The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat
The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The sensory drive theory of speciation predicts that populations of the same species inhabiting different environments can differ in sensory traits, and that this sensory difference
can ultimately drive speciation. However, even in the best-known examples of sensory ecology driven speciation, it is uncertain whether the variation in sensory traits is the cause or the
consequence of a reduction in levels of gene flow. Here we show strong genetic differentiation, no gene flow and large echolocation differences between the allopatric Myanmar and Thai
populations of the world's smallest mammal, _Craseonycteris thonglongyai_, and suggest that geographic isolation most likely preceded sensory divergence. Within the geographically
continuous Thai population, we show that geographic distance has a primary role in limiting gene flow rather than echolocation divergence. In line with sensory-driven speciation models, we
suggest that in _C. thonglongyai_, limited gene flow creates the suitable conditions that favour the evolution of sensory divergence via local adaptation. SIMILAR CONTENT BEING VIEWED BY
OTHERS EVOLUTION OF COMMUNICATION SIGNALS AND INFORMATION DURING SPECIES RADIATION Article Open access 02 October 2020 DIVERGENT DYNAMICS OF SEXUAL AND HABITAT ISOLATION AT THE TRANSITION
BETWEEN STICK INSECT POPULATIONS AND SPECIES Article Open access 13 March 2024 COUNTERACTING FORCES OF INTROGRESSIVE HYBRIDIZATION AND INTERSPECIFIC COMPETITION SHAPE THE MORPHOLOGICAL
TRAITS OF CRYPTIC IBERIAN _EPTESICUS_ BATS Article Open access 08 July 2022 INTRODUCTION Theoretical models1,2 and a few empirical studies3,4 have suggested the role of divergent ecology and
sensory perception in speciation. Despite classical studies investigating the role of sensory ecology in speciation,3,5 it has not yet been possible to confirm whether sensory divergence is
initially responsible for limiting gene flow or results as a consequence of limited gene flow6. Typically, most classical research on speciation follows a retrospective 'spyglass'
approach, whereby the causes and mechanisms of speciation are inferred retrospectively after the speciation process is finished7. Although this approach has undeniably improved our
understanding of speciation, it often lacks resolution at the early stages of the process, either because after speciation is completed, the signal has been confounded by post-speciation
changes or simply because of the lack of historical ecological and/or behavioural data when the process was initiated8. Templeton9 suggested that 'The closer to the speciation process,
the greater the ability to focus upon the genetics of speciation', leading to the view that research is needed at the population/species interface to uncover how speciation
occurs7,8,10. Therefore, studying the population/species interface, using a 'magnifying glass' approach, can reveal important aspects of speciation and how ecology, behaviour and
genetics interact in different situations to cause the evolution of barriers to gene flow7,11, particularly important in the case of sensory ecology driven speciation. The majority of bats
use sound (echolocation) to orient themselves in their environment, locate their food and communicate12. A bat's echolocation signal and its ability to perceive this signal are
functionally linked, as the bat must be physically able to hear the echoes of its outgoing calls13. Therefore, studying the signal or echolocation call in bats will inform our understanding
of their sensory system and perception capabilities11,13. Currently the genetic mechanisms that control echolocation are being uncovered and studies are starting to explore the role that
echolocation has in communication and sexual selection14. Recent studies have suggested that changes in echolocation frequency (for example, _Rhinolophus_) are associated with assortative
mating, reproductive isolation and ultimately speciation, regardless of external barriers to gene flow5. Therefore, bat species are an excellent mammalian model to study the role of sensory
drive (that is, echolocation calls) in the speciation process11,15. One such species is _Craseonycteris thonglongyai_, a rare and endangered, charismatic bat species, considered as the
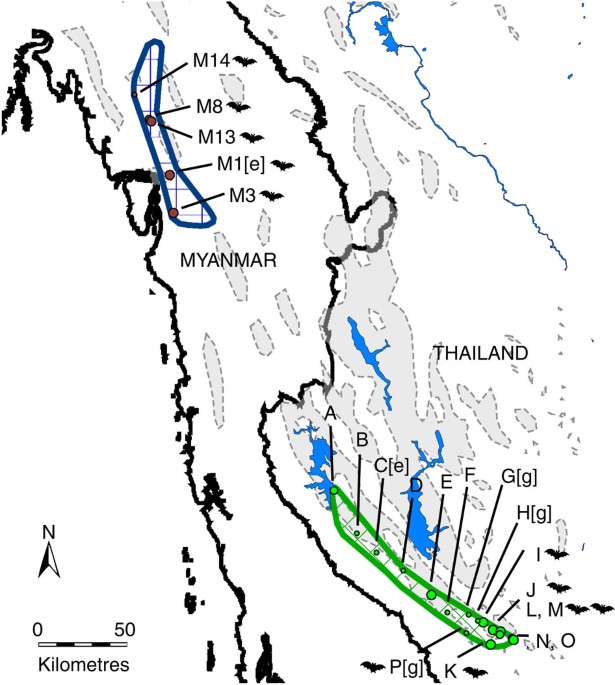
World's smallest mammal and restricted to a 2,000-km2 region that straddles the Thai–Myanmar border16. Currently, the Thai and Myanmar populations are disjointed, although the long-term
presence of suitable intervening karst landscape suggests that this may once have been a continuous distribution (Fig. 1). Despite being morphologically indistinguishable17, observed
variation in echolocation calls between Thailand18 and Myanmar19 suggested that more than one species might be present. Therefore, studying both the allopatric and sympatric populations
offers a unique opportunity to study the factors that influence population structure, gene flow and sensory trait's divergence across an entire species range and at different
evolutionary timeframes. Here we used both a 'spyglass' and 'magnifying glass' approach to untangle the early drivers of speciation in allopatric and sympatric
populations of _C. thonglongyai_, the sole representative of the monotypic bat family Craseonycteridae. To illuminate the early stages of the speciation process, we examined the ecological
and genetic factors that could have limited gene flow and driven local adaptation in these unique populations. We specifically focus on the role that echolocation has in this process. We
show that currently no gene flow is occurring between the geographically isolated allopatric populations in Thailand and Myanmar, and infer that geographic isolation most likely preceded the
echolocation divergence. Within the sympatric populations in Thailand, we identified geographic distance rather than echolocation divergence as the major factor shaping population structure
across the entire range. We suggest that echolocation divergence within Thailand, most likely resulting from interspecific competition, evolved in a context of limited gene flow, which
itself resulted from the extreme localized dispersal of this species. RESULTS THE SPYGLASS APPROACH To reconstruct the spatio-temporal history of these populations and determine the role
that echolocation call variation may have in speciation, we generated and analysed a multi-genic data set (Cytochrome _b_ and partial D-loop, X- and Y-introns and microsatellites; GenBank
accession no. GU247601–247751, Supplementary Tables S1 and S2) and collected acoustic data from throughout the species' range (Fig. 1). Intensive trapping, sampling and acoustic
