Overcoming the limitations of directed c–h functionalizations of heterocycles
Overcoming the limitations of directed c–h functionalizations of heterocycles"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
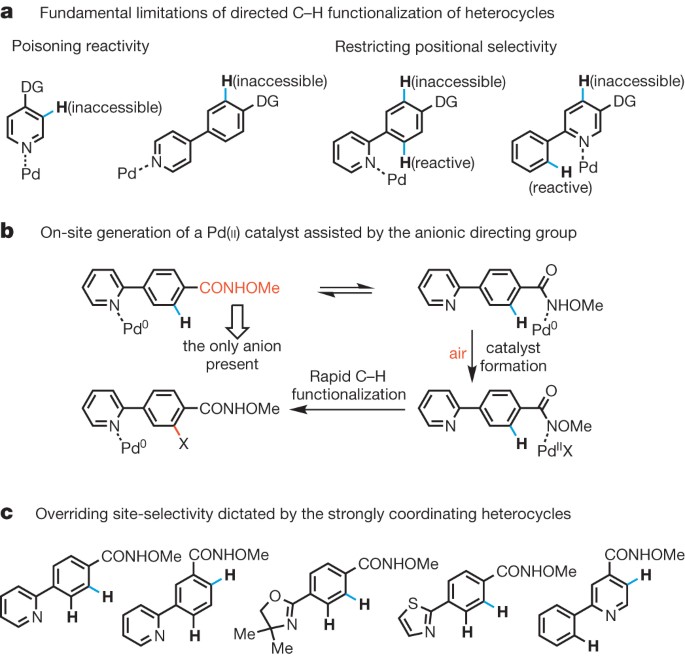
ABSTRACT In directed C–H activation reactions, any nitrogen or sulphur atoms present in heterocyclic substrates will coordinate strongly with metal catalysts. This coordination, which can
lead to catalyst poisoning or C–H functionalization at an undesired position, limits the application of C–H activation reactions in heterocycle-based drug discovery1,2,3,4,5, in which regard
they have attracted much interest from pharmaceutical companies3,4,5. Here we report a robust and synthetically useful method that overcomes the complications associated with performing C–H
functionalization reactions on heterocycles. Our approach employs a simple _N_-methoxy amide group, which serves as both a directing group and an anionic ligand that promotes the _in situ_
generation of the reactive PdX2 (X = ArCONOMe) species from a Pd(0) source using air as the sole oxidant. In this way, the PdX2 species is localized near the target C–H bond, avoiding
interference from any nitrogen or sulphur atoms present in the heterocyclic substrates. This reaction overrides the conventional positional selectivity patterns observed with substrates
containing strongly coordinating heteroatoms, including nitrogen, sulphur and phosphorus. Thus, this operationally simple aerobic reaction demonstrates that it is possible to bypass a
fundamental limitation that has long plagued applications of directed C–H activation in medicinal chemistry. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only
$3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TRANSITION METAL-CATALYSED
DIRECTED C–H FUNCTIONALIZATION WITH NUCLEOPHILES Article 27 October 2022 REMOTE SITE-SELECTIVE ARENE C–H FUNCTIONALIZATION ENABLED BY N-HETEROCYCLIC CARBENE ORGANOCATALYSIS Article 11 July
2024 NICKEL-MEDIATED AEROBIC C(_SP_2)–NUCLEOPHILE COUPLING REACTIONS FOR LATE-STAGE DIVERSIFICATION OF ARYL ELECTROPHILES Article 16 January 2025 REFERENCES * Meanwell, N. A. Improving drug
candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. _Chem. Res. Toxicol._ 24, 1420–1456 (2011) Article CAS Google Scholar
* Ritchie, T. J., Macdonald, S. J. F., Young, R. J. & Pickett, S. D. The impact of aromatic ring count on compound developability: further insights by examining carbo- and
hetero-aromatic and -aliphatic ring types. _Drug Discov. Today_ 16, 164–171 (2011) Article CAS Google Scholar * Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery
and a call for new C–H methylation reactions. _Angew. Chem. Int. Edn Engl._ 52, 12256–12267 (2013) Article Google Scholar * Bryan, M. C. et al. Sustainable practices in medicinal
chemistry: current state and future directions. _J. Med. Chem._ 56, 6007–6021 (2013) Article CAS Google Scholar * Davies, I. W. & Welch, C. J. Looking forward in pharmaceutical
process chemistry. _Science_ 325, 701–704 (2009) Article ADS CAS Google Scholar * Snieckus, V. Directed _ortho_ metalation. Tertiary amide and O-carbamate directors in synthetic
strategies for polysubstituted aromatics. _Chem. Rev._ 90, 879–933 (1990) Article CAS Google Scholar * Kakiuchi, F. et al. Catalytic addition of aromatic carbon–hydrogen bonds to olefins
with the aid of ruthenium complexes. _Bull. Chem. Soc. Jpn_ 68, 62–83 (1995) Article CAS Google Scholar * Jun, C.-H., Hong, J.-B. & Lee, D.-Y. Chelation-assisted hydroacylation.
