Blue cone monochromatism: a phenotype and genotype assessment with evidence of progressive loss of cone function in older individuals
Blue cone monochromatism: a phenotype and genotype assessment with evidence of progressive loss of cone function in older individuals"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT _AIM_ To perform a detailed clinical and psychophysical assessment of the members of three British families affected with blue cone monochromatism (BCM), and to determine the
molecular basis of disease in these families. _METHODS_ Affected and unaffected members of three families with BCM were examined clinically and underwent electrophysiological and detailed
psychophysical testing. Blood samples were taken for DNA extraction. The strategy for molecular analysis was to amplify the coding regions of the long wavelength-sensitive (L) and middle
wavelength-sensitive (M) cone opsin genes and the upstream locus control region by polymerase chain reaction, and to examine these fragments for mutations by direct sequencing. _RESULTS_ We
have confirmed the reported finding of protan-like D-15 arrangements of patients with BCM. In addition, we have demonstrated that the Mollon–Reffin (MR) Minimal test is a useful
colour-discrimination test to aid in the diagnosis of BCM. Affected males were shown to fail the protan and deutan axes, but retained good discrimination on the tritan axis of the MR test, a
compelling evidence for residual colour vision in BCM. This residual tritan discrimination was also readily detected with HRR plates. In two families, psychophysical testing demonstrated
evidence for progression of disease. In two pedigrees, BCM could be linked to unequal crossovers within the opsin gene array that resulted in a single 5′-L/M-3′ hybrid gene, with an
inactivating Cys203Arg mutation. The causative mutations were not identified in the third family. _CONCLUSIONS_ The MR test is a useful method of detecting BCM across a wide range of age
groups; residual tritan colour discrimination is clearly demonstrated and allows BCM to be distinguished from rod monochromatism. BCM is usually classified as a stationary cone dysfunction
syndrome; however, two of our families show evidence of progression. This is the first report of progression associated with a genotype consisting of a single 5′-L/M-3′ hybrid gene carrying
an inactivating mutation. We have confirmed that the Cys203Arg inactivating mutation is a common sequence change in blue cone monochromats. SIMILAR CONTENT BEING VIEWED BY OTHERS PHENOTYPIC
CHARACTERIZATION OF AUTOSOMAL DOMINANT PROGRESSIVE CONE DYSTROPHIES ASSOCIATED WITH A HETEROZYGOUS VARIANT C.2512C>T OF _GUCY2D_ GENE IN A LARGE KINDRED Article 12 December 2022 GENETIC
CHARACTERISTICS OF 234 ITALIAN PATIENTS WITH MACULAR AND CONE/CONE-ROD DYSTROPHY Article Open access 08 March 2022 CONE DYSTROPHY ASSOCIATED WITH AUTOIMMUNE POLYGLANDULAR SYNDROME TYPE 1
Article Open access 11 July 2023 INTRODUCTION In order to derive colour vision, the normal human visual system compares the rate of quantum catches in three classes of cone, the short (S or
blue) wavelength-sensitive, middle (M or green) wavelength-sensitive and long (L or red) wavelength-sensitive cones, which are maximally sensitive to light at 430, 535, and 565 nm,
respectively. This triad of cone types provides the physiological substrate for trichromacy.1, 2, 3 Each cone class contains its own visual pigment composed of an opsin protein linked by a
Schiff base to the chromophore retinal. In humans, the L- and M-opsins are encoded by genes on the X chromosome, and the S-opsin by a gene located on chromosome 7.4 There exist three types
of inherited colour vision deficiency in which vision is dichromatic; each type corresponds to a selective defect in one of the three receptor mechanisms.1, 2 Protanopia and deuteranopia are
characterised by defects of the red- and green-sensitive mechanisms, respectively. They are among the common X-linked disorders of colour vision whose association with alterations in the
visual pigment gene cluster at Xq28 identified those genes as encoding the red- and green-sensitive visual pigments.4, 5 The wild-type arrangement of the L- and M-opsin genes consists of a
head-to-tail tandem array of two or more repeat units of 39 kb on chromosome Xq28 that are 98% identical at the DNA level.5 This high level of identity would appear to pre-dispose the L and
M-opsin genes to unequal inter- and intragenic recombination. Transcriptional regulation of the L and M genes is controlled by an upstream locus control region (LCR).6 In contrast,
tritanopia is an autosomal dominant disorder that is characterised by a selective defect of the blue-sensitive mechanism and is due to dominant mutations in the S-cone opsin gene. Blue cone
