The involvement of foxo1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers
The involvement of foxo1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The role of transcriptional factor FOXO1 in the mechanism of drug-resistance in ovarian cancer has not been elucidated. In ovarian cancer cell lines, FOXO1 expression and its
correlation with paclitaxel treatment was investigated by cytotoxic assay and silencing experiment. Clinical ovarian cancer samples were also examined for FOXO1 expression by
immunohistochemistry. FOXO1 expression was distinctively upregulated in paclitaxel-resistant cell line, and enhanced by exposure to paclitaxel. FOXO1 overexpression was frequently observed
in tissue samples from chemoresistant patients compared to chemosensitive patients. FOXO1 silencing in paclitaxel-resistant cell line decreased its resistance. Modification of oxidative
stress by co-treatment with pharmacologic modulators of reactive oxygen species attenuated cytotoxicity of paclitaxel. Downstream targets of FOXO1 involving oxidative stress were also
attenuated in silencing experiment, suggesting its involvement in altered sensitivity to paclitaxel. These results indicate that FOXO1 links to cytotoxic stress induced by paclitaxel and
contributes to the drug-resistance in ovarian cancers. SIMILAR CONTENT BEING VIEWED BY OTHERS FAM83B INHIBITS OVARIAN CANCER CISPLATIN RESISTANCE THROUGH INHIBITING WNT PATHWAY Article Open
access 09 January 2021 STUB1 SUPPRESSES PACLITAXEL RESISTANCE IN OVARIAN CANCER THROUGH MEDIATING HOXB3 UBIQUITINATION TO INHIBIT PARK7 EXPRESSION Article Open access 05 November 2024 A
TARGETABLE OSGIN1 − AMPK − SLC2A3 AXIS CONTROLS THE VULNERABILITY OF OVARIAN CANCER TO FERROPTOSIS Article Open access 14 January 2025 MAIN Paclitaxel is one of the most active cancer
chemotherapeutic agents known. It is effective against a variety of human tumours, including ovarian carcinomas (Rowinsky and Donehower, 1995; McGuire et al, 1996; Wiseman and Spencer,
1998). The efficacy of paclitaxel is limited by intrinsic or acquired drug resistance in a population of surviving malignant cells. One molecular mechanisms for acquired tumour cell
resistance to paclitaxel is explained by overexpression of the drug efflux pump MDR-1; however, the role of this is still undetermined. The mammalian FOXO family of Forkhead transcription
factors, consisting of FOXO1, FOXO3a, and FOXO4, is a direct downstream target of the PI3K/Akt pathway (Brunet et al, 1999; Kops et al, 1999). Post-translational modification of FOXO
proteins is an important mechanism that regulates the ability of different transcription factors to activate distinct gene sets, involved in cell cycle inhibition (Dijkers et al, 2000),
apoptosis (Sunters et al, 2003), defense against oxidative stress and DNA repair (Kops et al, 2002; Nemoto et al, 2004). Since FOXO proteins were reported to be critical mediators of
apoptosis in cytotoxicity inducing drugs in many cells (Sunters et al, 2003; Kajihara et al, 2006; Goto et al, 2008), we postulated that FOXO expression or transcriptional activity could be
important event in the drug sensitivity in cancer cells. In the present study, we examined the consequence of FOXO1 expression correlating with paclitaxel cytotoxicity and sensitivity in
ovarian cancer cell lines using parent cells and paclitaxel-resistant derivative cells, and confirmed its expression in clinical samples from chemosensitive and resistant patients.
Furthermore, we explored the possible underlying mechanism in involvement of FOXO1 with paclitaxel resistance. MATERIALS AND METHODS CELL LINES, CULTURE CONDITIONS AND TREATMENT KF28 is a
single-cell clone of the human ovarian carcinoma cell line KF. KFr13 is a cisplatin-resistant subline derived from KF28 cells as described previously (Kikuchi et al, 1986), and KFr13Tx is a
paclitaxel-resistant subline derived from KFr13 cells (Yamamoto et al, 2000a). These cell lines were grown as monolayer cultures in RPMI-1640 (Immuno-Biological Laboratories Co. Ltd, Gunma,
Japan) medium supplemented with 10% fetal bovine serum (Invitrogen Japan KK, Tokyo, Japan), 2 mM glutamine, 100 U penicillin per ml, and 100 _μ_g streptomycin per ml (Invitrogen Japan KK) in
a humidified atmosphere of 5% CO2 at 37°C, and routinely tested for mycoplasma infection. Paclitaxel was obtained from Bristol Meier's Squib Oncology (Tokyo, Japan) and dissolved in
dimethylsulphoxide . CELL PROLIFERATION AND CYTOTOXICITY ASSAY Ovarian cancer cells were seeded onto 96-well plates, at approximately 2 × 103 or 10 × 103 cells cm−2 for proliferation or
cytotoxicity assays, respectively, and allowed to attach overnight. Cell viability was determined by MTS assay using the CellTiter 96 aqueous one solution cell proliferation assay (Promega
KK Japan, Tokyo, Japan) according to the manufacturer’s instructions. To study the effects of paclitaxel on cell proliferation, cells were treated with various doses of paclitaxel for 24 h.