recording were carried out in Thailand and Myanmar (Fig. 1). These data confirmed the marked and significant difference in echolocation calls between Thailand and Myanmar (Thailand:
_N_=4,188, 70.125–79.875 kHz and Myanmar: _N_=1,472, 76.875–83.625 kHz, generalized linear mixed model, _P_=0.002; Fig. 2a; Supplementary Fig. S1). Bayesian phylogenetic analyses based on a
1.8-kb mitochondrial fragment of 602 samples and two different NUMTs (mitochondrial pseudogenes) showed that the Thai and Myanmar populations were reciprocally monophyletic with a posterior
probability of 1.00 (Fig. 2b). This clear Thai–Myanmar split was confirmed at the nuclear level by screening all individuals (_n_=659) for 15 microsatellites (_F__st_=0.49, _P_<0.001;
Fig. 2c–d) and five nuclear single-nucleotide polymorphisms (SNPs), which showed almost complete lineage sorting (Supplementary Fig. S2). The overall strong differentiation of all markers
investigated combined with the complete lineage sorting of the mitochondrial fragment (Fig. 2b), two nuclear SNPs (Supplementary Fig. S2) and the absence of shared alleles at one
microsatellite locus, strongly advocate for a total absence of gene flow between the allopatric Thai and Myanmar populations. Relying on the molecular clock, the split between the two
populations is dated to approximately 0.4 Mya (95% highest probability density, 0.268–0.545 Mya). Genetic diversity indices of the Myanmar population are significantly reduced at the
mitochondrial (Thailand, Hs=0.92; Myanmar, Hs=0.36; permutation test, _P_=0.006) and nuclear (Thailand, Hs=0.56; Myanmar, Hs=0.43; permutation test, _P_=0.014) levels compared with the Thai
population. Similarly, nucleotide diversity of the mitochondrial DNA fragment calculated per site in Thailand and Myanmar differ strongly with more than 10-fold differences (Thailand:
average _π_=0.0034, min–max: 0.0028–0.0048; Myanmar: average _π_=0.00027, min–max: 0.00013–0.00050), indicating that the Myanmar population probably originated from a small number of
individuals, either as a consequence of a strong bottleneck or more likely via a founder effect. However, to fully elucidate whether echolocation variation drove the isolation of the two
populations or evolved after their split requires knowledge about distribution of species and the echolocation frequency of the population at the time of divergence. As this is not
available, we turned to the sympatric Thai population to address this question. THE MAGNIFYING GLASS APPROACH As the first steps of speciation occur at the population/species
interface7,8,9,20, we investigated the recent spatio-temporal history of the Thai population and the relationship between genetic structure and echolocation frequency at the population
level. To investigate past changes in distribution, we assessed whether the Thai population has been stable, increased in size (demographic expansion) or spatially expanded (spatial
expansion)21 using mismatch distribution analyses of mitochondrial fragments (_n_=462). A model of stable population size was rejected for all Thai colonies (non-overlapping 99% confidence
intervals of θ0 and θ1, two mutation parameters that are related to the effective size of the population before and after a putative expansion, respectively). While the Southern Thai
colonies (H, I, J and L, M, N, O, Fig. 1) fitted both models of demographic and spatial expansion equally well, the Central (E, F and G, Fig. 1) and Northern colonies (A, B and D, Fig. 1)
fitted a model of spatial expansion but not a model of pure demographic expansion (Supplementary Fig. S3). Principal component analysis of microsatellite data showed a strong population
structure orientated along the Northwest–Southeast direction (Fig. 3a–d). This structure was confirmed by a Mantel test showing a very strong correlation between geographic and genetic
distances (Mantel test; _r_=0.95, _R_2=0.90, _P_<0.001; Fig. 3e). This strong correlation could theoretically result from different evolutionary histories such as secondary contact
between two previously isolated populations or spatial expansion from a single population. Although the secondary contact scenario cannot be excluded, mismatch distributions do not support
the presence of two isolated populations, but rather suggest that a single population was most likely present in the Southern part of their range and recently expanded northwards. Analyses
that simulate the Thai population spatially expanding under a wide range of migration rate and population growth rate show that the strong isolation by distance pattern observed (Fig. 3e)
can result from a recent spatial expansion when gene flow is restricted to neighbouring populations (Supplementary Table S3 and Fig. S4). The average dispersal distance estimated from the
slope of the regression between genetic distance and geographic distance was 2.2–5.7 km, indeed demonstrating extreme localized dispersal. The geographically continuous Thai population
showed an abrupt change in echolocation frequency between colonies having similar echolocation calls (range: 73.35–73.96 kHz) for over 100 km in the Northern/Central part of their range and
then a sudden increase (∼3 kHz) in <20 km in the Southern part (Fig. 4). As body size and echolocation frequency are correlated across species22, we used forearm length as a proxy of body
size and showed that body size differences are unlikely to explain the observed echolocation difference (Spearman's rank correlation, _ñ_=−0.17, _P_=0.53). We then used causal
modelling on resemblance matrices23,24 to investigate the combination of factors driving genetic differentiation between colonies. We first evaluated the set of diagnostic statistical tests
of the seven possible organizational models, which included geographic distance, the presence of barriers (areas without limestone), echolocation distance (measured by the absolute
echolocation difference) and genetic distance (measured by the _F__st_ index calculated on 14 microsatellites; Supplementary Information). Only one organizational model, which included
geographic distance, echolocation distance and genetic distance, was fully supported by all statistical expectations (Supplementary Table S4). The six possible models of causal relationships
between geographic distance, echolocation distance and genetic distance were then compared with the predictions of the models23. Five models were not supported by the data, as one or
several of their predictions were not realized (Supplementary Table S5), while one model was fully supported. This latter model supports the primary influence of geographic distance on
