Fundamental role for hif-1α in constitutive expression of human β defensin-1
Fundamental role for hif-1α in constitutive expression of human β defensin-1"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Antimicrobial peptides are secreted by the intestinal epithelium to defend from microbial threats. The role of human β defensin-1 (hBD-1) is notable because its gene (beta-defensin
1 (_DEFB1_)) is constitutively expressed and its antimicrobial activity is potentiated in the low-oxygen environment that characterizes the intestinal mucosa. Hypoxia-inducible factor (HIF)
is stabilized even in healthy intestinal mucosa, and we identified that epithelial HIF-1α maintains expression of murine defensins. Extension to a human model revealed that basal HIF-1α is
critical for the constitutive expression of hBD-1. Chromatin immunoprecipitation identified HIF-1α binding to a hypoxia response element in the _DEFB1_ promoter whose importance was
confirmed by site-directed mutagenesis. We used 94 human intestinal samples to identify a strong expression correlation between _DEFB1_ and the canonical HIF-1α target _GLUT1_. These
findings indicate that basal HIF-1α is critical for constitutive expression of enteric _DEFB1_ and support targeting epithelial HIF for restoration and maintenance of intestinal integrity.
SIMILAR CONTENT BEING VIEWED BY OTHERS BILE ACID-DEPENDENT TRANSCRIPTION FACTORS AND CHROMATIN ACCESSIBILITY DETERMINE REGIONAL HETEROGENEITY OF INTESTINAL ANTIMICROBIAL PEPTIDES Article
Open access 22 August 2023 IDENTIFICATION OF HUMAN HOST FACTORS REQUIRED FOR BETA-DEFENSIN-2 EXPRESSION IN INTESTINAL EPITHELIAL CELLS UPON A BACTERIAL CHALLENGE Article Open access 04 July
2024 _SHIGELLA_ INFECTION IS FACILITATED BY INTERACTION OF HUMAN ENTERIC Α-DEFENSIN 5 WITH COLONIC EPITHELIAL RECEPTOR P2Y11 Article 03 February 2025 INTRODUCTION The intestinal epithelium
lines the entire gastrointestinal tract, covering a surface area of approximately 300 m2 in the adult human and forming an essential barrier to the outside world. This single layer of
epithelium must also defend this expanded interface from invasion by luminal microbiota. The anatomy of the intestine provides a fascinating metabolic profile. For example, it is well
documented that a steep oxygen gradient exists from the anaerobic lumen of the intestine across the epithelium into the highly vascularized sub-epithelium. From this perspective, it is
perhaps not surprising that the epithelium has evolved a number of features to cope with this metabolic setting. In fact, studies comparing functional responses between epithelial cells from
different tissues have revealed that intestinal epithelial cells seem to be uniquely resistant to hypoxia and that an extremely low level of oxygenation within the normal intestinal
epithelial barrier (so-called “physiological hypoxia”) may be a regulatory adaptation mechanism to the steep oxygen gradient.1 As a result, the intestinal epithelium has evolved coping
mechanisms that include basal regulation of hypoxia-inducible factor (HIF), which is central for aspects of physiology and immunity. For example, iron absorption is dependent on multiple
HIF-2α target genes.2 Likewise, epithelial HIF-1α regulates the multidrug resistance gene,3 mucin-3,4 and the intestinal trefoil factor gene5 important in maintenance of epithelial barrier
function. Antimicrobial peptides constitute a substantial part of the mucosal barrier. β-Defensins are the dominant class of antimicrobial peptide secreted by the epithelium. The four
well-characterized human β defensins (hBD-1–4, encoded by beta-defensin 1 (_DEFB1_), _DEFB4_, _DEFB103_, and _DEFB104_) are small (30–47 amino acid), cationic, cysteine-rich peptides that
possess broad antimicrobial activity.6, 7 In the gut, hBD-1 has two characteristics that confer prominence. First, its antimicrobial activity is potentiated under reducing conditions that
exist in the hypoxic gut lumen, whereas other antimicrobial peptides, such as hBD-3, are diminished in the reduced state.8 Second, expression of hBD-1 is constitutive, whereas other
defensins are expressed in response to microbial and inflammatory stimuli.9, 10, 11 Given these properties, it is not surprising that defective expression of hBD-1 is associated with mucosal
diseases, such as inflammatory bowel disease,12, 13, 14 candidia carriage,15 periodontitis,16 and dental carries.17 Despite the homeostatic role of HIF in maintaining other aspects of the
gut barrier, defensin expression has not previously been linked to HIF signaling. In the present study, we report that HIF-1α selectively regulates basal murine and human defensin
expression. This work identified and characterized a critical role for basal HIF-1α in _DEFB1_ expression that is dependent on a hypoxia response element (HRE) consensus sequence in the
_DEFB1_ promoter. Because species-specific expression of _DEFB1_ precludes _in vivo_ models, we used human intestinal samples to correlate the expression between _DEFB1_ and a canonical
HIF-1α target gene. These findings are the first to link HIF to defensin expression and illuminate a mechanism for constitutive _DEFB1_ expression that provide rationale for targeting
epithelial HIF for therapeutic treatment of mucosal disease. RESULTS BASAL REGULATION OF DEFENSINS BY HIF IN MURINE COLONIC EPITHELIA Initially, we confirmed previous observations of
