Neuronal survival in the brain: neuron type-specific mechanisms
Neuronal survival in the brain: neuron type-specific mechanisms"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Neurogenic regions of mammalian brain produce many more neurons that will eventually survive and reach a mature stage. Developmental cell death affects both embryonically produced
immature neurons and those immature neurons that are generated in regions of adult neurogenesis. Removal of substantial numbers of neurons that are not yet completely integrated into the
local circuits helps to ensure that maturation and homeostatic function of neuronal networks in the brain proceed correctly. External signals from brain microenvironment together with
intrinsic signaling pathways determine whether a particular neuron will die. To accommodate this signaling, immature neurons in the brain express a number of transmembrane factors as well as
intracellular signaling molecules that will regulate the cell survival/death decision, and many of these factors cease being expressed upon neuronal maturation. Furthermore, pro-survival
factors and intracellular responses depend on the type of neuron and region of the brain. Thus, in addition to some common neuronal pro-survival signaling, different types of neurons possess
a variety of 'neuron type-specific' pro-survival constituents that might help them to adapt for survival in a certain brain region. This review focuses on how immature neurons
survive during normal and impaired brain development, both in the embryonic/neonatal brain and in brain regions associated with adult neurogenesis, and emphasizes neuron type-specific
mechanisms that help to survive for various types of immature neurons. Importantly, we mainly focus on _in vivo_ data to describe neuronal survival specifically in the brain, without
extrapolating data obtained in the PNS or spinal cord, and thus emphasize the influence of the complex brain environment on neuronal survival during development. SIMILAR CONTENT BEING VIEWED
BY OTHERS FORMATION AND INTEGRATION OF NEW NEURONS IN THE ADULT HIPPOCAMPUS Article 25 February 2021 NEURONAL MATURATION AND AXON REGENERATION: UNFIXING CIRCUITRY TO ENABLE REPAIR Article
20 August 2024 HUMAN NEURONAL MATURATION COMES OF AGE: CELLULAR MECHANISMS AND SPECIES DIFFERENCES Article 23 November 2023 FACTS * During development neurons express a set of
pro-survival/death molecules that are not present in adult brain. * Neuronal survival in the brain often relies on different external factors in comparison with the spinal cord and PNS. *
Different types of neurons in the brain possess some common, but also distinct components of pro-survival signaling. * Immature neurons are more vulnerable to stress factors that trigger
neuronal death than mature neurons. OPEN QUESTIONS * How abundant are distinct components of pro-survival signaling in different types of neurons that might adapt neuronal survival to the
region of the brain, that is, neuron type-specific survival? * How do survival mechanisms of embryonically and adult-born neurons differ, that is, survival in immature _versus_ mature brain?
* During what period of brain development do the various types of neurons die? * What mechanisms account for higher vulnerability of immature neurons to stress factors? During brain
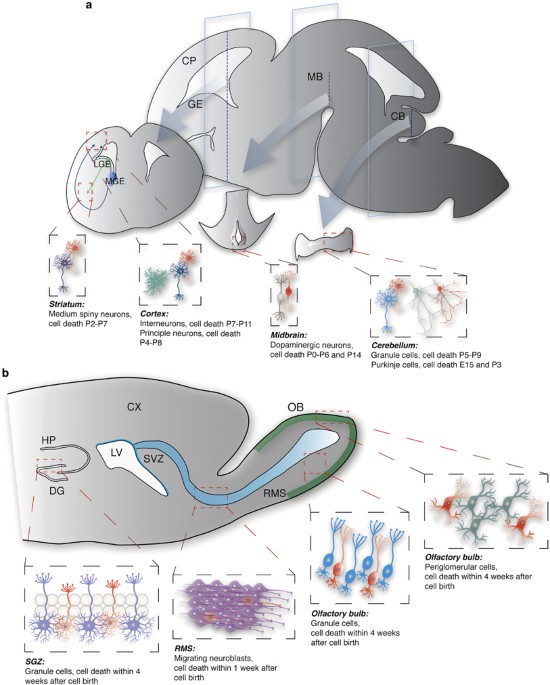
development, an excessive number of neurons is generated and, depending on the region and neuronal type, a varying number of neurons die before they mature.1, 2, 3, 4, 5 A high rate of
neuronal death also occurs in the regions of adult neurogenesis.6, 7, 8, 9 The process of neuronal overproduction and elimination is necessary to optimize brain connectivity. Disturbances in
regulating developmental neuronal death not only change cell composition and connectivity within local neuronal networks, but also alter global brain activity and, thus, cognition. Several
types of brain disorders enhance the death of immature neurons (i.e., postmitotic neurons, but before complete maturation) during brain development that could lead to decline in cognitive
abilities. After maturation, neurons become resistant to the signaling that was involved in the life/death decision at immature stages since, once neurogenesis is halted, it is advantageous
to protect mature neurons that cannot be produced again (protection of immature and mature neurons is compared in Benn and Woolf10 and Kole _et_ _al._11). There are two distinct modes of
neurogenesis – although the majority of neurons are generated during the embryonic period and their production is discontinued either in the embryonic brain or early postnatally (later
referred to as embryonic neurogenesis),12 some populations of neurons are continuously generated throughout the life of an animal (later referred to as adult neurogenesis)13, 14 (see Figures
1a and b, respectively). The death of neurons that are born embryonically reaches a peak in the neonatal brain and affects neurons that are still immature,15, 16, 17 and the critical period
for survival of adult-generated neurons is within 4 weeks after their birth; following this period of maturation, they become resistant to cell death.8, 9, 18 Principles of neuronal
survival are often generalized and data from different areas of the CNS are extrapolated to the CNS as a whole. Indeed, pro-survival signaling does converge on some common core components
(Figure 2). However, data accumulated over the recent years show that different types of neurons in the brain might use different pro-survival mechanisms as there are a variety of routes by
which core pro-survival components could be activated. Thus, we propose 'neuron type-specific' pro-survival mechanisms that will heavily rely upon (1) composition of extracellular
pro-survival factors that are available in a certain brain area at a certain time period, (2) composition of transmembrane molecules (e.g. receptors or ion channels) that are expressed on
distinct types of neurons and (3) composition of cytosolic molecules that could propagate pro-survival signaling from the cell membrane toward common core components (Figure 2). It should be
noted that not only pro-survival, but also pro-death pathways could be neuron type specific. In general terms, it is pro-survival signaling that blocks intrinsic pro-death signaling, and
when there is a lack of pro-survival signaling, pro-death pathways are triggered. However, in a recent paper19 it was shown that survival of CNS neurons during development is regulated by
'dependence receptors' that activate pro-death signaling when not bound to their ligands (reviewed in Dekkers _et al._20). Although the extent of expression and the number of
dependence receptors still remain to be determined in the developing brain, the presence of such a mechanism indicates that neuron type-specific pro-death pathways do exist. NEURON
TYPE-SPECIFIC PRO-SURVIVAL MECHANISMS As different types of neurons survive in different brain areas and at different periods of brain development, the transcriptome of the surviving neuron
should 'prepare' the neuron to survive in a certain environment. The preparation is coordinated by distinct sets of transcription factors that are involved in differentiation of
specific types of neurons. These transcription factors drive expression of transmembrane and intracellular molecules that are necessary to recognize and respond to the local environment.
Neurons failing to differentiate properly are less likely to respond to signals from local brain environment and could be eliminated during maturation. Interestingly, the period of
developmental cell death differs across types of neurons/brain areas. For instance, GABAergic interneurons of the cortex and medium spiny neurons exhibit one peak of cell death at P7-P111
and P2-P7,21 respectively, whereas two distinct peaks of developmental cell death have been observed for dopaminergic neurons, at P0-P6 and ~P14,2 and for Purkinje cells, at ~E15 and ~P3.22
The difference in survival mechanisms between embryonically and adult-born neurons illustrates the importance of time period of neuronal survival with regard to brain maturation, since
embryonically born _immature_ neurons must survive in _immature_ brain, whereas adult-born _immature_ neurons must survive in _mature_ brain. Thus, there is high pressure for adult-born
neurons to integrate into the pre-existing mature circuits, which is absent for embryonically born neurons. This is supported, for instance, by a higher vulnerability of adult-born neurons
to impairment in NMDA receptor (NMDAR) expression, since ablation of NR1 or NR2B subunit markedly augments death of adult-born neurons during maturation,23, 24, 25 whereas studies of global
or early postnatal knockout of these subunits do not report increase in apoptosis of embryonically produced neurons.26, 27, 28 The effect of brain maturation on neuronal survival might also
be illustrated by a decrease in survival of small axonless neurons – a type of neurons that is generated both during embryonic and adult neurogenesis.29 The majority of these neurons survive
in the deep cortical layers when circuits are still immature, and gradual maturation of the brain correlates with a decreased number of newly added neurons,29 although the number of these
neurons could be increased by pathological conditions such as stroke.30 Support of neuronal survival by the local environment depends on whether a specific factor itself and its receptor are
expressed in the region. Availability of pro-survival factors varies within the brain and even cortical layers,31, 32, 33 and response to different pro-survival factors markedly changes
over a course of neuronal maturation.34 Moreover, certain intracellular pro-survival molecules are present only in some types of neurons, but not in others. For instance, BDNF promotes
survival of dopaminergic neurons, medium spiny neurons and cerebellar granule cells,35, 36, 37 but it is dispensable for survival of GABAergic neurons in the cortex1 although the latter
express TrkB receptor and BDNF is available in the surrounding environment.31, 38 In the following, we summarize the evidence for neuron type-specific pro-survival mechanisms during
embryonic and adult neurogenesis (see overview in Table 1). EMBRYONIC NEUROGENESIS: GLUTAMATERGIC NEURONS The most information regarding survival of glutamatergic neurons in the brain was
obtained by studying cerebellar granule cells and principal neurons of the hippocampus and cortex (Figure 3a). The peak of cortical principal neuron cell death is at P4–P8,39 whereas the
majority of immature cerebellar granule cells die at P5–P9.40 Although knockout of a single neurotrophic factor or its receptor does not have large effects on neuronal survival during brain
development,41 double knockout of _Ntrk2_ and _Ntrk3_ (genes coding for TrkB and TrkC, respectively) results in the massive death of immature granule cells in the cerebellum and dentate
gyrus.42 This could be explained either by redundancy of intracellular pro-survival pathways that are triggered by each of the receptors or by compensatory effects in knockout mice.