After completion of the treatment, the percentage absorbance was calculated against untreated cells. For growth curve analysis and Trypan blue exclusion test, ovarian cancer cells were
plated in 24-well plates (2 × 103 or 10 × 103 cells cm−2). At the indicated time points, cells were trypsinised to detach from the plates and stained with Trypan blue (Doujin, Kumamoto,
Japan), and cell number was counted under a microscopy using a haemocytometer. Each experiment was performed in quadruplicate. Flow cytometry analysis was used to quantify apoptosis in
ovarian cancer cells by evaluating the sub-G1 fraction (<2 N) after propidium iodide (PI) staining of ethanol-fixed cells. WESTERN BLOTTING ANALYSIS Protein concentrations were determined
by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) and equal amounts of whole cell extracts or nuclear and cytoplasmic protein fractions were separated on a 10% SDS–polyacrylamide
gel before electrotransfer onto a polyvinylidene diflouride membrane (Hybond P, GE Healthcare UK Ltd, England, UK). Nonspecific binding sites were blocked by overnight incubation with 5%
dried skimmed milk in Tris-buffered saline (130 mM NaCl, 20 mM Tris, pH 7.6). Primary antibodies to FOXO1, phospho-FOXO1 (Ser256), Akt, phospho-Akt (Ser473) (Cell Signaling Technology,
Boston, MA, USA), FOXO3a (Upstate, Temecula, CA, USA), FOXO4, GADD45_α_, MnSOD, catalase, p27Kip1, Lamin B1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and PARP cleavage site
(214/215) (Biosource, Carmavillo, CA, USA) were used at 1 : 1000 whereas the antibody to _β_-actin (Abcam, Cambridge, UK) was diluted 1 : 100 000. Primary antibodies were detected using
horseradish peroxidase linked anti-mouse, anti-goat or anti-rabbit conjugates as appropriate (Dako Cytomation, Kyoto, Japan), and visualised using the ECL detection system (GE Healthcare UK
Ltd, England, UK). REAL-TIME QUANTITATIVE–PCR Total RNA was extracted from ovarian cancer cell lines by a ready-to-use reagent (TRIZOL, Invitrogen Japan KK) according to the manufacturer’s
instructions and reverse transcribed using the Superscript III reverse transcriptase (Invitrogen Japan KK), and resulting first-strand cDNA was used as template in the real-time
quantitative–PCR (RTQ–PCR) analysis. The following gene-specific primer pairs were used: L19-sense (5′-GCGGAAGGGTACAGCCAAT-3′) and L19-antisense (5′-GCAGCCGGCGCAAA-3′); FOXO1-sense
(5′-TGGACATGCTCAGCAGACATC-3′) and FOXO1-antisense (5′-TTGGGTCAGGCGGTTCA-3′). L19, a non-regulated ribosomal housekeeping gene, served as an internal control and was used to normalise for
differences in input RNA. Detection of the transcripts was performed with Power SYBR Green (Applied Biosystems, Foster City, CA, USA) and an ABI PRISM 7700 sequence detection system
according to the manufacturer's recommended protocol (Applied Biosystems). All measurements were performed in triplicate. PATIENT SELECTION FOR IMMUNOHISTOCHEMICAL STAINING Of patients
with primary epithelial ovarian cancer treated at the National Defense Medical College Hospital (Saitama, Japan), the following patients were selected: (a) patients who received no
chemotherapy before any surgical therapy; (b) patients who harboured measurable residual tumours after initial debulking surgery; (c) patients who were treated with six courses of adjuvant
chemotherapy using paclitaxel (180 mg m−2) and carboplatin (AUC=5) chemotherapy after the initial surgery and (d) patients who agreed to participate in the current study with written
informed consent. The patients were divided into the following four groups according to their response to chemotherapy measured with CT or MRI: (a) CR (complete response) group; (b) PR
(partial response) group; (c) SD (stable disease) group and (d) PD (progressive disease) group. Responders were defined as patients with CR or PR, and non-responders were defined as those
with SD and PD. A total of 13 responders and 10 non-responders were included in the study. IMMUNOHISTOCHEMISTRY After reviewing the haematoxylin-stained sections, a paraffin block of the
most representative sections were selected and cut into a 4-_μ_m thickness. All of the sections were deparaffinised and rehydrated with xylene and a graded alcohol series. To inactivate
endogenous peroxidase activity, sections were immersed in methanol containing 0.3% hydrogen peroxide for 30 min at room temperature, then incubated in 2.0% blocking serum for the reduction