“physiological hypoxia” in the murine colon.1 To visualize regions of low pO2 in the colonic mucosa in healthy mice (C57BL/6), we utilized HypoxyProbe-1, a pimonidazole HCl adduct-forming
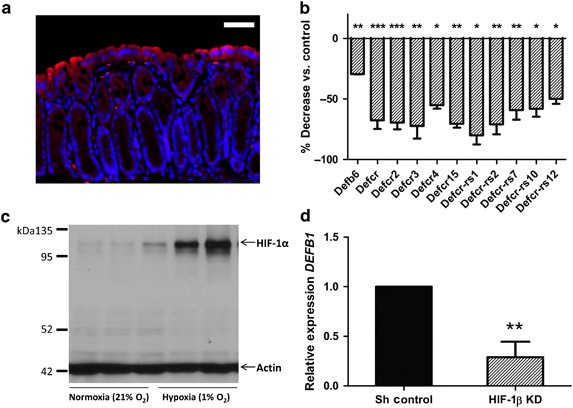
hypoxia marker that enables fluorescent antibody–mediated visualization of tissues experiencing pO2⩽10 mm Hg. As shown in Figure 1a, and consistent with previous observations,18 we observed
prominent localization of the pimonidazole adducts in the brush-border epithelial cells that line the colonic mucosa, with graded decreases toward the base of the crypt. From such
observations, we sought to determine how basal HIF signaling influences the transcriptional profile of the intestinal epithelium. To do this, we performed gene expression arrays using
epithelial-rich mucosal scrapings from mice with a targeted intestinal epithelial _Hif1a_ knock out. This analysis revealed a cluster of 11 defensin-related genes (murine β defensin-6,
various defensin cryptdins, and cryptdin-related sequences) that exhibited reduced expression with epithelial _Hif1a_ knock out (Figure 1b). SELECTIVE REGULATION OF HUMAN _DEFB1_ BY HIF-1Α
IN HUMAN INTESTINAL EPITHELIA Similar to “physiological hypoxia” _in vivo_, basal HIF-1α is detectable in Caco-2 cells cultured in normoxic conditions (Figure 1c). To determine whether basal
HIF contributes to human defensin expression, we generated Caco-2 intestinal epithelial cells with lentiviral knock down of HIF-1β (encoded by _ARNT_), which serves as the binding partner
of HIF-1α and HIF-2α required for the transcriptionally active heterodimer. We then used real-time PCR to screen expression of hBDs in these cells. Although some targets were unchanged
(_DEFB103_ and _DEFB104_) or undetectable (_DEFB4_), _ARNT_ knock down significantly reduced expression of human _DEFB1_ (Figure 1d). To determine the specificity of _DEFB1_ regulation by
HIF, we used lentiviral knockdown to target either HIF-1α or HIF-2α in Caco-2 as previously published19 and T84 intestinal epithelial cell lines with confirmation of knock down shown in
Figure 2a. This analysis revealed that _DEFB1_ is regulated selectively by HIF-1α in both cell lines (Figure 2b,c). Interestingly, exposure to 1% O2 for 2, 4, or 8 h did not influence
_DEFB1_ expression in wild-type or knockdown cells, suggesting that such regulation is basally controlled. Co-transfection of a plasmid encoding stabilized HIF-1α or HIF-2α, despite marked
stimulation of a HRE-containing promoter control (Figure 2d), did not increase DEFB1 promoter activity (Figure 2e). To define whether basal HIF-1α activity accounted for the maintenance of
_DEFB1_ expression, we reduced basal activity of HIF and examined _DEFB1_ expression. To reduce baseline HIF activity, we supplemented cell media with ascorbate (1 mM), a cofactor for the
oxygen-dependent hydroxylation of HIF by prolyl-hydroxylase enzymes that initiate degradation.20 Culture of wild-type Caco-2 cells in supplemented media for 24 h had no effect on nuclear
factor-κB p65, indicating a degree of specificity for ascorbate-dependent pathways (Figure 2f). Supplementation reduced cytoplasmic and nuclear HIF-1α by ∼25% (Figure 2g) and decreased
_DEFB1_ expression >40% (Figure 2h), suggesting at least some role for basal HIF-1α in _DEFB1_ expression. INFLUENCE OF HIF-1Α ON HBD-1 PROTEIN EXPRESSION We next used immunofluorescence
staining to determine whether lentiviral-mediated HIF-1α knock down influenced hBD-1 protein expression. As shown in Figure 3a, hBD-1 was expressed diffusely in the cytoplasm of both Caco-2
and T84 cells. Prominent decreases in hBD-1 were noted in epithelial cells lacking HIF-1α relative to either short hairpin controls or epithelial cells lacking HIF-2α (Figure 3a). Likewise,
examination of hBD-1 secreted into cell supernatants by enzyme-linked immunosorbent assay revealed a 48±6% decrease in hBD-1 secreted by HIF-1α knockdown cells relative to short hairpin
control and HIF-2α knockdown cells (_P_<0.01, Figure 3b). To define the functional relevance of a 50% decrease in hBD-1 on bacterial survival, we developed a killing assay with
_Escherichia coli_ Nissle 1917. This bacterium was selected because it is sensitive to oxidized recombinant hBD-1 and it is resistant to low osmolarity to which it was subject during
experimental incubation with salt-sensitive hBD-1.21 As shown in Figure 3c, increasing concentrations of recombinant hBD-1 (rhBD-1; range 0–8 μg ml−1) revealed marked increase in _E. coli_
killing by rhBD-1 at concentrations >2 μg ml−1. These results indicate that at such concentrations, a 50% decrease in hBD-1 expression could increase the _E. coli_ burden by several
orders of magnitude (Figure 3c). MOLECULAR MECHANISM OF REGULATION OF _DEFB1_ BY HIF-1Α Analysis of the proximal 1.5 kb of the _DEFB1_ promoter revealed a single HRE consensus sequence at
position −463 relative to the transcription start site. We used chromatin immunoprecipitation with Caco-2 cells cultured in normoxic conditions to evaluate binding of HIF-1α to this HRE. As
shown n Figure 4a, PCR amplification with HRE-spanning primers demonstrated a 7.8±1.4-fold enrichment over immunoglobulin G (IgG) control following precipitation of sheared DNA with