genetic distance, which itself influences echolocation distance. ECHOLOCATION AND DRIFT To investigate if this change in echolocation could result from echolocation drift, we assessed the
short- and long-term population sizes in the North/Centre and South of Kanchanaburi. We used the actual average colony size16 as a proxy for short-term population size, and mitochondrial and
nuclear genetic diversity found within colonies as a proxy for long-term population size; high genetic diversities are being associated with large effective population sizes and reduced
drift, whereas low genetic diversities are being associated with reduced population sizes and increased drift25. The actual average colony size (South: 275; North/Centre: 248) did not
significantly differ between Northern/Central and Southern colonies (Wilcoxon Mann–Whitney rank-sum test, _n_=20, _P_=0.19). A one-sided permutation test showed that the Northern/Central
colonies had significantly reduced mitochondrial (North/Centre, Hs=0.87; South, Hs=0.94; _P_=0.01) and nuclear (North/Centre, Hs=0.52; South, Hs=0.57; _P_=0.04) gene diversities when
compared with the Southern colonies. These short-term and long-term population sizes suggest that the Northern/Central colonies, which most likely spatially expanded, were equally or more
prone to drift than the Southern colonies, and therefore, if drift had a role in echolocation frequency change, the Northern/Central colonies should show similar or higher variation across
colonies than their Southern counterparts. Contrary to this expectation, _C. thonglongyai_ in the Northern/Central part of the range shows little echolocation variation over 100 km (colonies
A to F, Fig. 4). Therefore, this observation of a nonrandom pattern of echolocation call variation (Fig. 4) supports the view that variation in echolocation within the Thai population may
have resulted from adaptation as opposed to drift. ECHOLOCATION AND GENOMIC SELECTION There are marked differences in echolocation call between the North/Centre and South of Kanchanaburi in
Thailand (Fig. 4). If natural selection is driving the echolocation divergence within this population, then genes underlying echolocation frequency should show signs of divergent selection
on either side of the area of abrupt echolocation change (Fig. 4). This type of divergent selection has been shown in coat colour genes in dark- and light-coloured populations of
_Peromyscus_ living on dark and light soil, respectively26. The 15 microsatellites from 462 _Craseonycteris_ individuals were tested for selection based on the F_st_ outlier approach27. One
microsatellite locus (CTC1; arrow in Fig. 5a–c) showed strong signs of divergent selection when comparing colonies on either side of the area of abrupt echolocation change (Lositan;
_P_<0.001, Fig. 5a,d,e), but not when comparing colonies within each side (Lositan; _P_>0.05, Fig. 5b,c). The identification of CTC1 as being under selection was also recovered when
testing for selection using a hierarchical framework integrating population structure (Supplementary Information). We then identified the genomic region containing the microsatellite locus
under selection, which was located on the 5′-side of the recombination signal–binding protein for immunoglobulin kappa J region (_RBP-J_ gene; Supplementary Table S6). The _RBP-J_ gene has
been shown to be involved in hair cell formation in the cochlea28,29. As bats are using the highest frequencies of all terrestrial mammals, their hearing system and particularly the hair
cells in the organ of Corti, where the sound is received and amplified, need to be well adapted22. The microsatellite itself is most likely not under selection, but may be linked to parts of
the genome that are under selection, and the _RBP-J_ gene may be another candidate 'echolocation' gene, although further investigations are needed to confirm this. ECOLOGICAL
FACTORS DRIVING DIVERGENT ECHOLOCATION From an ecological perspective, the reasons for this sudden change in echolocation peak frequency are not well known. Under an adaptive hypothesis, it
would be driven by abiotic or biotic factors11. Abiotic factors such as temperature and humidity affect the attenuation of sound (echolocation call) in the air in a frequency-dependant
manner, and therefore influence the maximum distance of prey detection30, a process that could be responsible for differences in echolocation frequency between populations living in
different areas and facing dissimilar climatic conditions11. Nevertheless, the resulting interplay of temperature and humidity conditions throughout the _Craseonycteris_ range in Thailand
shows little variation, suggesting that attenuation should be constant throughout the range and therefore have a limited role in shaping the echolocation call's frequency (Fig. 6).
Furthermore, the observed differences in echolocation frequency result in high sound attenuation values in the Southern colonies (colonies I to O, Fig. 6). If the Southern colonies had a
peak frequency similar to Northern/Central colonies (∼74 kHz, colonies A to F), they would benefit from an increased detection distance and the attenuation would be very similar across
colonies. Rather, the difference in echolocation frequency seems to render the Southern colonies less-optimal in terms of prey detection distance (for example, increased sound attenuation;
Fig. 6). Therefore, it seems unlikely that echolocation frequency has changed to keep attenuation constant across the different colonies. Response to different biotic factors (such as
interspecific competition) could explain the pattern of echolocation call variation31. A shift in peak frequency (character displacement) may act to avoid using the same frequency bandwidth
as another small sympatric bat species, _Myotis siligorensis_. Despite being phylogenetically distant, _C. thonglongyai_ and _M. siligorensis_ produce similar echolocation calls at ∼70 kHz
(ref. 18; Fig. 7), are comparable in size, forage in the same habitat and exhibit similar hunting behaviour18. This hypothesis would be supported if _M. siligorensis_ was more abundant in
the south of Kanchanaburi, where _C. thonglongyai_ has a high echolocation call, than in the North/Centre, where _C. thonglongyai_ peak frequency is lower (Fig. 4). To test this character
displacement hypothesis, we visually screened 1,775 recordings from the surrounding areas of 14 caves, where we caught _C. thonglongyai_. Indeed, in six out of eight sites surveyed in the
South, _M. siligorensis_ was recorded multiple times (230/1,327 recordings), whereas in the North/Centre, no individual was recorded at any of the six sites surveyed (0/448 recordings; Fig.
