Phase ii study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer
Phase ii study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT This multicentre phase II study evaluated the efficacy and safety of preoperative capecitabine plus oxaliplatin and radiotherapy (RT) in patients with locally advanced rectal cancer
(T3/T4 rectal adenocarcinoma with or without nodal involvement). Treatment consisted of one cycle of XELOX (capecitabine 1000 mg m−2 bid on days 1–14 and oxaliplatin 130 mg m−2 on day 1),
followed by RT (1.8 Gy fractions 5 days per week for 5 weeks) plus CAPOX (capecitabine 825 mg m−2 bid on days 22–35 and 43–56, and oxaliplatin 50 mg m−2 on days 22, 29, 43 and 50). Surgery
was recommended 5 weeks after completion of chemoradiotherapy. The primary end point was pathological complete tumour response (pCR). Sixty patients were enrolled. In the intent-to-treat
population, the pCR rate was 23% (95% CI: 13–36%). 58 patients underwent surgery; R0 resection was achieved in 57 (98%) patients, including all 5 patients with T4 tumours. Sphincter
preservation was achieved in 49 (84%) patients. Tumour and/or nodal downstaging was observed in 39 (65%) patients. The most common grade 3/4 adverse events were diarrhoea (20%) and
lymphocytopaenia (43%). Preoperative capecitabine, oxaliplatin and RT achieved encouraging rates of pCR, R0 resection, sphincter preservation and tumour downstaging in patients with locally
advanced rectal cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS RETROSPECTIVE STUDY OF PREOPERATIVE CHEMORADIOTHERAPY WITH CAPECITABINE VERSUS CAPECITABINE PLUS OXALIPLATIN FOR LOCALLY
ADVANCED RECTAL CANCER Article Open access 27 July 2020 EFFICACY AND SAFETY OF PD-1 BLOCKADE PLUS LONG-COURSE CHEMORADIOTHERAPY IN LOCALLY ADVANCED RECTAL CANCER (NECTAR): A MULTI-CENTER
PHASE 2 STUDY Article Open access 11 March 2024 A PHASE II RANDOMISED TRIAL OF INDUCTION CHEMOTHERAPY FOLLOWED BY CONCURRENT CHEMORADIOTHERAPY IN LOCALLY ADVANCED PANCREATIC CANCER: THE
TAIWAN COOPERATIVE ONCOLOGY GROUP T2212 STUDY Article 17 December 2021 MAIN In recent years, considerable progress has been made in the treatment of locally advanced rectal cancer, mainly
due to improvements in the type and quality of surgery, better staging methods and regular use of chemoradiation (CRT) or radiation therapies. Although the use of preoperative CRT for
resectable rectal cancer remains a controversial issue, preoperative CRT is clearly preferred when tumour shrinkage is required before surgery, that is, in locally advanced T4 disease and
low-lying tumours when sphincter preservation is attempted (Sauer et al, 2004; Bosset et al, 2006; Gérard et al, 2006). Furthermore, preoperative CRT improves local disease control with less
toxicity compared with postoperative CRT (Sauer et al, 2004). Many attempts have been made to increase the convenience and activity of preoperative 5-fluorouracil (5-FU)-based CRT. Evidence
from phase II trials suggests that the oral fluoropyrimidine capecitabine (Xeloda®; F Hoffmann-La Roche Ltd, Basel, Switzerland) has similar activity to that of protracted 5-FU infusional
CRT regimens (Glynne-Jones et al, 2006a). Combining different chemotherapy agents, such as oxaliplatin or irinotecan, with fluoropyrimidines has a clear rationale based on a plethora of data
in the metastatic colorectal setting and the potential to further improve efficacy in patients receiving preoperative CRT. Oxaliplatin (Eloxatin®; Sanofi-Aventis, Bridgewater, NJ, USA) is
an ideal candidate for inclusion into neoadjuvant CRT regimens because of its radiosensitising capabilities and synergy with fluoropyrimidines. Capecitabine has been tested in combination
with oxaliplatin and radiotherapy in several different regimens (for review see Glynne-Jones et al, 2006a). These include continuous capecitabine (7 days per week) with oxaliplatin given on