of nonspecific binding. The sections were incubated with primary antibodies against FOXO1 (1 : 50 dilution; Cell Signaling Technology, Boston, MA, USA) and MnSOD (1 : 50 dilution; Santa Cruz
Biotechnology Inc.) in humid chamber for 60 min at room temperature, followed by washing with PBS. For the visualisation of FOXO1 and MnSOD, the EnVision+™ system (Dako Cytomation) was
applied to the sections for 2 h at room temperature, and diaminobenzidine hydrochloride was used. These sections were counterstained with Meyer’s haematoxylin. Cytoplasmic staining was
considered as positive expression. The proportion of positive-stained cells was counted in more than 10 high power fields by two investigators who were blinded to the data of patient
characteristics. Immunostaining for the specimen was classified as positive when >10% of cells were positive. TRANSIENT TRANSFECTION For silencing experiments, KFr13Tx cells cultured in
six-well plates were transfected with 50 nM of FOXO1 si_GENOME_ SMARTpool or non-targeting siRNA pool (Dharmacon, Lafayette, CO, USA) using Lipofectamine 2000 (Invitrogen Japan KK) according
to the manufacturer’s specifications. FOXO1 knockdown was confirmed by western blot analysis in all the experiments. INTRACELLULAR REACTIVE OXYGEN SPECIES MEASUREMENT Levels of
intracellular H2O2 were assessed spectrofluorimetrically using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen Japan KK) according to the
manufacturer’s instructions. Briefly, cells were seeded and attached overnight on 96-well plates (2 × 104 cells cm−2) and were washed with PBS and initially incubated with 10 _μ_ M
carboxy-H2DCFDA in PBS for 30 min, then changed to paclitaxel or H2O2 at the indicated concentrations with carboxy-H2DCFDA in PBS concomitantly. After 4 h incubation, cells were washed with
PBS and fluorescence intensity was measured by spectrofluorometry. Excitation and emission wavelengths used were 485 and 525 nm, respectively. The relative H2O2 production induced by
paclitaxel or H2O2 was expressed as the ratio between fluorescence intensity in cells treated with paclitaxel or H2O2 and with PBS alone. STATISTICAL ANALYSIS All values are presented as
mean±s.d. Statistical significance between two groups was determined by use of a two-tailed _t_-test and values of _P_<0.05 were considered significant. RESULTS CELLULAR CHARACTERISATION
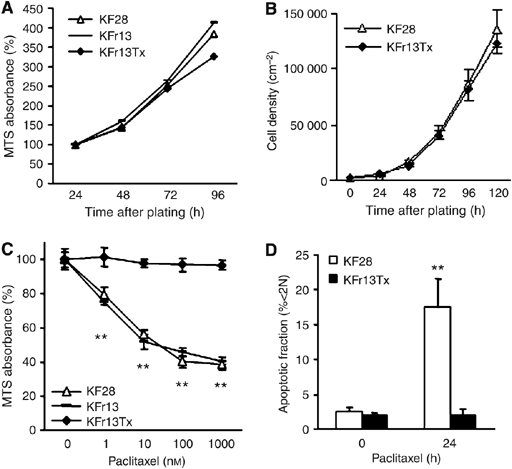
OF OVARIAN CANCER CELL LINES To examine the role of FOXO transcriptional factor in ovarian cancer cells, cellular characteristics, such as proliferation ability and drug sensitivity, were
first confirmed in three representative ovarian cancer cell lines, parent cells KF28, cisplatin-resistant derivative and paclitaxel-resistant derivative cells, KFr13 and KFr13Tx. Cellular
proliferation abilities in three cell lines were comparable as determined by MTS assay (Figure 1A), which was confirmed by growth curve analysis for KF28 and KFr13Tx (Figure 1B). Drug
sensitivity to paclitaxel was re-examined by MTS assay after 24 h exposure, which revealed considerable acquired resistance only in KFr13Tx cells (Figure 1C). These findings were also
confirmed for KF28 and KFr13Tx cells treated with 10 nM paclitaxel for 24 h by flow cytometry of PI-stained cells (Figure 1D). DIFFERENTIAL EXPRESSION OF FOXO1 IN OVARIAN CANCER CELL LINES
To clarify the role of FOXO transcriptional factor in ovarian cancer, screening of FOXO protein expression was performed using western blotting. Among these cells, KFr13Tx,
paclitaxel-resistant cell line, only showed marked FOXO1 expression in protein level (Figure 2A). Comparing to FOXO1, FOXO3a and FOXO4 did not show much difference among these cell lines. As
speculated, PI3K/Akt activity was considerably lower in KFr13Tx, as reflected by the phosphorylated Akt levels. For further analysis, the transcript levels were also examined by RTQ–PCR,
which revealed FOXO1 mRNA level was 15-fold highly expressed in KFr13Tx cells compared to KF28 cells (Figure 2B). These results prompted us to hypothesise that overexpression of FOXO1 in
these cell lines correlates especially with the mechanism of paclitaxel resistance. IMMUNOHISTOCHEMICAL ANALYSIS OF FOXO1 EXPRESSION IN OVARIAN CANCER SAMPLES FROM CHEMOTHERAPY-RESPONDED AND