1). DISCUSSION The extent of gene flow is of crucial importance during the speciation process; for example, the complete absence of gene flow between two populations of the same species will
lead these populations to accumulate genetic differences through time while keeping a pool of complementary genes within each population. The Bateson–Dobzhansky–Muller model32, recently
supported by empirical evidence33, proposes that given enough time, hybrid sterility and inviability will evolve between these two populations due to negative genetic interactions between
two or more loci that have accumulated substitutions, ultimately leading to speciation. This process is most likely ongoing between the allopatric Thai and Myanmar populations. The Thai and
Myanmar populations of _C. thonglongyai_ are genetically isolated from each other and show marked echolocation differences. Whether these changes in echolocation have had a major role in the
separation of the two populations remains difficult to test. The significantly reduced diversity of the Myanmar population suggests that either the population was previously more diverse
and went through a bottleneck or that the population originated from the Thai population via a founder effect. It is difficult to rule out the bottleneck hypothesis, but if this scenario was
correct, it would mean that the population was severely reduced to a point where all individuals shared the same haplotype. Although not impossible, this scenario seems unlikely. The recent
origin of the Myanmar population from the Thai population via a founder effect is more likely and would explain the reduced level of diversity, whereby only a few individuals founded the
Myanmar population. Although possible, an active dispersal of a few _Craseonycteris_ from Thailand to Myanmar (200 km) is unlikely given the very short dispersal distance estimated for
_Craseonycteris_ (about 2–5 km) and its very localized foraging activity (about 1 km from the colony)34. A more plausible scenario would be a 'sweepstakes' dispersal event35 of a
few _Craseonycteris_ via storms, cyclones or typhoons. The winter monsoon winds, blowing from East-South-East to West-North-West, could easily transport _Craseonycteris_ over the 200 km
separating the two populations. Episodes of strong winter monsoon winds correspond to orbital periods and have happened every 100-kyr in the past 600-kyr (refs. 36, 37). This
'sweepstakes' event would have been immediately followed by isolation and the absence of gene flow between the two populations. The date of the split was dated between 268,000 and
545,000 years BP, which spans over four periods of high winter monsoon activity36. It thus seems that although echolocation differences match perfectly with genetic differences, echolocation
probably did not have a role in the separation of the Thai and Myanmar populations. Interestingly, _M. siligorensis_ was captured and/or recorded at all study sites in Myanmar (Fig. 1),
further supporting the possible link between the presence of _M. siligorensis_ and high-frequency echolocation calls. The analyses at the population level within Thailand further illuminate
the contribution of different factors during the speciation continuum7. Even in the best known examples of 'sympatric' speciation, driven by sensory ecology (for example, the
Cichlids in Lake Victoria3), it is uncertain whether the variation in sensory traits is the cause or the consequence of the populations' isolation6. The quasi-linear distribution of
_Craseonycteris_ along the Kwae river valley16 combined with limited dispersal abilities have shaped a unique system in which to study the contribution of various factors to population
structure and gene flow. Within Thailand, isolation by geographic distance seems to be the main factor driving the genetic isolation of populations, a result consistent with recent
simulations38. If echolocation differences were the primary driver of the populations' isolation, they should explain a much higher percentage of genetic variance and also consistently
match the genetic distance, which was not the case. Our results rather support that echolocation differences evolved secondarily in a context of limited gene flow. Limited gene flow can
enhance rapid local adaptation39 and even lead to parapatric speciation in the absence of any environmental variability38. We however suggest that in _C. thonglongyai_, limited gene flow
creates the conditions that favour the evolution of echolocation differences via local adaptation and we predict that reinforcement-like selection might drive the speciation process through
acoustic isolation limiting mate choice to similar acoustic types5,15 (Supplementary Note 1). Further studies investigating mate-choice preference and survival of individuals in relation to
echolocation call frequency are needed to test if this predicted reinforcement-like selection would occur. The 'echolocation' displacement observed could have two functions, which
are not necessarily mutually exclusive (Supplementary Note 2 for further details); species echolocating at different frequencies can in theory detect different sizes of insect prey40,
thereby, partition food resources, especially if the prey size distribution is skewed towards small prey41. However, this hypothesis has received little empirical support in the literature,
especially when the echolocation frequency differences between species are less than 10 kHz as in the present case42 (see ref. 43). Alternatively, this character displacement could enable
the two species to avoid jamming each other's echolocation system by each having a private bandwidth44, and therefore reduce interference competition. In echolocating bats, the onset of
the emitted call activates a neuronal gating mechanism that establishes a time window during which pulse-echo pairs are processed for target distance determination44. If an echolocation
call of a similar frequency is played to an echolocating bat during this time window, the bat is unable to accurately determine target range45. Therefore, given the similarity in calls
between _C. thonglongyai_ and _M. siligorensis_, an upward shift in frequency, as seen in the Southern _C. thonglongyai_ population where _M. siligorensis_ is present, could have occurred to
avoid acoustic jamming. We note that this idea is consistent with the fact that _C. thonglongyai_'s calls are also emitted at high frequencies in Myanmar, where _M. siligorensis_
occurs. This work shows that sensory ecology and genetic divergence are associated at the early stage of population differentiation, and that echolocation divergence most likely occurs in a
context of limited gene flow, in response to a competitor bat species. We posit that this echolocation divergence may later act to further drive population differentiation within Thailand.
Our work highlights the importance of environmental factors (biotic and abiotic) in contributing to population structure and shows the strength of studying the speciation process using both
a 'spyglass' and 'magnifying glass' approach. Ongoing studies of these unique Thai and Myanmar populations of _C. thonglongyai_ will provide an opportunity to study the
long-term interaction between gene flow and sensory ecology to fully understand the intricacies of the speciation process in nature. Owing to the central role of echolocation in bat biology
and its dual role in foraging and communication, further studies using echolocating bats as models to investigate the interplay of sensory ecology, mate choice and gene flow will undeniably
advance our understanding of sensory-driven speciation. METHODS ECHOLOCATION CALLS Free flying bats were recorded at dusk for 1 h outside the cave's entrance using a D1000X ultrasound
detector (Pettersson Elektronik AB) and automatically stored in a memory card (Compaq Flash, SanDisk Ultra II, 1 or 4 GB). A sampling frequency of 384 kHz was used, enabling aliasing-free
sounds to be recorded up to 153 kHz. For analysis, calls were processed through a Fast Fourier Transformation (1,024 points, Hanning window, fast Fourier transformations calculated with 90%
time overlap; software BatSound version 3.31, Pettersson Elektronik AB). Peak frequency (that is, frequency with most energy) was measured using the function 'Pulse Characteristics
Analysis' available in BatSound. The 'mark positioning' was enabled and measurements were visually checked. Each individual's recording contained multiple calls. Our
echolocation call data set consisted of 5,650 calls from 376 recordings. To compare the peak frequency between Thailand and Myanmar, we used a generalized linear mixed model via the
'lmer' function implemented in the 'lme' package in R 2.12.0 (ref. 46). This model considers the nested structure of individuals within sites and sites within countries.