days 1 and 29 (Glynne-Jones et al, 2006c), continuous capecitabine (5 days per week) with weekly doses of oxaliplatin (Machiels et al, 2005; Rutten et al, 2006) and discontinuous
capecitabine (days 1–14 and 22–35) with oxaliplatin on days 1, 8, 22 and 29 (Roedel et al, 2003, 2007). The aim of the present multicentre phase II study was to evaluate the efficacy,
tolerability and feasibility of preoperative capecitabine plus oxaliplatin in combination with radiotherapy as described by Roedel et al (2003, 2007), and to investigate the contribution of
an additional single cycle of neoadjuvant capecitabine and oxaliplatin (XELOX regimen) (Díaz-Rubio et al, 2002; Cassidy et al, 2004) before the start of radiotherapy. MATERIALS AND METHODS
PATIENT POPULATION Patients entering the study had histologically confirmed rectal adenocarcinoma. Evidence of T3 or T4 disease with or without perirectal nodal involvement by endorectal
ultrasound or magnetic resonance imaging (MRI) of the pelvis was required. Further inclusion criteria were an Eastern Cooperative Oncology Group performance status 0–2 and adequate
haematological (neutrophils ⩾1.5 × 109 l−1 and platelets ⩾100 × 109 l−1), renal (calculated creatinine clearance >50 ml min−1) and liver function (serum bilirubin ⩽1.5 upper limit of
normal range, liver transaminase or alkaline phosphatase concentrations ⩽2.5 upper limit of normal range). No upper age limit was defined. Exclusion criteria were metastatic disease,
previous chemotherapy for colorectal cancer or prior radiotherapy to the pelvis, history of another malignancy within the last 5 years, any contraindication to radiotherapy, clinically
significant cardiac disease, malabsorption syndrome, peripheral neuropathy ⩾grade 1 according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC version
3.0), serious uncontrolled infection, concomitant treatment with any nucleoside analogue, known dihydropyrimidine dehydrogenase deficiency and psychiatric disorders or conditions interfering
with compliance for oral drug intake. Pregnant or lactating woman were excluded. All patients provided written informed consent. The study protocol was approved by a local independent
ethics committee and conducted in accordance with the Declaration of Helsinki. PRETREATMENT EVALUATION Before study entry, all patients were assessed by a multidisciplinary team comprising
medical, radiation and surgical oncologists, gastroenterologists and radiologists. Patients underwent a medical history, physical examination, biopsy, ECG and staging studies (chest X-ray,
abdominal–pelvis computed tomography scan, colonoscopy and endorectal ultrasound). Pelvic MRI was optional but recommended for all patients with low-lying or T4 tumours. Complete laboratory
tests included a full blood count, electrolytes, creatinine, liver transaminases, alkaline phosphatase, total bilirubin and carcinoembryonic antigen measurement. TREATMENT RADIOTHERAPY
Megavoltage equipment was used with 6–18 MV. Radiotherapy was delivered through three to four portal fields to the tumour and lymphonodal regions, and perirectal soft tissue structures at
risk of microscopic disease. All patients received 45 Gy, with a daily fraction of 1.8 Gy given 5 days per week for 5 consecutive weeks. If treatment was interrupted, the dose was increased
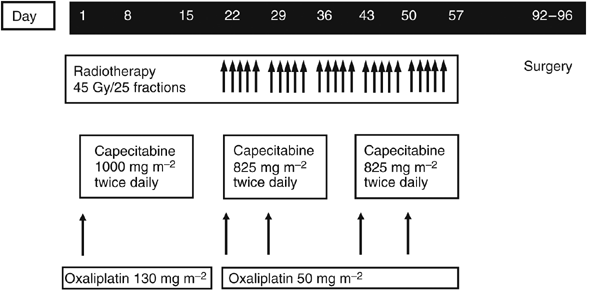
by 1–2 fractions. CHEMOTHERAPY Treatment consisted of a single cycle of XELOX (oral capecitabine 1000 mg m−2 twice daily on days 1–14 plus a 2-h intravenous infusion of oxaliplatin 130 mg
m−2 on day 1), followed by CAPOX combined with RT (capecitabine 825 mg m−2 twice daily on days 22–35 and 43–56, and oxaliplatin 50 mg m−2 on days 22, 29, 43 and 50; Figure 1). Chemotherapy