NON-RESPONDED PATIENTS To investigate whether our _in vitro_ data are relevant to clinical practice, immunohistochemical reactivities of FOXO1 in ovarian cancer samples, obtained at surgery
before chemotherapy, with different chemotherapeutic response to paclitaxel-based chemotherapy, were examined. Representative immunohistological staining of responder and non-responder are
shown in Figure 2C. FOXO1 overexpression with strong cytoplasmic staining was observed in 5 of 10 non-responders (50%), whereas it was less frequently detected in 2 of 13 responders (15%)
(_P_=0.073). Immunoreactivity was not correlated with stage or histological grade (data not shown). INDUCTION OF FOXO1 BY PACLITAXEL IN OVARIAN CANCER CELL LINES To investigate further the
correlation of FOXO1 and paclitaxel, FOXO1 expression was examined in KF28 and KFr13Tx cells treated with paclitaxel at the increased concentrations for 24 h. Western blotting showed strong
induced FOXO1 expression in KFr13Tx cells by paclitaxel treatment, whereas its induction was very weak in KF28 cells (Figure 3A). Conversely, cleaved PARP expression as apoptosis marker was
distinctively induced in KF28 cells, whereas its expression was almost undetectable in KFr13Tx cells even at 100 nM concentration, supporting the previous results (Figure 1C and D). Again,
RTQ–PCR was performed to examine transcript levels of FOXO1 in both cells treated with 10 nM paclitaxel at the indicated time points. FOXO1 mRNA expression was induced in both cells, which
were peaked after 24 h, especially marked in KFr13Tx cells (Figure 3B). For further analysis, translocation of FOXO1 was also investigated using protein fraction by western blotting. Nuclear
translocation of FOXO1 was clearly observed in both cells, which were again peaked after 24 h treatment (Figure 3C). The nuclear decrease after 48 h correlates with increase in
phosphorylated (Ser256) FOXO1 levels in cytosol. Notably, FOXO1 expression in the cytoplasmic fraction was increased in KFr13Tx cells compared to KF28 cells, whereas nuclear FOXO1 levels
were comparable in both cells, which were compatible with the previous results (Figure 2C). FOXO1 ATTENUATES SENSITIVITY TO PACLITAXEL-INDUCED CELL DEATH IN PACLITAXEL-RESISTANT CELL LINES
To clarify the role of FOXO1 in ovarian cancer cells, gene-silencing experiment was performed in KFr13Tx cells. After transfection with either non-targeting siRNA or FOXO1 siRNA, cellular
proliferation was monitored by MTS assay at the indicated time points. FOXO1 siRNA slightly promoted cellular proliferation, whose effect was not quite remarkable (Figure 4A). The same
silencing experiment was carried out before paclitaxel treatment at the indicated concentrations for 24 h. FOXO1 siRNA considerably increased the sensitivity to paclitaxel as determined by
MTS assay (Figure 4B). These findings were again confirmed by FACS analysis using PI staining for 24 h treatment at 10 nM paclitaxel (Figure 4C). FOXO1 silencing followed by paclitaxel
treatment in KFr13Tx cells was again performed and putative FOXO target genes involving with cell cycle inhibition (p27Kip1), defence against oxidative stress (MnSOD, catalase), and DNA
repair (GADD45_α_) were examined by western blotting. Transfection with FOXO1 siRNA decreased expression levels of these target genes, especially in p27Kip1 and MnSOD, regardless of
paclitaxel treatment (Figure 4D). Notably, cleaved PARP was detectable by paclitaxel treatment only in FOXO1 silencing cells, supporting the previous results (Figure 4B and C). ATTENUATION
OF OXIDATIVE STRESS BY PACLITAXEL AND FOXO1 IN OVARIAN CANCER CELL LINES To investigate the possible underlying mechanism that FOXO1 attenuates paclitaxel sensitivity in ovarian cancer
cells, intracellular reactive oxygen species (ROS) induced by paclitaxel was measured in KF28 cells and KFr13Tx cells. As assessed by C-H2DCFDA fluorescence, intracellular H2O2 levels were
increased in KF28 cells when exposed for 4 h to increasing concentrations of paclitaxel or H2O2 as indicated, whereas those changes were not marked in KFr13Tx cells exposed with paclitaxel
(Figure 5A). To study further the role of ROS accumulation in paclitaxel cytotoxicity, the effects of co-incubation of 10 nM paclitaxel or 500 _μ_ M H2O2 for 24 h with antioxidant, 400 _μ_M
