Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart
Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Klotho is a membrane protein predominantly produced in the kidney that exerts some antiageing effects. Ageing is associated with an increased risk of heart failure; whether Klotho is
cardioprotective is unknown. Here we show that Klotho-deficient mice have no baseline cardiac abnormalities but develop exaggerated pathological cardiac hypertrophy and remodelling in
response to stress. Cardioprotection by Klotho in normal mice is mediated by downregulation of TRPC6 channels in the heart. We demonstrate that deletion of Trpc6 prevents stress-induced
exaggerated cardiac remodelling in Klotho-deficient mice. Furthermore, mice with heart-specific overexpression of TRPC6 develop spontaneous cardiac hypertrophy and remodelling. Klotho
overexpression ameliorates cardiac pathologies in these mice and improves their long-term survival. Soluble Klotho present in the systemic circulation inhibits TRPC6 currents in
cardiomyocytes by blocking phosphoinositide-3-kinase-dependent exocytosis of TRPC6 channels. These results provide a new perspective on the pathogenesis of cardiomyopathies and open new
avenues for treatment of the disease.
Klotho is an antiageing protein predominantly produced in the kidney and several other tissues including parathyroid glands and epithelial cells of the choroids plexus1. Mice homozygous for
a hypomorphic Klotho allele (kl/kl) manifest multiple ageing-related phenotypes including skin and muscle atrophy, hyperphosphatemia, osteoporosis and vascular calcification, and die
prematurely at around 2–3 months of age. The full-length Klotho protein is a type-1 membrane protein with a large extracellular domain of 952 amino acids in human, a membrane-spanning
segment, and a short 11 amino acids intracellular carboxyl terminus1. Membranous Klotho associates with fibroblast growth factor (FGF) receptors to form co-receptors for the ligand FGF23, a
bone-derived circulating hormone that lowers serum phosphate levels by increasing renal phosphate excretion, suppressing 1,25-dihyroxyvitamin D synthesis, and decreasing gastrointestinal
phosphate absorption2,3,4,5. Klotho-deficient mice have severe hyperphosphatemia due to defects in the Klotho-FGF23-vitamin D regulatory axis5,6,7. This phosphate retention is pivotal for
growth retardation and premature death of Klotho-deficient mice. Dietary phosphate restriction rescues growth defects and premature death of the mice5,6,7. The notion that FGF receptor and
Klotho form obligatory co-receptors for FGF23 is supported by the demonstration that systemic injection of bioactive FGF23 decreases serum levels of phosphate in wild–type (WT) mice, but not
in Klotho-deficient mice8.
The extracellular domain of Klotho is composed of two internal repeats, KL1 and KL2, each sharing amino-acid sequence homology to family 1 glycosidases1. The extracellular domain of Klotho
is shed into the systemic circulation, urine and cerebrospinal fluid9. In urine, soluble Klotho regulates several ion transporters in the apical membrane of kidney tubules10,11,12. The
physiological function of soluble Klotho present in the systemic circulation is mostly unknown.
The heart responds to injury and stress signals by pathological growth and remodelling that often progresses to heart failure and sudden death13. One key regulatory step in the development
of pathological cardiac growth and remodelling is activation of calmodulin-dependent serine–threonine protein phosphatase calcineurin by abnormal calcium signalling14. Once activated by
increases in intracellular calcium, calcineurin dephosphorylates and causes nuclear translocation of nuclear factor of activated T cells (NFAT) transcription factors, which bind the
regulator regions of cardiac genes and in conjunction with other transcription factors induce gene expression and promote hypertrophic growth and remodelling.
Extracellular stimuli increase intracellular Ca2+ levels by either promoting its release from intracellular organelles or its entry across the plasma membrane. The TRPC family channels are
Ca2+-permeable cation channels expressed in the plasma membrane of many tissues including the heart15. The TRPC family includes seven members, and is divided into two groups based on
structural and functional similarities: TRPC1/4/5, which are not sensitive to diacylglycerol (DAG), and TRPC3/6/7, which are activated by DAG. TRPC2 is not expressed in humans. Evidence
indicates that Ca2+ influx through cardiac TRPC channels—including TRPC1, 3, 4, 5 and 6—is important in calcineurin signalling and hypertrophic growth of hearts16,17,18,19,20,21,22. The
expression of TRPC1, 3, 4, 5 and/or 6 is increased in hypertrophic hearts stimulated by various types or forms of stresses and their downregulation protects against cardiac hypertrophy. Some
members of TRPC family channels, such as TRPC6, contain NFAT-responsive elements in their promoters, which have a pivotal role in amplifying and sustaining gene expression through a
feed-forward circuit16. Thus, TRPC6 is an important modulator of cardiac hypertrophy and a potential target for treatment. However, physiological function of TRPC6 in the heart and its
regulation remain poorly understood, limiting therapeutic strategies for targeting the pathway. Here we show that soluble Klotho inhibits cardiac TRPC6 channels and protects the heart
against stress-induced pathological hypertrophy and remodelling.