monochromatism (BCM), or X-linked incomplete achromatopsia, is a rare congenital stationary cone dysfunction syndrome, affecting less than 1 in 100 000 individuals, characterised by the
absence of L- and M-cone function.2 Thus, blue-cone monochromats possess rod vision and a normal short-wavelength-sensitive cone mechanism. Photopic vision is mediated by the blue cones, and
without a comparison between different classes of the cone photoreceptor, affected individuals are reported to have poor colour discrimination. Mutations in the L- and M-opsin gene array
that result in the lack of functional L- and M- pigments, and thus inactivate the corresponding cones, have been identified in the majority of BCM cases studied.6, 7 As in rod monochromacy
(RM), BCM typically presents with reduced visual acuity, pendular nystagmus, and photophobia. Visual acuity is of the order of 6/24–6/60. Eccentric fixation may be present and myopia is a
common finding.8 BCM is distinguished from RM via psychophysical or electrophysiological testing. The photopic electroretinogram (ERG) is profoundly reduced in both, but the S-cone ERG is
well preserved in BCM.9 BCM patients have high Farnsworth–Munsell 100-Hue scores, but have fewer errors in the vertical (tritan) axis when compared to RM. At the molecular level, mutation
analyses have proved highly efficient at establishing the molecular basis for BCM.6, 7 The mutations in the L- and M-opsin gene array that cause BCM fall into two classes. In the first
class, a normal L- and M-opsin gene array is inactivated by a deletion in the LCR, located upstream of the L-opsin gene. A deletion in this region would appear to abolish transcription of
all genes in the opsin gene array and therefore inactivates both L- and M-cones.10 In the second class of mutations, the LCR is preserved, but changes within the L- and M-pigment gene array
lead to loss of functional pigment production. The most common genotype in this class consists of a single inactivated L/M hybrid gene. The first step in this second mechanism is thought to
be unequal crossing over that reduces the number of genes in the array to one, followed by a mutation that inactivates the remaining gene. A frequent inactivating mutation that has been
described is the thymine-to-cytosine transition at nucleotide 648, which results in a cysteine-to-arginine substitution at codon 203, a mutation known to disrupt the folding of cone opsin
molecules.11 Our aim was to perform a detailed clinical and psychophysical assessment of members of three British families affected with BCM, and subsequently to determine the molecular
basis of BCM in these families. PATIENTS AND METHODS Affected and unaffected members of three families with BCM were examined clinically and underwent psychophysical and electrophysiological
testing. After informed consent was obtained, blood samples were taken, genomic DNA was isolated from whole blood using an extraction kit (Nucleon®Biosciences), and molecular genetic
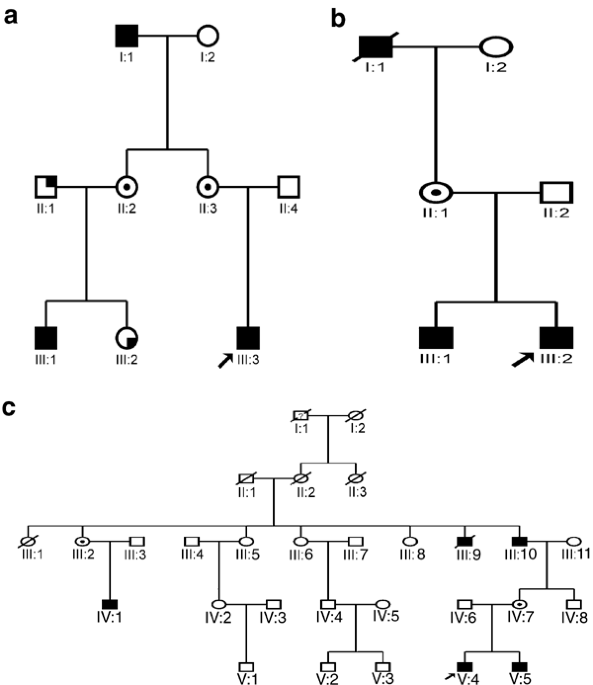
analysis was performed. CLINICAL ASSESSMENT The pedigrees of the families studied can be seen in Figures 1a–c. A full medical and ophthalmic history was taken in all examined members. A full
ophthalmological examination was performed. All affected individuals had an ERG performed. Adults had an ERG that conformed to the ISCEV standard, but the children had a modified protocol
using skin electrodes. Colour vision testing included the use of Hardy, Rand and Rittler (HRR) plates, SPP2 plates for acquired colour deficiency, Farnsworth–Munsell (FM) 100-hue test,
Farnsworth D-15, the Mollon-Reffin (MR) minimal test,12 a computerised colour vision test,13, 14 and anomaloscopy. The FM 100-hue, Farnsworth D-15 and the MR test were all performed under
CIE Standard Illuminant C from a MacBeth Easel lamp. The MR minimal test is a saturation discrimination-type test.12 The caps used in this test are of a similar design to those in the D-15
and FM 100-hue tests. The test features a series of caps that lie along protan, deutan, and tritan lines, respectively. In addition, there is one demonstration cap, which does not lie along
a dichromatic confusion axis. The remainder of the caps are all neutral, but have varying lightness (there are a total of nine grey caps). The examiner places one coloured cap among a group
of neutral caps and asks the subject to tap the side of the cap that is coloured. The coloured caps vary in their saturation, so the severity of the defect can be assessed. It can be
performed by children as young as 5 years. MOLECULAR GENETIC ANALYSIS The underlying strategy for molecular analysis was to amplify the coding regions of the L- and M-cone opsin genes and
the upstream LCR by PCR, and to examine these fragments for mutations by direct sequencing. Exonic sequences of the L- and M-genes are 98% identical at the nucleotide level and, although
