation designs
in dose-finding phase I trials of molecularly targeted agents. _PLoS ONE_ 7, e51039 (2012). Article CAS PubMed PubMed Central Google Scholar * Jaki, T., Clive, S. & Weir, C. J.
Principles of dose finding studies in cancer: a comparison of trial designs. _Cancer Chemother. Pharmacol._ 71, 1107–1114 (2013). Article PubMed PubMed Central Google Scholar * Rogatko,
A. _ et al_. Translation of innovative designs into phase I trials. _J. Clin. Oncol._ 25, 4982–4986 (2007). Article PubMed Google Scholar * Wood, L. D. _ et al_. The genomic landscapes of
human breast and colorectal cancers. _Science_ 318, 1108–1113 (2007). Article CAS PubMed Google Scholar * Wong, K. M., Hudson, T. J. & McPherson, J. D. Unraveling the genetics of
cancer: genome sequencing and beyond. _Annu. Rev. Genomics Hum. Genet._ 12, 407–430 (2011). Article CAS PubMed Google Scholar * Gerdes, M. J. _ et al_. Emerging understanding of
multiscale tumor heterogeneity. _Front. Oncol._ 4, 366 (2014). Article PubMed PubMed Central Google Scholar * Ludwig, J. A. & Weinstein, J. N. Biomarkers in cancer staging, prognosis
and treatment selection. _Nat. Rev. Cancer_ 5, 845–856 (2005). Article CAS PubMed Google Scholar * Henry, N. L. & Hayes, D. F. Cancer biomarkers. _Mol. Oncol._ 6, 140–146 (2012).
Article CAS PubMed PubMed Central Google Scholar * Gonzalez de Castro, D., Clarke, P. A., Al-Lazikani, B. & Workman, P. Personalized cancer medicine: molecular diagnostics,
predictive biomarkers, and drug resistance. _Clin. Pharmacol. Ther._ 93, 252–259 (2013). Article CAS PubMed PubMed Central Google Scholar * Hollebecque, A. _ et al_. Modifying phase I
methodology to facilitate enrolment of molecularly selected patients. _Eur. J. Cancer_ 49, 1515–1520 (2013). Article PubMed Google Scholar * Kwak, E. L. _ et al_. Anaplastic lymphoma
kinase inhibition in non-small-cell lung cancer. _N. Engl. J. Med._ 363, 1693–1703 (2010). Article CAS PubMed PubMed Central Google Scholar * Shaw, A. T. _ et al_. Ceritinib in
_ALK_-rearranged non-small-cell lung cancer. _N. Engl. J. Med._ 370, 1189–1197 (2014). Article CAS PubMed PubMed Central Google Scholar * Seto, T. _ et al_. CH5424802 (RO5424802) for
patients with _ALK_-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. _Lancet Oncol._ 14, 590–598 (2013). Article CAS PubMed
Google Scholar * Flaherty, K. T. _ et al_. Inhibition of mutated, activated BRAF in metastatic melanoma. _N. Engl. J. Med._ 363, 809–819 (2010). Article CAS PubMed PubMed Central Google
Scholar * Dancey, J. E. _ et al_. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. _Clin. Cancer Res._ 16, 1745–1755 (2010).
