Negative regulation of autophagy
Negative regulation of autophagy"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Autophagy is an evolutionarily conserved catabolic process that involves the invagination and degradation of cytoplasmic components through an autophagosomelysosome track. Autophagy
functions as a quality control of cellular milieu and is implicated in a wide variety of pathological conditions. However, excessive or imbalanced autophagic flux may also be associated
with cellular toxicity and may potentially contribute to the development of pathological conditions. Just as all membrane trafficking systems need to constantly strike a balance in their
level of activation and inhibition to ensure proper spatial and temporal delivery of their cargo, autophagy must also be tightly regulated. Here, we provide an overview of the current
knowledge regarding the negative regulation of mammalian autophagy in an effort to understand its physiological relevance and potential clinical importance. SIMILAR CONTENT BEING VIEWED BY
OTHERS AUTOPHAGY: REGULATOR OF CELL DEATH Article Open access 04 October 2023 THE EMERGING MECHANISMS AND FUNCTIONS OF MICROAUTOPHAGY Article 12 September 2022 MACHINERY, REGULATION AND
PATHOPHYSIOLOGICAL IMPLICATIONS OF AUTOPHAGOSOME MATURATION Article 23 July 2021 MAIN Autophagy, originally described as a lysosome-dependent bulk degradation of cytoplasmic components on
starvation, has since been shown to influence diverse aspects of homeostasis and is implicated in many diseases.1 The autophagic cascade is initiated by the engulfment of cytoplasmic
cargoes, including long-lived or aggregated proteins, defective organelles, and various soluble molecules, by a double-membraned autophagosome. The autophagosome is then progressively
acidified by fusion with the late endosome and the lysosomal compartment to form the autolysosome, exposing the inner compartment to lysosomal hydrolases. Eventually, the inner membrane of
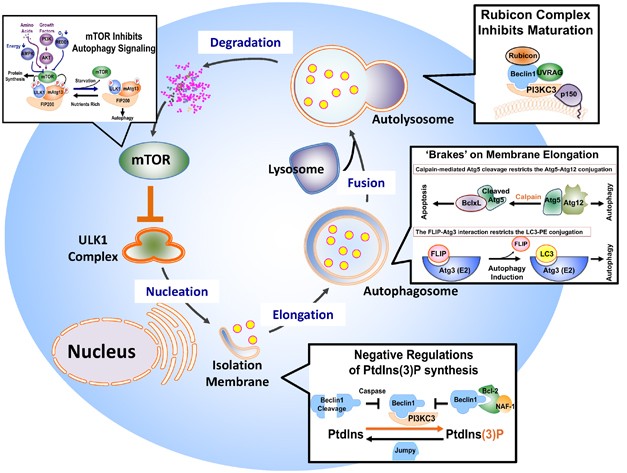
the autophagosome and its sequestered contents are degraded, with the resulting macromolecules recycled (Figure 1).1, 2 Over 30 distinct autophagy-related (_Atg_) genes have been identified
so far in yeast.3 Many of the genes have their orthologs in mammals.1, 3Classical autophagy can be largely divided into several major steps that require specific _Atg_ genes.1 These include
(a) target of rapamycin (TOR)-mediated induction of autophagy through an Atg1-kinase complex, (b) initiation of autophagosome formation through a Beclin1-associated class III
phosphoinositide (PI) 3-kinase (PI3KC3) complex, (c) autophagosome membrane elongation and completion, involving two evolutionarily conserved ubiquitin-like conjugation systems–the
Atg12–Atg5 and the LC3 (the mammalian ortholog of yeast Atg8)-PE (phosphatidylethanolamine) systems, (d) autophagosome maturation through fusion with lysosomes, and (e) cargo degradation
through lysosomal enzymes and recycling (Figure 1).1 It is important to note that it is the combined signaling cascades that regulate autophagy, coupled with the concerted action of these
distinct steps, that lead to the overall cellular autophagic response. Recently, an alternative autophagy pathway inducible by cellular stress was discovered in mouse embryonic fibroblasts
(MEFs) depleted of the key autophagosomal proteins, Atg5 and Atg7.4 Interestingly, this Atg5- and Atg7-independent autophagy pathway is not associated with LC3 lipidation but seems to
specifically involve membranes derived from _trans_-Golgi and late endosomes.4 Thus, autophagy seems to be far more complex than was previously realized, demanding sophisticated
autophagosomal sorting, conceivably encompassing multiple routes of trafficking while orchestrating regulation of membrane dynamics.1, 5 The activation of autophagy is a double-edged sword.
On the one hand, autophagy is essential for provoking a protective response and enhancing the cells’ adaptation to metabolic stresses and immunological challenges.6, 7 In particular,
infection, inflammation, neurodegeneration, and cancer can result from pathogen-induced or inherited disruption of the autophagy pathway.6, 8 On the other hand, excessive autophagic
activation, derailed autophagic trafficking, or imbalanced degradation and recycling can give rise to pathogenic conditions by straining critical cellular constituents, leading to cell
degeneration and toxicity.9 An example is cardiac myocyte death during ischemia/reperfusion, which involves excessively stimulated autophagy and can be alleviated with treatment of
3-methyladenine, an autophagy inhibitor, or knockdown of _beclin1_, an essential autophagy effector.10, 11 Moreover, although autophagy has been found to retard cardiac hypertrophy by
increasing protein degradation, prolonged autophagy seems to have a crucial part in the transition from cardiac hypertrophy to decompensated heart failure, likely because of loss of cardiac
mass.11 Indeed, overwhelming autophagic turnover or the build-up of a ‘traffic jam’ in autophagic vacuoles predominates the pathology of many types of myopathy.12 In such conditions,
autophagy is not simply a by-product of the cells’ recovery program, but rather a phenomenon that can directly contribute to cell pathologies. Similarly, neuronal atrophy, neurite
degeneration, and cell death can be exacerbated by the upregulation of autophagy, but are partially reversed by the inactivation of some essential autophagy genes, at least in certain
contexts.13 Autophagy may also facilitate pathogenesis of infections.14 Prominent examples include poliovirus, which intercepts autophagy to provide a membrane scaffold for viral
replication,15 and HIV, which hijacks autophagy as a bystander death program for CD4+ T-cell depletion and immunodeficiency.16 Although it is still a matter of debate whether autophagy is
ultimately a protective response or a detrimental process, it seems clear that, if left unchecked, autophagy can lead to adverse effects in cells under certain pathological conditions.
