Fine-scale spatial genetic dynamics over the life cycle of the tropical tree prunus africana
Fine-scale spatial genetic dynamics over the life cycle of the tropical tree prunus africana"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Studying fine-scale spatial genetic patterns across life stages is a powerful approach to identify ecological processes acting within tree populations. We investigated spatial
genetic dynamics across five life stages in the insect-pollinated and vertebrate-dispersed tropical tree _Prunus africana_ in Kakamega Forest, Kenya. Using six highly polymorphic
microsatellite loci, we assessed genetic diversity and spatial genetic structure (SGS) from seed rain and seedlings, and different sapling stages to adult trees. We found significant SGS in
all stages, potentially caused by limited seed dispersal and high recruitment rates in areas with high light availability. SGS decreased from seed and early seedling stages to older juvenile
stages. Interestingly, SGS was stronger in adults than in late juveniles. The initial decrease in SGS was probably driven by both random and non-random thinning of offspring clusters during
recruitment. Intergenerational variation in SGS could have been driven by variation in gene flow processes, overlapping generations in the adult stage or local selection. Our study shows
that complex sequential processes during recruitment contribute to SGS of tree populations. SIMILAR CONTENT BEING VIEWED BY OTHERS STRONGER GENETIC DIFFERENTIATION AMONG WITHIN-POPULATION
GENETIC GROUPS THAN AMONG POPULATIONS IN SCOTS PINE PROVIDES NEW INSIGHTS INTO WITHIN-POPULATION GENETIC STRUCTURING Article Open access 01 February 2024 SPATIAL GENETIC STRUCTURE AND SEED
QUALITY OF A SOUTHERNMOST _ABIES NEPHROLEPIS_ POPULATION Article Open access 27 October 2023 GENETIC DIVERSITY IN NORTH AMERICAN _CERCIS CANADENSIS_ REVEALS AN ANCIENT POPULATION BOTTLENECK
THAT ORIGINATED AFTER THE LAST GLACIAL MAXIMUM Article Open access 08 November 2021 INTRODUCTION Gene flow via pollen and seeds is a key factor in determining the genetic make-up of plant
populations (Loveless and Hamrick, 1984). Restricted gene flow is probably one of the most prevalent factors influencing fine-scale spatial genetic structure (SGS), that is, a decrease in
relatedness with increasing spatial distance between individuals (Vekemans and Hardy, 2004). It results in spatial aggregations of siblings in close vicinity of the mother trees and, thus,
in high offspring’s SGS (for example, Chung et al., 2003). Within generations, that is, among different juvenile life stages stemming from the same adult population, the persistence of the
initial genetic make-up is influenced by various processes. As only few individuals from the seed rain survive, SGS is likely to weaken in older stages (Chung et al., 2003). This effect is
expected even if mortality is random in space (random thinning). Yet, if mortality is non-random and driven by selection, the patterns of SGS can change more profoundly (Hamrick et al.,
1993; Jones and Hubbell, 2006). In addition, because of density- and distance-dependent predation and pathogen pressure, juveniles may encounter high mortality in the vicinity of the mother
tree (Janzen, 1970; Connell, 1971), leading also to a strong thinning of the sib clusters that dilutes SGS. Across generations, that is, between adults and juveniles, the level of SGS may
vary because of different demographical processes: historic factors can lead to differences in underlying patterns of gene flow between adults and juveniles. For example, if the extent of
seed dispersal has declined across generations, owing to, for example, anthropogenic disturbance, juveniles may show stronger SGS than adults (for example, Farwig et al., 2008). In contrast,
if the adult population results from a generation with few founder trees it may show stronger levels of SGS than the present day juveniles (Jones et al., 2006; Pardini and Hamrick, 2008).
If the population is in equilibrium and adults of the previous generation are representative of the currently establishing generation, SGS should weaken from juvenile to adults owing to
random and non-random mortality (Zhou and Chen, 2010). Studying genetic diversity and SGS of different life stages and across generations of a species helps to understand the formation of
genetic structure within adult plant populations (for example, Hamrick et al., 1993; Latouche-Hallé et al., 2003; Jacquemyn et al., 2006; Zhou and Chen, 2010). Owing to overlapping
generations, which are common in tree species, individuals from a younger life stage grow besides closely related individuals from older generations (Jones and Hubbell, 2006). Thus, SGS
analyses on pooled life stages of trees can be misleading. Hence a multistage perspective can lead to a higher resolution of the processes underlying the formation of SGS in trees (Chung et
al., 2003; Jones et al., 2006; Pardini and Hamrick, 2008). Only a few studies that assessed SGS from a multistage perspective incorporated the initial seed rain stage in their analyses (for
example, Jones and Hubbell, 2006; Zhou and Chen, 2010). Including this stage may reveal how seed dispersal leads to the formation of the initial genetic template. Here, we investigated the
development of SGS in the insect-pollinated and vertebrate-dispersed tropical tree _Prunus africana_ (Hook. f.) Kalkman (Rosaceae) in Kakamega Forest, western Kenya. _P. africana_ is a
common species in Kakamega Forest, considered a late pioneer or an early climax tree species (for example, Swaine and Whitmore, 1988). Farwig et al. (2008) studied genetic structure of _P.