A model with calls nested within individuals was too large to be run; therefore, to remove any potential bias caused by non-independence between calls from the same recording, we generated
10,000 data sets by randomly picking only one call per recording, when performing the generalized linear mixed model. The generalized linear mixed model was performed 10,000 times resulting
in10,000 _P_-values from which we calculated the median. SAMPLE COLLECTION AND DNA EXTRACTION _C. thonglongyai_ of both sexes were sampled from 15 colonies in Thailand (_n_=468) and 4
colonies in Myanmar (_n_=202; Fig. 1). Bats were captured either with a hand-net, while they were roosting during the day, or with mist-nets on emergence at dusk. Bats were placed in cloth
bags and immediately released after processing. Two skin biopsies were taken and stored in 200-μl pure ethanol or dried with silica-gel until extraction. DNA was extracted from wing biopsy
punches using a modified salt/chloroform extraction protocol47 that included an additional chloroform/isoamyl alcohol (24/1) step after adding the saturated NaCl solution. DNA samples were
used at a final concentration of 2–5 ng · μl−1 (Supplementary Methods). MICROSATELLITE GENOTYPING AND ANALYSIS The 15 microsatellite primers used in this study were specifically developed
for _C. thonglongyai_ and were amplified and genotyped following Puechmaille _et al_.48 (locus CTD114 not used). To check for genotyping consistency, 10% of samples were amplified and
genotyped twice. A total of 670 samples were genotyped for 15 microsatellite loci. Samples were collected on different dates and therefore it was possible that the same individual had been
sampled twice. We thus compared multilocus genotypes using GeneCap49 to identify and remove duplicates from the data set50. Out of 670 samples, 11 samples were duplicates (6 in Myanmar and 5
in Thailand), leaving a total of 659 individuals for the analysis (463 and 196 for Thailand and Myanmar, respectively). No departures from Hardy–Weinberg and linkage equilibrium were
detected at the colony level after Bonferroni correction using Fstat version 2.9.3.2. For the entire data set, population structure was investigated using the program STRUCTURE version 2.2
(ref. 51). We used a burn-in length of 10,000 and a run length of 100,000 without prior population information. The burn-in and run length were chosen after an initial test, whereby we
looked at the convergence of the values of summary statistics and consistency between runs. All other parameters were left as by default. Ten independent runs were undertaken for _K-_values
ranging from 1 to 10, reflecting the minimum and maximum number of populations suspected. The number of populations was inferred from the _ΔK_ statistic as developed in Evanno _et al_.52
Using the 659 individual genotypes, a phylogenetic tree was constructed by the neighbour-joining method with the Da distance. The tree construction was carried out using the program
Populations 1.2.30. Within Thailand (463 individuals), _F__st_ and population differentiation were calculated and tested using Genepop v 4.0.6 (ref. 53). MITOCHONDRIAL DNA AND PHYLOGENETIC
RECONSTRUCTION A 1,840-bp mitochondrial DNA fragment encompassing the entire _Cytb_, tRNA Threonine, tRNA Proline and part of the control region was amplified by polymerase chain reaction in
three overlapping fragments (Supplementary Fig. S5 and Supplementary Tables S7 and S8). Two NUMTs (nuclear pseudogenes) were also amplified and used in the phylogenetic analysis. Using the
mitochondrial DNA and the two NUMTs, phylogenetic reconstruction was undertaken using the Bayesian inference in BEAST54 (Supplementary Methods). NUCLEAR INTRONS PRIMERS, POLYMERASE CHAIN
REACTION AND GENOTYPING Five SNPs were identified on four nuclear introns (Supplementary Table S8), two found on the X chromosome (_BGN_ and _PHKA2_), the Y chromosome (_SMCY7_) and one on
chromosome 16 for _Homo sapiens_ (_ZP2_). For these five SNPs, we designed custom TaqMan SNP assays (Applied Biosystems; Supplementary Table S9) and screened the 659 individuals
(Supplementary Methods). GENETIC DIVERSITY INDICES To test for differences in genetic diversity between the Thai and Myanmar populations, we grouped colonies into two groups corresponding to
Thailand and Myanmar and compared their genetic diversity at the nuclear and mitochondrial level in Fstat version 2.9.3.2. Significance was tested using 10,000 permutations. Samples were
randomly allocated to the two groups, and the genetic diversity was calculated for each permutation. The _P_-value of the test was the proportion of randomized data sets giving a larger
value for the genetic index than the observed one (one-sided test). POPULATION HISTORY AND STRUCTURE The Thai population's demographic history was examined using the mismatch
distribution of 462 mitochondrial DNA sequences55. Population structure was analysed using principal component analysis on microsatellite data with R version 2.12.0 (ref. 46) using the
adegenet package version 1.3-0 (ref. 56). Simulations of a spatially expanding population mimicking the expansion of the Thai population were carried out in SPLATCHE57, and outputs analysed
in Arlequin ver 3.5 (ref. 58; _via_ arlecore) and displayed in R 2.12.0 (ref. 46). A 100×10 lattice was used, representing the distribution of _Craseonycteris_ along the Kwae River valley16.
Each deme was considered to be sending migrants to its neighbours at a rate _m_. Once a deme was colonized, its population size grew following a logistic model with rate _r_ and carrying
capacity _C_. The initial population size was set to 2,000 and the 'AllowSourcePopulationOverflow' parameter was set to '0'. A homogene friction map was used. A set of 12
locations spaced evenly along the 'lat' axis of the lattice (starting at deme 4 and every 8 demes) were sampled for 20 individuals. Sampling was carried out just after the entire
lattice was completely filled in. A set of 14 unlinked microsatellite loci with a mutation rate of 5×10−4 were simulated. On the basis of available information on _Craseonycteris_ colony
size16, the simulations were run for two values of the carrying capacity _C_, 100 and 250. For each value of _C_, 12 combinations of the _m_ (0.1, 0.3, 0.5, 0.7) and _r_ (0.1, 0.3, 0.5)
parameters were simulated. One hundred replicates were analysed for each combination of parameters. A simulation was considered to give an isolation by distance pattern similar to the
observed, if it contained at least one replicate with a maximal _F__st_ comprised between 0.2 and 0.3 and a Mantel _r_ greater than 0.9 (Supplementary Table S3). CAUSAL MODELLING Causal
modelling on resemblance matrices23,24 was used to investigate the combination of factors driving genetic differentiation between colonies and the causal relationships between these factors.