Furthermore, often data obtained _in vivo_ differs from _in vitro_ experiments, highlighting importance of brain environment for action of a particular pro-survival factor. For instance,
BDNF was shown to promote neuronal survival in the culture,43 but deletion of _Bdnf_ in all postmitotic neurons in the brain did not have a large effect on their survival.44 Granule cells of
the cerebellum represent a population of glutamatergic neurons that could be a target of pro-survival action of BDNF. Deleting _Camk4_ and _Camkk2_ genes in mice enhances apoptosis in
immature granule cells in the cerebellum, which is associated with a decrease in levels of CREB1 and BDNF expression.37 It was proposed that Ca2+ entering immature granule cells triggers
activation of the calmodulin/CaMKK2/CaMKIV cascade, which, in turn, activates CREB1 and transcription of _Bdnf_ gene.37 Survival of granule cells is also promoted by IGF1 that enhances
expression of Bcl-2 and Bcl-xL thus inhibiting caspase-3 activity.45 The existence of neuron type-specific pro-survival mechanisms in glutamatergic neurons was recently highlighted by the
identification of a pro-survival pathway that was largely restricted to cortical principal neurons of layer V, which require trophic support from microglia to survive during early postnatal
development.46 Microglia secrete IGF1, which binds to IGF1R on immature layer V neurons and activates the IRS1/PI3K/Akt1 cascade inhibiting caspase-3-dependent apoptosis.46 Microglia are
activated via CX3CL1, which is released from layer V neurons and interacts with CX3CR1 on microglia. Interestingly, caspase-3-dependent apoptosis of cortical excitatory, but not inhibitory,
neurons was shown to be activated by Rho GTPase RhoA.47 Inhibiting RhoA signaling in the developing brain rescues up to 25% of cortical neurons from apoptosis. EMBRYONIC NEUROGENESIS:
GABAERGIC NEURONS Only few studies have investigated developmental death of GABAergic neurons, and these were mainly focused on Purkinje cells of the cerebellum and medium spiny neurons of
the striatum that exhibit a peak of cell death at ~E15 and ~P3,22 and at P2–P7,21 respectively (Figure 3b). Lhx1/Lhx5 transcription factors together with their co-activator Ldb1 promote
survival of postmitotic Purkinje cells at E13.5–E15.5.48 Interestingly, two members of the EBF (early B-cell factor) family of transcription factors – EBF1 and EBF2 – are involved in
survival of medium spiny49 and Purkinje neurons,50 respectively, during perinatal development. In Purkinje cells, EBF2 binds to _Igf1_ promoter and activates _Igf1_ expression that results
in local IGF1 secretion and potentiation of Akt1-dependent pro-survival signaling.51 All the aforementioned transcription factors were also shown to be involved in differentiation and/or
migration of medium spiny and Purkinje neurons, and thus immature neurons might die because they are not able to complete their differentiation programs. Although, overall, neurotrophins do
not have a large role in survival of immature GABAergic neurons, BDNF and NT-3 were shown to enhance survival of immature medium spiny neurons, as they are secreted by midbrain dopaminergic
neurons during a critical period of striatal neuron survival and activate pro-survival signaling via TrkB and TrkC receptors.35 Recently, it was shown that around 40% of immature cortical
GABAergic interneurons die during the first two postnatal weeks (with the peak at P7–P11).1 Their survival did not depend on TrkB expression, but was regulated by either cell-autonomous or
population-autonomous mechanisms that activated pro-apoptotic Bax signaling. EMBRYONIC NEUROGENESIS: DOPAMINERGIC NEURONS Apoptosis of immature dopaminergic neurons occurs at two
developmental stages – at P0–P6 and ~P14.2 Three main transcription factors involved in specification dopaminergic neurons – _Nurr1_, _Pitx3_ and _En1_ – also regulate their survival.52, 53,
54, 55 Both Nurr1 and Pitx3 were shown to activate expression of BDNF,56, 57 which promotes survival of a subpopulation of dopaminergic neurons from E16 onward36 via TrkB receptors58, 59
(Figure 3c). Another BDNF receptor, low-affinity neurotrophin receptor p75NTR, promotes cell death of immature dopaminergic neurons.60 Expression of p75NTR is repressed by En1/2,60 and as
En1 was also proposed to co-activate expression of Nurr1-dependent genes,61 En1 could enhance survival of immature dopaminergic neurons via two pathways – enhancing BDNF expression (via
Nurr1) and repressing p75NTR expression. Pro-death signaling from p75NTR suppresses ERK1/2 activity and likely inhibits anti-apoptotic activity of Bcl-2 family members,60 thus activating a
classical apoptosis pathway via Bax, caspase-3 and caspase-9.62 Caspase-3/-9 activation is inhibited by dual-specificity tyrosine-phosphorylation regulated kinase 1A (Dyrk1a), a Down
syndrome-associated gene.63 Involvement of neuron type-specific signaling in survival of dopaminergic neurons is highlighted by inhibition of developmental apoptosis by TGF_β_-Smad-Hipk2
pathway.64 Interestingly, although transforming growth factor (TGF) _β_1 and _β_2 had little effect on modulation of survival of immature dopaminergic neurons, stimulation by TGF_β_3 led to
activation of Smad2/3 that directly interacted with Hipk2 and inhibited caspase-3-dependent apoptosis. ADULT NEUROGENESIS: SUBVENTRICULAR ZONE (SVZ) Survival of postnatally born neurons in
the olfactory bulb is regulated by neuronal activity (Figure 4a). Ablation or enhancement of olfactory activity onto maturing granule cells decreases or increases their survival,
respectively.65, 66 However, similar enhancement does not affect periglomerular neurons,9, 66 which could be explained by neuron type-specific pro-survival mechanisms. Furthermore,
stimulation of periglomerular neurons by a single odorant decreases their survival in the region that is activated by the odorant.9 Apoptosis is stimulated by connective tissue growth factor
(CTGF) that, in combination with TGF_β_2, activate TGF_β_Rs and Smads in immature periglomerular neurons.9 Few neurotransmitter receptors on newborn SVZ neurons mediate pro-survival effects
of neuronal activation. Glutamate NMDAR activity is required for survival of neuroblasts during their migration from the SVZ through the RMS and when maturing in the olfactory bulb.23, 67
This pro-survival effect likely depends on Ca2+ that enters into neuroblasts via NMDAR. When already in the olfactory bulb, expression of nicotinic acetylcholine receptor (nAChR) subunit
_β_2 regulates apoptosis in newborn granule cells.68 Knockout of the subunit results in 50% increase in survival of immature neurons, and stimulation of nAChR could be considered as another
'negative' regulator of immature neuronal survival in postnatal neurogenesis, similar to CTGF. Phosphorylation of CREB1 was shown to promote survival of SVZ-derived neuroblasts,69,
70 where CREB1 might be activated by Ca2+ signaling via calmodulin and CaMKIV.71, 72 As NMDAR are involved in survival of SVZ neuroblasts,23, 67 and upon opening they allow Ca2+ entry into
neuroblasts,67 it is likely that Ca2+ entry via NMDAR triggers CREB1-dependent pro-survival cascade (although other receptors on neuroblasts could also mediate Ca2+ entry).72, 73 Knockout of