anti-HIF-1α. To experimentally demonstrate the relevance of this HRE, we mutated three bases of the core HRE sequence (depicted in Figure 4b) in a _DEFB1_ luciferase reporter plasmid. We
transfected the wild-type and HRE mutant plasmid into Caco-2 cells and observed a 43±13% decrease in luciferase activity in the mutant plasmid (Figure 4b). Taken together, these studies
demonstrate that direct HIF-1α binding to the _DEFB1_ HRE contributes to the maintenance of _DEFB1_ expression in intestinal epithelial cells. CORRELATION OF _DEFB1_ EXPRESSION WITH
CANONICAL HIF-1Α TARGET GENE IN HUMAN TISSUE There are several challenges to studying _DEFB1_ regulation by HIF-1α _in vivo_. First, model organisms, including mice, do not express hBD-1.
Amino-acid sequence homology is only 53% comparing mouse with hBD-1, and there is also dissimilarity in promoter sequences.22, 23 Second, because HIF-1α is primarily regulated through
post-translational, oxygen-dependent degradation, changes in RNA expression do not reflect cellular protein. Therefore, to test the hypothesis that _DEFB1_ is a HIF-1α target in human
tissue, we used real-time PCR to correlate expression of _DEFB1_ with a canonical HIF-1α target gene, _GLUT1_.24 Human intestinal samples were obtained from the TissueScan quantitative PCR
gene expression arrays containing cDNA from normal and inflamed human intestinal tissues obtained during surgical resection and biopsy. PCR amplification of both _DEFB1_ and _GLUT1_ was
detected in 94 of 95 unique patient samples on the arrays. Correlation analysis revealed a significant association between _DEFB1_ and _GLUT 1_ expression in these 94 samples (_P_<0.0001,
Figure 5a). _DEFB1_ and _GLUT1_ expression are reported to be greater in the large intestine than in the small intestine.25, 26 This raises the possibility that combining samples from both
the locations might confound the correlation. Therefore, we correlated samples from the small intestine and the large intestine independently and found that the correlation was not
influenced by this distinction (_P_<0.0001, Figure 5a). We preformed similar analyses with samples from patients with no disease, Crohn’s disease, and ulcerative colitis separately and
found a positive correlation independent of disease status (_P_<0.0001, Figure 5b). Finally, to ensure that the association between _DEFB1_ and _GLUT1_ was not an artifact based on
varying epithelial content of the samples, we analyzed expression as a function of mucosal fraction. Pathologist reports that accompanied 47 of these samples provide information on the
percentage of mucosa, submucosa, and muscle of each specimen. No correlation was found between the percentage of mucosa and _DEFB1_ or _GLUT1_, indicating that variation in sample
composition cannot explain the correlation of these genes (Figure 5c). Taken together, such findings strongly implicate a common mechanism of regulation for _DEFB1_ and _GLUT1_ in the
samples tested here. Finally, immunofluorescence staining of hBD-1 in human colonic biopsies revealed positive staining within the epithelium, with greatest intensity in the epithelium
adjacent to the lumen and less intensity within the crypt (Figure 5d). This staining pattern mirrors the low pO2 microenvironment of the colon _in vivo_ as revealed by oxygen-sensitive
pimonidazole staining (Figure 1a). DISCUSSION The functional anatomy of the intestinal mucosa provides a particularly interesting oxygenation profile, wherein even under physiological
conditions, the intestinal mucosa experiences profound fluctuations in blood flow, oxygenation, and metabolism.27 Studies comparing functional responses between epithelial cells from
different tissues, for example, have revealed that intestinal epithelial cells appear to be uniquely resistant to even severe hypoxia and that low pO2 within the normal intestinal epithelial
barrier may be a regulatory adaptation mechanism to the steep oxygen gradient.5 Thus, both the absorptive and barrier properties of the intestinal epithelium are regulated by the
availability of O2 in both health and disease.27 Here, we describe a prominent physiological role for low pO2 in the basal regulation of _DEFB1_ by HIF-1α. Oxygen-sensitive staining of the
mouse colon enabled us to visualize a distinct layer of tissue subjected to low pO2 affecting the colonic epithelium (Figure 1a). This finding confirms previous work18 and underlies our
interest in the role of baseline hypoxia and resulting HIF on epithelial immunity. Utilizing an established mouse model wherein intestinal epithelial cells lacked _Hif1a_ expression,18 our
expression array identified a provocative cluster of 11 defensin-related genes that were significantly abrogated with loss of epithelial HIF-1α expression. Characterization of these animals
have found them more susceptible to experimental colitis, likely related to multiple defects in barrier function.18 The pleiotropic role of intestinal HIF-1α has made it difficult to
identify the particular contribution of HIF-regulated defensins in normal physiological function. It is notable that the reduced expression of murine defensin-related genes in mice lacking
intestinal epithelial HIF-1α was identified in healthy animals in the absence of hypoxia-inducing insult. This underscores the homeostatic role for basal HIF-1α signaling in the intestinal