Therefore, autophagy signaling and functions are necessities, but ones that must be under tight negative regulation. A series of negative regulators of autophagy have been identified in the
past 10 years (Table 1), and a picture of how the balance of autophagy in mammals is achieved is beginning to emerge. It should be noted that these negative regulators do not work as a
single entity, but are orchestrated to function at multiple levels within the autophagy cascade. The timing of autophagy induction is stringently controlled by the nutrient sensor, the
mammalian TOR (mTOR) kinase, which ensures the faithful application of autophagy in the appropriate physiological setting by preventing its direct activation.17 Once autophagy is provoked,
the autophagy flux can be further restrained at each stage of membrane dynamics: nucleation, elongation, maturation, and degradation.2 The overall magnitude of the entire autophagic ‘wave’
is determined by the activities associated with each of the steps illustrated in Figure 1. The end products of autophagy can serve as a negative-feedback regulatory mechanism to preclude
prolonged activity and to synchronize the positive and negative regulation of signal transduction.18 Moreover, the post-translational modification of key autophagosomal proteins provides
additional forms of regulation.19, 20, 21 Understanding how autophagy is regulated is important, as it will facilitate the development of new strategies for the control of
autophagy-associated pathologies. As many reviews have discussed the positive regulation of autophagy,22 and as recently negative regulation of autophagy has been intensively studied, we
herein summarize our current knowledge of the negative regulation of mammalian autophagy and discuss its physiological relevance and potential clinical importance (also shown in Figure 1).
MTOR: THE GATEKEEPER OF AUTOPHAGY SIGNALING The kinase mTOR of the phosphoinositide 3-kinase-related kinase (PIKK) family serves as a key signaling ‘station’ in the regulation of cellular
metabolism, which promotes protein synthesis and suppresses the induction of autophagy.23 mTOR is found to be part of at least two functionally distinct complexes: the rapamycin-sensitive
mTOR complex 1 (mTORC1) and the rapamycin-insensitive mTOR complex 2 (mTORC2). mTORC1 contains mTOR, along with the subunits regulatory-associated protein of mTOR (Raptor), lethal with SEC13
protein 8 (LST8), DEPTOR, and proline-rich AKT-substrate (PRAS40). This complex integrates a plethora of signaling pathways that respond to nutrition status and growth factors.24 The second
complex, mTORC2, contains mTOR along with the subunits rapamycin-insensitive companion of mTOR (Rictor), stress-activated-protein-kinase-interacting protein 1 (SIN1), LST8, and DEPTOR. This
complex is less understood, but has been shown to be involved in the regulation of actin organization and activation of AKT.25 When nutrients are plentiful, the PI3K-Akt signaling pathway,
stimulated by growth factors, affects mTORC1 (Figure 1).24 This triggers a cascade of anabolic processes for cell growth and proliferation, mainly mediated by the ribosomal S6 kinase (p70S6
K) and eukaryotic translation initiation factor 4E (eIF4E)-binding protein (4E-BP), two direct downstream substrates of mTORC1.24 Concomitantly, activation of mTORC1 leads to autophagy
suppression.23 This is achieved by direct inhibition of the Atg1Atg13–Atg17 complex, which is required for the initiation of autophagosome formation in both yeast and mammals.23, 26 In
yeast, TOR activation leads directly to phosphorylation of Atg13, precluding its interaction with Atg1 to thereby inhibit the biogenesis of autophagosomes.27 In support of this view,
expression of an unphosphorylatable form of Atg13 induces autophagy regardless of TOR activation.27 In mammals, the analogous mTORC1–Atg1 complex axis shows slightly different properties:
mTOR interacts with the mammalian Atg1 complex, the ULK1 complex, and phosphorylates ULK1 and mammalian Atg13 to inhibit autophagy, whereas dissociation of mTORC1 leads to activation of ULK1
(Figure 1).28, 29 As activation of the Atg1/ULK1 complex is essential for most, if not all, cases of autophagy initiation, induction of autophagy can occur autonomously in the absence of
any exogenous or endogenous stimuli when mTORC1 is inactivated or inhibited, as occurs with rapamycin treatment.30 Therefore, mTOR may constitute an early means of limiting autophagy
signaling in favor of maximizing the synthesis of proteins, lipids, and nucleic acids for cell growth, most notably in conditions of nutrient availability.24 Under conditions of low energy
or hypoxia, mTORC1 is repressed by AMP-activated protein kinase (AMPK), which responds to an increased AMP/ATP ratio under low-energy conditions, and REDD1, a transcriptional target of HIFα
that is stimulated by hypoxia.23, 31 Thus, mTORC1 inactivation releases the Atg1/ULK1 complex from a repressed state, leading to the stimulation of autophagy.32 In this context,
autophagy-mediated breakdown and recycling of cellular components becomes the preferred route of energy production. On the basis of the central role of mTORC1 in regulating cell growth and
proliferation, it is perhaps unsurprising that metabolic signaling upstream of mTORC1 seems profoundly altered in many tumors.33 The oncogenic components in the PI3K–AKTmTOR-mediated
anabolic pathway are constitutively active in most cancers, whereas molecules known to suppress mTORC1, including PTEN, AMPK, TSC1/TSC2, and p53, are often mutated.34 Accordingly, the mTORC1
inhibitor, rapamycin, and its analogs are actively pursued as a treatment for human malignancies. Despite gaps in our understanding, it is already clear that mTORC1 signaling and its
manipulation of the autophagic process represent plausible targets for cancer therapy. PHOSPHATIDYLINOSITOL-3-PHOSPHATE (PTDINS(3)P): A SPATIAL CONTROL OF AUTOPHAGY NUCLEATION Following
autophagy induction, signals originating from mTORC1 are resolved in space, to a membrane nucleation site rich in PtdIns(3)P. Recent studies by Axe _et al._35 have shown that these
PtdIns(3)P-enriched structures (denoted omegasomes because of their Ωlike shape) seem generated at the ER membrane, which provides a docking site for assembly of the autophagy machinery.