and radiotherapy were interrupted if grade 3 or 4 toxicity was encountered (except for anaemia). Study treatment was restarted when toxicity had resolved to grade ⩽1. Dose reductions were
required after grade 3–4 toxicity. If treatment was delayed for longer than 3 weeks, the patient was withdrawn from the study. SURGERY Five to six weeks after completion of CRT, total
mesorectal excision (TME) with sphincter preservation was performed whenever feasible according to standardised technique as the preferred type of radical resection. Assessment of the
intended surgical procedure (TME or abdominoperineal resection) was performed by the treating surgeon before registration. Administration of adjuvant chemotherapy was left to the treating
oncologist's discretion. EVALUATION OF EFFICACY AND SAFETY The extent of residual tumour in the resected specimen was classified according to the TNM staging system of the American
Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC). Semiquantitative evaluation of histological regression was performed according to the grading criteria established
by Mandard et al (1994) and Dworak et al (1997) in oesophageal carcinomas, which was subsequently adapted by Bouzourene et al (2002) (see Table 1 for summary). At each site, one preselected
pathologist evaluated the tumour tissue of all patients participating in the study. A second-opinion pathology review was performed in all tumours categorised as Dworak grade 2 or 3 by a
pathologist from another centre participating in the study. Safety was assessed through documenting adverse events and clinical laboratory tests performed at screening, during treatment and
the surgery period. Adverse events were graded using NCI CTC version 3.0. STATISTICAL ANALYSIS The intent-to-treat population (ITT) consisted of all patients who received at least one dose
of study medication whether or not they were eligible. All efficacy analyses were performed on this population. Patients not undergoing surgery or who were not evaluable for response were
considered nonresponders. The safety population consisted of patients who received at least one dose of any study drug and who had a baseline assessment and at least one safety follow-up.
The primary end point was pathological complete tumour response (pCR) prospectively defined as grade 3 or 4 according to the Dworak classification (DC) system (Dworak et al, 1997). The pCR
rate was presented with 95% confidence intervals (CIs) using the Pearson–Clopper method. Secondary end points were rate of sphincter preservation, R0 resection in patients with T4 tumours,
downstaging (defined as a decrease of ⩾1 point(s) in T and/or N value) and safety. An exploratory analysis using the Fisher exact test was performed to test for an association between the
presence and absence of a complete tumour response (DC grade 3/4 _vs_ 0/1/2), and site, location and size of primary tumour at screening, u/cTNM classification at screening, time between
radiotherapy and surgery (⩽40 _vs_ >40 days), age (⩽60 _vs_ >60 years) and treatment-related lymphocytopaenia. This study was designed as a one-stage phase II trial using pCR rate as
the primary efficacy criterion. A pCR of 22% was considered acceptable and a rate of ⩽7% was ruled out as futile. With a total of 48 evaluable patients (and a response rate of at least 22%),
a power of 86% and a type-I error of 4.8% was achieved. The planned sample size was increased to 60 patients to allow for dropouts. RESULTS PATIENT CHARACTERISTICS A total of 60 patients
were enrolled between March 2005 and July 2006 from six cancer centres in Switzerland. Patient characteristics are summarised in Table 2. All 60 patients were included in the safety and ITT
populations, including 2 patients who were ineligible (one patient because of cT2 rectal cancer, and the other patient because of an urothelial cancer 4 years before the start of the study).