_Creb1_ was shown to decrease expression of the polysialylated isoform of the neural cell adhesion molecule (PSA-NCAM),70 which, in turn, could promote survival of immature olfactory bulb
neurons by inhibiting p75NTR expression.74 Among p75NTR activating neurotrophins only the role of BDNF in postnatal SVZ neurogenesis has been studied, and _Ntrk2_ knockout decreases the
survival of dopaminergic periglomerular neurons, but not any other cells.75, 76 Mammalian target of rapamycin (mTOR) pathway promotes the survival of SVZ neuroblasts via hypoxia-inducible
factor 1a (HIF1A).77 Tuberous sclerosis proteins 1 and 2 (TSC1/2) inhibit mTOR, and HIF1A is strongly upregulated in _Tsc1−/−_ neuroblasts, thereby increasing their survival.77 mTOR is most
likely activated by PI3K/Akt1 signaling as many components of this pathway were shown to be present in SVZ neuroblasts.72, 78 Finally, pro-survival signaling in newborn SVZ neurons converges
on Bcl-2 family members and caspase−3/−9.7, 79 ADULT NEUROGENESIS: SUBGRANULAR ZONE (SGZ) Less is known regarding neuronal survival in the SGZ in comparison with the SVZ. Activation of
NMDAR on newborn SGZ neurons enhances their survival,24 and it is likely that the pro-survival effect depends on Bcl-2 stimulation (Figure 4b).80 Protection of newborn dentate gyrus neurons
by Bcl-2 signaling was also shown in transgenic mice that overexpress Bcl-2.81 Bcl-2 activity might be stimulated by Akt1 signaling, which was shown to enhance neuronal survival in the
SGZ.82 Cyclin-dependent kinase-like 5 (CDKL5) activates Akt1 and also inhibits Gsk-3_β_ thus activating CREB1-dependent gene expression. Similar to the SVZ, apoptosis in newborn SGZ neurons
converges on Bcl-2/Bax activity.6 Two growth factors promote survival of granule cells in the SGZ – TGF_β_1 and IGF1.83, 84 Importantly, both factors have little (if any) contribution to
survival of adult-born neurons in the olfactory bulb,9, 85 indicating neuron type-specific role of TGF_β_1 and IGF1 in survival of adult-born neurons. COMMON SIGNALING THAT REGULATES
NEURONAL SURVIVAL IN THE BRAIN Many neuron type-specific pro-survival pathways eventually converge on pro-apoptotic and pro-survival members of Bcl-2 family and caspase-3/caspase-9 (Figure
2). Neuronal apoptosis in the brain is inhibited by Bcl-2 and Bcl-xL pro-survival proteins,86, 87, 88, 89 whereas pro-apoptotic proteins, mainly Bax and Bak, promote neuronal death.87, 88
Massive death of immature neurons in the brain of _Bcl2l1−/−_ (gene name for Bcl-xL) mice suggests that Bcl-xL is the major neuronal pro-survival protein of Bcl-2 family,86, 87 and it
becomes important for survival only at the stage of postmitotic neurons, but not before.88 Another anti-apoptotic member of the Bcl-2 family, myeloid cell leukemia 1 (Mcl-1), was also shown
to be critical for survival of immature neurons during embryonic development.90 Several transcription factors promote neuronal survival, most likely by activating transcription of
pro-survival genes and/or inhibiting pro-apoptotic genes. A family of myocyte enhancer factor 2 (MEF2) transcription factors, MEF2A, 2C and 2D, are expressed in the mouse brain during
development and are critical for the survival of immature neurons.91 Widespread loss of neurons was also reported for knockout of another transcription factor – p73 (a member of p53 family
proteins).92 The loss of neurons started to be visible during second postnatal week, and was attributed to the anti-apoptotic role of the truncated form of p73, ΔNp73, which antagonizes p53
function and inhibits Bax and caspase-3/-9-dependent apoptosis.93 Finally, members of the CREB family of transcription factors, CREB1 and CREM, activate pro-survival signaling in postmitotic
neurons around the time of perinatal development (E16.5-P0).94 Activity-dependent survival of immature neurons via action of GABA and/or glutamate neurotransmitters was proposed for many
neuronal subtypes.95 For instance, deletion of syntaxin-binding protein 1 (_Stxbp1_) that is required for synaptogenesis and neurotransmission results in widespread neuronal death during
brain development.96 Furthermore, pharmacological inhibition of NMDAR leads to a pronounced decrease in survival of neurons during postnatal brain development.97, 98, 99 However, as
discussed above, knockouts of genes coding for NMDAR subunits show marked increase in neuronal death only during adult neurogenesis.26, 27, 28, 100 Neuronal activity also generates reactive
oxygen species (ROS) that could damage maturing neurons and trigger apoptosis. Protection from ROS is particularly important for immature neurons since they are often easier to excite than
mature ones.101, 102 It was recently shown that knockout of the gene coding for the antioxidant protein lanthionine synthetase C-like protein 1 (LanCL1) causes massive neuronal death in the
brain due to reduced glutathione-mediated antioxidant defense and via Bax activation.103 SURVIVAL OF NEURONS IN INJURED BRAIN Immature neurons are more vulnerable to stress factors than
mature neurons, as it is easier for external stimuli to trigger neuronal death during development than in adult brain.11 Although the exact mechanisms of such vulnerability are unknown, it
is likely that neurons over maturation devise a highly protective strategy against any external stress. Furthermore, expression of some pro-death molecules, for example, dependence
receptors,19, 20 could be limited to immature neurons. Therefore, similar stress factors might be more potent enhancers of neuronal death during development than in adult brain. In addition
to common stress factors that stimulate neuronal death both during development and in adult, few factors are specific for the developing brain – for instance, misplacement of neurons could
trigger their death due to impairment in neuronal connectivity. Certain types of immature neurons are more strongly affected by the stress than the others highlighting neuron type-specific
mechanisms of survival. Below we discuss factors that affect survival of neurons during abnormal brain development. OXIDATIVE STRESS Oxidative stress contributes to severe neurodevelopmental
deficits in the developing mammalian brain caused by chronic exposure to either reduced (hypoxia–ischemia) or elevated (hyperoxia) levels of oxygen (Figure 5). Perinatal hypoxia–ischemia or
neonatal stroke is the main cause of neurodevelopmental deficits in newborns. It is accompanied by an overall decrease in cortical and hippocampal volumes due to neuronal death and atrophy.
One of the major causes of neuronal death is excitotoxicity due to overactivation of NMDAR on immature neurons by the release of glutamate.104, 105 Pathological influx of Ca2+ via NMDAR is
followed by aberrant production of free radicals and mitochondrial dysfunction, which leads to the release of cytochrome C and, consequently, neuronal death.106, 107 Importantly,
interneurons were shown to be less susceptible to hypoxic cell death – although neonatal hypoxia slows maturation of interneurons, it does not affect their survival.108 A
glutamate-independent mechanism contributing to hypoxia–ischemia-induced neuronal death reveals transient receptor potential melastatin 7 (TRPM7) as a key factor.109 As early as 24 h after
neonatal ischemic insult, TRPM7 protein levels were upregulated, which might lead to increase in caspase-3-dependent apoptosis by inhibiting Akt1 and promoting Bax _versus_ Bcl-2 expression.