Subsequent study by Halley _et al._36 suggested that the outer membranes of mitochondria in mammalian cells can serve as an alternative origin of the autophagosomal membranes, in particular,
during starvation-induced autophagy, by providing PE, the lipid target of the autophagosome marker LC3, highlighting multiple sites of autophagosome nucleation and an intimate connection
between ER and mitochondria in autophagy. Notably, production of PtdIns(3)P is mainly governed by the Beclin1-associated PI3KC3 complex, whereas PtdIns(3)P phosphatases mediate PtdIns(3)P
hydrolysis to maintain a balanced reservoir of PtdIns(3)P in the cell.37 In addition, there is a level of inhibitory control over the function of the Beclin1–PI3KC3 complex that links the
production of PtdIns(3)P with cell survival and apoptosis regulation, exemplified by the roles of Bcl-2 and the recently identified nutrient-deprivation autophagy factor-1 (NAF-1) in
autophagy regulation.38, 39 The level of PtdIns(3)P in a cell must be carefully maintained, as the subversion of PtdIns(3)P homeostasis may be a fundamental mechanism in the development of
congenital myopathies.37 JUMPY _Jumpy_, also known as MTMR14, encodes a PtdIns(3)P phosphatase that belongs to the myotubularin (MTM) family. This family contains 15 members of lipid
phosphatases characterized by a conserved protein tyrosine phosphatase (PTP) domain, with specificity for PtdIns(3)P and PtdIns(3,5)P2 in mammalian cells.40 In a siRNA-based screen of MTM
family members for autophagy regulation, Vergne _et al._41 found that depletion of _jumpy_, but not of other _MTM_ members, significantly increases both basal and starvation-induced
autophagy. Furthermore, Jumpy associates with isolation membranes and promotes the PtdIns(3)P-dependent recruitment of the early autophagic protein, WIP-1(mammalian Atg18). In contrast, the
overexpression of Jumpy delays traffic out of the autophagosomal compartment.41 Although the details remain hazy, these results show that Jumpy is an effective negative regulator of
autophagy that drives the reverse reaction of PtdIns(3)P hydrolysis to control excessive PtdIns(3)P-mediated signaling. _In vivo_, _jumpy_-deficient mice display muscle weakness and fatigue,
a phenotype similar to that seen in patients with centronuclear myopathy (CNM, a severe congenital muscular disorder characterized by prominent myonuclei internalization and
centralization).42 Consistent with this, Jumpy and another myotubularin, MTM1, have been genetically linked to CNM.43, 44 Notably, siRNA knockdown of _MTM1_ in myocytes fails to give rise to
enhanced autophagy, as seen with _jumpy_,41 suggesting that the subversion of the autophagosomal PtdIns(3)P balance does not explain all cases of CNM, and disparate pathomechanisms might be
responsible for similar clinical features of CNM. As PtdIns(3)P homeostasis is also critical for endosome trafficking and Ca2+ metabolism, whether Jumpy-associated myopathies are solely due
to an overwhelmed autophagic self-consumption and/or aberrant endosome function remains to be tested. Nevertheless, expression of the R336Q missense mutant of Jumpy found in CNM patients
completely abrogates Jumpy phosphatase activity and results in an increased level of autophagy _in vitro_, indicating that aberrant recycling of autophagosomal PtdIns(3)P may contribute, at
least in part, to disease etiologies.44 Recently, MTMR3 has been found to regulate constitutive autophagy initiation and the size of autophagic vacuoles in epithelial cells, favoring the
idea that autophagosomal PtdIns(3)P regulation might be a shared property of the MTM family.37 BCL-2 AND ITS ASSISTANT, NAF-1 Unlike Jumpy, which promotes the PtdIns(3)P hydrolysis to
negatively regulate autophagy, antiapoptotic Bcl-2 proteins such as Bcl-2, Bcl-xL, and Bcl-w inhibit autophagy by targeting the ER-associated PI3KC3 complex to restrict PtdIns(3)P
production.38, 45 The core PI3KC3 complex, including Beclin1, PI3KC3, and p150, exists in at least three configurations: the Atg14 complex, which contains an additional subunit of Atg14,
functions at the early stage of autophagosome formation;26, 46 the UVRAG-Bif-1 complex, which has UVRAG and Bif-1 (also called endophilin B1) instead, facilitates membrane curvature of
autophagosomes;26, 47, 48 and the Rubicon complex, which harbors Rubicon and UVRAG, functions in autophagosome maturation as discussed below.26, 46, 49 Although the specific function of each
individual subunit remains to be established, Beclin1 expression and its interaction with PI3KC3 have been shown to be indispensable for autophagosome formation.50, 51 Antiapoptotic Bcl-2,
Bcl-xL, and Bcl-w bind and hold Beclin1 at bay, thereby preventing Beclin1 from assembling the autophagy-inducing PI3KC3 complex.38, 50, 51 Consistent with this view, ER-localized Bcl-2, but
not mitochondrial-localized Bcl-2, confers autophagy inhibition, most notably when nutrients are readily available.38 After stimulation with stress, the negative regulation of Bcl-2 can be
overcome by contextually different mechanisms, including the phosphorylation of Bcl-2 or Beclin1 by JNK or DAP-kinase (DAPk), respectively. In addition, the competitive binding of BH3-only
proteins (e.g., Bad) or pharmacological BH3 mimetic agents (e.g., ABT737) to Bcl-2 releases Beclin1 from Bcl2 inhibition.51, 52 Indeed, a Beclin1 mutant that no longer binds to Bcl-2 induces
a detrimental autophagy response.38 Thus, the status of the Bcl-2–Beclin1 interaction might be a switch for the Beclin1–PI3KC3-mediated autophagic response in the ER. Shore _et al._ have
recently added another twist to the Bcl-2–Beclin1 crosstalk by showing that ER-associated NAF-1 (CISD2: CDGSH iron sulfur domain 2; synonyms: ZCD2, Noxp70, and Miner1) is required for
Bcl-2–Beclin1 interaction and Bcl-2 inhibition of autophagy at the ER.53 Knockdown of _NAF-1_ diminishes the Bcl-2 binding to Beclin1 and triggers autophagy.50, 53 Unlike Beclin1, NAF-1 does
not engage the conventional hydrophobic BH3-binding pocket on the Bcl-2 surface for binding. Moreover, the ER-restricted proapoptotic BH3-only protein, Bik, can displace NAF-1 from Bcl-2
and induce autophagy.50, 53 A plausible explanation of this finding is that NAF1 might bind to a hitherto unknown site on Bcl-2 to facilitate a conformational change needed for an efficient
Beclin1 interaction. This may be reversed by Bik because of its competitive affinity for Bcl-2. Of special note, depletion of _NAF-1_ seems to have a minimal effect on the steady-state level
of autophagy, but autophagy is strongly evoked in starved cells with an unusual distribution of LC3 at membrane blebs and filopodia.53 This indicates that the loss of NAF-1 might influence
both the magnitude and spatial control of Beclin1-mediated autophagy flux. Consistently, _Cisd2_ (_naf-1_) deficiency in mice causes autophagic cell death in multiple tissues accompanied by
a panel of premature aging phenotypes.54 Similar to Bcl-2,55 NAF-1 is found in physical association with the inositol 1,4,5trisphosphate (IP3) receptor (IP3R), an IP3 sensor and an ER-Ca2+