there may be less evolutionary pressure for intronic sequences to remain stable, the introns of the two genes have also remained almost identical (>99.9% identical at the nucleotide
level). It was not possible, therefore, to design PCR primers that amplify only L- or only M-opsin exonic fragments from genomic DNA. Consequently, the primer pairs used in this study
amplified L and M sequences simultaneously, and individual nucleotide differences between the genes were subsequently utilised for differentiation between the genes in sequence analysis. (i)
PCR amplification of the L- and M- opsin genes: Intronic forward and reverse PCR primers (Table 1) were designed to amplify the LCR, and all six exons of the L- and M-genes from genomic
DNA. LCR primers were designed based on the published sequence,10 and encompassed the LCR core sequence. Primer pairs that would co-amplify both L- and M-opsin exonic sequences were designed
within each intron approximately 50 bp from the intron–exon junction, in order that the whole of each exon, some flanking DNA, and the splice sites were amplified. The design of these
primers was based on the published sequences of the L- and M-opsin genes sequence.5 PCR reactions (50 _μ_l) were performed as follows: 1 × NH4 buffer, 1 mM MgCl2, 200 _μ_M each dNTP, 10
pmols each of sense and antisense primers, 200 ng-1 _μ_g DNA, 1 U Bio_Taq_, _X_ _μ_l dH2O — with the exception that the concentrations of MgCl2 used in LCR, exon 3 and 6 PCR reactions were
optimised at 0.5 mM, 0.5 mM and 2 mM respectively — and with annealing at the exon-specific temperatures (Table 1). PCR products were visualised by electrophoresis using low melting
temperature agarose gel. Target products were then excised and eluted. (ii) Mutation analysis of the PCR-amplified exons and LCR was carried out by direct sequencing using a cycle-sequencing
kit. After ethanol precipitation, the DNA products were analysed on an ABI 373A automated DNA sequencer and examined for alterations, utilising _Sequencing Analysis_ (ABI PrismTM) and
_GeneWorks__TM_ software. RESULTS PHENOTYPE _FAMILY A_ ( _Figure 1a_ ) Patient III:3—This 7-year-old boy (proband) was originally seen at 1 year of age. He was found to have horizontal
pendular nystagmus, normal fundi, and clear media. A family history of nystagmus, photophobia, and ‘colour blindness’ affecting males of the family, including his grandfather (I:1) and male
cousin (III:1), was established. ERG testing revealed absent cone responses, but normal rod responses. He had a visual acuity of 3/36 in each eye with his myopic correction. Psychophysical
testing on the MR test and HRR plates revealed reasonable discrimination only along the tritan axis. On computerised testing, his colour-discrimination ellipses were oriented along the angle
that one would expect of someone making colour discriminations based on a comparison of quantum catches in the rods and S-cones. Patient I:1—This 60-year-old man was found to have a visual
acuity of 6/36 in the right eye and 6/60 in the left. He was found to have clear lenses and mild macular retinal pigment epithelial (RPE) changes. As a child, he had obvious nystagmus, but
this had improved throughout life, to the point that it was not at all noticeable. He felt that his vision had continued to slowly deteriorate throughout life. Cone ERG responses were
absent, but rod responses were normal. On the basis of his results on the HRR plates, the D-15, the MR minimal test, and Nagel anomaloscope, it was concluded that he had no residual colour
vision. His 12-year-old grandson (III:1) had a visual acuity of 6/60 in the right eye and 6/36 in the left and displayed evidence of residual colour discrimination. He had clear media and
normal fundi. He showed reasonable discrimination along both the tritan line of MR test and also on the SPP2 tritan plates. He displayed a protan ordering of the D-15. On computerised
testing his ellipses were oriented along the angle that is expected for colour discriminations based solely upon a comparison of quantum catches in the rods and the S-cones (Figures 2, 4 and
5). Patient III:2—This 14-year-old daughter of II:2 was asymptomatic. On the Nagel anomaloscope, she was found to be deuteranomalous. On the MR minimal test, she showed good discrimination
on the tritan axis, but was badly impaired on both protan and deutan axes. Patients II:2 and II:3 were both asymptomatic, and on detailed psychophysical testing were found to have normal
colour vision. The father of patients III:1 and III:2 was not available for psychophysical testing, but was reported to be ‘colour blind’. A consistent psychophysical hypothesis from these
observations would be that the proband III:3 and III:1 have inherited from their maternal grandfather I:1, via their mothers, an X-chromosome with an altered opsin array that has led to BCM.