Overexposure to oxygen could cause hyperoxia in the brain, which was shown to affect preterm born neonates receiving oxygen supplementation.110 Hyperoxia mainly affects cortical areas and
in mice the effect on neuronal survival is most pronounced between P3 and P7.111 Apoptosis is caspase-3 dependent and could be enhanced because of decreased pro-survival signaling from Akt1
and Erk1/2.112 Importantly, the effect is limited to immature neurons, as hyperoxia at later ages does not affect neuronal survival. Hyperoxia also triggers an inflammatory response that
could further promote neuronal death via increased levels of several interleukins - IL-1_β_, IL-18 and IL-18 receptor _α_ (IL-18R_α_).113 FETAL ALCOHOL SPECTRUM DISORDERS (FASDS) FASDs are
triggered by gestational alcohol exposure and lead to impaired brain development accompanied by deficits in cognitive functions.114 Data from animal models of prenatal alcohol exposure
suggest that neuronal cell death is one of the major effects contributing to the disease phenotype (Figure 6).115 Early postnatal (P7) exposure of rats to EtOH induces widespread apoptosis,
indicated by increased activation of caspase-3 as early as 8 h and neurodegeneration within less than 24 h after EtOH treatment.116 Differential susceptibility of immature neurons to
alcohol-induced stress is underlined by variability of the extent of neuronal death in different brain regions. Thus, the retrosplenial cortex and hippocampus were most affected, whereas the
olfactory bulb and piriform cortex exhibited much less apoptosis.116 In another study, the overall architecture of mouse brains exposed to alcohol at P7 appeared to be unaltered, but the
number of calretinin-positive and parvalbumin-positive GABAergic neurons was strongly reduced, indicating that they are more prone to alcohol-induced cell death when immature.117 Misplacing
GABAergic neurons could contribute to their death since low doses of prenatal alcohol increase ambient GABA levels in the extracellular space and upregulate GABAA receptors on neuroblasts
that lead to aberrant neuroblast migration.118 Ethanol possesses NMDA antagonist and GABAA agonist activities and both activities could induce apoptosis during brain development.97, 119
Thus, apoptotic effects of ethanol exposure are closely related to those observed with either disrupted NMDA or elevated GABA signaling. The former has been extensively studied in immature
neurons using NMDAR inhibitors causing rapid neuronal death of both excitatory and inhibitory neurons associated with decreased Bcl-2, Erk1/2 and CREB1 and increased activated caspase-3
levels.120, 121, 122 Embryonically administered EtOH was also shown to decrease activation of pro-survival PI3K/Akt1 signaling and increase activation of glycogen synthase kinase-3_β_
(GSK-3_β_).123 The latter could stimulate neuronal death by activating Bax and, thus, caspase-3-dependent apoptosis.124 Neuronal cell loss as a consequence of alcohol exposure in models of
FASD can be attributed in part to oxidative stress. Analysis of the cerebella of P1 rats chronically exposed to ethanol from E6 shows a decrease in mRNA levels of mitochondrial respiration
complex genes in granule cells, combined with increased expression of pro-apoptotic p53 and oxidative stress markers.125 EtOH also inhibits nuclear translocation of nuclear factor erythroid
2-related factor 2 (Nrf2), a transcription factor that is responsible for expression of those genes that protect against oxidative stress and reduce production of ROS.126 In the cerebellum,
ROS can activate c-jun N-terminal kinase (JNK) at P4, but not at P7 rats, highlighting a time window in immature granule cells when they are most vulnerable to the oxidative stress.127, 128
JNK, in turn, removes pro-survival 14-3-3 protein from its dimer with Bax, thus making it possible for cytosolic Bax to translocate into the mitochondria leading to mitochondrial dysfunction
and neuronal apoptosis via release of cytochrome C. TRAUMATIC BRAIN INJURY (TBI) Although brain injury due to physical trauma is common in both developing and adult brains, the effect of
such injury on the immature brain is much more devastating.129 Strikingly, in a rat model of the disorder, the extent of neuronal apoptosis is age-related, with the P3–P7 brains being most
vulnerable.130 Apoptosis of immature neurons was associated with enhanced expression of c-Jun and reduced expression of Bcl-2 and Bcl-xL leading to the release of cytochrome C and neuronal
cell death.130, 131 Caspase-1 was shown to promote neuronal death by activating two proinflammatory cytokines, IL-1_β_ and IL-18, acting via IL-18 R on neurons.113, 132 Interestingly,
immature neurons are also the most affected by TBI population in the regions of adult neurogenesis in mice.133, 134 OTHER DISEASES Neuronal death contributes to phenotypic effects observed
in several other neurodevelopmental disorders. Defects in microtubules because of mutations in tubulin alpha or beta genes are often associated with cortical malformations (e.g.,
lissencephaly or polymicrogyria) because of neuronal misplacement and subsequent death of misplaced neurons.135 For instance, deletion of _Tubb2_ gene during brain development causes
aberrant neuronal migration and arrest of cells near the ventricles that eventually leads to enhanced neuronal apoptosis.135, 136 Although apoptosis was proposed to be augmented in a variety
of psychiatric disorders, including schizophrenia and autism spectrum disorders (ASDs), the data were often obtained by analyzing adult brains. Experimental evidence in younger brains is
rather limited to gene expression measurements using western blot or PCR.137 Furthermore, it remains to be investigated whether a reduction in the number of GABAergic neurons that was
reported in postmortem brains of patients with schizophrenia, bipolar disorder and ASDs138, 139 occurs before neuronal maturation is finished. In addition, it might be that the strength of
marker expression rather than the number of neurons is affected.140 Although knockout/knockdown of genes that are associated with psychiatric disorders has been reported to decrease the
number of immature neurons in mice,141 other studies showed that maturation rather than survival of immature neurons is affected.142, 143, 144 CONCLUSIONS The mammalian brain is the most
complex tissue and includes many more neuronal subtypes than other parts of the nervous system. During perinatal development and in the regions of adult neurogenesis, neurons in the brain
are overproduced and multitudes of immature neurons die before they reach maturity. Although there are certain core components of survival/apoptotic machinery in immature neurons, it seems
that various types of neurons also exploit pro-survival pathways that are specific only for one or few type(s) and not utilized in others. Such _neuron type-specific_ components of
pro-survival signaling could indicate adaptation toward an optimal survival rate of overproduced neurons according to type of neuron and brain region. The number, type and position of
neurons that survived should affect both local neuronal circuits and higher brain activities, for example, oscillations. As there is increasing evidence that some types of neurons are more
susceptible to certain injuries in the developing brain, more targeted therapeutic strategies might be needed to treat such brain disorders. The advantage of targeting neuron type-specific
pro-survival pathways is to avoid side effects of the therapy on other neuron/cell types that are not affected in the disorder. Future studies will determine the extent to which neuron
type-specific pro-survival signaling is utilized in normal brain and in pathological conditions and how it contributes to brain information processing. REFERENCES * Southwell DG, Paredes MF,
Galvao RP, Jones DL, Froemke RC, Sebe JY _et al_. Intrinsically determined cell death of developing cortical interneurons. _Nature_ 2012; 491: 109–113. Article CAS PubMed PubMed Central
Google Scholar * Oo TF, Burke RE . The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. _Brain Res Dev Brain Res_ 1997; 98:
191–196. Article CAS PubMed Google Scholar * Burek MJ, Oppenheim RW . Programmed cell death in the developing nervous system. _Brain Pathol_ 1996; 6: 427–446. Article CAS PubMed
Google Scholar * White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD . Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. _J Neurosci_ 1998; 18:
1428–1439. Article CAS PubMed PubMed Central Google Scholar * Lossi L, Merighi A . _In vivo_ cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. _Prog
Neurobiol_ 2003; 69: 287–312. Article CAS PubMed Google Scholar * Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW . Programmed cell death of adult-generated hippocampal neurons
is mediated by the proapoptotic gene Bax. _J Neurosci_ 2004; 24: 11205–11213. Article CAS PubMed PubMed Central Google Scholar * Kim WR, Kim Y, Eun B, Park OH, Kim H, Kim K _et al_.
Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. _J Neurosci_ 2007; 27: 14392–14403.
Article CAS PubMed PubMed Central Google Scholar * Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM . Learning and survival of newly generated neurons: when time
matters. _J Neurosci_ 2008; 28: 11511–11516. Article CAS PubMed PubMed Central Google Scholar * Khodosevich K, Lazarini F, von Engelhardt J, Kaneko H, Lledo PM, Monyer H . Connective
tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. _Neuron_ 2013; 79: 1136–1151. Article CAS PubMed Google Scholar * Benn SC, Woolf CJ
. Adult neuron survival strategies—slamming on the brakes. _Nat Rev Neurosci_ 2004; 5: 686–700. Article CAS PubMed Google Scholar * Kole AJ, Annis RP, Deshmukh M . Mature neurons:
equipped for survival. _Cell Death Dis_ 2013; 4: e689. Article CAS PubMed PubMed Central Google Scholar * Buss RR, Sun W, Oppenheim RW . Adaptive roles of programmed cell death during
nervous system development. _Annu Rev Neurosci_ 2006; 29: 1–35. Article CAS PubMed Google Scholar * Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH . Regulation and function of
adult neurogenesis: from genes to cognition. _Physiol Rev_ 2014; 94: 991–1026. Article CAS PubMed PubMed Central Google Scholar * Khodosevich K, Alfonso J, Monyer H . Dynamic changes in
the transcriptional profile of subventricular zone-derived postnatally born neuroblasts. _Mech Dev_ 2013; 130: 424–432. Article CAS PubMed Google Scholar * Ferrer I, Bernet E, Soriano
E, del Rio T, Fonseca M . Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. _Neuroscience_ 1990; 39: 451–458. Article CAS
PubMed Google Scholar * Ferrer I, Soriano E, del Rio JA, Alcantara S, Auladell C . Cell death and removal in the cerebral cortex during development. _Prog Neurobiol_ 1992; 39: 1–43.
Article CAS PubMed Google Scholar * Finlay BL, Slattery M . Local differences in the amount of early cell death in neocortex predict adult local specializations. _Science_ 1983; 219:
1349–1351. Article CAS PubMed Google Scholar * van Praag H, Kempermann G, Gage FH . Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. _Nat Neurosci_
1999; 2: 266–270. Article CAS PubMed Google Scholar * Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L _et al_. Neurotrophin receptors TrkA and TrkC cause
neuronal death whereas TrkB does not. _Nature_ 2010; 467: 59–63. Article CAS PubMed Google Scholar * Dekkers MP, Nikoletopoulou V, Barde YA . Cell biology in neuroscience: death of
developing neurons: new insights and implications for connectivity. _J Cell Biol_ 2013; 203: 385–393. Article CAS PubMed PubMed Central Google Scholar * Fishell G, van der Kooy D .