efflux channel, and is required for the Bcl-2-mediated depression of ER Ca2+ store.53 It should be noted that IP3R also regulates autophagy and that the antagonists (e.g., xestospongins) of
IP3R strongly induce autophagy.56 Intriguingly, such an effect of IP3R on autophagy seems to be irrelevant to its regulation of intracellular Ca2+ levels but is dependent on Beclin1
interaction.57 Whether the dual roles of NAF-1 in Bcl-2-mediated Beclin1 inhibition and IP3 receptor activity represent two sides of the same coin or are functionally independent regulatory
mechanisms remains to be further tested. Nevertheless, the coordinated regulation of autophagic flux with Ca2+ metabolism by the NAF-1/Bcl-2 complex and IP3 receptor integrates autophagy
into the broader cellular signaling network. Not surprisingly, tumors exploit these mechanisms to gain a selective advantage. For example, cancer cells often express more Bcl-2 to prolong
the inhibition of apoptosis and autophagy to ensure cancer cell proliferation, in much the same way as herpesviruses, such as γ-Herpesvirus 68, maintain their latent infections in host
cells.52, 58 Recent study by Furuya _et al._59 adds another piece to the regulation of the PtdIns(3)P production, showing that cycline-dependent kinases (Cdks) including Cdk1 and Cdk5 can
phosphorylate PI3KC3 on Thr159, which inhibits PI3KC3 interaction with Beclin1 and negatively regulates autophagy. This study thus provides a mechanism by which autophagy is controlled in
cell cycle progression. FLIP (FLICE-LIKE INHIBITORY PROTEIN) AND CLEAVED ATG5: BRAKES FOR MEMBRANE ELONGATION Autophagosome membrane elongation involves two ubiquitin-like conjugation
systems: LC3 (mammalian Atg8)-PE (phophatidylethanolamine) conjugation and Atg12–Atg5 conjugation.60 After proteolytic cleavage of its carboxy terminus by the Atg4 cysteine protease, LC3 is
conjugated to the membrane lipid PE after sequential processing by Atg7 (E1-like enzyme) and Atg3 (E2-like enzyme).60 In this conjugation process, FLIP tunes the interaction between
ubiquitin-like LC3 and Atg3.61 Meanwhile, Atg12 is conjugated to Atg5 in a similar manner, except that Atg10 instead of Atg3 is used as the E2 enzyme. Furthermore, under the control of
calpain-mediated cleavage, the membrane elongation factor, Atg5, can switch from an autophagy mode to an apoptosis mode on apoptotic stimulation.19 FLIP'S FLIPPING AUTOPHAGY Cellular
caspase-8 (FLICE)-like inhibitory protein (cFLIP) was originally identified as an inhibitor of cell-surface death-receptor signaling induced by tumor necrosis factor (TNF), CD95 ligand (CD95
L; also known as FASL), and TNF-related apoptosis-inducing ligand (TRAIL).62 Two predominant splice variants of the _FLIP_ gene, the long form (_cFLIPL_) and the shorter form (_cFLIPS_),
have been identified. Both forms are capable of protecting cells from apoptosis through competition with procaspase-8 for the recruitment to FAS-associated death domain (FADD), thereby
preventing initiator caspase-8 activation.62 In addition, both cFLIP and viral FLIP orthologs (vFLIP) have a prominent role in potently inducing NF_κ_B signaling through the engagement of
the IKK_γ_ regulatory subunit of the I_κ_B kinase (IKK) complex.63, 64 These observations seem to indicate that various primary tumor cells and tumors with resistance to death ligands, such
as TRAIL, will benefit from enhanced expression of the antiapoptotic and proinflammatory FLIP protein.65 Recent studies by Lee _et al._61 have revealed a second important function of both
c-FLIP and v-FLIP as effective regulators of autophagy. _FLIP_ depletion by siRNA exaggerates the effect of rapamycin and results in autophagic cell death, whereas the overexpression of FLIP
inhibits both starvation and rapamycin-induced autophagy.61 The mechanism of inhibition was found to involve the altering of Atg3 and LC3 interactions during autophagosome elongation. The
presence of FLIP competes with LC3 for Atg3 binding and thereby perturbs the lipidation of LC3, but this can be alleviated under stress conditions. As with Bcl-2, this action of FLIP might
help maintain the level of autophagy within a physiological range compatible with cell survival and clearance of defective organelles. Interestingly, they identified a mutant FLIP that is
defective in both NF_κ_B activation and apoptosis inhibition but remains capable of blocking autophagy as efficiently as wild-type FLIP, suggesting that the antiautophagic role of FLIP is
genetically separable from its previously identified antiapoptotic and NF_κ_B activation functions. Thus, it is conceived that autophagy inhibition by FLIP represents a ‘direct hit’ on
essential autophagy proteins such as Atg3, rather than an ‘indirect output’ by way of interfering with other signaling pathways.61 CALPAIN-MEDIATED ATG5 CLEAVAGE: TWO BIRDS, ONE STONE
Post-translational modification of Atg5, a key autophagy effector protein, provides additional insight into the molecular mechanisms of how autophagy is negatively regulated under normal
nutritional conditions and in response to stress stimuli.19 Atg5 forms a high-order protein conjugation complex with Atg12 and Atg16, which is essential for autophagosome membrane
elongation. Surprisingly, Yousefi _et al._19 found that Atg5 also sensitizes tumor cells to various apoptotic stimuli that are not due to enhanced autophagy. In an effort to understand how
Atg5 exerts this effect, Yousefi _et al._19 identified a cleaved form of Atg5 produced from full-length Atg5 by calpain, a family of Ca2+-dependent non-lysosomal cysteine proteases, in cells
exposed to apoptotic stimuli. Atg5 cleavage obliterates its autophagy activity. Instead, the truncated Atg5 translocates to the mitochondria, wherein it associates with Bcl-xL and somehow
induces apoptosis. This mechanism has a dual effect in signaling: it enables the direct attenuation of the autophagy response by downregulating Atg5–Atg12 conjugation, while generating
unique signals that propagate to mitochondria for the induction of apoptosis. On the basis of this observation, Atg5 cleavage seems to function as a critical switch from protective autophagy
to cell death in the presence of apoptotic stimuli. Congruous results were recently provided by Xia _et al._66 further demonstrating that calpain1-mediated cleavage of Atg5 can modulate
autophagy by adjusting the level of Atg5–Atg12 conjugation in normal cells. Besides Atg5, Beclin1 has also been found to be cleaved by caspases during apoptosis, blunting the autophagy
process to promote the apoptotic response.20, 21 Taken together, these findings indicate a critical role for autophagy proteins Atg5 and Beclin1 in the crosstalk between autophagy and
apoptosis, while adding calpain and caspases to the growing list of negative regulators of the autophagy track. RUBICON: GATEKEEPER OF AUTOLYSOSOMAL TRAFFIC The autophagosome ‘matures’
through multiple transient interactions with endosomal compartments and lysosomes, to form a hybrid-like organelle called the autolysosome. The primary function of the autolysosome is to
degrade autophagocytosed materials. Along this line, maturation can be an inherently dangerous business, as any misdelivery of cargo to the lysosome can lead to improper proteolytic