III:2 has also inherited this altered X-chromosome from her mother, and an X-chromosome that leads to a deuteranomalous phenotype from her father. Since III:3 and III:1 both have some
residual colour discrimination and their grandfather has none, it would appear that their condition is not stationary. III:3 and III:1 can be labelled blue-cone monochromats, whereas their
grandfather behaves as a rod monochromat, presumably as a result of continued S-cone loss. The lack of colour vision seen in the grandfather is highly unlikely to be due to lenticular
changes, since his lenses were found to be clear. _FAMILY B_ ( _Figure 1b_ ) Patient III:2—This 12-year-old boy (proband) originally presented with pendular nystagmus, poor visual acuity
(6/24 in both eyes), and myopia. Ocular media were clear with normal fundi. ERG revealed absent cone but normal rod responses. On psychophysical testing, the anomaloscope and Sloan's
test for achromatopsia suggested a rod-dominated spectral sensitivity function. On the D-15, he showed the protan-like pattern reported for BCM by Weis & Biersdorf.15 On the computer
test, he failed completely on the protan and deutan lines, but scored nearly normally along the tritan line, which modulates the blue cones. In addition, his ellipses were well aligned to
the theoretical S-cone/rod confusion axis, consistent with the mechanism whereby colour discriminations are based upon a comparison between rod and S-cone quantum catches (Figures 3, 4 and
5). Exactly the same pattern was exhibited on the MR minimalist test: he could not find saturated protan and deutan probes among the grey distractors, but could find the least saturated
tritan cap. On the SPP2 plates for acquired colour deficiencies, he passed the plates that are failed by those with purely scotopic vision (RM). Patient III:1—This 14-year-old brother of the
proband also presented with pendular nystagmus, poor visual acuity (6/24 in the right eye and 6/36 in the left), myopia, and photophobia. Ocular media were clear with normal fundi. ERG
revealed absent cone responses but normal rod function. He showed reasonable discrimination only along the tritan axis on HRR testing. He declined further psychophysical testing. Patient
II:1—The 50-year-old mother of III:1 and III:2 was asymptomatic. ERG and colour vision testing was normal. Patient I:1—The maternal grandfather of the propositus was said to have had poor
eyesight since birth and to have always had great problems with colour vision. The grandfather had an elder brother who had also worn glasses and had suffered with poor vision since infancy.
Both were deceased at the time of investigation. _FAMILY C_ ( _Figure 1c_ ) Patient V:4 This 7-year-old boy (proband) originally presented as an infant with nystagmus and photophobia. His
current visual acuity was recorded at 6/24 in both eyes. He had clear ocular media with normal fundi. He had a family history of nystagmus, poor visual acuity, and colour vision affecting
males of the family including his grandfather (III:10) and uncle (IV:1). ERG revealed absent cone responses but normal rod function. On the D-15, he showed confusions characteristic of
congenital red-green deficiency or BCM rather than a scotopic axis (RM). On the MR minimal test, he failed the protan and deutan axes, but discriminated on the tritan axis. On the HRR
plates, he passed all the tritan plates and failed all the protan/deutan plates. Patient V:5 This 6-year-old boy had nystagmus and a visual acuity of 6/36 in the right eye and 6/24 in the
left. He had clear ocular media with normal fundi. On the MR test, he failed the protan and deutan axes, but discriminated well on the tritan axis. Computerised testing corroborated these
findings; he failed completely on the deutan line and discriminated very poorly on the protan line, which hold almost constant the blue cone signal, but scored almost normally along the
tritan line, which modulates the blue cones. Patient III:10 This 70-year-old grandfather of V:4 and V:5 has had poor vision and nystagmus since childhood. The nystagmus had become less
prominent over time. He felt that his vision had gradually deteriorated since childhood. He had early lens opacities with minimal yellowing of the lens and mild macular RPE changes. ERG
revealed absent cone responses and normal rod function. On detailed psychophysical testing, there was little evidence of any residual colour vision. On anomaloscopy, he displayed the
classical brightness-matching function of an achromat. On the D-15, his responses were anarchic. He failed all plates on the SPP2 series. On the MR minimal test, he failed the protan and
deutan axes completely, but did show some residual discrimination on the tritan axis, detecting the most saturated cap. Patient IV:1 This 50-year-old man who is registered partially sighted
was found to have a visual acuity of 6/36 in both eyes, with no nystagmus. He complained of poor vision since childhood and that he had always had trouble with colour vision. He had clear
lenses and normal fundi. Little residual colour vision could be detected. On the MR minimal test, he failed completely on all the three axes with both eyes. On anomaloscopy, he behaved like
an achromat. Patients IV:6 and IV:7—Both are asymptomatic and have entirely full colour vision on detailed testing. Since the grandfather (III:10), uncle (IV:1), and two grandchildren (V:4
and V:5) share the same genotype, the condition appears to be progressive. The children show a residual colour discrimination that is lacking in both older men. There is convincing
psychophysical evidence to suggest that the children have functional S-cones. The loss of residual colour vision seen in the two older men in this family is highly unlikely to be due to