Article CAS PubMed Google Scholar * [No authors listed] 2012 best practices for repositories collection, storage, retrieval, and distribution of biological materials for research
international society for biological and environmental repositories. _Biopreserv. Biobank._ 10, 79–161 (2012). * Chau, C. H., Rixe, O., McLeod, H. & Figg, W. D. Validation of analytic
methods for biomarkers used in drug development. _Clin. Cancer Res._ 14, 5967–5976 (2008). Article PubMed PubMed Central Google Scholar * Wagner, J. A. Strategic approach to
fit-for-purpose biomarkers in drug development. _Annu. Rev. Pharmacol. Toxicol._ 48, 631–651 (2008). Article CAS PubMed Google Scholar * Topalian, S. L. _ et al_. Safety, activity, and
immune correlates of anti-PD-1 antibody in cancer. _N. Engl. J. Med._ 366, 2443–2454 (2012). Article CAS PubMed PubMed Central Google Scholar * Falchook, G. S. _ et al_. Activity of the
oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. _Lancet Oncol._ 13, 782–789 (2012). Article CAS PubMed PubMed Central Google Scholar
* Rodon, J. _ et al_. Molecular prescreening to select patient population in early clinical trials. _Nat. Rev. Clin. Oncol._ 9, 359–366 (2012). Article CAS PubMed Google Scholar * Manji,
A. _ et al_. Evolution of clinical trial design in early drug development: systematic review of expansion cohort use in single-agent phase I cancer trials. _J. Clin. Oncol._ 31, 4260–4267
(2013). Article CAS PubMed Google Scholar * Bugano, D. _ et al_. Impact of phase 1 expansion cohorts on probability of success in phase 2 and time-to-drug-approval: analysis of 385 new
drugs in oncology [abstract 237]. _Eur. J. Cancer_ 50, 79–80 (2014). Article Google Scholar * Shea, M. B., Roberts, S. A., Walrath, J. C., Allen, J. D. & Sigal, E. V. Use of multiple
endpoints and approval paths depicts a decade of FDA oncology drug approvals. _Clin. Cancer Res._ 19, 3722–3731 (2013). Article PubMed Google Scholar * Garon, E. B. _ et al_.
Pembrolizumab for the treatment of non-small-cell lung cancer. _N. Engl. J. Med._ 372, 2018–2028 (2015). Article PubMed Google Scholar * Brahmer, J. R. _ et al_. Safety and activity of
anti-PD-L1 antibody in patients with advanced cancer. _N. Engl. J. Med._ 366, 2455–2465 (2012). Article CAS PubMed PubMed Central Google Scholar * Robert, C. _ et al_. Nivolumab in
previously untreated melanoma without _BRAF_ mutation. _N. Engl. J. Med._ 372, 320–330 (2015). Article CAS PubMed Google Scholar * Weber, J. S. _ et al_. Nivolumab versus chemotherapy in
patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. _Lancet Oncol._ 16, 375–384 (2015). Article
CAS PubMed Google Scholar * Lee, S. M. & Chow, L. Q. A new addition to the PD-1 checkpoint inhibitors for non-small cell lung cancer-the anti-PDL1 antibody-MEDI4736. _Transl. Lung
Cancer Res._ 3, 408–410 (2014). CAS PubMed PubMed Central Google Scholar * Parulekar, W. R. & Eisenhauer, E. A. Phase I trial design for solid tumor studies of targeted,
non-cytotoxic agents: theory and practice. _J. Natl Cancer Inst._ 96, 990–997 (2004). Article CAS PubMed Google Scholar * Jain, R. K. _ et al_. Phase I oncology studies: evidence that in
the era of targeted therapies patients on lower doses do not fare worse. _Clin. Cancer Res._ 16, 1289–1297 (2010). Article CAS PubMed PubMed Central Google Scholar * Postel-Vinay, S. _
et al_. Clinical benefit in phase-I trials of novel molecularly targeted agents: does dose matter? _Br. J. Cancer_ 100, 1373–1378 (2009). Article CAS PubMed PubMed Central Google
Scholar * Gupta, S. _ et al_. Meta-analysis of the relationship between dose and benefit in phase I targeted agent trials. _J. Natl Cancer Inst._ 104, 1860–1866 (2012). Article CAS PubMed
Google Scholar * Dienstmann, R., Brana, I., Rodon, J. & Tabernero, J. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer
drugs. _Oncologist_ 16, 1729–1740 (2011). Article CAS PubMed PubMed Central Google Scholar * Widakowich, C., de Castro, G. Jr, de Azambuja, E., Dinh, P. & Awada, A. Review: side
effects of approved molecular targeted therapies in solid cancers. _Oncologist_ 12, 1443–1455 (2007). Article CAS PubMed Google Scholar * Dy, G. K. & Adjei, A. A. Understanding,
recognizing, and managing toxicities of targeted anticancer therapies. _CA Cancer J. Clin._ 63, 249–279 (2013). Article PubMed Google Scholar * de Castro, G. Jr & Awada, A. Side
effects of anti-cancer molecular-targeted therapies (not monoclonal antibodies). _Curr. Opin. Oncol._ 18, 307–315 (2006). Article CAS PubMed Google Scholar * Lynch, T. J. Jr _ et al_.
Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. _Oncologist_ 12, 610–621 (2007). Article CAS PubMed Google Scholar
* Loriot, Y. _ et al_. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. _Nat. Clin. Pract. Oncol._ 5, 268–278 (2008). Article CAS
PubMed Google Scholar * Eaby, B., Culkin, A. & Lacouture, M. E. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor
inhibitors. _Clin. J. Oncol. Nurs._ 12, 283–290 (2008). Article PubMed Google Scholar * Grothey, A. Recognizing and managing toxicities of molecular targeted therapies for colorectal
cancer. _Oncology (Williston Park)_ 20, 21–28 (2006). Google Scholar * Workman, P. _ et al_. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing
clinical trials of innovative therapies. _J. Natl Cancer Inst._ 98, 580–598 (2006). Article CAS PubMed Google Scholar * Lorente, D., Mateo, J. & de Bono, J. S. Molecular
characterization and clinical utility of circulating tumor cells in the treatment of prostate cancer. _Am. Soc. Clin. Oncol. Educ. Book_ 2014, e197–e203 (2014). Article Google Scholar *
Diaz, L. A. Jr & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. _J. Clin. Oncol._ 32, 579–586 (2014). Article PubMed PubMed Central Google Scholar * Comets, E. &
Zohar, S. A survey of the way pharmacokinetics are reported in published phase I clinical trials, with an emphasis on oncology. _Clin. Pharmacokinet._ 48, 387–395 (2009). Article PubMed
PubMed Central Google Scholar * Goulart, B. H. _ et al_. Trends in the use and role of biomarkers in phase I oncology trials. _Clin. Cancer Res._ 13, 6719–6726 (2007). Article CAS PubMed
Google Scholar * Duffy, M. J. _ et al_. Validation of new cancer biomarkers: a position statement from the European group on tumor markers. _Clin. Chem._ 61, 809–820 (2015). Article CAS
PubMed Google Scholar * Josephs, D., Spicer, J. & O'Doherty, M. Molecular imaging in clinical trials. _Target Oncol._ 4, 151–168 (2009). Article PubMed Google Scholar *
Stephen, R. M. & Gillies, R. J. Promise and progress for functional and molecular imaging of response to targeted therapies. _Pharm. Res._ 24, 1172–1185 (2007). Article CAS PubMed
Google Scholar * Meric-Bernstam, F. & Mills, G. B. Overcoming implementation challenges of personalized cancer therapy. _Nat. Rev. Clin. Oncol._ 9, 542–548 (2012). Article CAS PubMed
Google Scholar * Hagemann, I. S., Cottrell, C. E. & Lockwood, C. M. Design of targeted, capture-based, next generation sequencing tests for precision cancer therapy. _Cancer Genet._
206, 420–431 (2013). Article PubMed Google Scholar * Andre, F. _ et al_. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a
multicentre, prospective trial (SAFIR01/UNICANCER). _Lancet Oncol._ 15, 267–274 (2014). Article CAS PubMed Google Scholar * Weiss, G. J. _ et al_. A pilot study using next-generation
sequencing in advanced cancers: feasibility and challenges. _PLoS ONE_ 8, e76438 (2013). Article CAS PubMed PubMed Central Google Scholar * Janku, F., Kaseb, A. O., Tsimberidou, A. M.,
Wolff, R. A. & Kurzrock, R. Identification of novel therapeutic targets in the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using targeted next generation sequencing. _Oncotarget_
5, 3012–3022 (2014). PubMed PubMed Central Google Scholar * Dienstmann, R. _ et al_. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I
clinical trials. _Mol. Cancer Ther._ 11, 2062–2071 (2012). Article CAS PubMed Google Scholar * Tuxen, I. V. _ et al_. Personalized oncology: genomic screening in phase 1. _APMIS_ 122,
723–733 (2014). Article PubMed Google Scholar * Tsimberidou, A. M. _ et al_. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. _Clin.