activation. Currently, little is known about what ‘prepares’ an autophagosome for fusion with a lysosome and what relevant ‘signals’ are involved. However, recent studies point to an
important negative regulatory mechanism in autophagosome maturation that is mediated by Rubicon (RUN domain and cysteine-rich domain containing, Beclin1-interacting protein). Rubicon, along
with Atg14, was independently identified by two laboratories46, 49 as a novel Beclin1 interactor. Although Atg14 seems conserved from yeast to humans, no yeast counterpart for Rubicon has
been found.67 Furthermore, unlike Atg14, which promotes the early stage of autophagosome biogenesis by forming a complex with Beclin1 and PI3KC3, Rubicon negatively regulates a downstream
stage of autophagosome maturation by joining the UVRAG, Beclin1, and PI3KC3 complex (Figure 1). Overexpression of Rubicon results in the accumulation of p62, an autophagy cargo, whereas
Rubicon depletion induces p62 degradation, suggesting that its overall function is in the inhibition of autophagy.49 In addition, both studies report that the acidification of
LC3-II-associated vesicles is severely impaired upon the overexpression of Rubicon,46, 49 indicating that Rubicon expression may restrict autophagic flux to lysosomes. It is currently not
clear how Rubicon affects autophagy maturation and its role in diseases remains to be explored. Although Rubicon is largely considered to be an autophagy-related protein, it is not
exclusively present in autophagosomes. Rather, a large portion of Rubicon is present in endosomal compartments.46, 49 Consistent with this observation, Matsunaga _et al._46 found that
Rubicon also has a role in endocytic trafficking. Overexpression of Rubicon causes defects in endocytic trafficking, whereas the knockdown of Rubicon is associated with enhanced trafficking
and degradation of epidermal growth factor receptor (EGFR). Given the previous finding that UVRAG facilitates lysosomal-directed membrane dynamics,68 one could speculate that Rubicon
potentially restricts the endocytic forward trafficking to the lysosome by antagonizing UVRAG. Alternatively or additionally, Rubicon may work in concert with UVRAG through different
mechanisms to maintain a net balance of overall autophagic flow. Thus, Rubicon may constitute an important component in both autophagic and endocytic trafficking. AMINO ACIDS: NEGATIVE
FEEDBACK LOOP OF AUTOPHAGY Autophagy is a unique process that degrades long-lived proteins, as well as protein aggregates, to replenish the intracellular amino acid pool, especially in
nutrient-deprived cells.23 Indeed, ablation of essential autophagy genes, such as _Atg7_, significantly dampens the level of intracellular amino acids, as is phenocopied in cells treated
with protein synthesis inhibitors.69 Conversely, increased intracellular free amino acids produced during autophagic degradation can reactivate the mTORC1 signaling and thus downregulate
autophagy, serving as a self-limiting feedback loop in autophagy regulation.70, 71 Using the _Xenopus laevis_ oocyte as a model, it has been shown that mTORC1 can sense small increases in
intracellular amino acids such as leucine, which leads to increased phosphorylation of both p70S6 kinase and its downstream target, ribosomal protein S6.72 Notably, such effects of amino
acids on mTORC1 can be independent of stimulation by insulin or other growth factors, but are dependent on autophagic proteolysis.73 A recent study by Yu _et al._71 also showed that mTOR
signaling is reactivated by prolonged starvation, which attenuates autophagy and restores lysosome homeostasis. This negative feedback loop is envisioned to be part of a homeostatic
mechanism required to prevent prolonged or overactivation of autophagy. THE ‘_YIN-YANG_’ FACE OF P53 IN AUTOPHAGY The tumor suppressor p53 is often considered the cellular gatekeeper because
of its role in sensing and synchronizing numerous stress conditions with cell cycle arrest, DNA repair, senescence, cellular metabolism, apoptosis, and autophagy, in both a
transactivation-dependent and/or -independent manner.74 Given the views of autophagy as a cellular stress response, it is unsurprising that several studies have shown that p53 can directly
activate autophagy.75, 76, 77 The exact mechanism by which p53 promotes autophagy is not clear, but relates to the ability of p53 to transactivate a group of critical factors that arrest
mTOR-mediated anabolic metabolism, including IGF-BP3, PTEN, TSC2, AMPK_β_1/_β_2, and sestrins1/2 (Figure 2).74, 75, 76 Each of these attenuates mTOR signaling and therefore counteracts its
repression of autophagy. Damage-regulated autophagy modulator (DRAM) is a p53 transcriptional target and has also been found to positively regulate autophagy.78 Along this line, any
impairment of p53 function leads to deregulation of the autophagy signaling pathway and may confer tumorigenesis. However, p53's role as an autophagy regulator remains enigmatic,
because recent studies by Tasdemir _et al._ have shown that, in addition to promoting autophagy, p53 also inhibits this process.79 Depletion or pharmacological inhibition of basal levels of
p53 in various cell lines, as well as _in vivo_ in mice and _Caenorhabditis elegans_, was found to induce autophagy, and this increased autophagy conferred resistance to metabolic stress in
p53-deficient cells. The mechanism by which p53 suppresses autophagy is not clear, but it seems independent of its transactivation activities.79 Further molecular dissection of p53 by
Tasdemir _et al._ revealed that it is the cytoplasmic p53, not nuclear p53, that mediates suppression of autophagy, reflecting the spatial control of p53 signaling (Figure 2). At first
glance, the antiautophagic effects of cytoplasmic p53 cannot be easily reconciled with the view of autophagy as a tumor suppressor mechanism. Yet, many tumor-derived, cytoplasm-restricted
forms of p53 are highly oncogenic, which may be partially due to their negative regulation of autophagy, at least in certain contexts.80 Thus, the regulation of autophagy by p53 is
multifaceted: stress-induced activation of p53 promotes autophagy, whereas physiological levels of p53 repress autophagy. The dual effects of p53 reflect the coordinated regulation of
cellular metabolism, including autophagy, which can be subverted (blunt the positive role _(‘yang’_) of nuclear p53) or capitalized on (amplify the negative role _(‘yin’_) of cytoplamsic
p53) in malignant transformation. CONCLUSIONS The pervasiveness and strength of autophagy necessitates the complexity of its negative regulation. Over the past 10 years, an extensive array
of negative regulators that limit the amplitude and duration of the autophagic response to confine it to a prescribed range has been identified.1, 2, 3 Defects in these endogenous autophagy
inhibitory processes can cause cell toxicity, tissue injury, and predispose the host to many atrophic diseases.12 Conversely, insufficient activation of autophagy causes the accumulation of
undesirable components, which can fuel inflammation, trigger necrosis, and induce genomic instability, features underlying the hallmarks of cancer, neurodegeneration, and aging.1, 6, 8 Thus,
