A toolkit of thread-based microfluidics, sensors, and electronics for 3d tissue embedding for medical diagnostics
A toolkit of thread-based microfluidics, sensors, and electronics for 3d tissue embedding for medical diagnostics"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Threads, traditionally used in the apparel industry, have recently emerged as a promising material for the creation of tissue constructs and biomedical implants for organ
replacement and repair. The wicking property and flexibility of threads also make them promising candidates for the creation of three-dimensional (3D) microfluidic circuits. In this paper,
we report on thread-based microfluidic networks that interface intimately with biological tissues in three dimensions. We have also developed a suite of physical and chemical sensors
integrated with microfluidic networks to monitor physiochemical tissue properties, all made from thread, for direct integration with tissues toward the realization of a thread-based
diagnostic device (TDD) platform. The physical and chemical sensors are fabricated from nanomaterial-infused conductive threads and are connected to electronic circuitry using thread-based
flexible interconnects for readout, signal conditioning, and wireless transmission. To demonstrate the suite of integrated sensors, we utilized TDD platforms to measure strain, as well as
gastric and subcutaneous pH _in vitro_ and _in vivo_. SIMILAR CONTENT BEING VIEWED BY OTHERS HIGH-SPEED, SCANNED LASER STRUCTURING OF MULTI-LAYERED ECO/BIORESORBABLE MATERIALS FOR ADVANCED
ELECTRONIC SYSTEMS Article Open access 31 October 2022 RECENT ADVANCES IN MICROSYSTEM APPROACHES FOR MECHANICAL CHARACTERIZATION OF SOFT BIOLOGICAL TISSUES Article Open access 07 July 2022
MICROFLUIDICS CHIPS FABRICATION TECHNIQUES COMPARISON Article Open access 20 November 2024 INTRODUCTION Implantable diagnostic devices (IDDs) and smart wearable systems (SWSs) that are
capable of _in situ_ sample collection and integration with the complex three-dimensional (3D) structure of biological tissues offer significant opportunities for diagnosing and treating
diseases. Recent technological advances in the miniaturization of sensors and the fabrication of smart materials have progressively changed the landscape of health care by providing the
ability to develop devices that can continuously monitor a patient’s health status1–5. Examples of such devices are electrocardiogram electrodes, temperature sensors, pH sensors, and
flexible batteries that have been used for real-time health monitoring6–12. The development of such devices requires overcoming the challenges associated with the mismatch between the
mechanical and topographical properties of the semiconductor-based electronics and the biological tissues. Flexibility and biocompatibility are other key characteristics needed for devices
in these applications. Materials such as polyimide13 and parylene14 have been extensively used as substrates for IDDs and SWSs. However, device microfabrication on such materials is
expensive because it requires clean room facilities and specialized processing. Paper has emerged as a promising substrate for implantable devices and wearable electronics because of its
universal availability, low cost, environmental friendliness, and ease of fabrication15–17. Several paper-based platforms have been developed recently, such as lateral flow immunoassays for
the detection of analytes18,19, detection of DNA or proteins20, and electrochemical biosensing21,22. Recently, nanofibrous polymeric substrates have been developed to fabricate elastic and
flexible electronics that can be sutured3. The fibrous microstructure of the substrate enables gas and liquid permeation and promotes the ability to pattern metallic electrodes on them.
Although these substrates hold great promise for the creation of wearable and implantable devices, their overall structure and form has essentially remained two-dimensional, limiting their
function to tissue surfaces, such as skin. However, the ability to integrate functional components, such as sensors, actuators, and electronics, in a way that they can penetrate multiple
layers of tissues in a 3D topology would be a significant advance. For example, wound fractures and orthopedic implants, which have complex 3D structures, would greatly benefit from the
implantation of physical (for example, strain) and chemical (for example, pH) sensors that can monitor the local tissue environment and provide valuable information to optimize
patient-specific treatments. IDDs and SWSs can also benefit from a system that enables the delivery of bodily fluids from different parts of the same tissue or from different tissues to the
sensing elements. Such a system for acquiring complex-sensing information distributed in space and time can provide more useful information about the function of an individual organ.
Microfluidic systems are promising platforms that allow the manipulation of minute amounts of liquid (~nl) in small footprints23. The ability to fashion microfluidic networks for
spatio-temporal chemical analysis in a tissue or organ environment in 3D would represent a significant advance. Microfluidic systems with integrated sensors have been widely used to perform
molecular assays on blood samples24, capture circulating tumor cells25, and detect cancer-specific biomarkers26. However, these systems are mainly limited to planar structures and are able
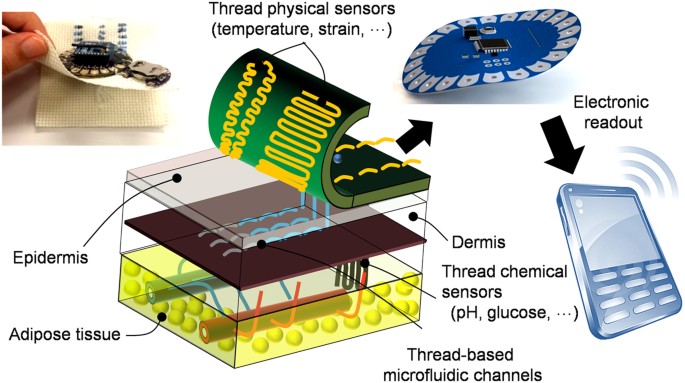
to transport liquid over small distances only. The overarching goal of this paper is to develop a toolkit of miniaturized sensors, electronics and microfluidic networks that addresses these
challenges for the next generation of IDDs and SWSs, using thread as a substrate for a completely thread-based diagnostic device (TDD) platform. Threads are naturally thin and flexible and
can be easily manipulated into complex shapes using well-known textile processing methods. Threads can be derived from natural materials, such as cotton and silk, or can be made using
synthetic biomaterials on a large scale using well-known spinning processes27. The mechanical and degradation properties of threads can be modified by changing the material composition28.