Pattern formation in the striatum: neurons with early projections to the substantia nigra survive the cell death period. _J Comp Neurol_ 1991; 312: 33–42. Article CAS PubMed Google
Scholar * Dusart I, Guenet JL, Sotelo C . Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. _Cerebellum_ 2006; 5: 163–173.
Article PubMed Google Scholar * Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C . Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain
circuits. _Neuron_ 2010; 65: 32–39. Article CAS PubMed PubMed Central Google Scholar * Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH . NMDA-receptor-mediated, cell-specific integration
of new neurons in adult dentate gyrus. _Nature_ 2006; 442: 929–933. Article CAS PubMed Google Scholar * Kelsch W, Li Z, Eliava M, Goengrich C, Monyer H . GluN2B-containing NMDA
receptors promote wiring of adult-born neurons into olfactory bulb circuits. _J Neurosci_ 2012; 32: 12603–12611. Article CAS PubMed PubMed Central Google Scholar * Forrest D, Yuzaki M,
Soares HD, Ng L, Luk DC, Sheng M _et al_. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. _Neuron_ 1994; 13: 325–338. Article CAS PubMed
Google Scholar * Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E _et al_. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD
in NMDA receptor epsilon 2 subunit mutant mice. _Neuron_ 1996; 16: 333–344. Article CAS PubMed Google Scholar * von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A _et
al_. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. _Neuron_ 2008; 60: 846–860. Article CAS PubMed Google
Scholar * Le Magueresse C, Alfonso J, Khodosevich K, Arroyo Martin AA, Bark C, Monyer H . "Small axonless neurons": postnatally generated neocortical interneurons with delayed
functional maturation. _J Neurosci_ 2011; 31: 16731–16747. Article CAS PubMed PubMed Central Google Scholar * Kreuzberg M, Kanov E, Timofeev O, Schwaninger M, Monyer H, Khodosevich K .
Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. _Exp Neurol_ 2010; 226: 90–99. Article CAS PubMed Google Scholar * Huang ZJ, Kirkwood A, Pizzorusso T,
Porciatti V, Morales B, Bear MF _et al_. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. _Cell_ 1999; 98: 739–755. Article CAS
PubMed Google Scholar * Katoh-Semba R, Takeuchi IK, Semba R, Kato K . Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. _J Neurochem_
1997; 69: 34–42. Article CAS PubMed Google Scholar * Patz S, Wahle P . Developmental changes of neurotrophin mRNA expression in the layers of rat visual cortex. _Eur J Neurosci_ 2006;
24: 2453–2460. Article PubMed Google Scholar * Catapano LA, Arnold MW, Perez FA, Macklis JD . Specific neurotrophic factors support the survival of cortical projection neurons at distinct
stages of development. _J Neurosci_ 2001; 21: 8863–8872. Article CAS PubMed PubMed Central Google Scholar * Baydyuk M, Xie Y, Tessarollo L, Xu B . Midbrain-derived neurotrophins
support survival of immature striatal projection neurons. _J Neurosci_ 2013; 33: 3363–3369. Article CAS PubMed PubMed Central Google Scholar * Baquet ZC, Bickford PC, Jones KR .
Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. _J Neurosci_ 2005; 25: 6251–6259.
Article CAS PubMed PubMed Central Google Scholar * Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR . BDNF-mediated cerebellar granule cell development is impaired in mice
null for CaMKK2 or CaMKIV. _J Neurosci_ 2009; 29: 8901–8913. Article CAS PubMed PubMed Central Google Scholar * Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A . Control of
cortical interneuron migration by neurotrophins and PI3-kinase signaling. _Development_ 2002; 129: 3147–3160. CAS PubMed Google Scholar * Verney C, Takahashi T, Bhide PG, Nowakowski RS,
Caviness VS Jr. . Independent controls for neocortical neuron production and histogenetic cell death. _Dev Neurosci_ 2000; 22: 125–138. Article CAS PubMed Google Scholar * Wood KA,
Dipasquale B, Youle RJ . _In situ_ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. _Neuron_ 1993; 11:
621–632. Article CAS PubMed Google Scholar * Henderson CE . Role of neurotrophic factors in neuronal development. _Curr Opin Neurobiol_ 1996; 6: 64–70. Article CAS PubMed Google
Scholar * Minichiello L, Klein R . TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons. _Genes Dev_ 1996; 10: 2849–2858.
Article CAS PubMed Google Scholar * Murase S, Owens DF, McKay RD . In the newborn hippocampus, neurotrophin-dependent survival requires spontaneous activity and integrin signaling. _J
Neurosci_ 2011; 31: 7791–7800. Article CAS PubMed PubMed Central Google Scholar * Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S _et al_. Global deprivation
of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. _J Neurosci_ 2010; 30: 1739–1749. Article CAS PubMed PubMed Central Google
Scholar * Chrysis D, Calikoglu AS, Ye P, D'Ercole AJ . Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in
a developmentally specific manner. _J Neurosci_ 2001; 21: 1481–1489. Article CAS PubMed PubMed Central Google Scholar * Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M _et
al_. Layer V cortical neurons require microglial support for survival during postnatal development. _Nat Neurosci_ 2013; 16: 543–551. Article CAS PubMed Google Scholar * Sanno H, Shen X,
Kuru N, Bormuth I, Bobsin K, Gardner HA _et al_. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. _J Neurosci_ 2010; 30: 4221–4231.
Article CAS PubMed PubMed Central Google Scholar * Zhao Y, Kwan KM, Mailloux CM, Lee WK, Grinberg A, Wurst W _et al_. LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1,
control Purkinje cell differentiation in the developing cerebellum. _Proc Natl Acad Sci USA_ 2007; 104: 13182–13186. Article CAS PubMed PubMed Central Google Scholar * Garel S, Marin F,
Grosschedl R, Charnay P . Ebf1 controls early cell differentiation in the embryonic striatum. _Development_ 1999; 126: 5285–5294. CAS PubMed Google Scholar * Croci L, Chung SH,
Masserdotti G, Gianola S, Bizzoca A, Gennarini G _et al_. A key role for the HLH transcription factor EBF2COE2,O/E-3 in Purkinje neuron migration and cerebellar cortical topography.
_Development_ 2006; 133: 2719–2729. Article CAS PubMed Google Scholar * Croci L, Barili V, Chia D, Massimino L, van Vugt R, Masserdotti G _et al_. Local insulin-like growth factor I
expression is essential for Purkinje neuron survival at birth. _Cell Death Differ_ 2011; 18: 48–59. Article CAS PubMed Google Scholar * Arenas E, Denham M, Villaescusa JC . How to make a
midbrain dopaminergic neuron. _Development_ 2015; 142: 1918–1936. Article CAS PubMed Google Scholar * Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M _et al_. Nurr1
is required for maintenance of maturing and adult midbrain dopamine neurons. _J Neurosci_ 2009; 29: 15923–15932. Article CAS PubMed PubMed Central Google Scholar * Sonnier L, Le Pen G,
Hartmann A, Bizot JC, Trovero F, Krebs MO _et al_. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. _J Neurosci_ 2007; 27:
1063–1071. Article CAS PubMed PubMed Central Google Scholar * van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF _et al_. Pitx3 is required for motor
activity and for survival of a subset of midbrain dopaminergic neurons. _Development_ 2003; 130: 2535–2542. CAS PubMed Google Scholar * Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N
_et al_. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. _J Neurosci_ 2011; 31: 12802–12815. Article CAS PubMed PubMed Central Google
Scholar * Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, Perrone-Capano C _et al_. Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons _in vitro_.
_J Neurochem_ 2007; 102: 441–453. Article CAS PubMed Google Scholar * Checa N, Canals JM, Gratacos E, Alberch J . TrkB and TrkC are differentially regulated by excitotoxicity during
development of the basal ganglia. _Exp Neurol_ 2001; 172: 282–292. Article CAS PubMed Google Scholar * Zaman V, Nelson ME, Gerhardt GA, Rohrer B . Neurodegenerative alterations in the
nigrostriatal system of trkB hypomorphic mice. _Exp Neurol_ 2004; 190: 337–346. Article CAS PubMed Google Scholar * Alavian KN, Sgado P, Alberi L, Subramaniam S, Simon HH . Elevated
P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. _Neural Dev_ 2009; 4: 11. Article CAS PubMed PubMed Central Google Scholar *
Veenvliet JV, Dos Santos MT, Kouwenhoven WM, von Oerthel L, Lim JL, van der Linden AJ _et al_. Specification of dopaminergic subsets involves interplay of En1 and Pitx3. _Development_ 2013;
140: 3373–3384. Article CAS PubMed Google Scholar * Yamaguchi Y, Miura M . Programmed cell death in neurodevelopment. _Dev Cell_ 2015; 32: 478–490. Article CAS PubMed Google Scholar
* Barallobre MJ, Perier C, Bove J, Laguna A, Delabar JM, Vila M _et al_. DYRK1A promotes dopaminergic neuron survival in the developing brain and in a mouse model of Parkinson's
disease. _Cell Death Dis_ 2014; 5: e1289. Article CAS PubMed PubMed Central Google Scholar * Zhang J, Pho V, Bonasera SJ, Holtzman J, Tang AT, Hellmuth J _et al_. Essential function of
HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. _Nat Neurosci_ 2007; 10: 77–86. Article CAS PubMed Google Scholar * Petreanu L, Alvarez-Buylla A . Maturation and death
of adult-born olfactory bulb granule neurons: role of olfaction. _J Neurosci_ 2002; 22: 6106–6113. Article CAS PubMed PubMed Central Google Scholar * Rey NL, Sacquet J, Veyrac A,
Jourdan F, Didier A . Behavioral and cellular markers of olfactory aging and their response to enrichment. _Neurobiol Aging_ 2012; 33: 626 e629–626 e623. Google Scholar * Platel JC, Dave
KA, Gordon V, Lacar B, Rubio ME, Bordey A . NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network.