it is important to envisage autophagy as a tightly regulated dynamic, not only for autophagy _per se_, but also in the context of its broad connection to other signaling or metabolic
pathways. Learning to manipulate this regulatory process of autophagy in the right contexts represents an exciting new challenge. It is likely to generate important advances in our
understanding of how autophagy is integrated at a broader level into the cellular network of signaling circuits and how this affects a range of cognitive processes, and is also critical for
devising successful therapies for autophagy-associated diseases. ABBREVIATIONS * TOR: target of rapamycin * PI3KC3: class III phosphoinositide (PI) 3kinase * PE: phosphatidylethanolamine *
MEFs: mouse embryonic fibroblasts * mTOR: mammalian TOR * PIKK: phosphoinositide 3-kinase-related kinase * mTORC1: mTOR complex 1 * mTORC2: mTOR complex 2 * Raptor: regulatory-associated
protein of mTOR * LST8: lethal with SEC13 protein 8 * PRAS40: proline-rich AKT-substrate * Rictor: rapamycin-insensitive companion of mTOR * SIN1: stress-activated-protein-kinase interacting
protein 1 * eIF4E: eukaryotic translation initiation factor 4E * 4E-BP: eIF4Ebidning protein * AMPK: AMP-activated protein kinase * AMPK_β_1/_β_2: the _β_ subunits of AMPK * PTEN:
phosphatase and tensin homolog * TSC: tuberous sclerosis complex * PtdIns(3)P: phosphatidylinositol-3-phosphate * NAF-1: nutrient-deprivation autophagy factor-1 * MTM: myotubularin * PTP:
protein tyrosine phosphatase * CNM: centronuclear myopathy * Rubicon, RUN domain and cysteine-rich domain containing: Beclin1 interacting protein * DAP: death-associated protein * DAPk:
DAP-kinase * IP3: inositol 1,4,5-triphosphate * IP3R: IP3 receptor * Cdks: cycline-dependent kinases * cFLIP: cellular FLICE-like inhibitory protein * TNF: tumor-necrosis factor * TRAIL:
TNF-related apoptosis-inducing ligand * FADD: FAS-associated death domain * IKK: I_κ_B kinase * EGFR: epidermal growth factor receptor * IGF-BP3: insulin-like growth factor binding protein 3
* DRAM: damage-regulated autophagy modulator REFERENCES * Levine B, Kroemer G . Autophagy in the pathogenesis of disease. _Cell_ 2008; 132: 27–42. Article CAS PubMed PubMed Central
Google Scholar * Yang Z, Klionsky DJ . An overview of the molecular mechanism of autophagy. _Curr Top Microbiol Immunol_ 2009; 335: 1–32. CAS PubMed PubMed Central Google Scholar * Xie
Z, Klionsky DJ . Autophagosome formation: core machinery and adaptations. _Nat Cell Biol_ 2007; 9: 1102–1109. Article CAS PubMed Google Scholar * Nishida Y, Arakawa S, Fujitani K,
Yamaguchi H, Mizuta T, Kanaseki T _et al_. Discovery of Atg5/Atg7-independent alternative macroautophagy. _Nature_ 2009; 461: 654–658. Article CAS PubMed Google Scholar * Klionsky DJ,
Lane JD . Alternative macroautophagy. _Autophagy_ 2010; 6: 201. Article PubMed Google Scholar * Deretic V . Autophagy in infection. _Curr Opin Cell Biol_ 2010; 22: 252–262. Article CAS
PubMed PubMed Central Google Scholar * Liang C, Jung JU . Autophagy genes as tumor suppressors. _Curr Opin Cell Biol_ 2010; 22: 226–233. Article CAS PubMed Google Scholar * Levine B,
Kroemer G . Autophagy in aging, disease and death: the true identity of a cell death impostor. _Cell Death Differ_ 2009; 16: 1–2. Article CAS PubMed Google Scholar * Levine B, Yuan J .
Autophagy in cell death: an innocent convict? _J Clin Invest_ 2005; 115: 2679–2688. Article CAS PubMed PubMed Central Google Scholar * Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H,
Asano T _et al_. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. _Circ Res_ 2007; 100:
914–922. Article CAS PubMed Google Scholar * De Meyer GR, Martinet W . Autophagy in the cardiovascular system. _Biochim Biophys Acta_ 2009; 1793: 1485–1495. Article CAS PubMed Google
Scholar * Sandri M . Autophagy in health and disease: 3. autophagy involvement in muscle atrophy. _Am J Physiol Cell Physiol_ 2010; 298: C1291–C1297. Article CAS PubMed Google Scholar *
Cherra SJ, Chu CT . Autophagy in neuroprotection and neurodegeneration: a question of balance. _Future Neurol_ 2008; 3: 309–323. PubMed PubMed Central Google Scholar * Virgin HW, Levine
B . Autophagy genes in immunity. _Nat Immunol_ 2009; 10: 461–470. Article CAS PubMed PubMed Central Google Scholar * Jackson WT, Giddings Jr TH, Taylor MP, Mulinyawe S, Rabinovitch M,
Kopito RR _et al_. Subversion of cellular autophagosomal machinery by RNA viruses. _PLoS Biol_ 2005; 3: e156. Article PubMed PubMed Central Google Scholar * Levine B, Sodora DL . HIV and
CXCR4 in a kiss of autophagic death. _J Clin Invest_ 2006; 116: 2078–2080. Article CAS PubMed PubMed Central Google Scholar * Jung CH, Ro SH, Cao J, Otto NM, Kim DH . mTOR regulation
of autophagy. _FEBS Lett_ 2010; 584: 1287–1295. Article CAS PubMed PubMed Central Google Scholar * Codogno P, Meijer AJ . Autophagy and signaling: their role in cell survival and cell
death. _Cell Death Differ_ 2005; 12 (Suppl 2): 1509–1518. Article CAS PubMed Google Scholar * Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L _et al_.
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. _Nat Cell Biol_ 2006; 8: 1124–1132. Article CAS PubMed Google Scholar * Djavaheri-Mergny M, Maiuri MC, Kroemer G .
Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. _Oncogene_ 2010; 29: 1717–1719. Article CAS PubMed Google Scholar * Luo S, Rubinsztein DC . Apoptosis
blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. _Cell Death Differ_ 2010; 17: 268–277. Article CAS PubMed Google Scholar * Heymann D . Autophagy: a
protective mechanism in response to stress and inflammation. _Curr Opin Investig Drugs_ 2006; 7: 443–450. CAS PubMed PubMed Central Google Scholar * Neufeld TP . TOR-dependent control of
autophagy: biting the hand that feeds. _Curr Opin Cell Biol_ 2010; 22: 157–168. Article CAS PubMed Google Scholar * Bjornsti MA, Houghton PJ . The TOR pathway: a target for cancer
therapy. _Nat Rev Cancer_ 2004; 4: 335–348. Article CAS PubMed Google Scholar * Huang J, Manning BD . The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. _Biochem J_
2008; 412: 179–190. Article CAS PubMed Google Scholar * Yang Z, Klionsky DJ . Mammalian autophagy: core molecular machinery and signaling regulation. _Curr Opin Cell Biol_ 2010; 22:
124–131. Article CAS PubMed Google Scholar * Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y . Tor-mediated induction of autophagy via an Apg1 protein kinase complex. _J
Cell Biol_ 2000; 150: 1507–1513. Article CAS PubMed PubMed Central Google Scholar * Chan EY, Longatti A, McKnight NC, Tooze SA . Kinase-inactivated ULK proteins inhibit autophagy via
their conserved C-terminal domains using an Atg13independent mechanism. _Mol Cell Biol_ 2009; 29: 157–171. Article CAS PubMed Google Scholar * Hara T, Takamura A, Kishi C, Iemura S,
Natsume T, Guan JL _et al_. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. _J Cell Biol_ 2008; 181: 497–510. Article CAS PubMed PubMed
Central Google Scholar * Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK _et al_. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway.