Fifty-eight patients (97% of all recruited patients) received CRT and underwent surgery; one patient withdrew consent and one patient died prior to surgery. DOSE INTENSITY AND SAFETY
Fifty-five patients (92%) received all three cycles of capecitabine (mean relative dose intensity 97%), and 52 patients (87%) received all five planned oxaliplatin doses (mean relative dose
intensity 97%). The mean relative dose intensity for capecitabine was 98% during XELOX and 96% during CAPOX-RT. For oxaliplatin, the mean relative dose intensity was 99% during XELOX and 93%
during CAPOX-RT. Fifty-six patients (93%) received at least 25 fractions (45 Gy) of radiotherapy as planned. Table 3 summarises grade 3/4 treatment-related nonhaematological toxicities
presented per treatment regimen (XELOX _vs_ CAPOX-RT). The most frequently occurring grade 3/4 adverse event was diarrhoea (20%); all other grade 3/4 events were uncommon (⩽5%). No grade 3/4
haematological toxicity was observed, except for lymphocytopaenia (43%). At least one serious adverse event was recorded in eight patients (13%) during the study. A total of 12 serious
adverse events (20%) were reported, the most common of which were diarrhoea (_n_=5) and colitis or proctitis (_n_=2). One patient developed severe neutropaenic infection and died on day 19
after the start of neoadjuvant XELOX. Four patients (7%) had one adverse event leading to discontinuation of capecitabine, and three patients (5%) had adverse events leading to
discontinuation of oxaliplatin. No patient required discontinuation of radiotherapy. EFFICACY AND SURGICAL PARAMETERS Surgery was performed in a total of 58 patients (TME in 47 patients
(81%), abdominoperineal extirpation in 9 patients (16%) and other type of surgery in 2 patients (3%)). The median time between the end of radiotherapy and surgery was 42 days (range: 24–59
days). In 57 patients (98%), including all 5 patients with c/uT4 tumours, R0 resection was achieved and sphincter preservation was achieved in 49 patients (84%). Comparing the baseline
tumour stage with the pathological stage in the ITT population, downstaging with respect to tumour stage was observed in 28 (47%) patients, and downstaging with respect to nodal stage was
observed in 29 (48%) patients. A detailed analysis is shown in Table 4. In the ITT population, complete tumour regression (ypT0 N0, DC regression grade 4) was achieved in seven patients. An
additional seven patients showed near-complete regression (DC regression grade 3) with only very few detectable tumour cells as assessed by two independent pathologists. According to
predefined criteria, the pCR rate was therefore 23% (95% CI: 13–36%). The corresponding pCR rate according to the Mandard regression grading system (grades 1 and 2) was 27% (grade 1, seven
patients; grade 2, nine patients). A second-opinion review of all specimens rated as DC grade 2 or 3 was necessary in 33 cases (57%). After the second opinion, the final DC grading remained
the same in 27 cases (82%), downgrading was deemed necessary in 5 cases (15%) and upgrading in 1 case (3%). Both tumour regression scales were compared using the final DC grades and Mandard
grades. The scales seemed to correspond well; all patients with DC grade 0 reported Mandard-tumour regression (M-TR) grade 5, 90% of patients with DC grade 1 reported M-TR grade 4, 74% of
patients with DC grade 2 reported M-TR grade 3, 86% of patients with DC grade 3 reported M-TR grade 2 and all patients with DC grade 4 reported M-TR grade 1. According to an exploratory
subgroup analysis, only upper location of the primary tumour (between 10 and 12 cm from anal verge) was found to be negatively correlated with pCR (_P_=0.0504). DISCUSSION Pathological
complete tumour response rates between 10 and 30% have been observed with combined preoperative chemotherapy and radiotherapy protocols. Pathological complete tumour response is a reliable
and reproducible surrogate for tumour response and is linked to improved outcome (Roh et al, 2004; Roedel et al, 2005). Although achievement of a pCR is not the primary goal of neoadjuvant