Such properties make threads an excellent choice for the development of IDDs and SWSs. Threads also have the potential for complex 3D physiochemical analysis due to the ability to suture
them in three dimensions through multiple layers of tissue. They can be used as microfluidic channels for injection and delivery of analytes by exploiting the natural capillary action, which
is similar to paper-based microfluidic devices29,30. The ability of a thread to be formed into an arbitrary 3D structure provides natural 3D microfluidic channels that, along with
thread-based sensors and electronics, can provide a 3D analytical platform. From the fabrication standpoint, photolithography, screen-printing, and stamping methods are common approaches for
the implementation of electrodes on planar substrates, which have also been used to realize functional sensing ‘tattoos’ on the body2,5,31. In these methods, the size of the electrodes
depends on the feature size on the photo mask, stencil or shadow mask. When working with threads, the fundamental limit is set by the thread diameter, which has already reached sub-micron
dimensions using conventional threading processes. The micropatterning of such threads using current textile technology, such as sewing, knitting, and embroidering to make functional devices
justifies the scalability of thread-based devices for different sizes and shapes for various applications. Our TDD is based on the use of functional threads as building blocks, which are
inherently flexible and can be fashioned on any flexible substrate or sutured into any biological tissues in an arbitrary 3D geometric form. We propose the fabrication of threads with
different physical, chemical, and biological functions to serve as sensors, microfluidics, and electronics, and to be integrated as a TDD (Figure 1). Hydrophilic threads were embroidered
onto a highly hydrophobic woven fabric to serve as microfluidic channels for the controlled delivery of bodily fluids to the sensing zones. Conductive threads infused with nanomaterials,
such as carbon nanotubes (CNTs), carbon nanopowders, polyaniline (PANI) and their combination, were used as thread-based electrodes for the _in vitro_ and _in vivo_ measurement of glucose,
pH, temperature, and strain as representative physiological properties. Outputs of the sensors were connected to readout electronics on a different layer, which consisted of electronics for
signal processing and wireless communication to a smartphone or a computer using conductive threads as interconnects. Although prior studies regarding thread as a substrate focused
exclusively on one aspect, such as microfluidics or sensors29,32 with a single thread type for _ex vivo_ low-cost diagnostic applications, we demonstrate an integrated TDD platform for
implantable applications with a diverse and greatly expanded toolkit of sensors, electronics, and microfluidic functions, such as custom-designed nano-infused threads as multiple chemical
and physical sensors. MATERIALS AND METHODS Phosphate-buffered saline solution (PBS), hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich, MA, USA. For
preparation of the electrodes, carbon ink (E3456 Graphite Erconinc, MA, USA), silver/silver chloride ink (AGCL-675C, Conductive Compound, Hudson, NH, USA), functionalized CNTs (Sigma
Aldrich), and PANI emeraldine base (Sigma Aldrich) were obtained. Polydimethylsiloxane (PDMS; Sigma Aldrich) and blue insulator (E6165, Erconinc, MA, USA) were utilized as dielectric covers
of the electrodes. DOPING OF THE PANI The PANI was doped based on a previously published procedure33. A PANI base (500 mg) was added to 20 ml of HCl (0.1 M). The solution was mixed for 5 h
at −4 °C inside an ice bath. The PANI-covered thread was kept in a desiccator before usage. CNT INK CNTs functionalized with a carboxylic group were purchased from Sigma Aldrich. The CNTs
were first premixed in IPA (2 mg ml−1) and stirred by a vortex, and then they were homogenized in an ultrasonic bath. SCANNING ELECTRON MICROSCOPY Scanning electron microscopy (SEM) images
were acquired using FESEM ultra55 (12 kV). The microparticles were dried on a paper substrate, mounted on aluminum stubs using conductive carbon paint and sputtered for SEM analysis.
CHARACTERIZATION OF SENSORS Characterization of the strain sensor was performed using a micro Instron 5542 mechanical tester (Norwood, MA, USA) with a 1-kN load cell. The two ends of the
thread with a length of 15 mm were connected to moving stages, and uniform strain was applied, whereas the resistance was measured using a source meter (Keithley source meter 2400). Data
were acquired after the output data were stabilized. The pH- and temperature-sensing measurements were conducted using a customized potentiometric board using off-the-shelf components,
including an LMP91200 as a main front-end amplifier component. For the pH readout, the voltage generated after transmission through an ultralow noise buffer was sampled and digitized by a
microcontroller and then transmitted to an XBee LilyPad or Bluetooth low-energy (BLE) wireless module, and the data were collected in real-time on a smartphone or computer. The BLE package
for cell phone communication is shown in Supplementary Figure S9. Glucose sensing measurements were also performed utilizing a customized printed circuit board with off-the-shelf components,
including a TI LMP91000 as a main component of the low-power potentiostat. The LMP91000 applied a voltage of 0.5 V to the glucose sensor, taking the current from the glucose sensor as input
and generating analog voltage as an output. The output voltage, similar to the pH readout after sampling and digitization using the microcontroller, was sent to a wireless transmitter. _IN
VIVO_ MEASUREMENT AND ANIMAL PROTOCOL The research protocol was approved and in compliance with Tufts University’s Institutional Animal Care and Use Committee (IACUC, protocol #M2013-53) in
accordance with the Office of Laboratory Animal Welfare at the National Institutes of Health. Briefly, animals were anesthetized using 2–3% isoflurane inhalation, and the incision site was
shaved and cleaned with alternating povidone–iodine and ethanol scrubs. For subcutaneous pH measurements, a small incision (<1 cm) was made, and the probes were inserted through the
wound. Alternatively, two 18-G needles were inserted under the skin, and the probes were fed through them, ensuring that the ends of the two probes did not touch each other. For gastric pH
measurement, the probes were fed through two 16-G oral gavage needles (Braintree Scientific, Braintree, MA, USA) and inserted through the mouth into the stomach. Last, for pH measurements of
the blood, the animals were sacrificed via thoracotomy while under anesthesia, and blood samples were taken from the heart and placed into green-top blood collection tubes containing