epithelium. This observation in murine tissues led us to investigate the contribution of HIF to human defensin expression in intestinal epithelia. Classically, HIF is induced in hypoxic
conditions, yet we detected basal HIF-1α in intestinal epithelial cells cultured in normoxic conditions (Figure 1c). Therefore, we sought to determine the role of basal and
hypoxia-stabilized HIF on defensin expression in this model. Initial studies indicated that human defensin expression was not changed by exposing cultured epithelia to hypoxia, yet targeted
knock down of HIF-1α and HIF-1β significantly decreased _DEFB1_ mRNA expression. This seemingly paradoxical finding, namely that exposure to even severe hypoxia (1% O2) did not change
_DEFB1_ expression despite the role of HIF-1α in maintenance of _DEFB1_ expression, is not without precedent. For example, one study analyzed expression array data sets and identified genes
differentially expressed in hypoxia that also contained at least one HRE within the proximal promoter. Of the top 200 predicted HIF target genes, only 81 responded to hypoxia in at least 3
of the 6 cell types evaluated.28 This finding provides evidence that a categorical response to hypoxia is not characteristic of all HIF target genes. It is possible that counter-regulatory
mechanisms in hypoxia might explain the absence of hypoxia induction of _DEFB1_. However, the well-characterized mechanism, in which hypoxia-induced microRNA-155 directly suppresses HIF-1α
transcript in Caco-2 cells, is unlikely to explain our results because such effects occur after prolonged hypoxia.29 The duration of hypoxia exposure in our experiments (8 h), while
sufficient to alter expression of HIF-target genes, obviate concern that microRNA-155 counter-regulation affected our results. To test the hypothesis that the transcriptional _DEFB1_
response was saturated by basal HIF-1α, we supplemented media with ascorbate to enhance HIF-1α degradation. This maneuver has precedent as an approach to reduce basal HIF.30 As a cofactor
for prolyl-hydroxylase enzymes that initiate the oxygen-dependent degradation of HIFα,20 ascorbate supplementation decreased both cytoplasmic and nuclear HIF-1α and _DEFB1_. Additional
evidence for HIF-mediated regulation was provided by chromatin immunoprecipitation assay and functional promoter site-directed mutagenesis of the classic HIF HRE. In conjunction with results
demonstrating no response to hypoxia, this finding strongly supports our hypothesis that basal HIF-1α saturates the _DEFB1_ transcriptional response through classic HIF-mediated molecular
mechanisms. Additionally, we found that co-transfection plasmid encoding stabilized HIF-1α or HIF-2α increase activity of a control (HRE-containing promoter reporter) but not the _DEFB1_
promoter reporter. This enabled us to selectively increase transcriptionally active HIF-1α and HIF-2α independent of hypoxia. These results argue that counter-regulation of HIF in hypoxia is
unlikely to explain the absence of _DEFB1_ induction by hypoxia in our experiments. The inability of oxygen-stable HIF-1α to induce _DEFB1_ promoter activity is consistent with our
conclusion that basal HIF-1α saturates the _DEFB1_ promoter in these intestinal epithelial cell lines. Because murine experiments were not tenable for reasons given previously, we sought to
examine the relationship between _DEFB1_ and HIF-1α in human tissue samples. We used _GLUT1_, a canonical HIF-1α target gene,24 as a proxy because HIF-1α is primarily regulated by
post-translational oxygen-dependent degradation; therefore HIF-1α transcript does not reflect the levels of HIF-1α protein. Results from 94 unique tissue samples from various locations in
the lower gastrointestinal tract indicated a significant correlation between _DEFB1_ and _GLUT1_, consistent with _in vitro_ findings that HIF-1α is critical for _DEFB1_ expression. Because
both _DEFB1_ and _GLUT1_ expression are reported to be higher in the large intestine than in the small intestine,25, 26 we analyzed samples from each location separately (Figure 5a) as well
as by disease status (Figure 5b), yet these factors did not abolish the correlation nor was the association an artifact of variation in the mucosal content in each sample (Figure 5c). The
information on the fraction of mucosa, submucosa, and muscularis was available for 47 of our samples, and this data was preferred over determining expression of epithelial markers, such as
EpCAM, that may be influenced by inflammation.31 Furthermore, intensity of immunofluorescence staining of hBD-1 in human colonic biopsies (Figure 5d) correlated with regions of hypoxic
epithelium identified by oxygen-sensitive staining in the mouse colon. Taken together, these observational analyses support our hypothesis that intestinal HIF-1α is critical for _DEFB1_
expression _in vivo_. These correlations also imply that, in the human intestine, HIF-1α signaling is within a dynamic range where _DEFB1_ expression could be influenced. If so, this adds to
growing interest in targeting epithelial HIF as a therapeutic target for restoration and maintenance of barrier integrity.32, 33, 34 Moreover, from the perspective of pathophysiology,