lenticular changes alone, since the lenses were found to be either clear (IV:1) or only minimally yellow (III:10). A markedly yellow lens would be required to result in any significant loss
of colour vision and such yellowing would be unlikely to result in the degree of loss of tritan function seen in this family. GENOTYPE A number of PCR primer pairs was used to amplify the
X-linked L- and M-opsin genes that underlie the L- and M-pigments and the LCR that controls the opsin gene array. The identification of L- or M-opsin gene depends on a number of sequence
differences that are confined to exons 2–5. _Family A_ Sequence analysis demonstrated the presence of a single M-opsin gene in the array of the affected subjects (Figure 6). However, no
further alterations of this gene were identified. Examination of the entire LCR revealed an unaltered sequence. The mutational basis of the colour vision defect in this family remains
uncertain. _Family B_ Sequence representing only the L gene was present for exons 2–4, while for exon 5 only sequence of the M gene was observed. This is consistent with the presence of only
a single 5′-L/M-3′ hybrid gene in affected members of family B in their opsin array. Moreover, alignment of exon 4 of the hybrid gene in these individuals with wild-type exon 4 sequence
showed that this opsin gene carries the previously reported T → C nucleotide alteration which encodes the Cys203Arg substitution (Figures 7 and 9). Sequence analysis of obligate carriers
demonstrated that both L- and M-opsin genes are present in the opsin gene array and that the T → C transition in exon 4 is also carried in the L-opsin genes in these individuals. _Family C_
Affected individuals of family C also carry the Cys203Arg mutation within a single 5′-L/M-3′ hybrid gene in the array. In this case, the crossover between the L- and M-genes, which is
required for generation of the hybrid gene, occurred within exon 3 (Figures 8 and 9). DISCUSSION Congenital. achromatopsia can be classified into three categories. (i) Typical rod
monochromats (complete achromatopsia) have normal rod function, but lack all sensitivity mediated by cone pigments. (ii) Atypical rod monochromats (incomplete achromatopsia) have some
residual cone function and consequently have better visual acuity and some residual colour vision. (iii) Blue-cone monochromats have rod and blue-cone function. Clinically, individuals in
all the three categories share a number of features. They have poor visual acuity, pendular nystagmus, photophobia, and in the majority of cases fundoscopy is unremarkable. Regardless of
category, rod monochromats classically have very poor or no colour discrimination, and on a Farnsworth D-15 or FM 100 Hue test show no clear axis, and therefore no preference for confusing
any subset of hues. Blue-cone monochromats can be distinguished from both types of rod monochromats by psychophysical threshold testing or electroretinographic studies at different
wavelengths.2, 9 Classification can also be aided by family history, because BCM is inherited as an X-linked recessive trait, whereas both types of rod monochromacy are autosomal recessive.
Rod monochromats cannot make colour judgements, but rather will use brightness cues to differentiate between colours. When confronted with tasks where luminance cues cannot be used, these
patients perform very poorly indeed (eg FM 100 hue scores are very high, D-15 arrangement patterns are characteristic and ordered along a scotopic axis). This contrasts with blue-cone
monochromats who do have access to colour discrimination, though this does depend upon the luminance of the task: at mesopic levels, they have rudimentary dichromatic colour discrimination
based upon a comparison of the quantum catches obtained by the rods and the S-cones, with their neutral point lying at about 460–470 nm.16, 17 Hue discrimination is reported to deteriorate
with increasing luminance.18 Blue-cone monochromats may be distinguished from rod monochromats by means of colour vision testing: blue-cone monochromats are reported to display fewer errors
along the vertical axis in the 100-Hue test (fewer tritan errors), and they may also display protan-like ordering patterns on the D-15.15 In addition, the Berson plates are claimed to
provide a good separation of blue cone monochromats from rod monochromats.19, 20 In our case series, we have confirmed the previously reported finding of protan-like patterns on D-15 testing
of patients with BCM.15 It should be noted, however, that this finding is not necessarily indicative of colour discrimination, since it is possible that BCM individuals order the chips of
this test according to their relative lightness to the S-cone mechanism.21 Relevant to this is our demonstration that affected males also retain reasonable discrimination on the tritan axis
of the MR test. Since this test utilises lightness randomisation, it should not be possible for those with BCM to discriminate the target chips by lightness cues. Furthermore, those patients
who were examined with a computer-controlled saturation discrimination task displayed discrimination ellipses with major axes well aligned to the theoretical S-cone/rod confusion axis. This
provides additional evidence that it is possible to derive colour discrimination at low photopic levels via a comparison of quantum catches in the S-cones and rods, as has been suggested
previously.16, 18 It is proposed that the latter two tests, which are suitable for use in children, can be readily employed in probing the differential diagnosis of congenital achromatopsia.