lithium heparin (Becton Dickinson). RESULTS AND DISCUSSION FABRICATION OF FUNCTIONAL THREADS Physical and chemical sensors, a microfluidic network, and interconnects are three components of
the envisioned 3D TDD platform for intimate tissue integration. Functional threads, such conductive threads, nano-infused threads and hydrophilic threads, serve as building blocks for the
realization of these components. Electrodes, which are the key constituents of any chemical and physical sensors, were fabricated by sequentially passing the core cotton threads through
multiple wells containing conductive inks (Figure 2a). Such a device was previously developed by our group to coat cell-laden hydrogels on suturing threads34. Conductive inks explored in
this work include silver/silver chloride, carbon, CNTs, and PANI prepared as described in the experimental section (Figures 2b–h). A dryer was utilized to cure the coating layer on the
thread whenever needed. In addition, an ultraviolet light was used for sterilization in animal studies. An image of the setup is shown in Supplementary Figure S1, and colorful dyes are added
for better visualization. Meters of functionalized threads were fabricated using the proposed manufacturing method and collected on rotating spools (Figures 2b, c, and f). For strain
sensors, nano-infused threads were made by coating CNT and PDMS layers on elastic threads (polyurethane (PU) threads). The use of PDMS enhanced the mechanical integrity of the conductive
layer and reduced the occurrence of delamination. For pH sensors, the working electrode was made from nano-infused threads coated with carbon and PANI, and the reference electrode was made
from silver/silver chloride (Supplementary Figure S2). PANI was chosen because of its biocompatibility, high electrical conductivity, and superior stability in electrolytes35. Because it has
acid/base-dependent oxidation states, it is an ideal material for pH sensors. Moreover, PANI forms a thin layer with a 3D network of interconnected nanofibrils (Figure 2d) that promotes its
mechanical flexibility and enhances the mechanical integrity of the coated layer. Carbon is selected because it enhances the electrochemical reactivity and promotes electron transfer due to
its unique physical and chemical structures36. Silver/silver chloride is a very common reference electrode for stabilizing the voltage level of the analyte solution. More conductive and
dielectric threads are shown in Supplementary Figure S2. The biocompatibility of the carbon/PANI, silver/silver chloride, and nafion utilized for the fabrication of chemical sensors has
already been demonstrated in prior work37. Physical sensors were covered with layers of PDMS, which is a well-known biocompatible material. Cotton and polyurethane threads have also been
shown to be biocompatible in previous studies38. Moreover, we also performed additional experiments to test the biocompatibility of cotton thread by culturing 3T3 cells (Supplementary Figure
S3). This demonstrated that cotton thread has excellent cell viability even after 7 days. To demonstrate the compatibility of the fabricated conductive threads with current textile
technologies, a programmable embroidering machine (Brother PE-500) was used to pattern conductive threads on a woolen fabric to illuminate a light-emitting diode with a coin cell battery
(Figure 2g). WICKING PROPERTIES OF THREADS Cotton threads commonly used in the apparel industry are often coated with wax and other additives to facilitate gliding and to prevent the thread
from tearing during the textile process39. Despite the thin wax layer on their surface, we noticed that the as-received threads have some wicking properties. This may be because the wax
layer only covers a thin layer on the surface of the thread and does not fill all the gaps between the strands. However, such fibers are not ideal for efficient wicking of liquid using
capillary action. We used plasma cleaning (50 W for 2 min) to remove the wax layer from the fiber strands, rendering them more hydrophilic. Oxygen plasma removes the wax from the thread
surface and adds the –OH bonds on the surface of the thread. As an alternative, if we intended to eliminate capillary action and render the threads hydrophobic, then we dipped the
as-received threads into a commercially available silicone lubricant. The water repellant material covered the surface of the thread and filled the pores between the strands, blocking any
possible liquid flow through them. We embroidered hydrophilic threads on a hydrophobic woven fabric (Figure 2h) to form patterned microfluidic channels for liquid delivery. We confirmed that
the liquid flow was restricted to within the patterned threads as channels and did not wick onto the water-repellent fabric (Supplementary Figure S4f). The wicking property of the
plasma-treated cotton threads was characterized by measuring the speed of the water-filling front (Figures 3a and b). The flow followed the standard Washburn relationship in which the
filling length is a function of the square root of time29,30,40,41 under controlled environmental humidity (relative humidity ~50%). Evaporation has a significant effect on the wicking
properties of the threads41. Evaporation can also affect the concentration of the analytes reaching the sensing zone and may cause errors in the sensing results. However, for implantable
devices, where the microfluidic system is embedded within tissue (which has a less differential saturated vapor pressure and comprises a more water-rich environment) evaporation has less of
an impact. We created a passive three-way microfluidic splitter by embroidering plasma-treated threads on a hydrophobic woven fabric (Figure 3c). Such a microfluidic splitter enables the
delivery of samples to three different sensors. In addition, we showed the ability to fabricate a topologically complex 3D microfluidic system for transporting liquid in three dimensions by
sewing a hydrophilic thread in a polyethylene terephthalate (PET) film (Figures 3d and e). Such a system can be used to deliver different samples on a single platform. To demonstrate the
ability of the thread-based microfluidic system to deliver samples in biologically relevant substrates, a hydrophilic thread was sutured on a chicken skin (Figure 3f). The sample wicked
along the suture without significant leakage due to the presence of a fat layer on the chicken skin. Capillary forces in the thread exist even after the initial thread wetting, which is also
promoted by the diffusion effects. See Supplementary Figure S5 for the fluidic flow after thread saturation by a liquid. Colorful dyes were used for better visualization. Supplementary