Cancer Res._ 18, 6373–6383 (2012). Article CAS PubMed PubMed Central Google Scholar * Le Tourneau, C. _ et al_. Molecularly targeted therapy based on tumour molecular profiling versus
conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. _Lancet Oncol._
http://dx.doi.org/10.1016/S1470-2045(15)00188-6. * Schwaederle, M. _ et al_. Molecular tumor board: the University of California-San Diego Moores Cancer Center experience. _Oncologist_ 19,
631–636 (2014). Article PubMed PubMed Central Google Scholar * Cronin, M. & Ross, J. S. Comprehensive next-generation cancer genome sequencing in the era of targeted therapy and
personalized oncology. _Biomark. Med._ 5, 293–305 (2011). Article CAS PubMed Google Scholar * Crockford, A., Jamal-Hanjani, M., Hicks, J. & Swanton, C. Implications of intratumour
heterogeneity for treatment stratification. _J. Pathol._ 232, 264–273 (2014). Article PubMed Google Scholar * Xuan, J., Yu, Y., Qing, T., Guo, L. & Shi, L. Next-generation sequencing
in the clinic: promises and challenges. _Cancer Lett._ 340, 284–295 (2013). Article CAS PubMed Google Scholar * Kruglyak, K. M., Lin, E. & Ong, F. S. Next-generation sequencing in
precision oncology: challenges and opportunities. _Expert Rev. Mol. Diagn._ 14, 635–637 (2014). Article CAS PubMed Google Scholar * McNeil, C. NCI-MATCH launch highlights new trial
design in precision-medicine era. _J. Natl Cancer Inst._ 107, djv193 (2015). Article PubMed Google Scholar * National Cancer Institute. _NCI-Molecular Analysis for Therapy Choice
(NCI-MATCH) Trial_ [online], (2015). * Roychowdhury, S. _ et al_. Personalized oncology through integrative high-throughput sequencing: a pilot study. _Sci. Transl. Med._ 3, 111ra121 (2011).
Article CAS PubMed PubMed Central Google Scholar * Meric-Bernstam, F., Farhangfar, C., Mendelsohn, J. & Mills, G. B. Building a personalized medicine infrastructure at a major
cancer center. _J. Clin. Oncol._ 31, 1849–1857 (2013). Article PubMed PubMed Central Google Scholar * Ocana, A., Freedman, O., Amir, E., Seruga, B. & Pandiella, A. Biological
insights into effective and antagonistic combinations of targeted agents with chemotherapy in solid tumors. _Cancer Metastasis Rev._ 33, 295–307 (2014). Article CAS PubMed Google Scholar
* Jia, J. _ et al_. Mechanisms of drug combinations: interaction and network perspectives. _Nat. Rev. Drug Discov._ 8, 111–128 (2009). Article CAS PubMed Google Scholar * Reinhardt, H.
C., Jiang, H., Hemann, M. T. & Yaffe, M. B. Exploiting synthetic lethal interactions for targeted cancer therapy. _Cell Cycle_ 8, 3112–3119 (2009). Article CAS PubMed Google Scholar
* Paller, C. J. _ et al_. Design of phase I combination trials: recommendations of the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee. _Clin. Cancer
Res._ 20, 4210–4217 (2014). Article PubMed PubMed Central Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ [online], (2015). * US National Library of Medicine.