africana_ seedlings and adults at a larger spatial scale among eight populations distributed over Kakamega Forest and adjacent fragments. They found stronger genetic differentiation among
seedling populations than among adults, showing that genetic diversity and structure of the species is sensitive to processes reducing gene flow, such as forest fragmentation and local
disturbance (Farwig et al., 2008). To better understand these patterns, we used parentage analyses to investigate the extent of gene flow across different life stages mediated by insect
pollinators and vertebrate seed dispersers (Berens et al., 2013). Our results suggested that seed dispersal was strongly distance restricted (mean distances of only 5 m), while pollen
dispersal reached over 23-fold longer distances. Yet, strong non-random mortality led to a disproportionate increase of realized maternal gene flow distances at later life stages, as well as
to strong variation in paternal gene flow distances (Berens et al., 2013). In this study, we used a multi-stage approach to determine whether the observed patterns of SGS are consistent
with expectations deriving from the observed biological processes. We hypothesize (i) strong SGS at the seed stage because of distance-restricted seed dispersal; (ii) a continuous decrease
of SGS across older recruitment stages because of mortality and the thinning of clusters of related individuals; and (iii) an increase in SGS across generations (between adult trees and
seedlings of the next generation) resulting not only from restricted dispersal but also from proposed changes in gene flow across generations (Farwig et al., 2008). MATERIALS AND METHODS
STUDY SPECIES _P. africana_ is a long-lived (>100 years) monoecious tree species whose range expands from East Africa to Madagascar and the Comores (Hall et al., 2000). Owing to bark
exploitation for medicinal use, the species is listed on CITES appendix II. _P. africana_ has small white hermaphroditic protogynous flowers. Main pollinators are hymenoptera and diptera
(Hall et al., 2000). The species is mainly outcrossing (Hall et al., 2000). Parentage analyses of the species revealed selfing rates of 5–13% (Berens, unpublished data). Main seed dispersers
of the one-seeded fleshy fruits are birds and monkeys (Hall et al., 2000). Secondary seed dispersal seems to be neglectible in the species, as experiments with thread-marked _P. africana_
seeds did not record any caching, but only later predation of seeds (Svd Gönna and M Melcher, personal communication). Survival to the sapling stage is very low, potentially because of high
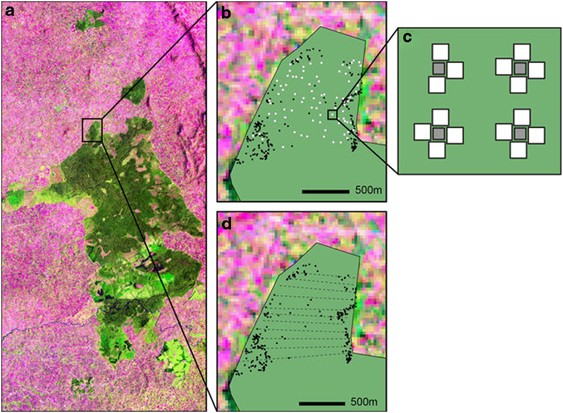
light requirement as well as strong seed predation and seedling herbivory (Tsingalia, 1989; Tesfaye et al., 2002). STUDY AREA Kakamega Forest is located in western Kenya (Figure 1). The
mid-altitudinal rainforest (1500–1700 m asl, KIFCON, 1994) comprises a main forest block and five fragments with a total area of 13 000 ha (Lung, 2004). Average annual temperature is 18.7 °C
(Mitchell et al., 2009) and average yearly precipitation is about 2000 mm per year (averaged from Forest Department records at Isecheno Forest Station from 1982 to 2005). The study area
within Kakamega Forest was a 120 ha forest peninsula in the northwest of the non-fragmented main forest block extending into the agricultural matrix (0°21′34.1′′ North, 34°51′31.0′′ East;
Figure 1). It is part of the official Kakamega Forest Nature Reserve, managed by the Kenya Wildlife Service (KWS) and established in 1967. The study area mostly comprises primary forest,
which has not been clear-cut for at least 100 years (Mitchell et al., 2009). Smaller secondary forest patches within the study area are _ca._ 50 years old. Owing to the strict protection by
the KWS, rates of human disturbance in terms of logging or bark harvesting of the species in the study area are low (Mitchell et al., 2009). Individuals of all life stages of _P. africana_
in the study area are spatially clustered and the degree of clumping decreases with life stage (see also ‘_Drivers of SGS change across life stages_’ for details on clustering): the density
of adult _P. africana_ trees (with a diameter at breast height >10 cm; hereafter ADLTS) within our study area is 0.00022 individuals m–2. Adult trees are clustered in areas of high light
availability, in particular near forest edges and in secondary regrowth in the west and east of the forest peninsula. Mean densities in our study plots decreased from 63.22 for propagules
(seeds and fruits, hereafter SDFRT), 40.06 for current year seedlings (SLYNG), 0.01 for seedlings up to 3-year (SLMID) to 0.004 seedlings older than 3-year (SLOLD) individuals per m2 (see
Plant material for life stage definition). PLANT MATERIAL In 2005, we established 22 parallel transects crossing the study area from the eastern to the western forest edge and covering _ca._
87 ha (Figure 1). Transects were between 694 and 1225 m long and were separated by 40 m. We recorded all _P. africana_ ADLTS in the transect area, as well as all trees up to 100 m distance
south of transect 1 and north of transect 22, as they may be potential parents of the offspring analyzed. In total, we detected 261 adult _P. africana_ trees. Global positioning system (GPS)
coordinates of each tree were taken. Leaf material of all adult trees was collected between January and May 2006. In the same year, 86 geopositioned sampling plots were randomly established
along the transects (Figure 1b). A number of 20 sampling plots were under _P. africana_ trees. Each plot consisted of an array of four seed traps (0.7 × 0.7 m) made of a wire frame covered
with mosquito mesh (Figure 1d). To avoid secondary dispersal from traps, traps were installed at a height of _ca._ 1 m above the ground. Propagules (SDFRT) were collected from seed traps