_Synlett_ 1–12 (1999) * Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. _Chem. Rev._ 110, 624–655 (2010)
Article CAS Google Scholar * Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. _Acc. Chem. Res._ 42, 1074–1086 (2009) Article
CAS Google Scholar * Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. _Chem. Rev._ 110, 1147–1169 (2010) Article CAS Google
Scholar * Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. _Acc. Chem. Res._ 45,
788–802 (2012) Article CAS Google Scholar * Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds.
_Chem. Rev._ 111, 1215–1292 (2011) Article CAS Google Scholar * Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote _meta_-C–H bonds assisted by an end-on template. _Nature_
486, 518–522 (2012) Article ADS CAS Google Scholar * Wasa, M., Worrell, B. T. & Yu, J.-Q. Pd(0)/PR3-catalyzed arylation of nicotinic acid and isonicotinic acid derivatives. _Angew.
Chem. Int. Edn Engl._ 49, 1275–1277 (2010) Article CAS Google Scholar * Ackermann, L. & Lygin, A. V. Ruthenium-catalyzed direct C–H bond arylations of heteroarenes. _Org. Lett._ 13,
3332–3335 (2011) Article CAS Google Scholar * Cho, J.-Y., Iverson, C. N. & Smith, M. R., III Steric and chelate directing effects in aromatic borylation. _J. Am. Chem. Soc._ 122,
12868–12869 (2000) Article CAS Google Scholar * Malik, H. A. et al. Non-directed allylic C–H acetoxylation in the presence of Lewis basic heterocycles. _Chem. Sci._ 5, 2352–2361 (2014)
Article CAS Google Scholar * Takagi, J., Sato, K., Hartwig, J. F., Ishiyama, T. & Miyaura, N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with
bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. _Tetrahedr. Lett._ 43, 5649–5651 (2002) Article CAS Google Scholar * Hurst, T. E. et al. Iridium-catalyzed C–H
activation versus directed _ortho_ metalation: complementary borylation of aromatics and heteroaromatics. _Chemistry_ 16, 8155–8161 (2010) Article CAS Google Scholar * Nakao, Y., Yamada,
Y., Kashihara, N. & Hiyama, T. Selective C-4 alkylation of pyridine by nickel/Lewis acid catalysis. _J. Am. Chem. Soc._ 132, 13666–13668 (2010) Article CAS Google Scholar * Tsai,
C.-C. et al. Bimetallic nickel aluminum mediated _para_-selective alkenylation of pyridine: direct observation of η2, η1-pyridine Ni(0)−Al(III) intermediates prior to C−H bond activation.
_J. Am. Chem. Soc._ 132, 11887–11889 (2010) Article CAS Google Scholar * Kwak, J., Kim, M. & Chang, S. Rh(NHC)-catalyzed direct and selective arylation of quinolines at the
8-position. _J. Am. Chem. Soc._ 133, 3780–3783 (2011) Article CAS Google Scholar * Wencel-Delord, J., Nimphius, C., Wang, H. & Glorius, F. Rhodium(III) and hexabromobenzene — a
catalyst system for the cross-dehydrogenative coupling of simple arenes and heterocycles with arenes bearing directing groups. _Angew. Chem. Int. Edn_ 51, 13001–13005 (2012) Article CAS
Google Scholar * Fu, H. Y., Chen, L. & Doucet, H. Phosphine-free palladium-catalyzed direct arylation of imidazo[1,2-a]pyridines with aryl bromides at low catalyst loading. _J. Org.
Chem._ 77, 4473–4478 (2012) Article CAS Google Scholar * Kuznetsov, A., Onishi, Y., Inamoto, Y. & Gevorgyan, V. Fused heteroaromatic dihydrosiloles: synthesis and double-fold
modification. _Org. Lett._ 15, 2498–2501 (2013) Article CAS Google Scholar * Wang, D.-H., Wasa, M., Giri, R. & Yu, J.-Q. Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and
sp3 boronic acids using air as the oxidant. _J. Am. Chem. Soc._ 130, 7190–7191 (2008) Article CAS Google Scholar * Campbell, A. N. & Stahl, S. S. Overcoming the “oxidant problem”:
strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu). _Acc. Chem. Res._ 45, 851–863 (2012) Article CAS Google Scholar * Lang, S.