Mantel and partial Mantel tests were used within the causal modelling framework to assess the support for all possible organizational models23,24. All tests were conducted with R version
2.12.0 (ref. 46) using the package ecodist59 version 1.1.4, and significance was assessed with 9,999 permutations (Supplementary Methods). SELECTION TESTS The 15 microsatellites from 463
individuals were tested for selection based on the _F__st_ outlier approach27 using two models, and island model and a hierarchical island model in LOSITAN60 and Arlequin58 version 3.5,
respectively (Supplementary Methods). MICROSATELLITE CTC1 GENOMIC POSITION Before a Blast search, the microsatellite sequence including the flanking regions (total of 599 nt) was
repeatmasked (RepeatMasker version open-3.2.8). Only simple repeats corresponding to the microsatellite motif were identified (from nt 347 to nt 408; TCCA motif). A Blastn search with the
default settings and 'word size' set to 7 was used to identify genomic regions similar to the microsatellite locus (CTC1) flanking regions. For the 5′-flanking region (346 nt), the
best match (_E_-value: 9e−39; coverage=84%, identities=221/302 (73%)) was much better than the second (_E_-value: 0.07; coverage=14%, identities=41/51 (80%)) or third match (_E_-value:
0.25; coverage=10%, identities=32/37 (86%)). Similarly, for the 3′-flanking region (191 nt), the best match (_E_-value: 2e−06; coverage=92%, identities=136/181 (75%)) was much better than
the second (_E_-value: 0.003; coverage=16%, identities=29/31 (93%)) or third match (_E_-value: 0.01; coverage=15%, identities=28/30 (93%)). Both flanking region's best matches were
situated on Human chromosome number 4 (GRCh37 reference primary), around 50 kb (53,653 for 3′-flanking region and 52,745 for 5′-flanking region) at 5′ side of the 'recombining binding
protein suppressor of hairless isoform 3′, also known as _RBP-J_ (_H. sapiens_ geneID: 3516; chromosome: 4, location: 4p15.2). As we do not know if there is synteny around _RBP-J_ between
_C. thonglongyai_ and _H. sapiens_, we then used Ensembl to identify potential synteny around _RBP-J_ between _H. sapiens_ and 12 other genomes sequenced at high coverage (Supplementary
Table S6). We showed that large syntenic blocks (average=30.2 M) around the _RBP-J_ gene in _H. sapiens_ were also present in all species investigated (Supplementary Table S6). Given the
conserved synteny observed in other species investigated, it is therefore likely that microsatellite CTC1 in _C. thonglongyai_ is also in the 5′-side of the _RBP-J_ gene. ADDITIONAL
INFORMATION ACCESSION CODES: The data have been deposited in the GenBank database under accession codes GU247601 to GU247751. HOW TO CITE THIS ARTICLE: Puechmaille, S.J. _et al_. The
evolution of sensory divergence in the context of limited gene flow in the bumblebee bat. _Nat. Commun._ 2:573 doi: 10.1038/ncomms1582 (2011). ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ *
GU247601 * GU247751 REFERENCES * Doebeli, M. & Dieckmann, U. Speciation along environmental gradients. _Nature_ 421, 259–264 (2003). Article ADS CAS Google Scholar * Kawata, M.,
Shoji, A., Kawamura, S. & Seehausen, O. A genetically explicit model of speciation by sensory drive within a continuous population in aquatic environments. _BMC Evol. Biol._ 7, 99
(2007). Article Google Scholar * Seehausen, O. et al. Speciation through sensory drive in cichlid fish. _Nature_ 455, 620–626 (2008). Article ADS CAS Google Scholar * Boughman, J. W.
Divergent sexual selection enhances reproductive isolation in sticklebacks. _Nature_ 411, 944–948 (2001). Article ADS CAS Google Scholar * Kingston, T. & Rossiter, S. J.
Harmonic-hopping in Wallacea's bats. _Nature_ 429, 654–657 (2004). Article ADS CAS Google Scholar * Nosil, P., Harmon, L. J. & Seehausen, O. Ecological explanations for
(incomplete) speciation. _Trends Ecol. Evol._ 24, 145–156 (2009). Article Google Scholar * Via, S. Natural selection in action during speciation. _Proc. Natl Acad. Sci. USA_ 106, 9939–9946
(2009). Article ADS CAS Google Scholar * Harrison, R. Molecular changes at speciation. _Annu. Rev. Ecol. Syst._ 22, 281–308 (1991). Article Google Scholar * Templeton, A. (ed). _The
Role of Molecular Genetics in Speciation Studies_ (Birkhauser-Verlag, 1998). * Losos, J. B. & Glor, R. E. Phylogenetic comparative methods and the geography of speciation. _Trends Ecol.
Evol._ 18, 220–227 (2003). Article Google Scholar * Endler, J. A. Signals, signal conditions, and the direction of evolution. _Am. Nat._ 139, S125–S153 (1992). Article Google Scholar *
Fenton, M. B. Eavesdropping on the echolocation and social calls of bats. _Mammal Rev._ 33, 193–204 (2003). Article Google Scholar * Thomas, J. A., Moss, C. F. & Vater, M. (eds)
_Echolocation in Bats and Dolphins_ (The University of Chicago Press, 2004). * Jones, G. & Siemers, B. The communicative potential of bat echolocation pulses. _J. Comp. Physiol. A_ 197,
447–457 (2011). Article Google Scholar * Boughman, J. W. How sensory drive can promote speciation. _Trends Ecol. Evol._ 17, 571–577 (2002). Article Google Scholar * Puechmaille, S. J. et
al. Population size, distribution, threats and conservation status of two endangered bat species: _Craseonycteris thonglongyai_ and _Hipposideros turpis_. _Endang. Species Res._ 8, 15–23
(2009). Article Google Scholar * Pereira, M. J. R. et al. Conservation of the world's smallest mammal, the bumble-bee bat _Craseonycteris thonglongyai_, in Myanmar. _Oryx_ 40, 456–463
(2006). Article Google Scholar * Surlykke, A. et al. Echolocation in two very small bats from Thailand: _Craseonycteris thonglongyai_ and _Myotis siligorensis_. _Behav. Ecol. Sociobiol._
33, 1–12 (1993). Article Google Scholar * Khin Mie Mie PhD dissertation, University of Yangon, 2004. * Templeton, A. Mechanisms of speciation--a population genetic approach. _Annu. Rev.
Ecol. Syst._ 12, 23–48 (1981). Article Google Scholar * Ray, N., Currat, M. & Excoffier, L. Intra-deme molecular diversity in spatially expanding populations. _Mol. Biol. Evol._ 20,
76–86 (2003). Article CAS Google Scholar * Neuweiler, G. _The Biology of Bats_ (Oxford University Press, 2000). * Legendre, P. & Trousselier, M. Heterotrophic bacteria: modeling in
the presence of spatial autocorrelation. _Limnol. Oceanogr._ 33, 1055–1067 (1988). Article ADS Google Scholar * Legendre, P. Autocorrelation: trouble or paradigm. _Ecology_ 74, 1659–1673
(1993). Article Google Scholar * Hartl, D. L. & Clark, A. G. _Principles of Population Genetics_ (Sinauer Associates, Inc., 1997). * Mullen, L. M. & Hoekstra, H. E. Natural
selection along an environmental gradient: a classic cline in mouse pigmentation. _Evolution_ 62, 1555–1570 (2008). Article CAS Google Scholar * Beaumont, M. A. & Nichols, R. A.