N-acetylcysteine (NAC), H2O2 scavenger, or 1 mM NaN3, inhibitor of catalase, were investigated in both cells by Trypan blue exclusion test. Co-treatment with NAC or NaN3 in KF28 cells
significantly decreased or increased paclitaxel or H2O2 induced cell death, whereas co-treatment with NaN3 in KFr13Tx cells also increased paclitaxel or H2O2 induced cell death (Figure 5B).
On the basis of these results, the possibility whether FOXO1 attenuates paclitaxel-induced cytotoxicity through oxidative stress was studied again by ROS measurement in KFr13Tx cells using
silencing experiment. As shown in Figure 5C, intracellular H2O2 levels were increased in KFr13Tx cells transfected with FOXO1 siRNA compared to those with NTsiRNA when exposed for 4 h to
increasing concentrations of paclitaxel as indicated. MNSOD EXPRESSION IN PACLITAXEL-SENSITIVE AND -RESISTANT OVARIAN CANCER CELL LINES AND OVARIAN CANCER SAMPLES To determine further the
relevance of FOXO1 target genes in ovarian cancer cells, we also compared the levels of p27Kip1, MnSOD, catalase and GADD45_α_ expression in KF28, KFr13 and KFr13Tx cells by western
blotting. As shown in Figure 6A, p27Kip1 and MnSOD were strongly expressed especially in paclitaxel-resistant cell line, whereas GADD45_α_ expression was also comparably observed in KFr13
cells and catalase expressions were almost the same among these three cell lines. Together with the previous results, we speculated that the FOXO1 attenuates paclitaxel sensitivity through
control of oxidative stress by regulation of MnSOD. Finally, again to investigate whether our _in vitro_ data is relevant to clinical practice, immunohistochemical reactivities of MnSOD in
the same ovarian cancer samples were examined. Representative immunohistological staining of responder and non-responder are shown in Figure 6B. MnSOD overexpression with strong cytoplasmic
staining was observed in 8 of 10 non-responders (80%), whereas it was less frequently detected in 5 of 13 responders (38%) (_P_=0.046). Furthermore, the cases with overexpression of FOXO1
also showed MnSOD overexpression in non-responder patients. DISCUSSION Although most ovarian cancers are responsive to paclitaxel-based chemotherapy, the emergence of drug-resistant cancer
clones can lead to treatment failure and disease relapse. There have been several reports regarding overexpression of genes related to paclitaxel resistance. MDR-1 overexpression in ovarian
cancer cell lines with paclitaxel resistance had been reported (Masanek et al, 1997; Duan et al, 1999). Similarly, we had also confirmed MDR-1 overexpression in paclitaxel-resistant
derivative ovarian cancer cell line, KFr13TX cells (Yamamoto et al, 2000b; Goto et al, 2006). The molecular mechanism of MDR-1 is still uncertain. Some studies showed MDR-1 as a predictive
marker of poor chemotherapeutic response (Yokoyama et al, 1999; Penson et al, 2004), but others did not show (Baird and Kaye, 2003; Vasey, 2003; Mozzetti et al, 2005). In the present study,
paclitaxel-resistant derivative cells showed increased expression of FOXO1, compared to parent cells and cisplatin-resistant derivative cells. Notably, cytoplasmic FOXO1, which is likely to
be inactive and should have no affect on expression of target genes in stress response, was strongly expressed in resistant cells both in cancer cell lines and clinical samples. In contrast,
induction and nuclear FOXO1 was markedly induced by 24 h exposure of paclitaxel in both sensitive and resistant cells. It is possible that acute exposure to paclitaxel leads to
FOXO1-dependent activation of a proapoptotic gene programme, and that prolonged or chronic exposure promotes selection of cells with another transcriptionally activated gene settings by
FOXO1, which are involved in cellular survival and drug resistance. For instance, despite of the several reports showing reduction in Akt phosphorylation in response to paclitaxel,
phosphorylation and nuclear exclusion of FOXO1 was clearly observed after 48 h in our experiments, especially stronger in resistant cells. Since FOXO1 has recently been shown to enhance Akt
phosphorylation in hepatocytes by repressing the expression of tribble 3 (Trb3), a pseudokinase capable of binding Akt and inhibiting its phosphorylation (Matsumoto et al, 2006), it seems to
be interesting to investigate whether feedback to PI3K/AKT pathway by FOXO1 could contribute to the survival advantage and development of drug resistance. Although drug resistance in cancer