therapy, it has become a commonly used end point in many phase II trials aiming to improve the efficacy of rectal cancer treatment. In the present trial, we are able to demonstrate a pCR in
23% of patients, defined as grades 3 and 4 according to the Dworak classification (Dworak et al, 1997) following preoperative therapy with a single cycle of XELOX and two further cycles of
CAPOX given with radiotherapy. Recently, several different tumour regression scales (Mandard et al, 1994; Dworak et al, 1997; Bouzourene et al, 2002; Wheeler et al, 2004) have been proposed
for the measurement of regression after preoperative therapies independent of the ypTNM stage. Besides several differences in categorisation of tumour regression, all of the scales
acknowledge a distinctive group of tumours with only microscopic foci of remaining tumour cells. We have grouped patients with sterilised primary tumours and lymph nodes (DC grade 4)
together with DC grade 3 tumours. This is based on the observation that single residual tumour cells confer a significantly lower local relapse rate and a better prognosis (Wheeler et al,
2004) than tumours with remaining dominant disease. The second-opinion review was used for all specimens rated to be either DC grade 2 or 3 by the first pathologist. A high concordance rate
between independent pathologists of 82% suggests a reasonable capability to discriminate between very few and difficult-to-find tumour cells (DC grade 3) from easy-to-find few tumour cells
or groups of tumour cells (DC grade 2). In addition, applying the regression grading classification of Mandard et al (1994) adapted for rectal cancer (Bouzourene et al, 2002) and combining
Mandard's grade 1 (absence of residual tumour cells) and grade 2 (rare residual tumour cells), complete tumour response was achieved in 27% of patients. These almost identical results
from two different scoring systems indicate a high reproducibility in defining near-complete and complete sterilisation of tumour cells. Furthermore, and an important point with regard to
prognosis, we observed nodal downstaging in 48% of patients. The number of tumour-infiltrated lymph nodes (ypN status) following preoperative radiochemotherapy is a strong and independent
prognostic factor for survival. Sterilising lymph nodes reflects the impact of effective neoadjuvant treatment and consistently translates into improved long-term outcome (Bouzourene et al,
2002; Chan et al, 2005; Chapet et al, 2005; Roedel et al, 2005). In 98% of the 58 patients who underwent surgery and in all patients with T4 rectal cancer, R0 resection was possible.
Sphincter preservation was achieved in 84% of patients. This appears remarkable as 35% of our patients had low-lying tumours (0–5 cm from anal verge). The most common nonhaematological
toxicity in our trial was grade 3 or 4 diarrhoea, which occurred overall in 20% of patients (10% during XELOX and 10% during CAPOX-RT). This rate is slightly higher than that reported by
Roedel et al (2003, 2007) and suggests that the additional cycle of XELOX increased the toxicity of preoperative CRT. All other toxicities were in the range of other trials with the
exception of grade 3 or 4 lymphocytopaenia. Lymphocytopaenia is a negative prognostic factor in cancer patients and can be induced by either chemotherapy or pelvic radiotherapy. Decline in
total lymphocyte counts is obviously an underreported toxicity and seems to be negatively correlated with tumour regression following pelvic radiotherapy (Lissoni et al, 2005). The addition
of a single chemotherapy cycle before CRT does not appear to have substantially enhanced the overall antitumour activity and should, therefore, not be considered as an important treatment
element. In our trial, we added this chemotherapy cycle primarily to assure early start of therapy. Even though many patients reported improvement in symptoms before starting CRT (data not
shown), we did not consider achieving a relevant downsizing effect with a single chemotherapy cycle. The use of neoadjuvant chemotherapy prior to preoperative CRT in rectal cancer patients
is a matter of debate (Glynne-Jones and Sebag-Montefiore, 2006; Glynne-Jones et al, 2006b) primarily because satisfactory local control rates can be achieved with preoperative CRT alone.