_ClinicalTrials.gov_ [online], (2015). * US National Library of Medicine. _ClinicalTrials.gov_ [online], (2015). * US National Library of Medicine. _ClinicalTrials.gov_ [online], (2015). *
Cha, E., Wallin, J. & Kowanetz, M. PD-L1 inhibition with MPDL3280A for solid tumors. _Semin. Oncol._ 42, 484–487 (2015). Article CAS PubMed Google Scholar * Riviere, M. K., Dubois,
F. & Zohar, S. Competing designs for drug combination in phase I dose-finding clinical trials. _Stat. Med._ 34, 1–12 (2015). Article PubMed Google Scholar * Riviere, M. K., Le
Tourneau, C., Paoletti, X., Dubois, F. & Zohar, S. Designs of drug-combination phase I trials in oncology: a systematic review of the literature. _Ann. Oncol._ 26, 669–674 (2015).
Article PubMed Google Scholar * Hamberg, P., Ratain, M. J., Lesaffre, E. & Verweij, J. Dose-escalation models for combination phase I trials in oncology. _Eur. J. Cancer_ 46,
2870–2878 (2010). Article PubMed Google Scholar * Harrington, J. A., Wheeler, G. M., Sweeting, M. J., Mander, A. P. & Jodrell, D. I. Adaptive designs for dual-agent phase I
dose-escalation studies. _Nat. Rev. Clin. Oncol._ 10, 277–288 (2013). Article CAS PubMed Google Scholar * Mandrekar, S. J. Dose-finding trial designs for combination therapies in
oncology. _J. Clin. Oncol._ 32, 65–67 (2014). Article PubMed Google Scholar * Cannistra, S. A. Challenges and pitfalls of combining targeted agents in phase I studies. _J. Clin. Oncol._
26, 3665–3667 (2008). Article CAS PubMed Google Scholar * Dancey, J. E. & Chen, H. X. Strategies for optimizing combinations of molecularly targeted anticancer agents. _Nat. Rev.
Drug Discov._ 5, 649–659 (2006). Article CAS PubMed Google Scholar * Kummar, S. _ et al_. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent
requirements. _Nat. Rev. Drug Discov._ 9, 843–856 (2010). Article CAS PubMed Google Scholar * Pollyea, D. A. _ et al_. Safety, efficacy and biological predictors of response to
sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. _Leukemia_ 26, 893–901 (2012). Article CAS PubMed Google Scholar * Yoshioka, T. _ et al_. Phase
I/II study of sequential therapy with irinotecan and S-1 for metastatic colorectal cancer. _Br. J. Cancer_ 101, 1972–1977 (2009). Article CAS PubMed PubMed Central Google Scholar *
Bruce, J. Y. _ et al_. A phase I pharmacodynamic trial of sequential sunitinib with bevacizumab in patients with renal cell carcinoma and other advanced solid malignancies. _Cancer
Chemother. Pharmacol._ 73, 485–493 (2014). Article CAS PubMed PubMed Central Google Scholar * Sherman, R. E., Li, J., Shapley, S., Robb, M. & Woodcock, J. Expediting drug
development — the FDA's new “breakthrough therapy” designation. _N. Engl. J. Med._ 369, 1877–1880 (2013). Article CAS PubMed Google Scholar * Pignatti, F., Jonsson, B., Blumenthal,
G. & Justice, R. Assessment of benefits and risks in development of targeted therapies for cancer — the view of regulatory authorities. _Mol. Oncol._ 9, 1034–1041 (2015). Article PubMed
Google Scholar * US Food and Drug Administration. _Food and Drug Administration, Regulatory Information, Food and Drug Administration Safety and Innovation Act (FDASIA)_ [online], (2015).