BCM is generally accepted to be a stationary cone dysfunction syndrome, although Fleischman and O'Donnell reported one BCM family with macular atrophy and noted a slight deterioration
of visual acuity and colour vision during a 12-year follow-up period, as well as pigmentary changes in the fovea.22 There are two further reports of individuals with BCM displaying a
progressive retinal degeneration; in both cases, the genotype consisted of deletion of the LCR.6, 23 In two of our families (A and C), there has also been progression in the severity of the
condition. Since, in family A, the children have normal tritan function, and their grandfather has none, it would appear that the condition is not stationary. The two children can be
labelled blue-cone monochromats, whereas their grandfather behaves as a rod monochromat, presumably secondary to the death of S-cones. In family C, the grandfather, uncle, and two
grandchildren share the same genotype. Since these children show convincing psychophysical evidence that S-cones are still surviving, whereas both older men in this family lack residual
colour discrimination, the condition also appears to be progressive in this family. Moreover, the genotype identified in this family of a single 5′-L/M-3′ hybrid gene with an inactivating
mutation differs from previous reports of progression, where an LCR deletion was present. There is, therefore, no consistent genotype associated with progression of BCM. Combined results of
previous studies6, 7, 23, 24 provide evidence for the general conclusion, first put forward by Nathans _et al_,6 that there are different mutational pathways to BCM. The data suggest that
40% of blue-cone monochromat genotypes are the result of a one-step mutational pathway that leads to deletion of the LCR. The remaining 60% of blue-cone monochromat genotypes comprise a
heterogeneous group of multi-step pathways. The evidence thus far shows that many of these multi-step pathways produce visual pigment genes that carry the inactivating Cys203Arg mutation. In
our study, the genetic mechanism identified was unequal recombination resulting in an array comprising a single 5′-L/M-3′ hybrid gene carrying a Cys203Arg mutation (Figure 9). This genotype
has been reported as a cause of BCM in earlier studies.6, 7, 24 The cysteine residue at position 203 of the L/M-opsin protein is highly conserved among all visual pigments and several other
seven-transmembrane-spanning receptors.25 This cysteine residue is located in the second extracellular loop of the opsin and, together with a conserved cysteine residue at position 126 in
the first extracellular loop, forms a disulphide bond necessary for stabilisation of the tertiary structure of the protein.11 The data presented here confirm the incidence of the Cys203Arg
mutation in BCM. The affected subjects assessed in family A would appear to possess only an M-opsin gene in the visual pigment array, which would be expected to produce dichromacy rather
than monochromacy. Sequence analysis failed to reveal a mutation in any of the six opsin gene exons or the LCR. The diagnosis of BCM on clinical and psychophysical grounds is clear in this
family, as is the X-linked inheritance; so it would seem most likely that the inactivating mutation in this case has not been identified. Other studies have also failed to detect the genetic
alteration that would explain the BCM phenotype in all assessed individuals.7, 26 Indeed, Nathans _et al_7 reported nine subjects out of 33 individuals with BCM, where the structure of the
opsin array did not reveal the genetic mechanism of the phenotype. It is therefore also plausible that further genetic heterogeneity remains to be identified in BCM. In this report, we
present the phenotypic and genotypic findings in a case series of three families with BCM, usually regarded as a stationary disorder. However, two of our families demonstrate progression.
Thus, it cannot always be assumed that BCM will remain stationary. In addition, it is not generally recognised that nystagmus associated with BCM may become less prominent over time, as has
been observed in our study. We have also demonstrated that the MR minimal test and HRR plates are useful colour-discrimination tests to aid in the diagnosis of BCM. Affected males repeatedly
failed the protan and deutan axes, but retained good discrimination on the tritan axis of the MR test, whereas patients with RM perform poorly along all the three axes. It is suggested that
this test can be readily employed in probing the differential diagnosis of congenital achromatopsia. REFERENCES * Boynton RM . _Human Color Vision_. Holt, Rinehart & Winston: New York,
1979. Google Scholar * Pokorny J, Smith VC, Verriest G . Congenital color defects. In: Pokorny J, Smith VC, Verriest G, Pinckers AJ (eds) _Congenital and Acquired Color Vision Defects_.