Chau et al (2006) questioned this position by adding four cycles of neoadjuvant capecitabine and oxaliplatin before CRT with capecitabine in their trial. Most patients (86%) had symptomatic
responses, and the radiological response rate measured by MRI was 88%. Pathological complete tumour response was achieved in 24% of patients, which is clearly superior to the 11% DC
regression grade 4 in our trial. However, 4 out of 77 patients died during neoadjuvant chemotherapy. In the absence of a randomised phase III trial proving superior outcome, the addition of
primary chemotherapy to CRT should be only used in the context of clinical trials. A different treatment strategy, in an attempt to increase the quantity of systemic treatment, was studied
by Roedel et al (2007). After preoperative capecitabine/oxaliplatin radiotherapy, 60% of patients received all four cycles of adjuvant capecitabine/oxaliplatin underlining the feasibility of
delivering adequate doses of postoperative combination chemotherapy in rectal cancer patients. In conclusion, we demonstrated that preoperative XELOX followed by CAPOX-RT is feasible with
manageable toxicity and results in encouragingly high rates of pCR, R0 resection, sphincter preservation and tumour downstaging in patients with locally advanced rectal cancer. More
importantly, we were able to replicate, and thus confirm the findings from Roedel et al (2003, 2007) in a multicentre setting in Switzerland. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper
was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Bosset JF, Collette L, Calais G, Mineur L, Maingon P,
Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. _N Engl J Med_ 355: 1114–1123 Article CAS Google Scholar *
Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P (2002) Importance of tumor regression assessment in predicting the outcome of patients with locally advanced rectal carcinoma who are
treated with preoperative radiotherapy. _Cancer_ 94: 1121–1130 Article Google Scholar * Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J,
Sawada N, Schöffski P, Sobrero A, Van Cutsem E, Díaz-Rubio E (2004) XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. _J Clin
Oncol_ 22: 2084–2091 Article CAS Google Scholar * Chan AK, Wong A, Jenken D, Heine J, Buie D, Johnson D (2005) Posttreatment TNM staging is a prognostic indicator of survival and
recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. _Int J Radiat Oncol Biol Phys_ 61: 665–677 Article Google Scholar * Chapet O, Romestaing
P, Mornex F, Souquet JC, Favrel V, Ardiet JM, d'Hombres A, Gerard JP (2005) Preoperative radiotherapy for rectal adenocarcinoma: which are strong prognostic factors? _Int J Radiat Oncol
Biol Phys_ 61: 1371–1377 Article Google Scholar * Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A, Oates J (2006) Neoadjuvant
capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. _J Clin Oncol_ 24: 668–674
Article CAS Google Scholar * Díaz-Rubio E, Evans TR, Tabernero J, Cassidy J, Sastre J, Eatock M, Bisset D, Regueiro P, Baselga J (2002) Capecitabine (Xeloda) in combination with
oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. _Ann Oncol_ 13: 558–565 Article Google Scholar * Dworak O, Keilholz L, Hoffmann A (1997)
Pathological feature of rectal cancer after preoperative radiochemotherapy. _Int J Colorectal Dis_ 12: 19–23 Article CAS Google Scholar * Gérard TC, Conroy T, Bonnetain F, Bouché O,
Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L (2006) Preoperative radiotherapy with or without concurrent
fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. _J Clin Oncol_ 24: 4620–4625 Article Google Scholar * Glynne-Jones R, Dunst J, Sebag-Montefiore D (2006a) The
integration of oral capecitabine into chemoradiation regimens for locally advanced rectal cancer: how successful have we been? _Ann Oncol_ 17: 361–371 Article CAS Google Scholar *
Glynne-Jones R, Grainger J, Harrison M, Ostler P, Makris A (2006b) Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: should we be more cautious?