Evaluating loci for use in the genetic analysis of population structure. _Proc. R. Soc. Lond. B_ 263, 1619–1626 (1996). Article ADS Google Scholar * Yamamoto, N. et al. Inhibition of
Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. _J. Mol. Med._ 84, 37–45 (2006). Article CAS Google Scholar * Hori, R. et al. Pharmacological inhibition of
Notch signal in the mature guinea pig cochlea. _Neuroreport_ 18, 1911–1914 (2007). Article CAS Google Scholar * Lawrence, B. D. & Simmons, J. A. Measurements of atmospheric
attenuation at ultrasonic frequencies and the significance for echolocation by bats. _J. Acoust. Soc. Am._ 71, 585–590 (1982). Article ADS CAS Google Scholar * Heller, K.- G. & Von
Helversen, O. Resource partitioning of sonar frequency bands in rhinolophoid bats. _Oecologia_ 80, 176–186 (1989). Article ADS Google Scholar * Orr, H. The population genetics of
speciation: the evolution of hybrid incompatibilities. _Genetics_ 139, 1805–1813 (1995). CAS PubMed PubMed Central Google Scholar * Matute, D., Butler, I., Turissini, D. & Coyne, J.
A test of the snowball theory for the rate of evolution of hybrid incompatibilities. _Science_ 329, 1518–1521 (2010). Article ADS CAS Google Scholar * Duangkhae, S. Ecology and behaviour
of the Kitti's Hog-nosed bat (_Craseonycteris thonglongyai_) in Western Thailand. _Nat. Hist. Bull. Siam Soc._ 38, 135–161 (1990). Google Scholar * Simpson, G. G. Mammals and land
bridges. _J. Wash. Acad. Sci._ 30, 137–163 (1940). Google Scholar * Liu, Z. et al. Clay mineral assemblages in the northern South China Sea: implications for East Asian monsoon evolution
over the past 2 million years. _Mar. Geol._ 201, 133–146 (2003). Article ADS CAS Google Scholar * Beaufort, L., de Garidel-Thoron, T., Linsley, B., Oppo, D. & Buchet, N. Biomass
burning and oceanic primary production estimates in the Sulu Sea area over the last 380 kyr and the East Asian monsoon dynamics. _Mar. Geol._ 201, 53–65 (2003). Article ADS CAS Google
Scholar * Hoelzer, G. A., Drewes, R., Meier, J. & Doursat, R. Isolation-by-distance and outbreeding depression are sufficient to drive parapatric speciation in the absence of
environmental influences. _PLoS Comput. Biol._ 4, e1000126 (2008). Article ADS MathSciNet Google Scholar * Nosil, P. Adaptive population divergence in cryptic color-pattern following a
reduction in gene flow. _Evolution_ 63, 1902–1912 (2009). Article Google Scholar * Pye, J. D. Is fidelity futile? The true signal is illusory, especially with ultrasound. _Bioacoustics_ 4,
271–286 (1993). Article Google Scholar * Safi, K. & Siemers, B. M. Implications of sensory ecology for species coexistence: biased perception links predator diversity to prey size
distribution. _Evol. Ecol._ 24, 703–713 (2010). Article Google Scholar * Houston, R. D., Boonman, A. M. & Jones, G. Do echolocation signal parameters restrict bats' choice of
prey? In _Echolocation in Bats and Dolphins_ (eds Jeanette A. Thomas, Cynthia F. Moss, & Marianne Vater) 339–345 (University of Chicago Press, 2004). * Siemers, B. M. & Schniltzer,
H.- U. Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. _Nature_ 429, 657–661 (2004). Article ADS CAS Google Scholar * Roverud, R. C. &
Grinnell, A. D. Echolocation sound features processed to provide distance information in the CF/FM bat, _Noctilio albiventris_: evidence for a gated time window utilizing both CF and FM
components. _J. Comp. Physiol. A_ 156, 457–469 (1985). Article Google Scholar * Neuweiler, G. Auditory adaptations for prey capture in echolocating bats. _Physiol. Rev._ 70, 615–641
(1990). Article CAS Google Scholar * R Development Core Team. _R: a language and environment for statistical computing, http://cran.r-project.org/, Vienna (Austria)_ (R Foundation for
Statistical Computing, 2010). * Miller, S. A., Dykes, D. D. & Polesky, H. F. A simple salting out procedure for extracting DNA from human nucleated cells. _Nucleic Acids Res._ 16, 1215
(1988). Article CAS Google Scholar * Puechmaille, S. J. et al. Characterization and multiplex genotyping of 16 polymorphic microsatellite loci in the endangered bumblebee-bat,
_Craseonycteris thonglongyai_ (Chiroptera: Craseonycteridae). _Conserv. Genet._ 10, 1073–1076 (2009). Article CAS Google Scholar * Wilberg, M. J. & Dreher, B. P. GENECAP: a program
for analysis of multilocus genotype data for non-invasive sampling and capture-recapture population estimation. _Mol. Ecol. Notes_ 4, 783–785 (2004). Article Google Scholar * Puechmaille,
S. J. & Petit, E. J. Empirical evaluation of non-invasive capture-mark-recapture estimates of population size based on a single sampling session. _J. Appl. Ecol._ 44, 843–852 (2007).
Article Google Scholar * Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. _Genetics_ 155, 945–959 (2000). CAS PubMed
PubMed Central Google Scholar * Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. _Mol. Ecol._ 14,
2611–2620 (2005). Article CAS Google Scholar * Rousset, F. genepop'007: a complete re-implementation of the genepop software for Windows and Linux. _Mol. Ecol. Notes_ 8, 103–106
(2008). Article Google Scholar * Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. _BMC Evol. Biol._ 7, 214 (2007). Article Google Scholar *
Slatkin, M. & Hudson, R. R. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. _Genetics_ 129, 555–562 (1991). CAS PubMed PubMed
Central Google Scholar * Jombart, T. adegenet: a R package for the multivariate analysis of genetic markers. _Bioinformatics_ 24, 1403–1405 (2008). Article CAS Google Scholar * Ray, N.,
Currat, M., Foll, M. & Excoffier, L. SPLATCHE2: a spatially explicit simulation framework for complex demography, genetic admixture and recombination. _Bioinformatics_ 26, 2993–2994
(2010). Article CAS Google Scholar * Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and
Windows. _Mol Ecol Resour_ 10, 564–567 (2010). Article Google Scholar * Goslee, S. C. & Urban, D. L. The ecodist package for dissimilarity-based analysis of ecological data. _J. Stat.