during a fruiting peak of _P. africana_ in our study site on a weekly basis between January and April, 2006. They were assigned to fruits (with pulp) or seeds (without pulp) and pooled for
further analyses. Fruit pulp was manually removed for storage and all samples were dried with silica gel. In total, 8544 propagules were counted in the traps. We aimed at having similar
sample sizes (_ca._ 300 individuals) for each life stage, evenly distributed across the study site. For genotyping, we subsampled 180 propagules from sampling plots, which were situated
under _P. africana_ trees (representing 2% of all propagules found there) and 131 propagules from sampling plots away from _P. africana_ trees (representing 92% of all propagules in that
category), resulting in a total sample of 311 genotyped propagules (SDFRT). SLYNG were collected at the same 86 sampling plots between March and May, 2006. Fifteen 1 m2 subplots were placed
within each sampling plot (Figure 1c) and newly emerged seedlings were counted and collected on a biweekly basis. Newly emerged seedlings originated from the same fruiting period as SDFRT
and could be identified because of the presence of cotyledons. Leaf samples were dried with silica gel. Again, 180 seedlings were subsampled from sampling plots under _Prunus_ trees
(representing <1% of all seedlings under _Prunus_ trees), and the remaining 129 seedlings from plots away from _Prunus_ trees (representing 27% of seedlings found at these stations),
resulting in a total subsample of 309 out of the 48 991 seedlings for genetic analyses. As a result of lower density of older seedlings (SLMID and SLOLD) compared with SDFRT and SLYNG, they
were sampled, between January and February 2008, along every second of the 22 transects crossing the study area (Figure 1c). Each _P. africana_ seedling encountered in these plots was
recorded and the geographic position was taken. A leaf sample of each individual was taken and the seedling age was determined by an experienced field assistant considering the degree of
lignification of the stem axis. Although this procedure implies a risk of misassignment to the wrong germination year, the approach is still valid for a differentiation between younger and
older seedlings. Thus, a subsample of 298 out of the 861 SLMID and 301 out of the 368 SLOLD was taken for genetic analyses. SLMID were between 1 and 3 years old (average 3 years), and SLOLD
from 4 years up to >6 years (average 5 years). MOLECULAR ANALYSES All plant material (leaves of ADLTS, SLYNG, SLMID, SLOLD, and embryo tissue of SDFRT), was ground to fine powder with a
Retsch mixer mill (Type MM 301, Retsch, Haan, Germany). DNA was extracted following the protocol described by Wang et al. (1993). The standard protocol was modified by using 40 μl of NaOH
and 95 μl of Tris buffer for each sample. Samples were genotyped at six microsatellite loci that were originally developed for _Prunus avium_ and _Prunus persica_: _UDP97-403_ (Cipriani et
al., 1999), _P12A02_ (Sosinski et al., 2000), _BPPCT-002_ (Dirlewanger et al., 2002), _UDP96-005_ and _UDP98-410_ (Schueler et al., 2003), and _EMPaS06_ (Vaughan and Russell, 2004). Primers
were fluorescent 5′-end-labeled (6-FAM, NED, HEX; Applied Biosystems, Foster City, CA, USA). Polymerase chain reaction protocols followed Farwig et al. (2008) with annealing temperatures
ranging from 54 to 62 °C and the number of cycles ranging from 44 to 46. For loci _UDP97-403_ and _EMPaS06_, a final extension step of 60 °C for 30 min was added to the standard protocol.
Samples were genotyped on ABI 3730 and 3130xl capillary sequencers. Allele scoring was performed using the software GeneMapper 4.0 (Applied Biosystems). For all loci, base shifts between
scorings of the different sequencers were calibrated by running 48 samples per locus (3%) on both instruments. All samples except that of one adult tree were successfully amplified. CHANGES
IN CLUSTERING ACROSS LIFE STAGES In order to investigate the change in spatial clustering across life stages, we obtained the pairwise geographic distances between individuals in a fixed
distance category of up to 100 m. We compared these individual pairwise distances across life stages using a generalized linear model with quasi-Poisson-distributed errors. Significance of
the independent variable ‘life stage’ was determined by comparing the full model with a model only including the intercept via analysis of variance (ANOVA) and F-tests. Differences between
life stages were analyzed with _post-hoc_ pairwise Wilcoxon tests (with Bonferroni corrections) as implemented in R 3.0.1 (R Core Team, 2013). GENETIC DIVERSITY AND SGS Rarefied allelic
richness, which is a measure of the number of alleles per locus based on the minimum number of samples genotyped at all loci, as well as observed (_H_o) and expected heterozygosity (_H_e)
were assessed for each locus and life stage using the software FStat 2.9.3.2 (Goudet, 1995; El Mousadik and Petit, 1996). Null allele frequencies were obtained from CERVUS 3.0.3 (Kalinowski
et al., 2007). Differences between _H_o and _H_e within life stages were tested through assessing the significance of _F_is after Bonferroni correction in FStat. We conducted ANOVAs to test
for differences in allelic richness, _H_o and _H_e among life stages. ANOVAs were done in R 3.0.1 (R Core Team 2013). In order to test for a significant decrease in relatedness across
distance, a regression between the kinship coefficient _f_ij and the logarithm of pairwise geographic distances of individuals was computed using SpaGeDi (Hardy and Vekemans, 2002). Standard
errors of the regression slope, _b-log_, were computed using a jackknife procedure over loci. Values were tested for significant difference from a random pattern using 10 000 permutations.