_Br J Cancer_ 94: 363–371 Article CAS Google Scholar * Glynne-Jones R, Sebag-Montefiore D (2006) Role of neoadjuvant chemotherapy in rectal cancer: interpretation of the EXPERT study. _J
Clin Oncol_ 24: 4664–4665 Article Google Scholar * Glynne-Jones R, Sebag-Montefiore D, McDonald A, Maughan TS, Falk SJ, McDonald AC (2006c) A phase I dose escalation study of continuous
oral capecitabine in combination with oxaliplatin and pelvic radiation (XELOX-RT) in patients with locally advanced rectal cancer. _Ann Oncol_ 17: 50–56 Article CAS Google Scholar *
Lissoni P, Meregalli S, Bonetto E, Mancuso M, Brivio F, Colciago M, Gardani G (2005) Radiotherapy-induced lymphocytopenia: changes in total lymphocyte count and in lymphocyte subpopulations
under pelvic irradiation in gynecologic neoplasms. _J Biol Regul Homeost Agents_ 19: 153–158 CAS PubMed Google Scholar * Machiels JP, Duck L, Honhon B, Coster B, Coche JC, Scalliet P,
Humblet Y, Aydin S, Kerger J, Remouchamps V, Canon JL, Van Maele P, Gilbeau L, Laurent S, Kirkove C, Octave-Prignot M, Baurain JF, Kartheuser A, Sempoux C (2005) Phase II study of
preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. _Ann Oncol_ 16: 1898–1905 Article Google Scholar * Mandard AN,
Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G (1994) Pathological assessment of tumour regression after preoperative chemoradiotherapy of
esophageal carcinoma: clinico-pathologic correlations. _Cancer_ 73: 2680–2686 Article CAS Google Scholar * Roedel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R
(2003) Phase I/II trial of capecitabine, oxaliplatin and radiation for rectal cancer. _J Clin Oncol_ 21: 3098–3104 Article CAS Google Scholar * Roedel C, Liersch T, Hermann RM, Arnold D,
Reese T, Hipp M, Fürst A, Schwella N, Bieker M, Hellmich G, Ewald H, Haier J, Lordick F, Flentje M, Sülberg H, Hohenberger W, Sauer R (2007) Multicenter phase II trial of chemoradiation with
oxaliplatin for rectal cancer. _J Clin Oncol_ 25: 110–117 Article CAS Google Scholar * Roedel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W,
Raab R, Sauer R, Wittekind C (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. _J Clin Oncol_ 23: 8688–8696 Article Google Scholar
* Roh MS, Colangelo L, Wieand S, O'Connell M, Petrelli N, Smith R, Mamounas E, Hyams D, Wolmark N (2004) Response to preoperative multimodality therapy predicts survival in patients
with carcinoma of the rectum. _J Clin Oncol_ 22 (14S): (abstract 3505) * Rutten H, Sebag-Montefiore D, Glynne-Jones R, Rullier E, Peeters M, Brown G, Van Cutsem E, Ricci S, Van de Velde CJ,
Quirke P (2006) Capecitabine, oxaliplatin, radiotherapy, and excision (CORE) in patients with MRI-defined locally advanced rectal adenocarcinoma: results of an international multicenter
phase II study. _J Clin Oncol_ 24 (18S): (abstract 3528) * Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch
T, Schmidberger H, Raab R, German Rectal Cancer Study Group (2004) Preoperative _vs_ postoperative chemoradiotherapy for rectal cancer. _N Engl J Med_ 351: 1731–1740 Article CAS Google
Scholar * Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD, Jones AC, Mortensen NJ (2004) Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced
rectal cancer: correlation with rectal cancer regression grade. _Dis Colon Rectum_ 47: 2025–2031 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We greatly appreciate the
help of Mr Lee Miller in preparing this manuscript. The study was sponsored by Roche Pharma (Schweiz) AG and Sanofi-Aventis (Schweiz) AG. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Division Oncology/Hematology, Department of Internal Medicine, Kantonsspital St Gallen, St Gallen, CH-9007, Switzerland D Koeberle & T Ruhstaller * Department of Internal Medicine,
Stadtspital Triemli, Zürich, CH-8063, Switzerland R Burkhard * Department of Medical Oncology, Kantonsspital Graubünden, Chur, CH-7000, Switzerland R von Moos * Department of Internal
Medicine, Kantonsspital Luzern, Luzern, CH-6000, Switzerland R Winterhalder * Department of Medical Oncology, Universitätsspital Basel, Basel, CH-4031, Switzerland V Hess * Department of