should be multifactorial, it is well recognised that a slower growth rate represents one component of drug resistance. In our results, FOXO1 silencing decreased expression level of p27Kip1,
which is one FOXO1 target gene involving cell cycle inhibition (Dijkers et al, 2000). However, cellular proliferation was not actually attenuated in these cells, which suggests more
critical event other than cell growth retardation is involved in these settings. ROS are thought to play multiple roles including tumour initiation, progression and maintenance, and ROS
production is highly increased in cancer cells (Szatrowski and Nathan, 1991; Burdon, 1995). ROS levels fluctuate in response to intracellular as well as extracellular signals and, in turn,
stimulate specific signalling cascades, such as MAPKs, that regulate cell growth and cell death (Benhar et al, 2001; Davis et al, 2001; Tobiume et al, 2001). ROS levels are increased in
cells exposed to various stress agents, including paclitaxel and other anticancer drugs (Varbiro et al, 2001; Ramanathan et al, 2005). Agents that decrease ROS can suppress taxol-induced
cytotoxicity, whereas increase of ROS levels by inhibition of SOD or glutamylcysteine synthase can enhance taxol-induced cytotoxicity in cancer cell lines (Ramanathan et al, 2005). The
cellular responses to paclitaxel involve activation of MAPK pathways (Bacus et al, 2001). Higher ROS levels and SAPK (stress-activated protein kinases) JNK activity were measured in tumour
cells that were sensitive to anticancer agents than in those that were drug-resistant, suggesting that ROS-mediated JNK and p38 activation played a key role in the sensitisation to stress
signals and to anticancer drugs (Benhar et al, 2001; Davis et al, 2001). Thus, control of endogenous ROS level and the regulation of MAPK pathway may involve in proliferation and sensitivity
to stress stimuli including anticancer drugs in cancer cells. In the present study, the increase of intracellular H2O2 levels in ovarian cancer cells were observed by adding extracellular
H2O2 as well as paclitaxel. In addition, modifying intracellular ROS level by co-incubation with NAC or catalase inhibitor showed significant decrease or increase in cytotoxicity of H2O2 as
well as paclitaxel. Moreover, FOXO1 silencing attenuated intracellular H2O2 levels, and also decreased expression of its putative target gene, MnSOD and Gadd45_α_, simultaneously showing
increased paclitaxel-induced cytotoxicity, which collectively suggest one of possible explanation in transcriptional role of FOXO1 as redox mechanism to cytotoxic stimuli such as paclitaxel
in these cells. We also demonstrated in clinical samples that FOXO1 overexpression was correlated with paclitaxel resistance, although the number of samples was small and further analysis
will be required to confirm these findings. Among the FOXO1 target genes we examined, MnSOD was strongly expressed especially in paclitaxel-resistant cell line, which prompted us to
speculate that FOXO1 might attenuate paclitaxel sensitivity through control of oxidative stress by regulation of MnSOD, then confirmed its overexpression in the same samples showing FOXO1
overexpression from chemoresistant patients. There are far more mechanisms to elucidate although, together with our _in vitro_ data, FOXO1 might be the candidate to predict the
chemotherapeutic response and it could be a molecular target for the treatment of drug-resistant ovarian cancers. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after
initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Bacus SS, Gudkov AV, Lowe M, Lvass L, Yung Y, Komarov AP, Kevomarsi K, Yarden Y, Seger
R (2001) Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. _Oncogene_ 20: 147–155 Article CAS Google Scholar * Baird RD, Kaye SB (2003) Drug
resistance reversal – are we getting closer? _Eur J Cancer_ 39: 2450–2461 Article CAS Google Scholar * Benhar M, Dalyot I, Engelberg D, Levitzki A (2001) Enhanced ROS production in
oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. _Mol Cell Biol_ 21: 6913–6926
Article CAS Google Scholar * Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and
inhibiting a Forkhead transcription factor. _Cell_ 96: 857–868 Article CAS Google Scholar * Burdon RH (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation.