_Neuron_ 2010; 65: 859–872. Article CAS PubMed PubMed Central Google Scholar * Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM _et al_. Nicotinic receptors
regulate the survival of newborn neurons in the adult olfactory bulb. _Proc Natl Acad Sci USA_ 2004; 101: 9822–9826. Article CAS PubMed PubMed Central Google Scholar * Giachino C, De
Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G _et al_. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. _J
Neurosci_ 2005; 25: 10105–10118. Article CAS PubMed PubMed Central Google Scholar * Herold S, Jagasia R, Merz K, Wassmer K, Lie DC . CREB signalling regulates early survival, neuronal
gene expression and morphological development in adult subventricular zone neurogenesis. _Mol Cell Neurosci_ 2011; 46: 79–88. Article CAS PubMed Google Scholar * Khodosevich K, Monyer H
. Signaling in migrating neurons: from molecules to networks. _Front Neurosci_ 2011; 5: 28. Article PubMed PubMed Central Google Scholar * Khodosevich K, Seeburg PH, Monyer H . Major
signaling pathways in migrating neuroblasts. _Front Mol Neurosci_ 2009; 2: 7. Article CAS PubMed PubMed Central Google Scholar * Khodosevich K, Zuccotti A, Kreuzberg MM, Le Magueresse
C, Frank M, Willecke K _et al_. Connexin45 modulates the proliferation of transit-amplifying precursor cells in the mouse subventricular zone. _Proc Natl Acad Sci USA_ 2012; 109:
20107–20112. Article CAS PubMed PubMed Central Google Scholar * Gascon E, Vutskits L, Jenny B, Durbec P, Kiss JZ . PSA-NCAM in postnatally generated immature neurons of the olfactory
bulb: a crucial role in regulating p75 expression and cell survival. _Development_ 2007; 134: 1181–1190. Article CAS PubMed Google Scholar * Bergami M, Vignoli B, Motori E, Pifferi S,
Zuccaro E, Menini A _et al_. TrkB signaling directs the incorporation of newly generated periglomerular cells in the adult olfactory bulb. _J Neurosci_ 2013; 33: 11464–11478. Article CAS
PubMed PubMed Central Google Scholar * Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A . Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in
adult mice and rats. _J Neurosci_ 2008; 28: 13368–13383. Article CAS PubMed PubMed Central Google Scholar * Feliciano DM, Zhang S, Quon JL, Bordey A . Hypoxia-inducible factor 1a is a
Tsc1-regulated survival factor in newborn neurons in tuberous sclerosis complex. _Hum Mol Genet_ 2013; 22: 1725–1734. Article CAS PubMed PubMed Central Google Scholar * Khodosevich K,
Monyer H . Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons _in vitro_. _BMC Neurosci_ 2010; 11: 18. Article CAS PubMed PubMed Central Google
Scholar * Miwa N, Storm DR . Odorant-induced activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in the olfactory bulb promotes survival of newly formed
granule cells. _J Neurosci_ 2005; 25: 5404–5412. Article CAS PubMed PubMed Central Google Scholar * Mu Y, Zhao C, Toni N, Yao J, Gage FH . Distinct roles of NMDA receptors at different
stages of granule cell development in the adult brain. _Elife_ 2015; 4: e07871. Article PubMed PubMed Central Google Scholar * Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler
J . Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. _Eur J Neurosci_ 2005; 22: 1907–1915. Article
PubMed Google Scholar * Fuchs C, Trazzi S, Torricella R, Viggiano R, De Franceschi M, Amendola E _et al_. Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering
AKT/GSK-3beta signaling. _Neurobiol Dis_ 2014; 70: 53–68. Article CAS PubMed PubMed Central Google Scholar * Kandasamy M, Lehner B, Kraus S, Sander PR, Marschallinger J, Rivera FJ _et
al_. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. _J Cell Mol Med_ 2014; 18: 1444–1459. Article CAS PubMed PubMed
Central Google Scholar * Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR . Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate
granule neurons: insights into the regulation of adult hippocampal neurogenesis. _J Neurosci Res_ 2006; 83: 199–210. Article CAS PubMed Google Scholar * Hurtado-Chong A, Yusta-Boyo MJ,
Vergano-Vera E, Bulfone A, de Pablo F, Vicario-Abejon C . IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone.
_Eur J Neurosci_ 2009; 30: 742–755. Article PubMed Google Scholar * Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K _et al_. Massive cell death of immature hematopoietic cells
and neurons in Bcl-x-deficient mice. _Science_ 1995; 267: 1506–1510. Article CAS PubMed Google Scholar * Shindler KS, Latham CB, Roth KA . Bax deficiency prevents the increased cell
death of immature neurons in bcl-x-deficient mice. _J Neurosci_ 1997; 17: 3112–3119. Article CAS PubMed PubMed Central Google Scholar * Nakamura A, Swahari V, Plestant C, Smith I, McCoy
E, Smith S _et al_. Bcl-xL is essential for the survival and function of differentiated neurons in the cortex that control complex behaviors. _J Neurosci_ 2016; 36: 5448–5461. Article CAS
PubMed PubMed Central Google Scholar * Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM . Bcl-x is required for proper development of the mouse substantia nigra. _J Neurosci_ 2005; 25:
6721–6728. Article CAS PubMed PubMed Central Google Scholar * Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC _et al_. Mcl-1 is a key regulator of apoptosis
during CNS development and after DNA damage. _J Neurosci_ 2008; 28: 6068–6078. Article CAS PubMed PubMed Central Google Scholar * Akhtar MW, Kim MS, Adachi M, Morris MJ, Qi X,
Richardson JA _et al_. _In vivo_ analysis of MEF2 transcription factors in synapse regulation and neuronal survival. _PLoS ONE_ 2012; 7: e34863. Article CAS PubMed PubMed Central Google
Scholar * Pozniak CD, Barnabe-Heider F, Rymar VV, Lee AF, Sadikot AF, Miller FD . p73 is required for survival and maintenance of CNS neurons. _J Neurosci_ 2002; 22: 9800–9809. Article CAS
PubMed PubMed Central Google Scholar * Jacobs WB, Walsh GS, Miller FD . Neuronal survival and p73/p63/p53: a family affair. _Neuroscientist_ 2004; 10: 443–455. Article CAS PubMed
Google Scholar * Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A _et al_. Disruption of CREB function in brain leads to neurodegeneration. _Nat Genet_ 2002;
31: 47–54. Article CAS PubMed Google Scholar * Luhmann HJ, Sinning A, Yang JW, Reyes-Puerta V, Stuttgen MC, Kirischuk S _et al_. Spontaneous neuronal activity in developing neocortical
networks: from single cells to large-scale interactions. _Front Neural Circuits_ 2016; 10: 40. Article PubMed PubMed Central Google Scholar * Verhage M, Maia AS, Plomp JJ, Brussaard AB,
Heeroma JH, Vermeer H _et al_. Synaptic assembly of the brain in the absence of neurotransmitter secretion. _Science_ 2000; 287: 864–869. Article CAS PubMed Google Scholar * Ikonomidou
C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K _et al_. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. _Science_ 1999; 283: 70–74. Article CAS
PubMed Google Scholar * Heck N, Golbs A, Riedemann T, Sun JJ, Lessmann V, Luhmann HJ . Activity-dependent regulation of neuronal apoptosis in neonatal mouse cerebral cortex. _Cereb
Cortex_ 2008; 18: 1335–1349. Article PubMed Google Scholar * Wagner-Golbs A, Luhmann HJ . Activity-dependent survival of developing neocortical neurons depends on PI3K signalling. _J
Neurochem_ 2012; 120: 495–501. Article CAS PubMed Google Scholar * Maskos U, McKay RD . Neural cells without functional N-Methyl-D-Aspartate (NMDA) receptors contribute extensively to
normal postnatal brain development in efficiently generated chimaeric NMDA R1 -/- <—> +/+ mice. _Dev Biol_ 2003; 262: 119–136. Article CAS PubMed Google Scholar *
Schmidt-Hieber C, Jonas P, Bischofberger J . Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. _Nature_ 2004; 429: 184–187. Article CAS PubMed Google
Scholar * Wang XQ, Deriy LV, Foss S, Huang P, Lamb FS, Kaetzel MA _et al_. CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons. _Neuron_ 2006; 52: 321–333.