Softw._ 22, 1–19 (2007). Article Google Scholar * Antao, T., Lopes, A., Lopes, R. J., Beja-Pereira, A. & Luikart, G. LOSITAN: a workbench to detect molecular adaptation based on a
_Fst_-outlier method. _BMC Bioinformatics_ 9, 323 (2008). Article Google Scholar Download references ACKNOWLEDGEMENTS This paper is dedicated to the memory of Professor Daw Tin Nwe of
Yangon University for her encouragement and assistance with the research and for her kindness and humour, which she shared with all who knew her. We thank Sai Yok and Erawan National Park
(Thailand) for assisting us during the survey; the Institut pour la Recherche et le Développement, Unité de Recherche 178, and Mahidol University for their logistic support; Nu Nu Aye, Wai
Wai Myint, Aye Thida and Thida Tin for their help in the field. This project was funded by Science Foundation Ireland (RFP GEN0056) and supported by the Royal Irish Academy, the Darwin
Initiative (DEFRA) and the Royal Society (International project). AUTHOR INFORMATION Author notes * Sébastien J. Puechmaille Present address: Present address: Max Planck Institute for
Ornithology, Sensory Ecology Group, 82319 Seewiesen, Germany., * Tin Nwe: Deceased. AUTHORS AND AFFILIATIONS * School of Biology and Environmental Science & UCD Conway Institute of
Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland., Sébastien J. Puechmaille & Emma C. Teeling * Center of Excellence for Vector and
Vector-Borne Diseases, Faculty of Science, Mahidol University at Salaya, Nakhon Pathom, 73170, Thailand Meriadeg Ar Gouilh * Laboratory for Urgent Response to Biological Threats (CIBU),
Institut Pasteur, Paris, 75724, France Meriadeg Ar Gouilh * Faculty of Science, Prince of Songkla University, Hat-Yai, 90112, Thailand Piyathip Piyapan, Medhi Yokubol & Chutamas Satasook
* Zoology Department, Yangon University, Yangon, Myanmar Khin Mie Mie & Tin Nwe * Harrison Institute, Centre for Systematics and Biodiversity Research, Sevenoaks, Kent, TN13 3AQ, UK
Paul J. Bates * Hinthada University, Hinthada, Myanmar Si Si Hla Bu * Aberdeen Centre for Environmental Sustainability, Institute of Biological and Environmental Sciences, University of
Aberdeen, Aberdeen, AB24 2TZ, UK Iain J. Mackie * University Rennes 1/CNRS, UMR 6553 ECOBIO, Station Biologique, Paimpont, 35380, France Eric J. Petit Authors * Sébastien J. Puechmaille View
author publications You can also search for this author inPubMed Google Scholar * Meriadeg Ar Gouilh View author publications You can also search for this author inPubMed Google Scholar *
Piyathip Piyapan View author publications You can also search for this author inPubMed Google Scholar * Medhi Yokubol View author publications You can also search for this author inPubMed
Google Scholar * Khin Mie Mie View author publications You can also search for this author inPubMed Google Scholar * Paul J. Bates View author publications You can also search for this
author inPubMed Google Scholar * Chutamas Satasook View author publications You can also search for this author inPubMed Google Scholar * Tin Nwe View author publications You can also search
for this author inPubMed Google Scholar * Si Si Hla Bu View author publications You can also search for this author inPubMed Google Scholar * Iain J. Mackie View author publications You can
also search for this author inPubMed Google Scholar * Eric J. Petit View author publications You can also search for this author inPubMed Google Scholar * Emma C. Teeling View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.C.T. and P.J.B. conceived the study. E.C.T, P.J.B., C.S., T.N. and S.S.H.B. supervised the project
either during fieldwork in Thailand or Myanmar and/or in the laboratory. S.J.P., M.A.G., P.P., M.Y., I.J.M. and K.M.M. surveyed caves and collected genetic samples. S.J.P. collected the
acoustic data, generated the genetic data and performed all the analysis. E.J.P. and E.C.T. helped with the genetic analysis. S.J.P and E.C.T. wrote the paper. All authors discussed the
results, implications and edited the manuscript. CORRESPONDING AUTHORS Correspondence to Sébastien J. Puechmaille or Emma C. Teeling. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures S1-S5, Supplementary Tables S1-S9, Supplementary Notes 1-2, Supplementary
Methods and Supplementary References. (PDF 901 kb) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License.
To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Puechmaille, S., Gouilh, M., Piyapan, P.
_et al._ The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat. _Nat Commun_ 2, 573 (2011). https://doi.org/10.1038/ncomms1582 Download citation *
Received: 01 July 2011 * Accepted: 02 November 2011 * Published: 06 December 2011 * DOI: https://doi.org/10.1038/ncomms1582 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
How will the ukraine crisis play out in british politics? | thearticleToday had been billed as the day of reckoning in Ukraine. At the time of writing, reports of a Russian withdrawal have d...
Aarp vital voices: consumer fraud among u. S. Adults ages 45+: incidence, concern, protection, vulnerabilityThe Federal Trade Commission’s (FTC) 2020 Consumer Sentinel Report shows U.S. consumers filed more than 4.7 million repo...
Observation of an acoustic octupole topological insulatorABSTRACT Berry phase associated with energy bands in crystals can lead to quantised observables like quantised dipole po...
Visa applicants will now face social media background checksWant to come to America? Get your Twitter handle ready. The White House on Thursday approved a harsher visa vetting proc...
Cristiano ronaldo – latest news information updated on may 29, 2025 | articles & updates on cristiano ronaldo | photos & videos | latestlyCristiano Ronaldo – Latest News Information updated on May 29, 2025 | Articles & Updates on Cristiano Ronaldo | Phot...
Latests News
The evolution of sensory divergence in the context of limited gene flow in the bumblebee batABSTRACT The sensory drive theory of speciation predicts that populations of the same species inhabiting different envir...
You’ve Earned a Say - Protect Seniors, Retirement, Social SecurityFacebook Twitter LinkedIn After years of paying into Medicare and Social Security, you’ve earned a say in their future. ...
LG To Introduce ‘Pay Via Your TV’ System To WebOSLG Electronics is set to enable their Smart TV’s so that users can make purchases directly via their TV.The Korean Compa...
Madchen Amick on Being a Sandwich Generation Caregiver1:22 Mädchen Amick on Being a Sandwich Generation Caregiver Facebook Twitter LinkedIn The Riverdale actress describes th...
Mr j gentle v flare products ltd: 3313818/2019MR J GENTLE V FLARE PRODUCTS LTD: 3313818/2019 Employment Tribunal decision. Read the full decision in Mr J Gentle v Fla...