Analyses were conducted for each life stage separately across the full distance range. Spatial autocorrelation of genetic relatedness across geographic distance was assessed using GenAlEx
6.501 (Peakall and Smouse, 2006; Peakall and Smouse 2012). We performed random permutations (_n_=999), to test the null hypothesis that genotypes are spatially interchangeable (Smouse et
al., 2008). First, analyses were conducted for each life stage separately. Distance intervals were set at 100-m intervals (0–100, 101–200 and so on). Maximum distance intervals were 1100 for
SDFRT and SLYNG, 1600 for SLMID and SLOLD, and 1700 for ADLTS (for the mean number of pairs per distance interval see Table 1). We performed a heterogeneity test in order to evaluate the
significance of correlograms at a _P_-level of 0.01 (Banks and Peakall, 2012). Second, we conducted a heterogeneity test to see whether relatedness differed among life stages for each
distance interval and across distance intervals (Smouse et al., 2008). The maximum distance interval was set to 1000 m to assure comparability among life stages. Assuming that SGS has
reached equilibrium, we used an iterative procedure implemented in SpaGeDi to indirectly estimate the axial variance of gene dispersal distance σ for adult trees from it effective density
_D_e (Hardy and Vekemans, 2002). _D_e is normally unknown but different values of _N_e/_N_, whereby _N_e is the effective population size and _N_ the census one, can be used to approximate
this quantity (Vekemans and Hardy, 2004; Born et al., 2008). As the ratio _N_e/_N_ typically ranges between 0.1 and 0.5 (Frankham 1995; Born et al., 2008), we used the observed adult
density, 0.00022 individuals m–2 as an upper limit estimate, as well as 0.00022/10 as a lower limit estimate of the effective population density _D_e. The distance range for the regression
was set between σ and 20 σ, assuming a mutation rate _μ_ of 10−3 for nuclear microsatellites (see Heuertz et al., 2003). RESULTS CHANGES IN CLUSTERING ACROSS LIFE STAGES The clustering rate
of individuals in terms of the mean±s.e. distances of individual pairs in the smallest distance category (up to 100 m distance) was 56.2±0.5 m for ADLTS (_n_=2322) and increased from
21.4±0.4 m for SDFRT (_n_=5603), 23.4±0.3 m for SLYNG (_n_=6961), 39.2±0.4 m for SLMID (_n_=5543) to 41.2±0.3 m for SLOLD (_n_=8972). The differences in spatial distance between individuals
in the smallest distance category were significant between all life stages except for SLMID and SLOLD (pairwise Wilcoxon rank sum tests, all _P_-values <0.001 after Bonferroni
correction). GENETIC DIVERSITY The microsatellite loci were highly polymorphic with a mean±s.e. allelic richness of 18.7±1.5 (from 16.7 in SDFRT to 29.2 in ADLTS, based on a minimum number
of 252 diploid individuals; Table 1). Mean null allele frequency was 0.07 across loci and life stages and ranged from −0.06 to 0.26 (locus R1 in SLMID). Observed heterozygosity (_H_o) ranged
between 0.66 (SDFRT) and 0.77 (ADLTS), while gene diversity (expected heterozygosity, _H_e) was very similar (0.82–0.83) across all life stages (Table 1). In addition, _H_o was
significantly lower than _H_e in all cases (_P_-values for all _F_is-values <0.0017; Table 1). Neither allelic richness, nor _H_o or _H_e differed between life stages as shown by ANOVA
(all _P_-values >0.05). FINE-SCALE SGS We found significant SGS in all life stages (Figure 2, Table 1). Relatedness was positive and significant up to 200 m in ADLTS, SDFRT and SLMID, up
to 300 m for SLYNG and up to 100 m for SLOLD. The heterogeneity test revealed that across autocorrelograms, relatedness differed among all life stages, particularly in the first distance
interval (Table 2, Supplementary Table S1). Relatedness among individual pairs in the first distance interval up to 100 m was three times stronger for SDFRT and SLYNG than for SLMID and
SLOLD, indicating stronger SGS in the earlier life stages (Table 1). Relatedness was intermediate in ADLTS (Table 1). Historical neighborhood sizes and gene dispersal distances (based on the
adult life stage) were estimated to be 152 and 236 m for _D_e=0.00022 and 85 and 558 m for _D_e/10, respectively. DISCUSSION All life stages of _P. africana_ from Kakamega Forest showed
SGS. Although genetic diversity did not differ across the various life stages, the strength of SGS varied considerably. INBREEDING AND GENETIC DIVERSITY For all life stages, we found lower
observed (_H_o) than expected (_H_e) heterozygosity. The frequency of null alleles was problematically high only in one locus (R1) in two life stages (SLMID=0.26, SLOLD=0.21) and are thus
not considered to be the main cause for this pattern. The significant autocorrelation in the first distance interval for adults indicates a close genetic relationship of reproductive nearby
individuals. As a large proportion of pollination events normally occur within spatial clusters of conspecific trees (Berens et al., 2013), biparental inbreeding (that is, mating between
relatives) may explain the observed heterozygosity deficit. Genetic diversity, in terms of allelic richness and heterozygosity, was similar throughout the life stages in _P. africana_. It
shows that effective population sizes were large enough at each life stage to prevent the effects of genetic drift. It further indicates that the extent of gene flow is large for all life
stages, and that no historic events have led to a fundamental alteration of processes across life stages. This is in line with other studies that have also shown that genetic diversity is
similar across life stages within populations (for example, Chung et al., 2003; Jacquemyn et al., 2006). The levels of genetic diversity in our study were similar to those of another study
on _P. africana_ in Kakamega Forest (Farwig et al., 2008) and to those of European _Prunus_ species (Stoeckel et al., 2006, _P. avium_: _H_e: 0.448–0.918) but higher than the diversity in an
Asian _Prunus_ species (Pakkad et al., 2003, _P. cerasoides_: _H_e: 0.292–0.689). PREVALENCE OF SGS IN _P. AFRICANA_ The high relatedness we found in the smallest distance categories and
the significant decrease in relatedness across geographic distance in all stages can potentially be ascribed to limited seed dispersal in _P. africana_. Usually, seed dispersal by
frugivorous birds and large mammals should sustain gene flow across large distances, thereby leading to low levels of SGS. In fact, indirect estimations of gene dispersal distances (both
pollen and seeds combined) from microsatellites revealed distances of 236–558 m. Further, also a direct assessment of contemporary pollen and seed dispersal distances using parentage
analyses revealed seed dispersal distances of up to 700 m within our study population (Berens et al., 2013), with related individuals found even at far distances. However, direct estimates
of pollen and seed dispersal revealed that the majority of seeds were dispersed across short distances, leading to a mean seed dispersal distance of only 5 m (Berens et al., 2013). This low
average distance may result from the behavior of seed dispersers. Although numerous dispersers of _P. africana_ swallow the fruits, some drop them directly under the maternal tree (Farwig et
al., 2006). Especially monkeys spend a lot of time in the tree feeding on fruits and a large proportion of seeds is spit out and dropped under the crown (DG Berens, personal observation).