_Free Radic Biol Med_ 18: 775–794 Article CAS Google Scholar * Davis Jr W, Ronai Z, Tew KD (2001) Cellular thiols and reactive oxygen species in drug-induced apoptosis. _J Pharmacol Exp_
296: 1–6 CAS Google Scholar * Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmaters JA, Lammers JW, Koenderman L, Coffer PJ (2000) Forkhead transcription
factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). _Mol Cell Biol_ 20: 9138–9148 Article CAS Google Scholar * Duan Z, Feller AJ, Person RT, Chabner BA,
Seiden MU (1999) Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA microarray technology: Analysis of interleukin (IL) 6, IL-8, and monocyte
chemotactic protein 1 in the paclitaxel-resistant phenotype. _Clin Cancer Res_ 5: 3445–3453 CAS PubMed Google Scholar * Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B,
Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EW, Bamberger AM, Ghaem-Maghami S, Brosens JJ (2008) Mechanism and functional consequences of loss of FOXO1
expression in endometrioid endometrial cancer cells. _Oncogene_ 27 (1): 9–19 Article CAS Google Scholar * Goto T, Takano M, Sakamoto M, Kondo A, Hirata J, Kita T, Tsuda H, Tenjin Y,
Kikuchi Y (2006) Gene expression profiles with cDNA microarray reveals RhoGDI as a predictive marker for paclitaxel resistance in ovarian cancers. _Oncol Rep_ 15: 1265–1271 CAS PubMed
Google Scholar * Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ (2006) Differential expression of FOXO1 and FOXO3a
confers resistance to oxidative cell death upon endometrial decidualization. _Mol Endocrinol_ 20: 2444–2455 Article CAS Google Scholar * Kikuchi Y, Miyauchi M, Kizawa I, Oomori K, Katoh K
(1986) Establishment of a cisplatin-resistant human ovarian cancer cell line. _J Natl Cancer Inst_ 77: 1181–1185 CAS PubMed Google Scholar * Kops GJ, Dansen TB, Polderman PE, Saarloos I,
Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. _Nature_ 419: 316–321 Article CAS
Google Scholar * Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM (1999) Direct control of the Forkhead transcription factor AFX by protein kinase B. _Nature_
398: 630–634 Article CAS Google Scholar * Masanek U, Stammler G, Volm M (1997) Messenger RNA expression of resistance proteins and related factors in human ovarian carcinoma cell lines
resistant to doxorubicin, taxol and cisplatin. _Anticancer Drugs_ 8: 189–198 Article CAS Google Scholar * Matsumoto M, Han S, Kitamura T, Accili D (2006) Dual role of transcription factor
FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. _J Clin Invest_ 116: 2464–2472 CAS PubMed PubMed Central Google Scholar * McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Patridge EE, Look KY, Clarke-Pearson DL, Davidson M (1996) Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer.