Grune & Stratton: New York, 1979, p 183. Google Scholar * Young T . On the theory of light and colours. _Philos Trans R Soc Lond_ 1802; 92: 12–48. Article Google Scholar * Nathans J,
Piantanida TP, Eddy RL, Shows TB, Hogness DS . Molecular genetics of inherited variation in human color vision. _Science_ 1986; 232: 203–210. Article CAS Google Scholar * Nathans J,
Thomas D, Hogness DS . Molecular genetics of human colour vision: the genes encoding blue, green, and red pigments. _Science_ 1986; 232: 193–202. Article CAS Google Scholar * Nathans J,
Davenport CM, Maumenee IH, Lewis RA, Hejtmancik JF, Litt M et al. Molecular genetics of human blue cone monochromacy. _Science_ 1989; 245: 831–838. Article CAS Google Scholar * Nathans J,
Maumenee IH, Zrenner E, Sadowski B, Sharpe LT, Lewis RA et al. Genetic heterogeneity among blue-cone monochromats. _Am J Hum Genet_ 1993; 53: 987–1000. CAS PubMed PubMed Central Google
Scholar * Weleber RG, Eisner A . Cone degeneration (‘Bull's eye dystrophies’) and colour vision defects. In: Newsome DA (ed) _Retinal Dystrophies and Degenerations_. Raven Press: New
York, 1988, pp 233–256. Google Scholar * Gouras P, MacKay CJ . Electroretinographic responses of the short-wavelength-sensitive cones. _Invest Ophthalmol Vis Sci_ 1990; 31: 1203–1209. CAS
PubMed Google Scholar * Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J et al. A locus control region adjacent to the human red and green visual pigment genes. _Neuron_ 1992;
9: 429–440. Article CAS Google Scholar * Kazmi MA, Sakmar TP, Ostrer H . Mutation of a conserved cysteine in the X-linked cone opsins causes color vision deficiencies by disrupting
protein folding and stability. _Invest Ophthalmol Vis Sci_ 1997; 38: 1074–1081. CAS PubMed Google Scholar * Mollon JD, Astell S, Reffin JP . A minimalist test of colour vision. In: Drun
B, Moreland JD, Serra A (eds) _Colour Vision Deficiencies XI_. Kluwer Academic Publishers: Dordrecht, 1991, pp 59–67. Chapter Google Scholar * Mollon JD, Reffin JP . A computer-controlled
colour vision test that combines the principles of Chibret and of Stilling. _J Physiol_ 1989; 414: 5. Google Scholar * Regan BC, Reffin JP, Mollon JD . Luminance noise and the rapid
determination of discrimination ellipses in colour deficiency. _Vision Res_ 1994; 34: 1279–1299. Article CAS Google Scholar * Weis AH, Biersdorf WR . Blue cone monochromatism. _J Pediatr
Ophthalmol Strabismus_ 1989; 26: 218–223. Google Scholar * Reitner A, Sharpe LT, Zrenner E . Is colour vision possible with only rods and blue-sensitive cones? _Nature_ 1991; 352: 798–800.