This probably leads to aggregates of closely related offspring, which was in turn mirrored in marked SGS. Our assumption is supported by the fact that SGS was not significantly different
from zero in distance categories exceeding 300 m. Thus, individuals in larger spatial distances were not more related to one another than expected by chance. Thus, the patterns of SGS we
found in this study agree with our direct observations and are in line with several other studies that also found a correlation between restricted seed dispersal and SGS in tropical trees
(for example, Latouche-Hallé et al., 2003). In addition to distance-restricted dispersal, high light requirements of the species may also have contributed to the overall high levels of SGS
across stages. Adult trees are strongly clustered near forest edges and in secondary forest patches, leading to high densities of offspring in these areas. Enhanced recruitment potential in
areas of high light availability may again lead to a spatial clustering of related individuals, expressed in pronounced SGS. Previous studies have also shown that microhabitat selection can
lead to SGS in plant species (Kalisz et al., 2001; Ueno et al., 2002). Thus, a combination of distance-restricted seed dispersal and microhabitat requirements are potentially the main
drivers of SGS found in our study. SGS CHANGES ACROSS LIFE STAGES In contrast to genetic diversity, the strength of SGS differed across life stages in _P. africana_. SGS was pronounced in
early stages (SDFRT and SLYNG), but drastically decreased from young to older juvenile stages (SLMID, SLOLD). SGS in adults (ADLTS) was slightly stronger than in old juvenile stages. Other
studies looking at SGS from a multistage perspective have either found an increase (for example, Jacquemyn et al., 2006), decrease (for example, Zhou and Chen, 2010) or no change (for
example, Berg and Hamrick, 1995) in SGS with age. This absence of a common pattern highlights the importance of species- and site-specific postdispersal and early selection, as well as
differences across generations (for example, owing to different levels of gene flow). DECREASE OF SGS FROM EARLY TO LATE JUVENILE STAGES We consider several valid explanations for the
observed decrease in SGS from early to late recruitment stages. One likely cause could be an interannual variation in allele frequencies within the older juveniles and adults. Indeed, SLMID,
SLOLD and ADLTS life stages comprise individuals stemming from different recruitment years. Individual pollen and seed dispersal patterns within the _P. africana_ population may have varied
among these years, producing distinct SGS patterns. In addition, pooling individuals across different recruitment years may cause an overall veiling of the SGS in comparison with SGS formed
in progeny of a single year. A second likely factor causing the decrease in SGS could be offspring mortality (that is, stand thinning with age), which would reduce relatedness within
offspring clumps. In their study on _Jacaranda copaia_, Jones and Hubbell (2006) also detected a decrease in SGS from young seedlings to saplings. Comparable to many other tree species
(Petit and Hampe, 2006), recruitment rates of young _P. africana_ seedlings to older saplings are low, potentially because of high light requirements (Tsingalia, 1989; Tesfaye et al., 2002).
Thus, multiple mortality factors, both random and non-random, may lead to the observed thinning and drive the initial decrease in SGS. In fact, our analyses of spatial clustering revealed
thinning of individuals from offspring clusters across life stages and support these results. Specifically, differing pollen and seed dispersal distances directly estimated at different
recruitment stages by means of parentage analyses indicated a non-random density and distant-dependent mortality in our study population, which could be driving the observed patterns (Berens
et al., 2013). STRONGER SGS IN ADULTS THAN IN LATE JUVENILES Contrary to our expectations, SGS was stronger in adult trees than in late juvenile stages. We consider several valid
explanations for this pattern. First, one has to consider that differences in SGS between juvenile stages and the adult stage may actually mirror differences between generations, for
example, in gene flow and demographic processes. Jones and Hubbell (2006) attributed the decrease in SGS from adults to subadults in _J. copaia_ to non-equilibrium demographic processes. _J.