transcriptional regulation by HIF-1α may represent a mechanism whereby _DEFB1_ could be induced when a tissue normally exposed to an oxygen-rich environment becomes hypoxic. One potential
organ is the lung, where _DEFB1_ was recently found to be inversely associated with functional measures in chronic obstructive pulmonary disease.35 It is tempting to speculate that
upregulation of _DEFB1_ in the diseased lung is mediated by hypoxia and HIF-1α, but this remains to be determined in future studies. In summary, we show, for the first time, that basal
HIF-1α is critical for the constitutive expression of _DEFB1_ in intestinal epithelial cells and that _DEFB1_ correlates with a canonical HIF-1α target in the human intestine. These findings
exemplify a unique epithelial response to physiological hypoxia, support therapeutic targeting of epithelial HIF, and provide the basis for investigating the _DEFB1_–HIF-1α relationship in
other tissues. METHODS ANIMALS. HypoxyProbe-1 was used to visualize tissue hypoxia according to the manufacturer’s instructions (Hypoxyprobe, Burlington, MA) in healthy 8-week-old C57BL/6J
mice. Intestinal epithelial HIF-1α null mice were generated using Cre-lox technology (_Fabpl_-Cre and _Hif1a__lox_P/_lox_P mice) on a C57BL/6-129/SvJ background as previously described.18
RNA was isolated from colonic mucosal scrapings (three animals per group), reverse transcribed, and hybridized with the PGA mouse v1.1 chip. Experiments were approved by the Institutional
Animal Care and Use Committee at the University of Colorado Denver in compliance with National Institutes of Health guidelines for use of live animals. CELL CULTURE. Caco-2 (ATCC# HTB-37)
and T84 (ATCC# CCL-248) human intestinal epithelial cells were obtained from ATCC (Manassas, VA) and maintained in 95% air with 5% CO2 at 37 °C according to ATCC’s instructions. Hypoxia (1%
O2) exposure was performed using a humidified O2 control glove box at 37 °C (Coy Labs, Grass Lake, MI) by displacement with 5% CO2, 95% N2. Ascorbate supplementation was done with L-ascorbic
acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich, St Louis, MO), a comparatively stable form that accumulates as ascorbate intracellularly.36 Lentiviral particles encoding a
panel of short hairpin RNAs directed against human HIF-1β, HIF-1α or HIF-2α (MISSION TRC, Functional Genomics, University of Colorado, Boulder, CO) were used to transduce Caco-2 cells using
standard protocols as described previously.37 TRANSCRIPTIONAL ANALYSIS. TRIzol (Invitrogen, Grand Island, NY) was used to isolate RNA from cultured cells. iScript cDNA Synthesis Kit (Bio-Rad
Laboratories, Hercules, CA) and SYBR Green (Applied Biosystems, Warrington, UK) were used for real-time PCR analysis using the following primer sequences: β-actin (sense
5′-CCTGGCACCCAGCACAAT, antisense 5′-GCCGATCCACACGGAGTACT), DEFB1 (sense 5′-ATACTTCAAAAGCAATTTTCCTTTAT, antisense 5′-TTGTCTGAGATGGCCTCAGGTGGTAAC), GLUT1 (sense 5′-CTTTGTGGCCTTCTTTGAAGT,
antisense 5′-CCACACAGTTGCTCCACAT). PROTEIN ANALYSIS. Secreted hBD-1 was measured using an enzyme-linked immunosorbent assay kit capable of relative quantification of hBD-1 (Adipo Bioscience,
Santa Clara, CA). HIF-1α was measured after nuclear and cytoplasmic extraction using the NE-PER kit (Thermo Scientific, Rockford, IL) using the Meso Scale Discovery Total HIF-1 α Kit
(Gaithersburg, MD). Sample protein was quantified for normalization using the Pierce BCA Protein Assay Kit (Thermo Scientific). Western blotting performed as previously described38 with
whole-cell lysate after overnight hypoxia/normoxia using mouse anti-human HIF-1α antibody 610959 (BD Biosciences, San Jose, CA). IMMUNOFLUORESCENCE. Cells were grown to confluence on
coverslips, fixed with ice-cold 100% ethanol, permeabilized with 0.2% Triton X 100 in phosphate-buffered saline, blocked with 5% normal goat serum and 1% bovine serum albumin in
phosphate-buffered saline. Primary antibodies were rabbit polyclonal IgG anti-hBD-1 (AbD Serotec, Raleigh, NC) and mouse monoclonal IgG anti-human ZO-1 (Invitrogen) both diluted to 1:100 in
blocking solution. Slides were washed three times in phosphate-buffered saline, and secondary antibodies (Alexa Fluor 555 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG
(Invitrogen)) were used in a 1:500 dilution in blocking solution before mounting with ProLong Gold antifade (Invitrogen). The rabbit polyclonal IgG anti-hBD-1 (AbD Serotec) and Alexa Fluor
488 goat anti-rabbit IgG (Invitrogen) were used at the same concentrations for staining human colonic biopsy tissue. BACTERIAL KILLING ASSAY. _E. coli_ Nissle 1917 (kind gift of Paul Cohen)
was grown in Luria Broth. Cultures in log-phase growth were diluted 1000 × in deionized water to reduce salt, which inhibits the antimicrobial activity of hBD-1.21 Bacteria were incubated
for 1 h at 37 °C with recombinant hBD-1 (ProSpec, East Brunswick, NJ) at a final concentration of 8, 4, 2, 1, 0.5, and 0.25 μg ml−1. In all, 10 μl samples were plated in serial dilutions on
agar in triplicate, grown at 37 °C for until colonies were visible for enumeration of colony-forming units. PROMOTER EXPERIMENTS. Chromatin-immunoprecipitation was performed with Caco-2
cells maintained in normoxic conditions as previously described.39 Rabbit polyclonal anti-HIF-1α antibody NB-100-134 (Novus Biologicals, Littleton, CO) was used for immunoprecipitation.