Article CAS PubMed Google Scholar * Huang C, Chen M, Pang D, Bi D, Zou Y, Xia X _et al_. Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required
for neuronal survival. _Dev Cell_ 2014; 30: 479–487. Article CAS PubMed PubMed Central Google Scholar * Gucuyener K, Atalay Y, Aral YZ, Hasanoglu A, Turkyilmaz C, Biberoglu G .
Excitatory amino acids and taurine levels in cerebrospinal fluid of hypoxic ischemic encephalopathy in newborn. _Clin Neurol Neurosurg_ 1999; 101: 171–174. Article CAS PubMed Google
Scholar * Pu Y, Li QF, Zeng CM, Gao J, Qi J, Luo DX _et al_. Increased detectability of alpha brain glutamate/glutamine in neonatal hypoxic-ischemic encephalopathy. _AJNR Am J Neuroradiol_
2000; 21: 203–212. CAS PubMed PubMed Central Google Scholar * Fiskum G, Murphy AN, Beal MF . Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. _J
Cereb Blood Flow Metab_ 1999; 19: 351–369. Article CAS PubMed Google Scholar * Kumar A, Mittal R, Khanna HD, Basu S . Free radical injury and blood-brain barrier permeability in
hypoxic-ischemic encephalopathy. _Pediatrics_ 2008; 122: e722–e727. Article PubMed Google Scholar * Komitova M, Xenos D, Salmaso N, Tran KM, Brand T, Schwartz ML _et al_. Hypoxia-induced
developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. _J Neurosci_ 2013; 33: 13375–13387. Article CAS PubMed PubMed
Central Google Scholar * Chen W, Xu B, Xiao A, Liu L, Fang X, Liu R _et al_. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. _Mol Brain_ 2015; 8: 11.
Article CAS PubMed PubMed Central Google Scholar * Deuber C, Terhaar M . Hyperoxia in very preterm infants a systematic review of the literature. _J Perinat Neonat Nur_ 2011; 25:
268–274. Article Google Scholar * Ikonomidou C, Kaindl AM . Neuronal death and oxidative stress in the developing brain. _Antioxid Redox Signal_ 2011; 14: 1535–1550. Article CAS PubMed
Google Scholar * Kaindl AM, Sifringer M, Zabel C, Nebrich G, Wacker MA, Felderhoff-Mueser U _et al_. Acute and long-term proteome changes induced by oxidative stress in the developing
brain. _Cell Death Differ_ 2006; 13: 1097–1109. Article CAS PubMed Google Scholar * Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, Mahler L _et al_.
Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. _Ann Neurol_ 2005; 57: 50–59. Article CAS PubMed Google Scholar * Riley EP, Infante MA, Warren
KR . Fetal alcohol spectrum disorders: an overview. _Neuropsychol Rev_ 2011; 21: 73–80. Article PubMed PubMed Central Google Scholar * Goodlett CR, Horn KH, Zhou FC . Alcohol
teratogenesis: mechanisms of damage and strategies for intervention. _Exp Biol Med (Maywood)_ 2005; 230: 394–406. Article CAS Google Scholar * Wilson DA, Peterson J, Basavaraj BS, Saito M
. Local and regional network function in behaviorally relevant cortical circuits of adult mice following postnatal alcohol exposure. _Alcohol Clin Exp Res_ 2011; 35: 1974–1984. Article
PubMed PubMed Central Google Scholar * Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN _et al_. Selective reduction of cerebral cortex GABA neurons in a late gestation
model of fetal alcohol spectrum disorder. _Alcohol_ 2015; 49: 571–580. Article CAS PubMed PubMed Central Google Scholar * Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH . Ethanol
consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. _J Neurosci_ 2008; 28: 1854–1864. Article CAS PubMed PubMed
Central Google Scholar * Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K _et al_. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. _Science_ 2000;
287: 1056–1060. Article CAS PubMed Google Scholar * Coleman LG Jr, Jarskog LF, Moy SS, Crews FT . Deficits in adult prefrontal cortex neurons and behavior following early post-natal
NMDA antagonist treatment. _Pharmacol Biochem Behav_ 2009; 93: 322–330. Article CAS PubMed PubMed Central Google Scholar * Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W
_et al_. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. _Neurobiol Dis_ 2004; 16: 440–453. Article CAS PubMed Google Scholar *
Lema Tome CM, Nottingham CU, Smith CM, Beauchamp AS, Leung PW, Turner CP . Neonatal exposure to MK801 induces structural reorganization of the central nervous system. _Neuroreport_ 2006;
17: 779–783. Article PubMed Google Scholar * de la Monte SM, Wands JR . Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in
cerebellar neurons. _Cell Mol Life Sci_ 2002; 59: 882–893. Article CAS PubMed Google Scholar * Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z _et al_. Overexpression of glycogen synthase
kinase 3beta sensitizes neuronal cells to ethanol toxicity. _J Neurosci Res_ 2009; 87: 2793–2802. Article CAS PubMed PubMed Central Google Scholar * Chu J, Tong M, de la Monte SM .
Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. _Acta Neuropathol_ 2007; 113: 659–673. Article CAS PubMed Google
Scholar * Kumar A, Singh CK, Lavoie HA, Dipette DJ, Singh US . Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal
alcohol spectrum disorders. _Mol Pharmacol_ 2011; 80: 446–457. Article CAS PubMed PubMed Central Google Scholar * Heaton MB, Paiva M, Kubovec S . Differential effects of ethanol on bid,
tBid, and Bax:tBid interactions in postnatal day 4 and postnatal day 7 rat cerebellum. _Alcohol Clin Exp Res_ 2015; 39: 55–63. Article CAS PubMed PubMed Central Google Scholar * Heaton
MB, Paiva M, Kubovic S, Kotler A, Rogozinski J, Swanson E _et al_. Differential effects of ethanol on c-jun N-terminal kinase, 14-3-3 proteins, and Bax in postnatal day 4 and postnatal day
7 rat cerebellum. _Brain Res_ 2012; 1432: 15–27. Article CAS PubMed Google Scholar * Giza CC, Prins ML . Is being plastic fantastic? Mechanisms of altered plasticity after developmental
traumatic brain injury. _Dev Neurosci-Basel_ 2006; 28: 364–379. Article CAS Google Scholar * Bittigau P, Sifringer M, Pohl D, Stadthaus D, Ishimaru M, Shimizu H _et al_. Apoptotic
neurodegeneration following trauma is markedly enhanced in the immature brain. _Ann Neurol_ 1999; 45: 724–735. Article CAS PubMed Google Scholar * Felderhoff-Mueser U, Sifringer M,
Pesditschek S, Kuckuck H, Moysich A, Bittigau P _et al_. Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. _Neurobiol Dis_ 2002; 11: 231–245.