Figure S5a shows an optical image of the thread after the addition of two subsequent dyes, and Supplementary Figure S5b shows that the flow rate is reduced by almost half after the thread
gets saturated, but the thread is still viable as a microfluidic channel. According to the Washburn equation, the fluidic flow rate in a known time is a function of the capillary diameter,
viscosity of the liquid, and contact angle of the liquid with the thread. Moreover, diffusion is another factor governing the flow of species and is a function of the concentration gradient
across the thread. The capillary diameter is expected to decrease due to the particle accumulation from the longer duration flow, consequently reducing the flow rate over time. However, in
our case, because we are only interested in facilitating the transfer of small sensing species, such as glucose and hydrogen ions, we believe that such a flow will be sustained over a longer
duration for different biological fluids (blood, serum, urine, and so on). PHYSICAL SENSORS Physical parameters such as pressure, stress, strain, and temperature are important indicators of
tissue health. Here we developed strain and temperature sensors as representative thread-based physical sensors for implantable devices. The basic operation of strain sensors is based on a
variation of electrical parameters, such as resistance because of mechanical deformation. The gauge factor (GF), that is, the relative change in electrical resistance due to a mechanical
strain, the response time, and the maximum detectable strain are critical parameters for the evaluation of strain sensors. A typical GF and maximum strain for conventional strain sensors are
~2 and ~5%, respectively42. Strain sensors were fabricated from PU threads coated with carbon ink and CNTs. Such materials are biocompatible and have been extensively used for the
fabrication of strain sensors12,43,44; they have been suggested as promising candidates due to their high elasticity and sensitivity12,45. PU is a thermoplastic that can be chemically
activated when plasma-treated. We observed that conductive threads made from PU have superior conductivity compared with other types of elastic threads. Carbon ink and CNTs, which were
functionalized with carboxylic groups, bind easily to plasma-treated threads using the method described previously. A thin layer of PDMS was coated on the threads to protect the conductive
layer from scratching and delaminating during cyclic stretching loads45. In addition, the integrity of the system in the sandwich structure and the strong adhesion between CNTs and the two
elastic layers prevented buckling and fracture of CNTs, providing excellent linearity and elasticity. The stretchable conductive threads were then embroidered on a woven construct and
connected to an electronic readout circuitry using silver/silver chloride conductive threads (Figure 4a). Figures 4b and c show SEM images of the uncoated and conductive threads,
respectively. The conductive particles covered most of the surface of the thread and infiltrated its pores, creating interconnected conductive materials. Although some particles are attached
to the outer layer, most of them are attached to the core thread, preventing them from breaking under higher strain. Figure 4d illustrates the sandwich structure of the CNT between the PU
thread and PDMS, confirming the complete coverage of the surface. Moreover, PDMS, as a dielectric material, has an important role in isolating the conductive thread from other wiring in the
system. To evaluate the performance of the strain sensors, they were stretched at a constant rate (0.1 mm min−1) with a tensile machine (Figure 4e). Strain gauges registered linear changes
in electrical resistance when the sensor was stretched. Figures 4f and g show the variation in the relative resistance versus strain for threads coated with carbon ink and CNTs,
respectively. The results indicate that the strain sensors made from CNTs were capable of measuring higher strains (up to 100%, GF~3) compared with those made from carbon ink (strains up to
8%, GF~2). This is because CNTs possess higher deformability due to their fibrous structure12. CNTs also offer faster response times and lower creep12. Another important marker for
monitoring tissue health is temperature, as temperature variations can be indicators of inflammation or bacterial infection46. Moreover, temperature measurement is crucial for precise
sensing in integrated systems as most chemical and physical biosensors are temperature dependent. Nickel and platinum are the best choices for materials among the readily available metals
for resistive temperature sensors. Moreover, they have a linear temperature coefficient of resistance over a long temperature range. However, coating the threads with such materials using
inexpensive methods is not feasible. Here we used CNT-coated threads as resistive temperature sensors. Forty centimeters of thread were embroidered in a meandering zigzag pattern on a woven
fabric. Such a pattern provided an electrical resistance of 100 Ω in a small footprint. Supplementary Figure S6 shows a linear change in resistance for temperatures ranging from 20 to 40 °C,
which is in the biological range of most viable tissues. CHEMICAL SENSORS pH is one of the most important parameters in the body and is an established indicator of health. Almost all
biochemical processes in the body are affected by pH. For example, the pH of a wound is an indication of the wound condition and can be correlated to angiogenesis, protease activity, and
bacterial infection47,48. The healing process occurs more readily in an acidic environment; however, extremely acidic pH values can indicate a bacterial infection47. Thus, monitoring the pH
of the tissue may provide a method for measuring the condition of the wound bed and ultimately enables determination of the wound’s response to treatment. Another application for pH sensing
is in the gastric environment, which is essential for the diagnosis of gastrointestinal diseases, such as inflammatory bowel disease and gastroesophageal reflux disease, or infection from
_Helicobacter pylori_49–51. Here we present a thread-based pH sensor consisting of conductive threads and a microfluidic splitter with three channels for the delivery of a sample to the
sensing chambers (Figures 5a and b). The microfluidic splitter was fabricated by patterning hydrophilic threads on a hydrophobic woven fabric. For pH measurements, a potentiometric approach
was used. In this method, the open-circuit potential of a working electrode was measured with respect to a reference electrode. CNT coated with doped PANI and silver/silver chloride threads