copaia_ shows strong temporal variation in reproduction, suggesting that adult trees are descendants of one or few past recruitment events (Jones and Hubbell, 2006). Although phenology data
do not suggest such irregular massive recruitment pulses in _P. africana_ (DG Berens, personal observation), the extent and spatial patterns of seed dispersal events and selection processes
may have been different at the time the adult generation established (Farwig et al., 2008). Although the study area nowadays is well protected, the extent of historic anthropogenic
influences, such as selective logging and habitat alteration are unknown. Past logging may have led to altered light regimes and may have affected seed disperser communities at the time the
adult generation recruited, before the establishment of the nature reserve in 1967. Furthermore, adjacent forest areas had potentially been deforested at the beginning of the last century
(Mitchell et al., 2009), which could have led to reduced gene flow at the time of the parental generation (the ADLTS class) establishment. Moreover, a founder effect with few, related
individuals in the founding generation could have led to stronger SGS in the adult population (Jones and Hubbell, 2006; Pardini and Hamrick, 2008). Few patches in our study area had been
clear-cut >50 years ago. Recolonization of formerly clear-cut patches by single individuals may have led to patterns similar to those deriving from a founder effect within the adult
population. However, an overall founder effect causing high levels of SGS in adults is unlikely because the _P. africana_ population we studied is situated in mostly primary forest (Mitchell
et al., 2009). Furthermore, if the adult population had established from a founder generation, adults should show a lower allelic richness than the younger stages, as allelic richness would
have increased with time because of gene flow (Jones and Hubbell, 2006). Finally, assuming that the population is in equilibrium and ADLTS of the previous generation are representative of
the establishing generation, the differences in SGS between adults and late juveniles in _P. africana_ could also be driven by microhabitat heterogeneity and local selection (Linhart and
Grant, 1996; Jones and Hubbell, 2006). Related individuals may have similar microhabitat requirements. Consequently, spatial heterogeneity in abiotic conditions, for example, soil nutrients
or especially light environment, would allow for the survival of different groups of related individuals in later life stages leading to significant SGS in adults despite lower SGS in late
juveniles (Jones and Hubbell, 2006). If related individuals require similar microhabitat conditions, and generations of related individuals overlap, neighboring individuals can be a mixture
of related parents and progeny, leading to pronounced SGS (Jones and Hubbell, 2006). LIMITATIONS SGS is a complex function of the density of individuals, behavior of pollinators and seed
dispersers, and site history (Jones et al., 2006). Moreover, the ability to detect SGS is highly influenced by sampling schemes (Vekemans and Hardy, 2004). Although we have a rather detailed
understanding on how the ecological processes pollination and seed dispersal may have driven the observed patterns of SGS, our knowledge on site history is rather scarce. Thus, conclusions
based on potential historic effects remain speculative, although site history is an important determinant of current SGS in tree populations. Moreover, one potential caveat of our study lies
in different sampling designs for different life stages (transects vs sampling stations). Naturally, older recruitment stages occur in low densities, which makes sampling along transects an
appropriate method to achieve a representative sample of the population. In contrast, young recruitment stages occur in high density. Therefore, we had to base our sampling scheme on
sampling stations to keep field work tractable. Consequently, sampling of older life stages mirrored the clustered distribution of _P. africana_ trees, while for younger stages sampling in
areas of _P. africana_ tree clusters may have been underrepresented. Moreover, for SDFRT and SLYNG, we used only a small proportion of the offspring found under _Prunus_ trees. However, this
sampling scheme should give conservative SGS estimates for these life stages. Thus, we still consider the conclusions drawn from our results to be valid. CONCLUSIONS Changes in genetic
structure have to be conceived as a multi-step process. Caused mostly by distance-restricted seed dispersal, the seed rain and early seedling stages of our study species showed significant
SGS, resulting in spatial clusters of closely related individuals. During the next transition to older seedling and sapling stages, random and non-random mortality have led to the thinning
of family clusters and, eventually, to the reduction of SGS. In addition, across generations, non-equilibrium processes, spatial heterogeneity in microhabitat conditions and overlapping
generations are potential drivers of changes in SGS between adults and juveniles of the next generation. Corroborating the findings of previous studies, our results highlight the importance
of a multistage perspective to understand genetic patterns within populations of trees. Further, the comparison of data on SGS and on contemporary gene flow across different life stages can
help to assess the development of SGS of trees. DATA ARCHIVING The genotype data of all individuals of the five life stages are available from the Dryad Digital Repository:
doi:10.5061/dryad.39fs7. REFERENCES * Banks SC, Peakall R . (2012). Genetic spatial autocorrelation can readily detect sex-biased dispersal. _Mol Ecol_ 21: 2092–2105. Google Scholar *
Berens DG, Griebeler EM, Braun C, Chityi BB, Nathan R, Böhning-Gaese K . (2013). Changes of effective gene dispersal by pollen and seeds across successive life stages in a tropical tree.
_Oikos_ 122: 1616–1625. Google Scholar * Berg EE, Hamrick JL . (1995). Fine-scale genetic-structure of a Turkey oak forest. _Evolution_ 49: 110–120. Google Scholar * Born C, Hardy OJ,
Chevallier M-H, Ossari S, Attéké C, Wickings EJ _et al_. (2008). Small-scale spatial genetic structure in the Central African rainforest tree species _Aucoumea klaineana_: a stepwise
approach to infer the impact of limited gene dispersal, population history and habitat fragmentation. _Mol Ecol_ 17: 2041–2050. Google Scholar * Chung MY, Epperson BK, Chung MG . (2003).
Genetic structure of age classes in _Camellia japonica_ (Theaceae). _Evolution_ 57: 62–73. Google Scholar * Cipriani G, Lot G, Huang WG, Marrazzo MT, Peterlunger E, Testolin R . (1999).
AC/GT and AG/CT microsatellite repeats in peach [_Prunus persica_ (L) Batsch]: isolation, characterisation and cross-species amplification in _Prunus_. _Theor Appl Genet_ 99: 65–72. Google
Scholar * Connell JH . (1971). On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR, (eds)
_Dynamics of Populations_. Centre for Agricultural Publishing and Documentations: Wageningen. pp 298–310. Google Scholar * Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto
A _et al_. (2002). Development of microsatellite markers in peach [_Prunus persica_ (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (_Prunus avium_ L.).
_Theor Appl Genet_ 105: 127–138. Google Scholar * El Mousadik A, Petit RJ . (1996). High level of genetic differentiation for allelic richness among populations of the argan tree [_Argania
spinosa_ (L.) Skeels] endemic to Morocco. _Theor Appl Genet_ 92: 832–839. Google Scholar * Farwig N, Böhning-Gaese K, Bleher B . (2006). Enhanced seed dispersal of _Prunus africana_ in
fragmented and disturbed forests? _Oecologia_ 147: 238–252. Google Scholar * Farwig N, Braun C, Böhning-Gaese K . (2008). Human disturbance reduces genetic diversity of an endangered
tropical tree, _Prunus africana_ (Rosaceae). _Conserv Genet_ 9: 317–326. Google Scholar * Frankham R . (1995). Effective-population size adult-population size ratios in wildlife—a review.
_Genet Res_ 66: 95–107. Google Scholar * Goudet J . (1995). FSTAT (Version 1.2): a computer program to calculate F-statistics. _J Hered_ 86: 485–486. Google Scholar * Hall JB, ÓBrien EM,
Sinclair FL . (2000) _Prunus africana_—a monograph. School of Agricultural and Forest Sciences. Publication Number 18, University of Wales, Bangor. * Hamrick JL, Murawski DA, Nason JD .