_Biochem Soc Trans_ 2009; 37: 232–236. Article CAS PubMed Google Scholar * Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS _et al_. AMPK phosphorylation of raptor
mediates a metabolic checkpoint. _Mol Cell_ 2008; 30: 214–226. Article CAS PubMed PubMed Central Google Scholar * Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y _et al_.
Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. _Mol Biol Cell_ 2009; 20: 1981–1991. Article CAS PubMed PubMed Central Google Scholar *
Shaw RJ, Cantley LC . Ras, PI(3)K and mTOR signalling controls tumour cell growth. _Nature_ 2006; 441: 424–430. Article CAS PubMed Google Scholar * Sabatini DM . mTOR and cancer:
insights into a complex relationship. _Nat Rev Cancer_ 2006; 6: 729–734. Article CAS PubMed Google Scholar * Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A _et al_.
Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. _J Cell Biol_ 2008; 182: 685–701.
Article CAS PubMed PubMed Central Google Scholar * Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK _et al_. Mitochondria supply membranes for autophagosome
biogenesis during starvation. _Cell_ 2010; 141: 656–667. Article CAS PubMed PubMed Central Google Scholar * Vergne I, Deretic V . The role of PI3P phosphatases in the regulation of
autophagy. _FEBS Lett_ 2010; 584: 1313–1318. Article CAS PubMed PubMed Central Google Scholar * Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N _et al_. Bcl-2 antiapoptotic
proteins inhibit Beclin 1-dependent autophagy. _Cell_ 2005; 122: 927–939. Article CAS PubMed Google Scholar * Long DX, Chang PA, Liang YJ, Yang L, Wu YJ . Degradation of neuropathy
target esterase by the macroautophagic lysosomal pathway. _Life Sci_ 2009; 84: 89–96. Article CAS PubMed Google Scholar * Clague MJ, Lorenzo O . The myotubularin family of lipid
phosphatases. _Traffic_ 2005; 6: 1063–1069. Article CAS PubMed Google Scholar * Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T _et al_. Control of autophagy
initiation by phosphoinositide 3-phosphatase jumpy. _Embo J_ 2009; 28: 2244–2258. Article CAS PubMed PubMed Central Google Scholar * Shen J, Yu WM, Brotto M, Scherman JA, Guo C,
Stoddard C _et al_. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis. _Nat Cell Biol_ 2009; 11: 769–776. Article CAS PubMed PubMed Central
Google Scholar * Laporte J, Bedez F, Bolino A, Mandel JL . Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases.
_Hum Mol Genet_ 2003; 12 Spec No. 2: R285292. * Tosch V, Rohde HM, Tronchere H, Zanoteli E, Monroy N, Kretz C _et al_. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating
variant in centronuclear myopathy. _Hum Mol Genet_ 2006; 15: 3098–3106. Article CAS PubMed Google Scholar * Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S _et al_.
Differential interactions between Beclin 1 and Bcl-2 family members. _Autophagy_ 2007; 3: 561–568. Article CAS PubMed Google Scholar * Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T,
Kurotori N _et al_. Two Beclin 1-binding proteins, Atg14 L and Rubicon, reciprocally regulate autophagy at different stages. _Nat Cell Biol_ 2009; 11: 385–396. Article CAS PubMed Google
Scholar * Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH _et al_. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. _Nat Cell Biol_ 2006; 8: 688–699.
Article CAS PubMed Google Scholar * Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y _et al_. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and
tumorigenesis. _Nat Cell Biol_ 2007; 9: 1142–1151. Article CAS PubMed PubMed Central Google Scholar * Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT _et al_. Distinct regulation of
autophagic activity by Atg14 L and Rubicon associated with Beclin 1phosphatidylinositol-3-kinase complex. _Nat Cell Biol_ 2009; 11: 468–476. Article CAS PubMed PubMed Central Google
Scholar * Maiuri MC, Criollo A, Kroemer G . Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. _Embo J_ 2010; 29: 515–516. Article CAS PubMed PubMed Central
Google Scholar * He C, Levine B . The Beclin 1 interactome. _Curr Opin Cell Biol_ 2010; 22: 140–149. Article CAS PubMed PubMed Central Google Scholar * Levine B, Sinha S, Kroemer G .
Bcl-2 family members: dual regulators of apoptosis and autophagy. _Autophagy_ 2008; 4: 600–606. Article CAS PubMed Google Scholar * Chang NC, Nguyen M, Germain M, Shore GC . Antagonism
of Beclin 1dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. _Embo J_ 2010; 29: 606–618. Article CAS PubMed Google Scholar * Chen YF, Kao CH, Chen YT, Wang CH, Wu
CY, Tsai CY _et al_. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. _Genes Dev_ 2009; 23: 1183–1194. Article CAS PubMed PubMed Central Google
Scholar * Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD _et al_. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from
the ER in response to inositol 1,4,5-trisphosphate. _J Cell Biol_ 2004; 166: 193–203. Article CAS PubMed PubMed Central Google Scholar * Criollo A, Maiuri MC, Tasdemir E, Vitale I,
Fiebig AA, Andrews D _et al_. Regulation of autophagy by the inositol trisphosphate receptor. _Cell Death Differ_ 2007; 14: 1029–1039. Article CAS PubMed Google Scholar * Vicencio JM,
Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L _et al_. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. _Cell Death Differ_ 2009; 16:
1006–1017. Article CAS PubMed Google Scholar * E X, Hwang S, Oh S, Lee JS, Jeong JH, Gwack Y _et al_. Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus
68. _PLoS Pathog_ 2009; 5: e1000609. Article PubMed PubMed Central Google Scholar * Furuya T, Kim M, Lipinski M, Li J, Kim D, Lu T _et al_. Negative Regulation of Vps34 by Cdk Mediated
Phosphorylation. _Mol Cell_ 2010; 38: 500–511. Article CAS PubMed PubMed Central Google Scholar * Khalfan WA, Klionsky DJ . Molecular machinery required for autophagy and the cytoplasm
to vacuole targeting (Cvt) pathway in S. cerevisiae. _Curr Opin Cell Biol_ 2002; 14: 468–475. Article CAS PubMed Google Scholar * Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR _et al_.