served as the working and reference electrodes, respectively. To evaluate the silver/silver-chloride and doped PANI as electrodes, their conductivity was measured. To perform the experiment,
the cotton threads were covered by silver/silver-chloride and doped PANI via several repetitions of dipping and drying. Then, the impedance was measured between two ends. The result is
shown in Supplementary Figures S8a, b. Conductivity and charge accumulation on the working electrode are dependent on the protonation and deprotonation of the doped PANI under different pH
conditions. The output voltage correlates to the pH value according to the Nernst equation: E = E 0 − K T / e ( p H ) where _E_0 is the standard reduction potential of hydrogen with respect
to the reference electrode silver/silver chloride, _e_ is the electron charge, and _K_ and _T_ are the Boltzmann constant and the temperature, respectively. The pH was measured in various
buffer solutions with pH values ranging from 3 to 8. All the solutions were prepared using hydrochloric acid and sodium hydroxide in PBS52. This range covers the physiological pH that occurs
in the body. Before measurement, each sensor was immersed in purified water to ensure similar starting conditions. The pH of the solution was independently measured using a pH meter
(Benchtop pH/MV Meter—860031). A hydrophilic thread was used to deliver the liquid sample from a buffer solution reservoir to the microfluidic thread channel. The thread was passed through a
chicken skin to mimic the subcutaneous measurements; the results demonstrate the confinement of biological fluids in the thread-based microfluidic channel without significant leakage onto
the chicken fat (Figure 5c and Supplementary Figure S7). Recorded data were sent wirelessly to a desktop computer (Figure 5d). Figure 5e shows the stability of the pH measurement at
different acidic/basic conditions. A rapid response time (<30 s) was achieved as the signal was stabilized in a few seconds after changing the pH. The voltage was found to be linearly
dependent on the pH value with the slope of −59.63 mV per pH, which exhibits near-ideal Nernstian behavior (Figure 5f). To assess the long-term signal stability, the voltage drop was
measured in a solution with a pH of 7.4 for 4 h. A 2.5-mV h−1 drift was observed, indicating the high stability of the fabricated pH sensors (Figure 5g). The concentration of glucose is an
important indicator of diabetes and has to be tightly monitored for the management of this disease. The ability to monitor the glucose level _in vivo_ over a long time would enable the
intensive control of blood glucose concentration in patients with diabetes. In addition, such long-lasting implantable sensors reduce the frequency of implantation and replacement, resulting
in less discomfort for the patients. Measuring glucose using thread-based sensors is promising for long-term monitoring as threads can be implanted in the body with minimum invasiveness.
Here we developed an amperometric glucose sensor consisting of carbon/functionalized CNT threads (working electrode), carbon threads (counter electrode), and silver/silver chloride threads
(reference electrode). Threads were patterned onto a woven fabric as shown in Figure 5h. Glucose oxidase enzyme solution (20 μl, 1 mg ml−1) and 2 ml of nafion (5%) were subsequently added to
the working thread. Nafion was used for immobilization of the enzyme53. Glucose solutions with concentrations in the range of 2–15 mM were prepared in PBS with a pH of 7.4. Potassium
ferricyanide in KCl (100 mM) was added to the solution as a mediator. Chronoamperometry was used for glucose measurements. Briefly, two pulses with voltages of 0.5 and 0 V with a 50% duty
cycle were applied, and the output current was measured. Figure 5i shows the sensor response to different concentrations of the glucose in the solution. Extremely rapid responses (~ms) were
observed, with the amplitude depending linearly on the glucose concentration (Figure 5j). _IN VIVO_ EVALUATION OF THE INTEGRATED THREAD-BASED MICROFLUIDICS, SENSORS, AND ELECTRONICS
INTEGRATED SYSTEM A sensing patch using the thread-based toolkit was developed. It consisted of a thread-based microfluidic system with integrated physical and chemical sensors, as described
earlier, and a separate readout electronics module. The integration of different components was easily achieved through sewing to form a TDD. The readout electronic circuitry consisted of
data acquisition from the sensors, followed by amplification and wireless transmission of the data to a cell phone or computer. A set of off-the-shelf components, including the Texas
Instruments LMP91200 and LMP91000 (Texas Instrument, Dallas, TX, USA), was used for measurement of the pH and strain. For measurement of pH-sensing results, the output voltages of the
electrodes were attached to an ultralow input current bias buffer amplifier. The output analog voltage was read by an Arduino (SparkFun, Niwot, CO, USA) microcontroller with a 1-Hz sampling
frequency. The data were sent to an XBee (SparkFun, Niwot, CO, USA) wireless transmitter. The electronics were implemented by sewing the circuit board on the fabric. Although the board
itself was rigid in this initial prototype, it was small and externally placed to not influence the sensor performance. Moreover, one can easily port the electronics onto a flexible printed
circuit board on a polyimide substrate through commercial vendors or explore emerging paper/textile substrates54–56. _IN VIVO_ MEASUREMENTS The gastric and subcutaneous pH values were
measured as a representative form of chemical sensing _in vivo_. For gastric pH measurements, the thread-based sensor was directly inserted into the stomachs of animals (rats) using oral
gavage needles as guides (Figure 6a). Subcutaneous measurements were performed by implanting the electrodes under the skin (Figure 6c), or by placing them under the skin using needles
(Figure 6d). The results obtained from measuring the pH subcutaneously and in the stomach are shown in Figure 6f. The pH measured under the skin was neutral and stable over 1 min of
measurement (pH=7.0±0.1). As expected, the pH value in the stomach was highly acidic and fluctuated from 1 to 3 over time. The _in vivo_ measurement of the pH was recorded for a short period
of time. It demonstrated the feasibility of the thread-based pH sensors as implantable devices. The characterization of the pH sensor in Figure 5g shows the stability and the drift of the
sensor over time. The performance of the sensor might degrade when used continuously for an _in vivo_ application due to the adsorption of proteins and other molecules. Antifouling-coating
material will be necessary to improve the lifetime of the sensor, but this is not the goal of this study, and an excellent review on the options available can be found elsewhere57. Suture