Article CAS Google Scholar * Alpern M, Lee GB, Maaseidvaag F, Miller SS . Colour vision in blue cone monochromacy. _J Physiol_ 1971; 212: 211–233. Article CAS Google Scholar * Young
RS, Price J . Wavelength discrimination deteriorates with illumination in blue cone monochromats. _Invest Ophthalmol Vis Sci_ 1985; 26: 1543–1549. CAS PubMed Google Scholar * Berson EL,
Sandberg MA, Rosner B, Sullivan PL . Color plates to help identify patients with blue cone monochromatism. _Am J Ophthalmol_ 1983; 95: 741–747. Article CAS Google Scholar *
Haegerstrom-Portnoy G, Schneck ME, Verdon WA, Hewlett SE . Clinical vision characteristics of the congenital achromatopsias. II. Color vision. _Optom Vis Sci_ 1996; 73: 457–465. Article CAS
Google Scholar * Hess RF, Mullen KT, Sharpe LT, Zrenner E . The photoreceptors in atypical achromatopsia. _Journal of Physiology_ 1989; 417: 123–149. Article CAS Google Scholar *
Fleischman JA, O'Donnell Jr FE . Congenital X-linked incomplete achromatopsia. Evidence for slow progression, carrier fundus findings, and possible genetic linkage with
glucose-6-phosphate dehydrogenase locus. _Arch Ophthalmol_ 1981; 99: 468–472. Article CAS Google Scholar * Ayyagari R, Kakuk LE, Coats EL, Bingham EL, Toda Y, Felius J et al. Bilateral
macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene. _Mol Vis_ 1999; 5: 13–18. CAS PubMed Google Scholar *
Reyniers E, Van Thienen MN, Meire F, De Boulle K, Devries K, Kestelijn P et al. Gene conversion between red and defective green opsin gene in blue cone monochromacy. _Genomics_ 1995; 29:
323–328. Article CAS Google Scholar * Karnik SS, Khorana HG . Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. _J Biol Chem_ 1990; 265:
17520–17524. CAS PubMed Google Scholar * Ayyagari R, Kakuk LE, Bingham EL, Szczesny JJ, Kemp J, Toda Y et al. Spectrum of color gene deletions and phenotype in patients with blue cone
monochromacy. _Hum Genet_ 2000; 107: 75–82. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The work was supported by grants from the British Retinitis Pigmentosa Society
and the Guide Dogs for the Blind Association. We are also grateful to the patients who kindly agreed to take part in this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of
Ophthalmology, University College London, London, UK M Michaelides, S Johnson, A T Moore & D M Hunt * Department of Experimental Psychology, University of Cambridge, Cambridge, UK M P
Simunovic & J D Mollon * Department of Ophthalmology, Addenbrooke's Hospital, Cambridge, UK K Bradshaw * Moorfields Eye Hospital, City Road, London, UK G Holder Authors * M
Michaelides View author publications You can also search for this author inPubMed Google Scholar * S Johnson View author publications You can also search for this author inPubMed Google
Scholar * M P Simunovic View author publications You can also search for this author inPubMed Google Scholar * K Bradshaw View author publications You can also search for this author
inPubMed Google Scholar * G Holder View author publications You can also search for this author inPubMed Google Scholar * J D Mollon View author publications You can also search for this
author inPubMed Google Scholar * A T Moore View author publications You can also search for this author inPubMed Google Scholar * D M Hunt View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D M Hunt. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Michaelides, M.,
Johnson, S., Simunovic, M. _et al._ Blue cone monochromatism: a phenotype and genotype assessment with evidence of progressive loss of cone function in older individuals. _Eye_ 19, 2–10
(2005). https://doi.org/10.1038/sj.eye.6701391 Download citation * Received: 19 August 2003 * Accepted: 10 November 2003 * Published: 16 April 2004 * Issue Date: 01 January 2005 * DOI:
https://doi.org/10.1038/sj.eye.6701391 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * monochromatism * cone * dysfunction * phenotype *
genotype
Trending News
Chelsea Hawley | VA Bedford Health Care | Veterans AffairsThe .gov means it’s official.Federal government websites often end in .gov or .mil. Before sharing sensitive information...
Boron containing polyvinyl alcohol/ polyethylene oxide/polyvinyl pyrrolidone composites: preparation, characterization, gamma radiation shielding andRADIATION PHYSICS AND CHEMISTRY Volume 214, January 2024, 111261 https://doi.org/10.1016/j.radphyschem.2023.111261Get ri...
Haleakalā national park: a visitor’s guide to planning a tripTwo or three hours from the luaus and swim-up bars at Maui’s beachside resorts, one of the world’s largest dormant volca...
Cult leader to appeal court loss, seeks new libel trial before new judgeAdNewsLocal NewsNewsLocal NewsNews HomeNewsSportCommunityTributes & FuneralsClassifiedsExplore TravelEntertainmentLifest...
Avaxtars tokens explained: $avxt, $enxt and $dgc_AVAXTARS BEGAN AS A BROWSER-BASED GAME PROJECT ON THE AVALANCHE BLOCKCHAIN NETWORK, WHICH WENT LIVE ON MARCH 31, 2021. ...
Latests News
Blue cone monochromatism: a phenotype and genotype assessment with evidence of progressive loss of cone function in older individualsABSTRACT _AIM_ To perform a detailed clinical and psychophysical assessment of the members of three British families aff...
Sea and inland waterway passenger rightsConsultation outcome SEA AND INLAND WATERWAY PASSENGER RIGHTS Get emails about this page This was published under the 20...
How much energy are we using? - GOV.UKNews story HOW MUCH ENERGY ARE WE USING? DFID has started publishing real-time information on energy and water use in it...
13 reasons why renewed for third season despite controversy over netflix series_13 Reasons Why _is getting a third season, Netflix confirmed Wednesday. The streaming service announced the news with a...
The handmaid's tale season 3 cast: who does emily althaus play?The Handmaid’s Tale season three has introduced a number of new actors to the dystopian drama so far this series. This i...