Amplification with both conventional and real-time PCR was done using HRE-spanning primers (sense 5′-TTCTGAGGAGTGCCCTTTGG, antisense 5′-CGGCTCTAAGCTGGTGTTGG). DEFB1 promoter luciferase
reporter plasmid (SwitchGear Genomics, Menlo Park, CA) was used with the QuickChange Lightning site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) with the following
primers: sense 5′-TTTCTGAGGAGTGCCCTTTG and antisense 5′-TAAGCTGGTGTTGGCCTCTT. Plasmid was transfected into subconfluent wild-type Caco-2 cells (six-well plate; 1 μg per well) using Fugene HD
transfection reagent (Promega, Madison, WI) and luciferase activity determined at 48 h. Experiments using co-transfection of stabilized HIF-1α and HIF-2α (containing a mutated
oxygen-dependent degradation domain) were performed with 200 ng per well ΔODD HIF-1α or HIF-2α plasmid and 1 ugper well _DEFB1_ promoter luciferase reporter plasmid or HRE-containing
luciferase reporter plasmid as control (kind gift of Dan Theodorescu).40 HUMAN TISSUE. De-identified human intestinal tissue cDNA was obtained using the TissueScan Crohn’s/Colitis I and II
arrays (OriGene Technologies, Rockville, MD). Of 95 cDNA samples, 94 amplified with reverse transcriptase–PCR corresponding to 45 female and 49 males, age range 19–89 years. Distribution of
diagnoses was 12 normal, 35 Crohn’s, and 47 colitis. Analysis of samples by classification of no disease, Crohn’s disease, and ulcerative colitis included 90 total samples because 4 samples
from patients with “colitis” were not specified as Crohn’s or ulcerative colitis. Complete patient/sample characteristics are accessible from the company website (www.origene.com).
Immunofluorescence staining of hBD-1 as described above was performed on human colon biopsy tissue obtained under University of Colorado Denver approved protocols. STATISTICAL ANALYSIS.
GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used to create figures and perform statistical analyses, including one-way analysis of variance with Tukey’s _post-hoc_ test, paired
Student’s _t_-test, and Pearson product-moment correlation coefficient. REFERENCES * Colgan, S.P. & Taylor, C.T. Hypoxia: an alarm signal during intestinal inflammation. _Nat. Rev.
Gastroenterol. Hepatol._ 7, 281–287 (2010). Article PubMed PubMed Central Google Scholar * Mastrogiannaki, M., Matak, P., Keith, B., Simon, M.C., Vaulont, S. & Peyssonnaux, C.
HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. _J. Clin. Invest._ 119, 1159–1166 (2009). Article CAS PubMed PubMed Central Google Scholar * Comerford, K.M., Wallace,
T.J., Karhausen, J., Louis, N.A., Montalto, M.C. & Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. _Cancer Res._ 62, 3387–3394
(2002). CAS PubMed Google Scholar * Louis, N.A., Hamilton, K.E., Canny, G., Shekels, L.L., Ho, S.B. & Colgan, S.P. Selective induction of mucin-3 by hypoxia in intestinal epithelia.
_J. Cell. Biochem._ 99, 1616–1627 (2006). Article CAS PubMed Google Scholar * Furuta, G.T. _et al_. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects
barrier function during hypoxia. _J. Exp. Med._ 193, 1027–1034 (2001). Article CAS PubMed PubMed Central Google Scholar * Pazgier, M., Hoover, D.M., Yang, D., Lu, W. & Lubkowski, J.
Human beta-defensins. _Cell. Mol. Life Sci._ 63, 1294–1313 (2006). Article CAS PubMed Google Scholar * Ganz, T. Defensins: antimicrobial peptides of innate immunity. _Nat. Rev.
Immunol._ 3, 710–720 (2003). Article CAS Google Scholar * Schroeder, B.O. _et al_. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. _Nature_
469, 419–423 (2011). Article CAS PubMed Google Scholar * Harder, J., Bartels, J., Christophers, E. & Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel
human inducible peptide antibiotic. _J. Biol. Chem._ 276, 5707–5713 (2001). Article CAS PubMed Google Scholar * O'Neil, D.A. _et al_. Expression and regulation of the human
beta-defensins hBD-1 and hBD-2 in intestinal epithelium. _J. Immunol._ 163, 6718–6724 (1999). CAS PubMed Google Scholar * Zhao, C., Wang, I. & Lehrer, R.I. Widespread expression of
beta-defensin hBD-1 in human secretory glands and epithelial cells. _FEBS Lett._ 396, 319–322 (1996). Article CAS PubMed Google Scholar * Peyrin-Biroulet, L. _et al_. Peroxisome
proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. _Proc. Natl. Acad. Sci. USA_ 107, 8772–8777 (2010). Article CAS
PubMed Google Scholar * Kocsis, A.K. _et al_. Association of beta-defensin 1 single nucleotide polymorphisms with Crohn's disease. _Scand. J. Gastroenterol._ 43, 299–307 (2008).