Article CAS PubMed Google Scholar * Sifringer M, Stefovska V, Endesfelder S, Stahel PF, Genz K, Dzietko M _et al_. Activation of caspase-1 dependent interleukins in developmental brain
trauma. _Neurobiol Dis_ 2007; 25: 614–622. Article CAS PubMed Google Scholar * Kim DH, Ko IG, Kim BK, Kim TW, Kim SE, Shin MS _et al_. Treadmill exercise inhibits traumatic brain
injury-induced hippocampal apoptosis. _Physiol Behav_ 2010; 101: 660–665. Article CAS PubMed Google Scholar * Zhou H, Chen L, Gao X, Luo B, Chen J . Moderate traumatic brain injury
triggers rapid necrotic death of immature neurons in the hippocampus. _J Neuropathol Exp Neurol_ 2012; 71: 348–359. Article PubMed Google Scholar * Jaglin XH, Poirier K, Saillour Y,
Buhler E, Tian G, Bahi-Buisson N _et al_. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. _Nat Genet_ 2009; 41: 746–752. Article CAS PubMed PubMed Central
Google Scholar * Stottmann RW, Donlin M, Hafner A, Bernard A, Sinclair DA, Beier DR . A mutation in Tubb2b, a human polymicrogyria gene, leads to lethality and abnormal cortical
development in the mouse. _Hum Mol Genet_ 2013; 22: 4053–4063. Article CAS PubMed PubMed Central Google Scholar * Wei H, Alberts I, Li X . The apoptotic perspective of autism. _Int J
Dev Neurosci_ 2014; 36: 13–18. Article PubMed Google Scholar * Pantazopoulos H, Wiseman JT, Markota M, Ehrenfeld L, Berretta S . Decreased numbers of somatostatin-expressing neurons in
the amygdala of subjects with bipolar disorder or schizophrenia: relationship to circadian rhythms. _Biol Psychiatry_ 2016; 81: 536–547. Article CAS PubMed PubMed Central Google Scholar
* Hashemi E, Ariza J, Rogers H, Noctor SC, Martinez-Cerdeno V . The number of parvalbumin-expressing interneurons is decreased in the medial prefrontal cortex in autism. _Cereb Cortex_
2016 (doi:10.1093/cercor/bhw021). * Chung DW, Fish KN, Lewis DA . Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. _Am J Psychiatry_
2016; 173: 1131–1139. Article PubMed PubMed Central Google Scholar * Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H _et al_. Absence of CNTNAP2 leads to epilepsy,
neuronal migration abnormalities, and core autism-related deficits. _Cell_ 2011; 147: 235–246. Article CAS PubMed PubMed Central Google Scholar * Kim JY, Liu CY, Zhang F, Duan X, Wen Z,
Song J _et al_. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. _Cell_ 2012; 148: 1051–1064. Article CAS PubMed PubMed Central
Google Scholar * Karayannis T, Au E, Patel JC, Kruglikov I, Markx S, Delorme R _et al_. Cntnap4 differentially contributes to GABAergic and dopaminergic synaptic transmission. _Nature_
2014; 511: 236–240. Article CAS PubMed PubMed Central Google Scholar * Watanabe Y, Khodosevich K, Monyer H . Dendrite development regulated by the schizophrenia-associated gene FEZ1
involves the ubiquitin proteasome system. _Cell Rep_ 2014; 7: 552–564. Article CAS PubMed Google Scholar * Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG _et al_. TrkB
regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. _Neuron_ 2008; 59: 399–412. Article CAS PubMed PubMed Central Google Scholar * Sairanen M, Lucas
G, Ernfors P, Castren M, Castren E . Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in
the adult dentate gyrus. _J Neurosci_ 2005; 25: 1089–1094. Article CAS PubMed PubMed Central Google Scholar * Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP . Nerve growth factor
promotes survival of new neurons in the adult hippocampus. _Neurobiol Dis_ 2007; 26: 47–55. Article CAS PubMed Google Scholar * Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J,
Clary DO _et al_. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons.
_J Neurosci_ 1995; 15: 2888–2905. Article CAS PubMed PubMed Central Google Scholar * Muller M, Triaca V, Besusso D, Costanzi M, Horn JM, Koudelka J _et al_. Loss of NGF-TrkA signaling
from the CNS is not sufficient to induce cognitive impairments in young adult or intermediate-aged mice. _J Neurosci_ 2012; 32: 14885–14898. Article CAS PubMed PubMed Central Google
Scholar * Scardigli R, Capelli P, Vignone D, Brandi R, Ceci M, La Regina F _et al_. Neutralization of nerve growth factor impairs proliferation and differentiation of adult neural
progenitors in the subventricular zone. _Stem Cells_ 2014; 32: 2516–2528. Article CAS PubMed Google Scholar * Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A _et al_. Severe
sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. _Nature_ 1994; 368: 246–249. Article CAS PubMed Google Scholar * Bartkowska K, Paquin A, Gauthier
AS, Kaplan DR, Miller FD . Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. _Development_ 2007; 134: 4369–4380. Article CAS
PubMed Google Scholar * Ma L, Harada T, Harada C, Romero M, Hebert JM, McConnell SK _et al_. Neurotrophin-3 is required for appropriate establishment of thalamocortical connections.
_Neuron_ 2002; 36: 623–634. Article CAS PubMed Google Scholar * Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R _et al_. NT-3 facilitates hippocampal plasticity and learning
and memory by regulating neurogenesis. _Learn Mem_ 2006; 13: 307–315. Article CAS PubMed PubMed Central Google Scholar * Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M .
Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. _Eur J Neurosci_ 1997; 9: 2045–2056. Article CAS PubMed Google
Scholar * Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F . Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal
parvalbumin-containing neurons. _Neuron_ 1995; 14: 717–730. Article CAS PubMed Google Scholar * Hodge RD, D'Ercole AJ, O'Kusky JR . Insulin-like growth factor-I (IGF-I)
inhibits neuronal apoptosis in the developing cerebral cortex _in vivo_. _Int J Dev Neurosci_ 2007; 25: 233–241. Article CAS PubMed PubMed Central Google Scholar * Harrist A, Beech RD,
King SL, Zanardi A, Cleary MA, Caldarone BJ _et al_. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor.
_Synapse_ 2004; 54: 200–206. Article CAS PubMed Google Scholar * Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N _et al_. Abnormal avoidance learning in mice lacking
functional high-affinity nicotine receptor in the brain. _Nature_ 1995; 374: 65–67. Article CAS PubMed Google Scholar * Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP . Increased
neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. _EMBO J_ 1999; 18: 1235–1244. Article CAS PubMed PubMed Central Google Scholar * Brionne TC, Tesseur
I, Masliah E, Wyss-Coray T . Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. _Neuron_ 2003; 40: 1133–1145. Article CAS PubMed Google Scholar *
Stritt C, Stern S, Harting K, Manke T, Sinske D, Schwarz H _et al_. Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. _Nat Neurosci_ 2009; 12:
418–427. Article CAS PubMed Google Scholar * Bernabeu RO, Longo FM . The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and
non-neuronal cell genesis. _BMC Neurosci_ 2010; 11: 136. Article CAS PubMed PubMed Central Google Scholar * McQuillen PS, DeFreitas MF, Zada G, Shatz CJ . A novel role for p75NTR in
subplate growth cone complexity and visual thalamocortical innervation. _J Neurosci_ 2002; 22: 3580–3593. Article CAS PubMed PubMed Central Google Scholar * Lotta LT, Conrad K,
Cory-Slechta D, Schor NF . Cerebellar Purkinje cell p75 neurotrophin receptor and autistic behavior. _Transl Psychiatry_ 2014; 4: e416. Article CAS PubMed PubMed Central Google Scholar
* Zanin JP, Abercrombie E, Friedman WJ . Proneurotrophin-3 promotes cell cycle withdrawal of developing cerebellar granule cell progenitors via the p75 neurotrophin receptor. _Elife_ 2016:
5. * Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ . The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. _Eur J Neurosci_ 2008; 28: 883–892.
Article PubMed Google Scholar * Adams SM . de Rivero Vaccari JC, Corriveau RA. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. _J
Neurosci_ 2004; 24: 9441–9450. Article CAS PubMed PubMed Central Google Scholar * Maskos U, Brustle O, McKay RD . Long-term survival, migration, and differentiation of neural cells
without functional NMDA receptors _in vivo_. _Dev Biol_ 2001; 231: 103–112. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Work in the Khodosevich lab is
supported by the Novo Nordisk Foundation (Hallas-Møller Investigator, NNF16OC0019920) and Agnes og Poul Friis Fond. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Biotech Research and
Innovation Centre (BRIC), University of Copenhagen, Copenhagen, Denmark Ulrich Pfisterer & Konstantin Khodosevich Authors * Ulrich Pfisterer View author publications You can also search
for this author inPubMed Google Scholar * Konstantin Khodosevich View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to
Konstantin Khodosevich. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by A Verkhratsky RIGHTS AND PERMISSIONS _Cell Death
and Disease_ is an open-access journal published by _Nature Publishing Group_. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative
Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pfisterer, U., Khodosevich, K. Neuronal survival in the brain: neuron type-specific mechanisms. _Cell Death Dis_ 8, e2643
(2017). https://doi.org/10.1038/cddis.2017.64 Download citation * Received: 17 October 2016 * Revised: 24 January 2017 * Accepted: 31 January 2017 * Published: 02 March 2017 * Issue Date:
March 2017 * DOI: https://doi.org/10.1038/cddis.2017.64 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Moment officer came face to face with blood-soaked killer who said 'we all die together' - YorkshireLiveNewsMoment officer came face to face with blood-soaked killer who said 'we all die together'Police Officer Jessica Witto...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Latests News
Neuronal survival in the brain: neuron type-specific mechanismsABSTRACT Neurogenic regions of mammalian brain produce many more neurons that will eventually survive and reach a mature...
Are you storing cheese all wrong?There’s a lot to love about cheese. It’s delicious, versatile and a great source of calcium and protein, which are good ...
Crédits | City of Paris Museum of Modern ArtIMAGES Reproductions of works in the online collection are intended for personal and private consultation only. In accor...
Budapest boat crash leaves 7 south korean tourists dead; cruise ship captain detainedReporting from BUDAPEST, Hungary — Rescue crews geared up Thursday to raise a sightseeing boat from the bottom of the Da...
"unconstitutional order... ": tmc mp and petitioner mahua moitra on sc verdict on 'nameplates' in kanwar yatra routesNew Delhi [India], July 22 (ANI): After the Supreme Court put an interim stay on 'nameplates' on eateries in K...