strain measurements were performed on three wound conditions to mimic the wound closure process during healing: (i) closed; (ii) semi-closed; and (iii) open. For this measurement, a 1-cm
incision was made on the back of the neck. The strain sensor was then passed through the wound and secured with a simple knot on each side. Strain-monitoring equipment was then attached
using alligator clips. For different wound conditions, the wound site was closed manually, and the strain was measured. The recorded data were then transferred to a mobile phone wirelessly
and saved for further analysis. Figure 6h shows the typical values measured by this system for the wound closure model. Note that the strain of the wound was created manually; therefore, the
indicated values in the _x_ axes are exact numbers, matching the value taken from the calibration plot of the strain sensor. CONCLUSION We have developed a toolkit of thread-based devices
to measure physical (strain and temperature) and chemical (pH and glucose) markers in an integrated TDD platform. _In vivo_ functionality of the system was evaluated by measurement of pH and
strain in different parts of the body. The primary benefit is that threads are low in cost and are biocompatible, having been widely used in the apparel industry. The electrical and surface
properties of the threads were tailored to transport fluids using capillary action and were infused with nanomaterials to perform electrochemical sensing using an inexpensive dipping
approach. The performances of sensors were evaluated and optimized individually. The integrated system was used to measure pH and strain _in vitro_ and _in vivo_. Our research suggests that
TDD has the potential to act as a part of human skin or clothing and to even be implanted. The ability to suture TDD intimately into a tissue or organ in three dimensions adds a unique
feature that is not available with other flexible diagnostic platforms. We believe that such a TDD could eventually find a wide range of applications, such as smart sutures for surgical
implants, smart bandages to monitor wound healing, integration with textile or fabric as personalized health monitors and point-of-care diagnostics, and embedding into engineered tissue
constructs for organ-on-a-chip platforms. We envision being able to extend the approach to more than the strain, pH, or glucose sensors mentioned here by functionalizing them with sensing
chemistries to measure proteins, DNA and other biomarkers directly in the tissues where they are implanted. Although we demonstrated thread-based interconnects, future efforts could be in
the area of integrating other electronic components, such as capacitors, diodes, and transistors, on threads, which will result in a truly self-contained integrated platform with unmatched
size, flexibility, and maneuverability. For thread-based devices to be used as long-term implantable devices, additional studies on the biocompatibility of the proposed cotton-based threads
and their functionalized forms may be required. Such studies may indicate that threads elicit an immune response when used long term, which would necessitate exploration of other
biocompatible materials for thread creation that have a more favorable immune response. REFERENCES * Hammock ML, Chortos A, Tee BCK et al. 25th Anniversary Article: The evolution of
electronic skin (E-Skin): A brief history, design considerations, and recent progress. _Advanced Materials_ 2013; 25: 5997–6038. Article Google Scholar * Kim DH, Lu N, Ma R et al.
Epidermal electronics. _Science_ 2011; 333: 838–843. Article Google Scholar * Najafabadi AH, Tamayol A, Annabi N et al. Biodegradable nanofibrous polymeric substrates for generating
elastic and flexible electronics. _Advanced Materials_ 2014; 26: 5823–5830. Article Google Scholar * Sun J-Y, Keplinger C, Whitesides GM et al. Ionic skin. _Advanced Materials_ 2014; 26:
7608–7614. Article Google Scholar * Windmiller JR, Wang J . Wearable electrochemical sensors and biosensors: A review. _Electroanalysis_ 2013; 25: 29–46. Article Google Scholar *
Guinovart T, Valdés-Ramírez G, Windmiller JR et al. Bandage-based wearable potentiometric sensor for monitoring wound pH. _Electroanalysis_ 2014; 26: 1345–1353. Article Google Scholar *
Kim DH, Song J, Choi WM et al. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. _Proceedings of the National
Academy of Sciences of the United States of America_ 2008; 105: 18675–18680. Article Google Scholar * Kim DH, Ghaffari R, Lu N et al. Electronic sensor and actuator webs for large-area
complex geometry cardiac mapping and therapy. _Proceedings of the National Academy of Sciences of the United States of America_ 2012; 109: 19910–19915. Article Google Scholar * Kim DH, Lu
N, Ghaffari R et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. _Nature Materials_ 2011; 10: 316–323.
Article Google Scholar * Mostafalu P, Sonkusale S . A high-density nanowire electrode on paper for biomedical applications. _RSC Advances_ 2015; 5: 8680–8687. Article Google Scholar * Xu
S, Zhang Y, Cho J et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. _Nature Communications_ 2013; 4: 1543. Article Google
Scholar * Yamada T, Hayamizu Y, Yamamoto Y et al. A stretchable carbon nanotube strain sensor for human-motion detection. _Nature Nanotechnology_ 2011; 6: 296–301. Article Google Scholar
* Viventi J, Kim DH, Vigeland L et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity _in vivo_. _Nature Neuroscience_ 2011; 14: 1599–1605.
Article Google Scholar * Mostafalu P, Sonkusale S . Flexible and transparent gastric battery: Energy harvesting from gastric acid for endoscopy application. _Biosensors and
Bioelectronics_ 2014; 54: 292–296. Article Google Scholar * Carrilho E, Martinez AW, Whitesides GM . Understanding wax printing: A simple micropatterning process for paper-based
microfluidics. _Analytical Chemistry_ 2009; 81: 7091–7095. Article Google Scholar * Li X, Tian J, Nguyen T et al. Paper-based microfluidic devices by plasma treatment. _Analytical
Chemistry_ 2008; 80: 9131–9134. Article Google Scholar * Martinez AW, Phillips ST, Wiley BJ et al. FLASH: A rapid method for prototyping paper-based microfluidic devices. _Lab on a Chip_
2008; 8: 2146–2150. Article Google Scholar * Liu H, Li X, Crooks RM . Paper-based slippad for high-throughput chemical sensing. _Analytical Chemistry_ 2013; 85: 4263–4267. Article Google
Scholar * Martinez AW, Phillips ST, Nie Z et al. Programmable diagnostic devices made from paper and tape. _Lab on a Chip_ 2010; 10: 2499–2504. Article Google Scholar * Scida K, Li B,
Ellington AD et al. DNA detection using origami paper analytical devices. _Analytical Chemistry_ 2013; 85: 9713–9720. Article Google Scholar * Dungchai W, Chailapakul O, Henry CS .