(1993). The influence of seed dispersal mechanisms on the genetic structure of tropical tree populations. _Vegetatio_ 107–108: 281–297. Google Scholar * Hardy OJ, Vekemans X . (2002).
SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. _Mol Ecol Notes_ 2: 618–620. Google Scholar * Heuertz M, Vekemans X,
Hausman JF, Palada M, Hardy OJ . (2003). Estimating seed vs. pollen dispersal from spatial genetic structure in the common ash. _Mol Ecol_ 12: 2483–2495. Google Scholar * Jacquemyn H, Brys
R, Vandepitte K, Honnay O, Roldan-Ruiz I . (2006). Fine-scale genetic structure of life history stages in the food-deceptive orchid _Orchis purpurea_. _Mol Ecol_ 15: 2801–2808. Google
Scholar * Janzen DH . (1970). Herbivores and number of tree species in tropical forests. _Am Nat_ 104: 501–528. Google Scholar * Jones FA, Hubbell SP . (2006). Demographic spatial genetic
structure of the neotropical tree _Jacaranda copaia_. _Mol Ecol_ 15: 3205–3217. Google Scholar * Jones FA, Hamrick JL, Peterson CJ, Squiers ER . (2006). Inferring colonization history from
analyses of spatial genetic structure within populations of _Pinus strobus_ and _Quercus rubra_. _Mol Ecol_ 15: 851–861. Google Scholar * Kalinowski ST, Taper ML, Marshall TC . (2007).
Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. _Mol Ecol_ 16: 1099–1106. Google Scholar * Kalisz S, Nason JD, Hanzawa FM,
Tonsor SJ . (2001). Spatial population genetic structure in _Trillium grandiflorum_: the roles of dispersal, mating, history, and selection. _Evolution_ 55: 1560–1568. Google Scholar *
KIFCON. (1994) Kakamega guide. The official guide. Kenya Indigenous Forest Conservation Programme, Nairobi. * Latouche-Hallé C, Ramboer A, Bandou E, Caron H, Kremer A . (2003). Nuclear and
chloroplast genetic structure indicate fine-scale spatial dynamics in a neotropical tree population. _Heredity_ 91: 181–190. Google Scholar * Linhart YB, Grant MC . (1996). Evolutionary
significance of local genetic differentiation in plants. _Annu Rev Ecol Syst_ 27: 237–277. Google Scholar * Loveless MD, Hamrick JL . (1984). Ecological determinants of genetic-structure in
plant populations. _Annu Rev Ecol Syst_ 15: 65–95. Google Scholar * Lung T . (2004) Landbedeckungsänderungen im Gebiet ‘Kakamega Forest und assoziierte Waldgebiete’
(Westkenia)—Multispektrale Klassifikation von Landsat-Satellitenbilddaten und Auswertung mittels Methoden im Raster-GIS. Karlsruher Geowissenschaftliche Schriften—Reihe A 15, Karlsruhe
University of Applied Sciences, Karlsruhe, Germany. * Mitchell N, Schaab G, Wägele JW . (2009) Kakamega Forest ecosystem: an introduction to the natural history and the human context.
Karlsruher Geowissenschaftliche Schriften, Reihe A, Band 17, Karlsruhe University of Applied Sciences, Karlsruhe. * Pakkad G, James C, Torre F, Elliott S, Blakesley D . (2003). Genetic
variation of _Prunus cerasoides_ D. Don, a framework tree species in northern Thailand. _New Forests_ 27: 189–200. Google Scholar * Pardini EA, Hamrick JL . (2008). Inferring recruitment
history from spatial genetic structure within populations of the colonizing tree _Albizia julibrissin_ (Fabaceae). _Mol Ecol_ 17: 2865–2879. Google Scholar * Peakall R, Smouse PE . (2006)
GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research _Mol Ecol Notes_ 6: 288–295. * Petit RJ, Hampe A . (2006). Some evolutionary consequences of being
a tree. _Ann Rev Ecol Evol Syst_ 37: 187–214. Google Scholar * Peakall R, Smouse PE . (2012). GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-
an update. _Bioinformatics_ 28: 2537–2539. Google Scholar * R Core Team. (2013) _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing: Vienna,
Austria. URL http://www.R-project.org/. * Schueler S, Tusch A, Schuster M, Ziegenhagen B . (2003). Characterization of microsatellites in wild and sweet cherry (_Prunus avium_ L.)—markers
for individual identification and reproductive processes. _Genome_ 46: 95–102. Google Scholar * Smouse PE, Peakall R, Gonzales E . (2008). A heterogeneity test for fine-scale spatial
genetic structure. _Mol Ecol_ 17: 3389–3400. Google Scholar * Sosinski B, Gannavarapu M, Hager LD, Beck LE, King GJ, Ryder CD _et al_. (2000). Characterization of microsatellite markers in
peach [_Prunus persica_ (L.) Batsch]. _Theor Appl Genet_ 101: 421–428. Google Scholar * Stoeckel S, Grange J, Fernandez-Manjarres JF, Bilger I, Frascaria-Lacoste N, Mariette S . (2006).
Heterozygote excess in a self-incompatible and partially clonal forest tree species–_Prunus avium_ L. _Mol Ecol_ 15: 2109–2118. Google Scholar * Swaine M, Whitmore TC . (1988). On the
definition of ecological species groups in tropical rain forests. _Vegetatio_ 75: 81–86. Google Scholar * Tesfaye G, Teketay D, Fetene M . (2002). Regeneration of fourteen tree species in
Harenna forest, southeastern Ethiopia. _Flora_ 197: 461–474. Google Scholar * Tsingalia MH . (1989). Variation in seedling predation and herbivory in _Prunus africana_ in the Kakamega
Forest, Kenya. _Afr J Ecol_ 27: 207–217. Google Scholar * Ueno S, Tomaru N, Yoshimaru H, Manabe T, Yamamoto S . (2002). Size-class differences in genetic structure and individual
distribution of _Camellia japonica_ L. in a Japanese old-growth evergreen forest. _Heredity_ 89: 120–126. Google Scholar * Vaughan SP, Russell K . (2004). Characterization of novel
microsatellites and development of multiplex PCR for large-scale population studies in wild cherry _Prunus avium_. _Mol Ecol Notes_ 4: 429–431. Google Scholar * Vekemans X, Hardy OJ .