* Kramer, D. B. & Kesselheim, A. S. User fees and beyond — the FDA Safety and Innovation Act of 2012. _N. Engl. J. Med._ 367, 1277–1279 (2012). Article CAS PubMed PubMed Central
Google Scholar * US Food and Drug Administration. _Guidance for Industry Expedited Programs for Serious Conditions — Drugs and Biologics_ [online], (2014). * Kesselheim, A. S. & Darrow,
J. J. FDA designations for therapeutics and their impact on drug development and regulatory review outcomes. _Clin. Pharmacol. Ther._ 97, 29–36 (2015). Article CAS PubMed Google Scholar
* US Food and Drug Administration. _Breakthrough Therapy Approvals_ [online], (2015). * Gadgeel, S. M. _ et al_. Safety and activity of alectinib against systemic disease and brain
metastases in patients with crizotinib-resistant _ALK_-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. _Lancet Oncol._ 15,
1119–1128 (2014). Article CAS PubMed Google Scholar * Khozin, S. _ et al_. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung
cancer. _Clin. Cancer Res._ 21, 2436–2439 (2015). Article CAS PubMed Google Scholar * Wong, K. M., Noonan, S., O'Bryant, C. & Jimeno, A. Alectinib for the treatment of
ALK-positive stage IV non-small cell lung cancer. _Drugs Today (Barc.)_ 51, 161–170 (2015). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Division of Medical Oncology/Department of Medicine, Developmental Therapeutics Program, University of Colorado Cancer Center, School of Medicine, University of Colorado Anschutz Medical
Campus, Aurora, 80045, Colorado, USA Kit Man Wong, Anna Capasso & S. Gail Eckhardt Authors * Kit Man Wong View author publications You can also search for this author inPubMed Google
Scholar * Anna Capasso View author publications You can also search for this author inPubMed Google Scholar * S. Gail Eckhardt View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS K.M.W. performed the literature search, planned the sections of the review, and wrote the entire manuscript and subsequent revisions, A.C. reviewed the
manuscript before submission. S.G.E. contributed to the contents of the manuscript and reviewed the manuscript before submission. CORRESPONDING AUTHOR Correspondence to S. Gail Eckhardt.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR TABLE 1 POWERPOINT SLIDE FOR
TABLE 2 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wong, K., Capasso, A. & Eckhardt, S. The changing landscape of phase I trials in oncology.
_Nat Rev Clin Oncol_ 13, 106–117 (2016). https://doi.org/10.1038/nrclinonc.2015.194 Download citation * Published: 10 November 2015 * Issue Date: February 2016 * DOI:
https://doi.org/10.1038/nrclinonc.2015.194 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
France: stop your regular taxe d’habitation paymentSome 80% of main home households will have zero to pay on this year’s taxe d’habitation bill, which will be sent out in ...
Analysts reduce their inflation forecast—and growthPrivate analysts consulted by Mexico’s central bank have lowered their forecasts for both economic growth and inflation ...
allopen3 | designboom.com* imprint * privacy policy * terms of use * cookies * copyright info * contribute * about us * contact us * newsletter *...
Something went wrong, sorry. :(The Paris mairie is cracking down on ‘voyeurs’ and similar sexual assaults in its public swimming pools, after many wome...
Soccer-bullet point preview of premier league fixtures, round 32Following are match-by-match facts and statistics ahead of round 32 of the Premier League fixtures on April 8-10 (1400 G...
Latests News
The changing landscape of phase i trials in oncologyKEY POINTS * Several aspects of the design of phase I trials have evolved in the era of molecular targeted agents to ena...
Portable covid-19 rapid-testing units helping keep calgary curling bubble safeSave for later The men curling in the Canadian championship don’t much care there’s a small, silver box processing their...
San pedro falls to franklin's aerial assaultSan Pedro High’s defense spent most of Saturday afternoon at Daniels Field cringing beneath an aerial barrage. The mad b...
College Football - Los Angeles TimesL.A. Times Archives Dec. 16, 1992 12 AM PT Share via Close extra sharing options Email Facebook X LinkedIn Threads Reddi...
Request for a default costs certificate: form n254Form REQUEST FOR A DEFAULT COSTS CERTIFICATE: FORM N254 Use this form to request that the court issues a certificate for...