Internal Medicine, Stadtspital Waid, Zurich, CH-8037, Switzerland F Heitzmann * Department of Clinical Pathology, Universitätsspital Basel, Basel, CH-4031, Switzerland L Terraciano *
Department of Pathology, Kantonsspital St Gallen, St Gallen, CH-9007, Switzerland J Neuweiler * Roche Pharma (Schweiz) AG, Reinach, CH-4153, Switzerland G Bieri & C Rust * Department of
Radio-Oncology, Kantonsspital St Gallen, St Gallen, CH-9007, Switzerland M Toepfer Authors * D Koeberle View author publications You can also search for this author inPubMed Google Scholar *
R Burkhard View author publications You can also search for this author inPubMed Google Scholar * R von Moos View author publications You can also search for this author inPubMed Google
Scholar * R Winterhalder View author publications You can also search for this author inPubMed Google Scholar * V Hess View author publications You can also search for this author inPubMed
Google Scholar * F Heitzmann View author publications You can also search for this author inPubMed Google Scholar * T Ruhstaller View author publications You can also search for this author
inPubMed Google Scholar * L Terraciano View author publications You can also search for this author inPubMed Google Scholar * J Neuweiler View author publications You can also search for
this author inPubMed Google Scholar * G Bieri View author publications You can also search for this author inPubMed Google Scholar * C Rust View author publications You can also search for
this author inPubMed Google Scholar * M Toepfer View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D Koeberle. RIGHTS
AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a
copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Koeberle, D., Burkhard, R., von Moos, R. _et al._
Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. _Br J Cancer_ 98,
1204–1209 (2008). https://doi.org/10.1038/sj.bjc.6604297 Download citation * Received: 15 November 2007 * Revised: 14 February 2008 * Accepted: 21 February 2008 * Published: 18 March 2008 *
Issue Date: 08 April 2008 * DOI: https://doi.org/10.1038/sj.bjc.6604297 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * rectal cancer *
radiochemotherapy * capecitabine * oxaliplatin
Trending News
Hydro-electric power and its utilizationABSTRACT In these days when the world is talking of power from fissionable, matter, one is apt to forget the inexhaustib...
Tryp® by wyndham discount, an aarp member benefitMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Rain and flood warnings increase in south-west france after stormsUP TO 100 MM OF RAIN FORECAST TO FALL IN SOME AREAS Heavy rain and flash flood warnings are in place in south-west Franc...
Shortage of staff caused by christmas party excessDentistry is facing a crisis according to a report in a national tabloid newspaper. Apparently there is an alleged short...
Comparative studies of gene expression and the evolution of gene regulationKEY POINTS * The hypothesis that differences in gene regulation have an important role in speciation and adaptation is m...
Latests News
Phase ii study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advancedABSTRACT This multicentre phase II study evaluated the efficacy and safety of preoperative capecitabine plus oxaliplatin...
A critical guide to the oscar best pic nominees – and why call me by your name is the standoutFilms nominated for Best Picture are often hit and miss – sometimes some are very good, often many are mediocre, reflect...
Travis kelce says he’s ‘heartbroken’ after grisly shooting at kansas city chiefs super bowl victory paradeTravis Kelce is “heartbroken” after at least 22 people were shot and one was killed during the Kansas City Chiefs’ Super...
Journal of Nutrition | NatureAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
Here’s shahid’s relationship advice for priyanka and nickWhile neither have confirmed or denied it, Shahid and Priyanka were rumoured to have dated each other during the filming...