Electrochemical detection for paper-based microfluidics. _Analytical Chemistry_ 2009; 81: 5821–5826. Article Google Scholar * Russo A, Ahn BY, Adams JJ et al. Pen‐on‐paper flexible
electronics. _Advanced Materials_ 2011; 23: 3426–3430. Article Google Scholar * Whitesides GM . The origins and the future of microfluidics. _Nature_ 2006; 442: 368–373. Article Google
Scholar * Sackmann EK-H, Berthier E, Schwantes EA et al. Characterizing asthma from a drop of blood using neutrophil chemotaxis. _Proceedings of the National Academy of Sciences of the
United States of America_ 2014; 111: 5813–5818. Article Google Scholar * Murlidhar V, Zeinali M, Grabauskiene S et al. A radial flow microfluidic device for ultra-high-throughput
affinity-based isolation of circulating tumor cells. _Small_ 2014; 10: 4895–4904. Article Google Scholar * Perozziello G, Candeloro P, Gentile F et al. Microfluidics & nanotechnology:
Towards fully integrated analytical devices for the detection of cancer biomarkers. _RSC Advances_ 2014; 4: 55590–55598. Article Google Scholar * Tamayol A, Akbari M, Annabi N et al.
Fiber-based tissue engineering: Progress, challenges, and opportunities. _Biotechnology Advances_ 2013; 31: 669–687. Article Google Scholar * Lendlein A, Langer R . Biodegradable, elastic
shape-memory polymers for potential biomedical applications. _Science_ 2002; 296: 1673–1676. Article Google Scholar * Li X, Tian J, Shen W . Thread as a versatile material for low-cost
microfluidic diagnostics. _ACS Applied Materials & Interfaces_ 2009; 2: 1–6. Article Google Scholar * Reches M, Mirica KA, Dasgupta R et al. Thread as a matrix for biomedical assays.
_ACS Applied Materials & Interfaces_ 2010; 2: 1722–1728. Article Google Scholar * Windmiller JR, Bandodkar AJ, Valdes-Ramirez G et al. Electrochemical sensing based on printable
temporary transfer tattoos. _Chemical Communications_ 2012; 48: 6794–6796. Article Google Scholar * Huang C-T, Shen C-L, Tang C-F et al. A wearable yarn-based piezo-resistive sensor.
_Sensors and Actuators A: Physical_ 2008; 141: 396–403. Article Google Scholar * Li J, Tang X, Li H et al. Synthesis and thermoelectric properties of hydrochloric acid-doped polyaniline.
_Synthetic Metals_ 2010; 160: 1153–1158. Article Google Scholar * Akbari M, Tamayol A, Laforte V et al. Composite living fibers for creating tissue constructs using textile techniques.
_Advanced Functional Materials_ 2014; 24: 4060–4067. Article Google Scholar * Liao C, Zhang M, Yao MY et al. Flexible organic electronics in biology: Materials and devices. _Advanced
Materials_ 2015; 27: 7493–7527. Article Google Scholar * Wang J . Carbon-nanotube based electrochemical biosensors: A review. _Electroanalysis_ 2005; 17: 7–14. Article Google Scholar *
Rahimi R, Ochoa M, Parupudi T et al. A low-cost flexible pH sensor array for wound assessment. _Sensors and Actuators B: Chemical_ 2016; 229: 609–617. Article Google Scholar * Akbari M,
Tamayol A, Bagherifard S et al. Textile technologies and tissue engineering: A path toward organ weaving. _Advanced Healthcare Materials_ 2016; 5: 751–766. Article Google Scholar * Karahan
HA, Özdoğan E . Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. _Fibers and Polymers_ 2008; 9: 21–26. Article Google Scholar * Nilghaz A, Wicaksono
DHB, Gustiono D et al. Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. _Lab on a Chip_ 2012; 12: 209–218. Article Google Scholar * Safavieh
R, Zhou GZ, Juncker D . Microfluidics made of yarns and knots: From fundamental properties to simple networks and operations. _Lab on a Chip_ 2011; 11: 2618–2624. Article Google Scholar *
Lu N, Lu C, Yang S et al. Highly sensitive skin-mountable strain gauges based entirely on elastomers. _Advanced Functional Materials_ 2012; 22: 4044–4050. Article Google Scholar * Lee D,
Hong HP, Lee MJ et al. A prototype high sensitivity load cell using single walled carbon nanotube strain gauges. _Sensors and Actuators A: Physical_ 2012; 180: 120–126. Article Google
Scholar * Li X, Zhang R, Yu W et al. Stretchable and highly sensitive graphene-on-polymer strain sensors. _Scientific Reports_ 2012; 2: 870. Article Google Scholar * Amjadi M,
Pichitpajongkit A, Lee S et al. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. _ACS Nano_ 2014; 8: 5154–5163. Article Google Scholar *
Collins A, Cosh J . Temperature and biochemical studies of joint inflammation. A preliminary investigation. _Annals of the Rheumatic Diseases_ 1970; 29: 386. Article Google Scholar *
Gethin G . The significance of surface pH in chronic wounds. _Wounds_ 2007; 3: 52. Google Scholar * Schneider LA, Korber A, Grabbe S et al. Influence of pH on wound-healing: A new
perspective for wound-therapy? _Archives of Dermatological Research_ 2007; 298: 413–420. Article Google Scholar * Klauser A, Schindlbeck N, Müller-Lissner S . Symptoms in
gastro-oesophageal reflux disease. _The Lancet_ 1990; 335: 205–208. Article Google Scholar * Podolsky DK . Inflammatory bowel disease. _New England Journal of Medicine_ 1991; 325: 928–937.