Article CAS PubMed Google Scholar * Wehkamp, J. _et al_. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. _Inflamm.
Bowel Dis._ 9, 215–223 (2003). Article PubMed Google Scholar * Jurevic, R.J., Bai, M., Chadwick, R.B., White, T.C. & Dale, B.A. Single-nucleotide polymorphisms (SNPs) in human
beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. _J. Clin. Microbiol._ 41, 90–96 (2003). Article CAS PubMed
PubMed Central Google Scholar * Schaefer, A.S. _et al_. A 3' UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. _Genes Immun._ 11, 45–54 (2010).
Article CAS PubMed Google Scholar * Ozturk, A., Famili, P. & Vieira, A.R. The antimicrobial peptide DEFB1 is associated with caries. _J. Dent. Res._ 89, 631–636 (2010). Article CAS
PubMed Google Scholar * Karhausen, J., Furuta, G.T., Tomaszewski, J.E., Johnson, R.S., Colgan, S.P. & Haase, V.H. Epithelial hypoxia-inducible factor-1 is protective in murine
experimental colitis. _J. Clin. Invest._ 114, 1098–1106 (2004). Article CAS PubMed PubMed Central Google Scholar * Keely, S. _et al_. Hypoxia-inducible factor-dependent regulation of
platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. _Mol. Biol. Cell_ 21, 538–546 (2010). Article CAS PubMed PubMed Central Google
Scholar * Flashman, E., Davies, S.L., Yeoh, K.K. & Schofield, C.J. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl
hydroxylase domain 2) on ascorbate and other reducing agents. _Biochem. J._ 427, 135–142 (2010). Article CAS PubMed Google Scholar * Goldman, M.J., Anderson, G.M., Stolzenberg, E.D.,
Kari, U.P., Zasloff, M. & Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. _Cell_ 88, 553–560 (1997). Article CAS
PubMed Google Scholar * Morrison, G.M. _et al_. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. _Mamm. Genome_ 9, 453–457 (1998). Article CAS PubMed Google
Scholar * Huttner, K.M., Kozak, C.A. & Bevins, C.L. The mouse genome encodes a single homolog of the antimicrobial peptide human beta-defensin 1. _FEBS Lett._ 413, 45–49 (1997). Article
CAS PubMed Google Scholar * Wood, S.M. _et al_. Selection and analysis of a mutant cell line defective in the hypoxia- inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of
hif- 1alpha-dependent and -independent hypoxia-inducible gene expression. _J. Biol. Chem._ 273, 8360–8368 (1998). Article CAS PubMed Google Scholar * Yoshikawa, T., Inoue, R.,
Matsumoto, M., Yajima, T., Ushida, K. & Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. _Histochem.
Cell Biol._ 135, 183–194 (2011). Article CAS PubMed Google Scholar * Malik, A.N. & Al-Kafaji, G. Glucose regulation of beta-defensin-1 mRNA in human renal cells. _Biochem. Biophys.
Res. Commun._ 353, 318–323 (2007). Article CAS PubMed Google Scholar * Taylor, C.T. & Colgan, S.P. Hypoxia and gastrointestinal disease. _J. Mol. Med. (Berl)_ 85, 1295–1300 (2007).
Article Google Scholar * Benita, Y., Kikuchi, H., Smith, A.D., Zhang, M.Q., Chung, D.C. & Xavier, R.J. An integrative genomics approach identifies hypoxia inducible factor-1
(HIF-1)-target genes that form the core response to hypoxia. _Nucleic Acids Res._ 37, 4587–4602 (2009). Article CAS PubMed PubMed Central Google Scholar * Bruning, U. _et al_.
MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. _Mol. Cell. Biol._ 31, 4087–4096 (2011). Article CAS PubMed PubMed Central Google
Scholar * Nytko, K.J., Maeda, N., Schlafli, P., Spielmann, P., Wenger, R.H. & Stiehl, D.P. Vitamin C is dispensable for oxygen sensing _in vivo_. _Blood_ 117, 5485–5493 (2011). Article
CAS PubMed PubMed Central Google Scholar * Gires, O. _et al_. Tumor necrosis factor alpha negatively regulates the expression of the carcinoma-associated antigen epithelial cell
adhesion molecule. _Cancer_ 92, 620–628 (2001). Article CAS PubMed Google Scholar * Cummins, E.P. _et al_. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model
of colitis. _Gastroenterology_ 134, 156–165 (2008). Article CAS PubMed Google Scholar * Hindryckx, P. _et al_. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial
damage by hypoxia-inducible factor-1-dependent repression of FADD. _J. Immunol._ 185, 6306–6316 (2010). Article CAS PubMed Google Scholar * Robinson, A., Keely, S., Karhausen, J.,
Gerich, M.E., Furuta, G.T. & Colgan, S.P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. _Gastroenterology_ 134, 145–155 (2008). Article CAS PubMed
PubMed Central Google Scholar * Andresen, E., Gunther, G., Bullwinkel, J., Lange, C. & Heine, H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary
disease. _PLoS One_ 6, e21898 (2011). Article CAS PubMed PubMed Central Google Scholar * Vislisel, J.M., Schafer, F.Q. & Buettner, G.R. A simple and sensitive assay for ascorbate
using a plate reader. _Anal. Biochem._ 365, 31–39 (2007). Article CAS PubMed PubMed Central Google Scholar * Keely, S. _et al_. Hypoxia-inducible factor-dependent regulation of
platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. _Mol. Biol. Cell_ 21, 538–546 (2009). Article PubMed Google Scholar * MacManus,
C.F., Campbell, E.L., Keely, S., Burgess, A., Kominsky, D.J. & Colgan, S.P. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. _FASEB J._ 25, 1856–1864
(2011). Article CAS PubMed PubMed Central Google Scholar * Rosenberger, P., Khoury, J., Kong, T., Weissmuller, T., Robinson, A.M. & Colgan, S.P. Identification of
vasodilator-stimulated phosphoprotein (VASP) as an HIF-regulated tissue permeability factor during hypoxia. _FASEB J._ 21, 2613–2621 (2007). Article CAS PubMed PubMed Central Google
Scholar * Sheta, E.A., Trout, H., Gildea, J.J., Harding, M.A. & Theodorescu, D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP
kinase mediated signaling pathways. _Oncogene_ 20, 7624–7634 (2001). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by National Institutes
of Health grants F30DK096709 (C.J.K.), TL1RR025778 (C.J.K.), DK50189 (S.P.C.), HL60569 (S.P.C.), DK095491 (S.P.C.) and by grants from the Crohn’s and Colitis Foundation of America. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine, Mucosal Inflammation Program, University of Colorado School of Medicine, Aurora, Colorado, USA C J Kelly, L E Glover, E L
Campbell, D J Kominsky, S F Ehrentraut, B E Bowers, A J Bayless, B J Saeedi & S P Colgan * Department of Anesthesiology, University of Colorado, Aurora, Colorado, USA D J Kominsky &
A J Bayless Authors * C J Kelly View author publications You can also search for this author inPubMed Google Scholar * L E Glover View author publications You can also search for this author
inPubMed Google Scholar * E L Campbell View author publications You can also search for this author inPubMed Google Scholar * D J Kominsky View author publications You can also search for
this author inPubMed Google Scholar * S F Ehrentraut View author publications You can also search for this author inPubMed Google Scholar * B E Bowers View author publications You can also
search for this author inPubMed Google Scholar * A J Bayless View author publications You can also search for this author inPubMed Google Scholar * B J Saeedi View author publications You
can also search for this author inPubMed Google Scholar * S P Colgan View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to S P Colgan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declared no conflict of interest. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT
SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works
3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kelly, C.,
Glover, L., Campbell, E. _et al._ Fundamental role for HIF-1α in constitutive expression of human β defensin-1. _Mucosal Immunol_ 6, 1110–1118 (2013). https://doi.org/10.1038/mi.2013.6
Download citation * Received: 11 October 2012 * Accepted: 08 January 2013 * Published: 06 March 2013 * Issue Date: November 2013 * DOI: https://doi.org/10.1038/mi.2013.6 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative
Trending News
Chelsea Hawley | VA Bedford Health Care | Veterans AffairsThe .gov means it’s official.Federal government websites often end in .gov or .mil. Before sharing sensitive information...
Haleakalā national park: a visitor’s guide to planning a tripTwo or three hours from the luaus and swim-up bars at Maui’s beachside resorts, one of the world’s largest dormant volca...
* congregation am hayam in oxnard will...* Congregation Am HaYam in Oxnard will present the concert “Songs of the Heart II--Shirav Ha Lev” at 8 tonightat Oxnard ...
Low risk waste positions: electrical equipment, including constituent parts and accessoriesGuidance LOW RISK WASTE POSITIONS: ELECTRICAL EQUIPMENT, INCLUDING CONSTITUENT PARTS AND ACCESSORIES The Environment Age...
Ford cto paul mascarenas on bridging the worlds of silicon valley and motor city [tctv] | techcrunchWhen you think about Detroit, you don’t often think first about the technology industry; you think about automobiles. Bu...
Latests News
Fundamental role for hif-1α in constitutive expression of human β defensin-1ABSTRACT Antimicrobial peptides are secreted by the intestinal epithelium to defend from microbial threats. The role of ...
404 - Page not foundHomeNG HindiIndiaIndiaAndhra PradeshArunachal PradeshAssamBiharChhattisgarhGoaGujaratHaryanaHimachal PradeshJharkhandKar...
What tier was london in before lockdown? Will london go into tier 3?She called for it to be placed in tier two at the “very least” when the new system is rolled out, adding: “Reopening in ...
The bee gees: how can you mend a broken heart, review – an enjoyable if sanitised retrospectiveEd Power 13 December 2020 11:10pm GMT THE BEE GEES: HOW CAN YOU MEND A BROKEN HEART (Sky Documentaries) was a solid docu...
Snp warns 'scotland is in trouble' as he admits biggest brexit fearSNP leader in Westminster Ian Blackford appeared on BBC News when he made the revelation - and host Clive Myrie reiterat...