Klotho expression is decreased in aging, a condition associated with increased risk for heart failure23,24. We examined the role of Klotho in protecting the heart using Klotho-hypomorphic
mice rescued by dietary phosphate restriction. To avoid potential variations caused by strain and gender differences, we studied male mice congenic for the 129/SvJ background by backcrossing
for >6 generations. As reported previously5,6,7, dietary phosphate restriction lowered serum phosphate levels and rescued growth defects and premature death of Klotho-deficient mice
(Supplementary Figs S1 and S2a). Serum levels of sodium, potassium, chloride, calcium, magnesium and urea nitrogen were not different between WT and kl/kl mice on a phosphate-restricted diet
(Supplementary Table S1). Phosphate restriction did not affect the growth of WT mice (Supplementary Fig. S1). Klotho-hypomorphic mice on low-phosphate diet remained markedly
Klotho-deficient (Supplementary Fig. S2b).
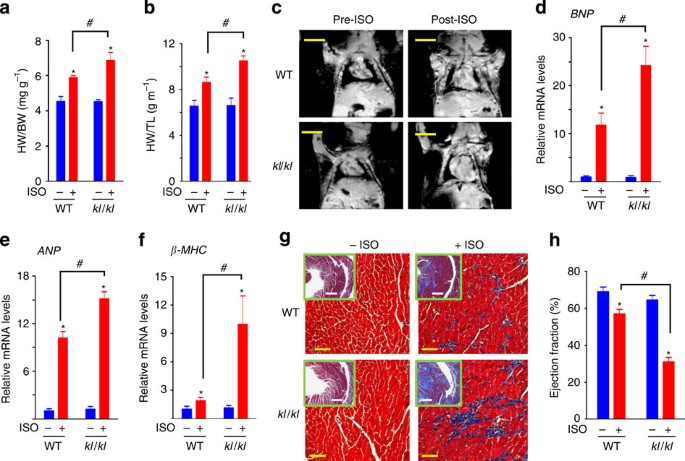
To investigate the potential cardioprotective effect of Klotho, we measured heart weight indices (heart weight normalized to body weight or tibia length) as well as the overall heart size in
WT and Klotho-deficient mice. Heart weight indices (Fig. 1a) and the overall heart size measured using magnetic resonance imaging (MRI) (Fig. 1c) were not different between WT and
Klotho-deficient mice at baseline. Overstimulation by isoproterenol (ISO) induced pathological hypertrophy in WT mice as reflected by increase in heart weight indices and the overall heart
size, and these ISO-induced changes were aggravated in Klotho-deficient mice (Fig. 1a–c). ISO overstimulation is a well-accepted experimental model of stress-induced cardiac
hypertrophy25,26. Phosphate restriction itself did not alter cardiac responses to stress, as baseline and ISO-induced increases in heart mass were not different between WT mice fed normal
and phosphate-restricted diets (Supplementary Fig. S2c).
(a,b) Heart weight/body weight (HW/BW) (a) and heart weight/tibia length (HW/TL) (b) ratios of WT and homozygous klotho-hypomorphic mice (kl/kl) treated with ISO or vehicle (PBS). Mice were
fed a low-phosphate diet after weaning. At the time of study (~3 months of age), body weight of WT and kl/kl mice were not different (25.1±0.76 g versus 24.2±1.22 g). Also, systemic blood
pressure of WT and kl/kl mice were not different (systolic BP: 122±4 mm Hg versus 119±5 mm Hg). Data were mean±s.e.m.; n=6 for each group. *P
Trending News
Happy Raksha Bandhan: Best Gift Ideas for Brothers and SistersRaksha Bandhan is just around the corner and siblings can't hold their excitement to meet their loved ones, shower them ...
Facebook Goes Vernacular, Allows Users to Type in Hindi on the AppFacebook is continuing to improve so it works seamlessly and easily for people in all parts of the world, regardless of ...
Redstone ‘Embarrassed’ by NotesWILMINGTON, Del. — Viacom Inc. Chairman Sumner Redstone on Monday called “embarrassing” company documents that said its ...
Photos: ‘the embrace,’ a memorial to martin luther king jr. And coretta scott king, celebrated on boston commonLocal News THE 20-FOOT-HIGH AND 40-FOOT-WIDE BRONZE SCULPTURE DEPICTS THE HANDS AND ARMS OF THE COUPLE IN AN EMBRACE AND...
Pug lovers doggedly seek answersFor what it’s worth, and that may not be much, I spoke to a woman the other day who identified herself as a psychic. She...
Latests News
Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heartKlotho is a membrane protein predominantly produced in the kidney that exerts some antiageing effects. Ageing is associa...
Notes - Los Angeles Times* Pets.com has named TBWA/Chiat/Day as its advertising agency of record. The companies did not disclose the size of the ...
Californians face an 'uphill battle' in challenging costly Trump tax law - CalMattersTime is running out for California taxpayers trying to recoup a valuable tax deduction lost in the Republican tax overha...
California confronts higher education issues, and Newsom is Iowa-bound - CalMattersWhat better day than today—#GivingTuesday—to donate to CalMatters’ nonprofit, nonpartisan newsroom. To show your support...
Justice Dept. Will Give $305 Million to Victim Aid ProgramsWASHINGTON — The Justice Department will distribute more than $305 million to state programs that help crime victims reb...