_N Engl J Med_ 334: 1–6 Article CAS Google Scholar * Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia
G (2005) Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. _Clin Cancer Res_ 11: 298–305 CAS Google Scholar * Nemoto S,
Fergusson MM, Finkel T (2004) Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. _Science_ 306: 2105–2108 Article CAS Google Scholar * Penson RT, Oliva E, Skates
SJ, Glyptis T, Fuller Jr AF, Goodman A, Seiden MV (2004) Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a
study in serial samples. _Gynecol Oncol_ 93: 98–106 Article CAS Google Scholar * Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS (2005) Resistance to paclitaxel is proportional to
cellular total antioxidant capacity. _Cancer Res_ 65: 8455–8460 Article CAS Google Scholar * Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). _N Engl J Med_ 332: 1004–1014 Article
CAS Google Scholar * Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW (2003) FOXO3a transcriptional regulation
of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. _J Biol Chem_ 278: 49795–49805 Article CAS Google Scholar * Szatrowski TP, Nathan CF (1991) Production of large
amounts of hydrogen peroxide by human tumour cells. _Cancer Res_ 51: 794–798 CAS PubMed Google Scholar * Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O,
Miyazono K, Noda T, Ichijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. _EMBO Rep_ 2: 222–228 Article CAS Google Scholar * Varbiro G, Veres B,
Gallyas F, Sumeqi B (2001) Direct effect of Taxol on free radical formation and mitochondrial permeability transition. _Free Radic Biol Med_ 31: 548–558 Article CAS Google Scholar *
Vasey PA (2003) Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. _Br J Cancer_ 89: S23–S28 Article CAS Google Scholar * Wiseman LR, Spencer CM
(1998) Paclitaxel: An update of its use in the treatment of metastatic breast and ovarian and other gynecological cancers. _Drugs Aging_ 12: 305–334 Article CAS Google Scholar * Yamamoto
K, Kikuchi Y, Kudoh K, Hirata J, Kita T, Nagata I (2000a) Treatment with paclitaxel alone rather than combination with paclitaxel and cisplatin may be selected for cisplatin-resistant
ovarian carcinoma. _Jpn J Clin Oncol_ 30: 446–449 Article CAS Google Scholar * Yamamoto K, Kikuchi Y, Kudoh K, Nagata I (2000b) Modulation of cisplatin sensitivity by taxol in
cisplatin-sensitive and -resistant human ovarian carcinoma cell lines. _J Cancer Res Clin Oncol_ 126: 168–172 Article CAS Google Scholar * Yokoyama Y, Sato S, Fukushi Y, Sakamoto T,
Futagami M, Saito Y (1999) Significance of multi-drug-resistant proteins in predicting chemotherapy response and prognosis in epithelial ovarian cancer. _J Obstet Gynaecol Res_ 25: 387–3894
Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge Professor Jan J Brosens and Professor Eric W-F Lam for their constructive and continuous support.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Gynaecologic Oncology, Saitama Medical University International Medical Centre Comprehensive Cancer Centre, 1397-1 Yamane, Hidaka
city, Saitama 350-1298, Japan, T Goto * Department of Obstetrics and Gynaecology, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan, M Takano & J Hirata
* Department of Pathology II, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan, H Tsuda Authors * T Goto View author publications You can also search for
this author inPubMed Google Scholar * M Takano View author publications You can also search for this author inPubMed Google Scholar * J Hirata View author publications You can also search
for this author inPubMed Google Scholar * H Tsuda View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to T Goto. RIGHTS
AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy
of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Goto, T., Takano, M., Hirata, J. _et al._ The
involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. _Br J Cancer_ 98, 1068–1075 (2008). https://doi.org/10.1038/sj.bjc.6604279 Download
citation * Revised: 17 December 2007 * Accepted: 24 January 2008 * Published: 04 March 2008 * Issue Date: 25 March 2008 * DOI: https://doi.org/10.1038/sj.bjc.6604279 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative KEYWORDS * ovarian cancer * paclitaxel * FOXO1 * drug resistance * reactive oxygen spices (ROS)
Trending News
Patient mutations alter atrx targeting to pml nuclear bodiesABSTRACT ATRX is a SWI/SNF-like chromatin remodeling protein mutated in several X-linked mental retardation syndromes. G...
Oat growers to gain from opening of new processing plant - farmers weeklyABSTRACT Powerful emissions from the centres of nearby galaxies may represent dead quasars. Access through your institut...
Location, location, location: trump has the best spot in american politicsHistory can weigh heavily on a filmmaker, and that is what happens with “Amelia,” a disappointing rendering of the remar...
Rear viewing system for safe reversing - farmers weekly26 OCTOBER 2001 ------------------------- REAR VIEWING SYSTEM FOR SAFE REVERSING SPALDINGS has now launched a full colou...
How to be happy: 5 easy ways to increase your happinessMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Latests News
The involvement of foxo1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancersABSTRACT The role of transcriptional factor FOXO1 in the mechanism of drug-resistance in ovarian cancer has not been elu...
Error 404Quiz News Business Sport Tech Hype Korea Life Health Community Regional RegionalIDN Times Jawa Barat Banten Jawa Tenga...
Mr n llewellyn v melin homes: 1600191/2021MR N LLEWELLYN V MELIN HOMES: 1600191/2021 Employment Tribunal decision. Read the full decision in Mr N Llewellyn v Meli...
Assam: suspended darrang asp, 3 doctors arrested in minor girl’s death caseGUWAHATI: The Criminal Investigation Department (CID) of Assam police has arrested a suspended police officer and three ...
Effects of vitamin e supplementation on intracellular antioxidant enzyme production in adolescents with type 1 diabetes and early microangiopathyABSTRACT Defective intracellular antioxidant enzyme production (IAP) has been demonstrated in adults with diabetic nephr...