(2004). New insights from fine-scale spatial genetic structure analyses in plant populations. _Mol Ecol_ 13: 921–935. Google Scholar * Wang H, Qi MQ, Cutler AJ . (1993). A simple method of
preparing plant samples for PCR. _Nucleic Acids Res_ 21: 4153–4154. Google Scholar * Zhou H-P, Chen J . (2010). Spatial genetic structure in an understorey dioecious fig species: the roles
of seed rain, seed and pollen-mediated gene flow, and local selection. _J Ecol_ 98: 1168–1177. Google Scholar Download references ACKNOWLEDGEMENTS We thank the KWS for granting access to
Kakamega Forest. Fieldwork greatly benefited from the assistance of BB Chityui, NK Sajita, S Tillmann, J Kitschke, R Berens, R Dohm and numerous other people. We also thank J Albrecht, N
Farwig, A Hampe, S Liepelt and three anonymous reviewers for useful input and E and N Fouts for English proofreading. Financial support was provided by the BMBF (01LC0625E1). RN gratefully
acknowledges support from the Israel Science Foundation (ISF-FIRST 1316/05 and ISF 150/07), the US National Science Foundation (DEB-0453665), the Adelina and Massimo Della Pergola Chair of
Life Sciences, and the Friedrich Wilhelm Bessel Research Award of the Humboldt Foundation. DGB is funded by the Robert Bosch Foundation. This study is part of the PhD thesis of DGB at the
University of Mainz. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ecology, Institute for Zoology, Johannes Gutenberg-University of Mainz, Mainz, Germany D G Berens, C Braun, E
M Griebeler & K Böhning-Gaese * Department of Ornithology, National Museums of Kenya, Nairobi, Kenya D G Berens & K Böhning-Gaese * Department of Forest Ecology and Genetics,
CIFOR-INIA, Madrid, Spain S C González-Martínez * Department of Ecology, Movement Ecology Lab, Evolution and Behavior, The Hebrew University of Jerusalem, Edmond J Safra Campus, Givat Ram,
Jerusalem, Israel R Nathan * Biodiversity and Climate Research Centre (BiK-F), Senckenberg Gesellschaft für Naturforschung, Frankfurt (Main), Germany K Böhning-Gaese * Department of
Biological Sciences, Goethe University, Frankfurt (Main), Germany K Böhning-Gaese Authors * D G Berens View author publications You can also search for this author inPubMed Google Scholar *
C Braun View author publications You can also search for this author inPubMed Google Scholar * S C González-Martínez View author publications You can also search for this author inPubMed
Google Scholar * E M Griebeler View author publications You can also search for this author inPubMed Google Scholar * R Nathan View author publications You can also search for this author
inPubMed Google Scholar * K Böhning-Gaese View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D G Berens. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on Heredity website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION (DOC 120 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Berens, D., Braun, C., González-Martínez, S. _et al._
Fine-scale spatial genetic dynamics over the life cycle of the tropical tree _Prunus africana_. _Heredity_ 113, 401–407 (2014). https://doi.org/10.1038/hdy.2014.40 Download citation *
Received: 30 June 2013 * Revised: 14 March 2014 * Accepted: 17 March 2014 * Published: 21 May 2014 * Issue Date: November 2014 * DOI: https://doi.org/10.1038/hdy.2014.40 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative
Trending News
The New Normal — AARP0:52 AARP Videos The New Normal — AARP If you knew that your children or grandchildren would live to 100, how would you ...
No differences with bjp, says bdjs president thushar vellapally* Home * News * No differences with BJP, says BDJS President Thushar Vellapally IT IS THE VIEW OF SNDP AND NOT THAT OF B...
Madhya pradesh assembly elections 2018: 745 evms, vvpat machines replaced; congress writes to cecJyotiraditya Scindia addressing media over EVM malfunctioning  |  Photo Credit: ANI BHOPAL: As m...
No, i believe it’s supposed to say, “bernie is going to win” but a few of those i’s were not…No, I believe it’s supposed to say, “Bernie is going to win” but a few of those i’s were not italicized...
Trading negative eu for positive nations is a big plus says ross clarkYesterday, the European Commission announced that it is taking Britain to court - its own court, the European Court of J...
Latests News
Fine-scale spatial genetic dynamics over the life cycle of the tropical tree prunus africanaABSTRACT Studying fine-scale spatial genetic patterns across life stages is a powerful approach to identify ecological p...
Warriors vs Dolphins, Cross Pool at Gqeberha, CSA 4-Day, Mar 16 2021 - Full ScorecardMatches (14)IPL (1)ENG vs WI (1)WCL 2 (1)ENG-W vs WI-W (1)Vitality Blast Men (3)WI-A vs SA-A (1)Kwibuka WT20 (4)Vitality...
Programmers can now access test io code qa service in jira development tool | techcrunchSoftware quality-assurance testing has sometimes taken a backseat in today’s rush-rush agile development environment. In...
Google and intel to team up on android phones and tabletsNaming a powerful ally in its quest to become the king of smartphones, Google Inc. said it was teaming up with Intel Cor...
Believe it or not, study shows bengaluru’s traffic jams decreased in 2021While traffic continues to be a hurdle, Bengaluru has seen a sharp increase in the number of electric vehicles (EV) on t...