Article Google Scholar * Verdu E, Armstrong D, Fraser R et al. Effect of Helicobacter pylori status on intragastric pH during treatment with omeprazole. _Gut_ 1995; 36: 539–543. Article
Google Scholar * Chung HJ, Sulkin MS, Kim JS et al. Stretchable, multiplexed pH sensors with demonstrations on rabbit and human hearts undergoing ischemia. _Advanced Healthcare Materials_
2014; 3: 59–68. Article Google Scholar * Ammam M, Easton EB . High-performance glucose sensor based on glucose oxidase encapsulated in new synthesized platinum nanoparticles supported on
carbon Vulcan/Nafion composite deposited on glassy carbon. _Sensors and Actuators B: Chemical_ 2011; 155: 340–346. Article Google Scholar * Jain K, Klosner M, Zemel M et al. Flexible
electronics and displays: high-resolution, roll-to-roll, projection lithography and photoablation processing technologies for high-throughput production. _Proceedings of the IEEE_ 2005; 93:
1500–1510. Article Google Scholar * Mostafalu P, Dokmeci MR, Ziaie B et al. Fully integrated oxygen sensor with four larger printed circuit electronics on paper. 18th International
Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2014); 26–30 Oct, 2014; San Antonio, TX, USA; 2014: 2128–2130. * Siegel AC, Phillips ST, Dickey MD et al.
Foldable printed circuit boards on paper substrates. _Advanced Functional Materials_ 2010; 20: 28. Article Google Scholar * Frost MC, Meyerhoff ME . Implantable chemical sensors for
real-time clinical monitoring: progress and challenges. _Current Opinion in Chemical Biology_ 2002; 6: 633–641. Article Google Scholar Download references ACKNOWLEDGEMENTS The National
Science Foundation partially funded this project under grant EFRI-1240443. SEM images were acquired at Harvard University Center for Nanoscale System. We also acknowledge Sara Mostafalu for
her help with embroidering and experiments. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Electrical and Computer Engineering, Nano Lab, Tufts University, Medford, 02155, MA,
USA Pooria Mostafalu & Sameer R. Sonkusale * Division of Biomedical Engineering, Department of Medicine, Biomaterials Innovation Research Center, Brigham and Women’s Hospital, Harvard
Medical School, Cambridge, 02139, MA, USA Mohsen Akbari & Ali Khademhosseini * Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge,
02139, MA, USA Mohsen Akbari & Ali Khademhosseini * Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, 02115, MA, USA Mohsen Akbari & Ali
Khademhosseini * Department of Mechanical Engineering, Laboratory for Innovation in MicroEngineering (LiME), University of Victoria, Victoria, BC V8P 2C5, USA Mohsen Akbari * Department of
Biomedical Engineering, Tufts University, Medford, 02155, MA, USA Kyle A. Alberti & Qiaobing Xu * Department of Physics, King Abdulaziz University, Jeddah, 21589, Saudi Arabia Ali
Khademhosseini Authors * Pooria Mostafalu View author publications You can also search for this author inPubMed Google Scholar * Mohsen Akbari View author publications You can also search
for this author inPubMed Google Scholar * Kyle A. Alberti View author publications You can also search for this author inPubMed Google Scholar * Qiaobing Xu View author publications You can
also search for this author inPubMed Google Scholar * Ali Khademhosseini View author publications You can also search for this author inPubMed Google Scholar * Sameer R. Sonkusale View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Sameer R. Sonkusale. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information for this article can be found on the _Microsystems & Nanoengineering_ website
(http://www.nature.com/micronano). SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 475 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0
International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the
material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mostafalu, P., Akbari, M., Alberti, K. _et al._ A toolkit of thread-based
microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics. _Microsyst Nanoeng_ 2, 16039 (2016). https://doi.org/10.1038/micronano.2016.39 Download citation *
Received: 02 March 2016 * Revised: 27 April 2016 * Accepted: 28 April 2016 * Published: 18 July 2016 * DOI: https://doi.org/10.1038/micronano.2016.39 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
Trending News
How to create a rest api — spring boot and ballerinaToday, there are numerous programming languages and frameworks for creating REST APIs and Microservices. Among such fram...
Italy told to expect ‘severe turbulence’ with trump ally meloni as pmGIORGIA MELONI SAYS THE EU HAS AN 'INADEQUACY TO RESPOND' Dr Marina Cino Pagliarello said Ms Meloni, leader of...
The lunch box latest news in hindi, photos, videos on the lunch box inextlive jagranब्रिटिश फिल्म अवॉर्ड्स के नॉमिनेशन में पहुंची इरफान की 'द लंचबॉक्स' i-exclusive10 years ago इरफान खान और निम...
Ventura : burning room puts sprinklers to testThe Ventura City Fire Department put people in a burning living room Tuesday to promote a proposed city ordinance requir...
Admissions experts unsurprised by elimination of sat subject tests and essay | news | the harvard crimsonSome admissions officers and college counselors said they were unsurprised by College Board’s decision to discontinue th...
Latests News
A toolkit of thread-based microfluidics, sensors, and electronics for 3d tissue embedding for medical diagnosticsABSTRACT Threads, traditionally used in the apparel industry, have recently emerged as a promising material for the crea...
The lunch box latest news in hindi, photos, videos on the lunch box inextlive jagranब्रिटिश फिल्म अवॉर्ड्स के नॉमिनेशन में पहुंची इरफान की 'द लंचबॉक्स' i-exclusive10 years ago इरफान खान और निम...
Ventura : burning room puts sprinklers to testThe Ventura City Fire Department put people in a burning living room Tuesday to promote a proposed city ordinance requir...
Admissions experts unsurprised by elimination of sat subject tests and essay | news | the harvard crimsonSome admissions officers and college counselors said they were unsurprised by College Board’s decision to discontinue th...
Researchers are hatching a low-cost coronavirus vaccine (published 2021)A new formulation entering clinical trials in Brazil, Mexico, Thailand and Vietnam could change how the world fights the...