Unravelling the labyrinth of palladium-catalysed reactions involving isocyanides. _Chem. Soc. Rev._ 42, 4867–4880 (2013) Article CAS Google Scholar * Ito, Y., Suginome, M., Matsuura, T.
& Murakami, M. Palladium-catalyzed insertion of isocyanides into the silicon-silicon linkages of oligosilanes. _J. Am. Chem. Soc._ 113, 8899–8908 (1991) Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We thank the following for financial support: the Shanghai Institute of Organic Chemistry, the Chinese Academy of Sciences, the CAS/SAFEA International
Partnership Program for Creative Research Teams, the National Natural Science Foundation of China (grant NSFC-21121062), the Recruitment Program of Global Experts, the Scripps Research
Institute and the NIH (NIGMS, 1R01 GM102265). AUTHOR INFORMATION Author notes * Yue-Jin Liu and Hui Xu: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * State Key
Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China , Yue-Jin Liu, Hui Xu, Wei-Jun Kong,
Ming Shang, Hui-Xiong Dai & Jin-Quan Yu * Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Jin-Quan Yu Authors *
Yue-Jin Liu View author publications You can also search for this author inPubMed Google Scholar * Hui Xu View author publications You can also search for this author inPubMed Google Scholar
* Wei-Jun Kong View author publications You can also search for this author inPubMed Google Scholar * Ming Shang View author publications You can also search for this author inPubMed Google
Scholar * Hui-Xiong Dai View author publications You can also search for this author inPubMed Google Scholar * Jin-Quan Yu View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS Y.-J.L. and H.X. performed the reaction discovery experiments and contributed equally. W.-J.K., H.X. and M.S. performed the reactions with the
heterocyclic substrates. H.-X.D. and J.-Q.Y. conceived the concept, directed the project and prepared this manuscript. CORRESPONDING AUTHORS Correspondence to Hui-Xiong Dai or Jin-Quan Yu.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Text,
Supplementary Methods, Supplementary Data and additional references - see Contents for details. (PDF 36081 kb) SUPPLEMENTARY DATA This zipped file contains the 'cif' files for the
X-ray crystallographic data for compounds: complex E, complex F, 3a, and 5i. (ZIP 98 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3
POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, YJ., Xu, H., Kong, WJ. _et al._ Overcoming the limitations of directed
C–H functionalizations of heterocycles. _Nature_ 515, 389–393 (2014). https://doi.org/10.1038/nature13885 Download citation * Received: 18 February 2014 * Accepted: 16 September 2014 *
Published: 10 November 2014 * Issue Date: 20 November 2014 * DOI: https://doi.org/10.1038/nature13885 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Channelnews : hisense tv’s: dumb.. But cheapHisense has unleashed 10 affordable new TV’s, starting from $299 for a 24″ to $1299 for a 55″ LED TV. The new thin LED ...
Today's pickup: digital brokerage app lacking traction, freight markets still volatile(Photo: TruckStockImages) Good day, FreightWaves Chad Prevost is reporting today on the UBS Evidence Lab's finding ...
TJLoading......
October 23, 2009 correspondence to a. Barry randCharles B. Rangel, New York, Chariman Fortney Pete Stark, California Sander M. Levin, Michigan Jim McDermott, Washington...
Unpacking the difference between feminist and women’s movements in africaWomen’s organisations have proliferated across Africa and are networking across the continent on an unprecedented scale ...
Latests News
Overcoming the limitations of directed c–h functionalizations of heterocyclesABSTRACT In directed C–H activation reactions, any nitrogen or sulphur atoms present in heterocyclic substrates will coo...
Harry maguire is mocked but euro 2024 absence robs gareth southgate of his one england constantJason Burt Chief Football Correspondent 07 June 2024 7:03am BST In the ever-evolving England team under Gareth Southgate...
Denise van outen enjoyed ‘ride’ to fame but now fears cancel cultureDenise van Outen, 47, has revealed her thoughts on being a celebrity nowadays, along with the risks of being “cancelled”...
C.H. Robinson profits surge - FreightWavesC.H. Robinson profits surge C.H. Robinson, the Minneapolis-based trucking and multimodal logistics provider, said Tue...
Moorpark : agreement near on teachers' contractMoorpark’s teachers and school district officials announced Thursday that they are close to reaching an agreement on a c...