FLIP-mediated autophagy regulation in cell death control. _Nat Cell Biol_ 2009; 11: 1355–1362. Article CAS PubMed PubMed Central Google Scholar * Safa AR, Day TW, Wu CH . Cellular
FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. _Curr Cancer Drug Targets_ 2008; 8: 37–46. Article CAS PubMed PubMed Central Google Scholar * Golks A, Brenner
D, Krammer PH, Lavrik IN . The c-FLIP-NH2 terminus (p22FLIP) induces NF-kappaB activation. _J Exp Med_ 2006; 203: 1295–1305. Article CAS PubMed PubMed Central Google Scholar *
Chaudhary PM, Jasmin A, Eby MT, Hood L . Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. _Oncogene_ 1999; 18: 5738–5746. Article CAS
PubMed Google Scholar * Yang JK . FLIP as an anti-cancer therapeutic target. _Yonsei Med J_ 2008; 49: 1927. Google Scholar * Xia HG, Zhang L, Chen G, Zhang T, Liu J, Jin M _et al_.
Control of basal autophagy by calpain1 mediated cleavage of ATG5. _Autophagy_ 2010; 6: 61–66. Article CAS PubMed Google Scholar * Zhong Y, Wang QJ, Yue Z . Atg14 L and Rubicon: yin and
yang of Beclin 1mediated autophagy control. _Autophagy_ 2009; 5: 890–891. Article PubMed Google Scholar * Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA _et al_. Beclin1-binding UVRAG
targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. _Nat Cell Biol_ 2008; 10: 776–787. Article CAS PubMed PubMed Central Google Scholar *
Onodera J, Ohsumi Y . Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. _J Biol Chem_ 2005; 280: 31582–31586. Article CAS PubMed
Google Scholar * Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N . Amino acid regulation of TOR complex 1. _Am J Physiol Endocrinol Metab_ 2009; 296: E592602. Article
Google Scholar * Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J _et al_. Termination of autophagy and reformation of lysosomes regulated by mTOR. _Nature_ 2010; 465: 942–946. Article
CAS PubMed PubMed Central Google Scholar * Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM . Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6
kinase in a target of rapamycin-dependent manner. _J Biol Chem_ 2002; 277: 9952–9957. Article CAS PubMed Google Scholar * Beugnet A, Tee AR, Taylor PM, Proud CG . Regulation of targets
of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. _Biochem J_ 2003; 372: 555–566. Article CAS PubMed PubMed Central Google Scholar * Vousden
KH, Ryan KM . p53 and metabolism. _Nat Rev Cancer_ 2009; 9: 691–700. Article CAS PubMed Google Scholar * Feng Z, Zhang H, Levine AJ, Jin S . The coordinate regulation of the p53 and mTOR
pathways in cells. _Proc Natl Acad Sci USA_ 2005; 102: 8204–8209. Article CAS PubMed PubMed Central Google Scholar * Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G .
Autophagy regulation by p53. _Curr Opin Cell Biol_ 2010; 22: 181–185. Article CAS PubMed Google Scholar * Feng Z . p53 Regulation of the IGF-1/AKT/mTOR Pathways and the Endosomal
Compartment. _Cold Spring Harb Perspect Biol_ 2010; 2: a001057. Article PubMed PubMed Central Google Scholar * Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR _et al_.
DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. _Cell_ 2006; 126: 121–134. Article CAS PubMed Google Scholar * Tasdemir E, Maiuri MC, Galluzzi L, Vitale I,
Djavaheri-Mergny M, D’Amelio M _et al_. Regulation of autophagy by cytoplasmic p53. _Nat Cell Biol_ 2008; 10: 676–687. Article CAS PubMed PubMed Central Google Scholar * Morselli E,
Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A _et al_. Mutant p53 protein localized in the cytoplasm inhibits autophagy. _Cell Cycle_ 2008; 7: 3056–3061. Article CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS This work was partly supported by United States Public Health Service Grants CA140964, AI083841, the Leukemia & Lymphoma Society, the Wright
Foundation, and the Baxter Foundation (C Liang). We thank Stacy Lee for her critical reading of the paper. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Microbiology
and Immunology, University of Southern California, Los Angeles, CA, USA C Liang Authors * C Liang View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to C Liang. ADDITIONAL INFORMATION Edited by D Klionsky RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liang, C.
Negative regulation of autophagy. _Cell Death Differ_ 17, 1807–1815 (2010). https://doi.org/10.1038/cdd.2010.115 Download citation * Received: 28 April 2010 * Revised: 08 July 2010 *
Accepted: 27 July 2010 * Published: 24 September 2010 * Issue Date: December 2010 * DOI: https://doi.org/10.1038/cdd.2010.115 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * autophagy * negative regulation * mTOR * Bcl-2 * FLIP * p53
Trending News
Three Seasons In, And Just Like That Is Finally Its Own Gloriously Preposterous ThingCarrie Bradshaw never stops starting over. After the original Sex and the City series ended with Sarah Jessica Parker’s ...
Hamas leader mohammed sinwar killed, netanyahu saysHamas’s de facto leader in Gaza, Mohammed Sinwar, was killed during a recent airstrike, Israeli Prime Minister Netanyahu...
Who are todd and julie chrisley, whom trump is pardoning?President Donald Trump announced Tuesday that he would be pardoning reality TV stars Todd and Julie Chrisley, a couple b...
Why the u. S. Is pausing student visa interviews at embassiesProspective international students to the U.S. were dealt another blow when U.S. embassies were ordered not to schedule ...
Should you take a vitamin d supplement?Vitamin D does a lot for your body, supporting strong bones, muscle movement, your immune system, and more. Taking a vit...
Latests News
Negative regulation of autophagyABSTRACT Autophagy is an evolutionarily conserved catabolic process that involves the invagination and degradation of cy...
The murder case against brian walshe, according to prosecutorsLocal News INVESTIGATORS BELIEVE THE BODY OF ANA WALSHE, A COHASSET MOTHER OF THREE, WAS THROWN INTO TRASH BAGS HER HUSB...
Coronation street: why did kym marsh leave coronation street?Opening up to The Mirror, Marsh said she wanted to be part of the show as she had been a victim of identity fraud hersel...
Jared goff reacts to los angeles rams win and makes super bowl claimThe 24-year-old became the youngest quarterback to win a NFC championship game. Goff threw 297 yards after completing ju...
Light Winds at 470 Worlds >> Scuttlebutt Sailing News: Providing sailing news for sailorsHaifa, Israel (October 13, 2015) – A lighter breeze filled in over the race course in Haifa Bay on day two of the 2015 4...