Loss of ep-cam (co17-1a) expression predicts survival in patients with gastric cancer
Loss of ep-cam (co17-1a) expression predicts survival in patients with gastric cancer"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Preoperative staging of gastric cancer is difficult and not optimal. The TNM stage is an important prognostic factor, but it can only be assessed reliably after surgery. Therefore,
there is need for additional, reliable prognostic factors that can be determined preoperatively in order to select patients who might benefit from (neo) adjuvant treatment. Expression of
immunohistochemical markers was demonstrated to be associated with tumour progression and metastasis. The expression of p53, CD44 (splice variants v5, v6 and v9), E-cadherin, Ep-CAM (CO17-1A
antigen) and c-erB2/_neu_ were investigated in tumour tissues of 300 patients from the Dutch Gastric Cancer Trial, investigating the value of extended lymphadenectomy compared to that of
limited lymphadenectomy). The expression of tumour markers was analysed with respect to patient survival. Patients without loss of Ep-CAM-expression of tumour cells (19%) had a significantly
better 10-year survival (_P_<0.0001) compared to patients with any loss: 42% (s.e.=7%) _vs_ 22% (s.e.=3%). Patients with CD44v6 (VFF18) expression in more than 25% of the tumour cells
(69% of the patients) also had a significantly better survival (_P_=0.01) compared to patients with expression in less than 25% of the tumour cells: 10 year survival rate of 29% (s.e.=3%)
_vs_ 19% (s.e.=4%). The prognostic value of both markers was stronger in stages I and II, and independent of the TNM stage. Ep-CAM and CD44v6-expression provides prognostic information
additional to the TNM stage. Loss of Ep-CAM-expression identifies aggressive tumours especially in patients with stage I and II disease. This information may be helpful in selecting patients
suitable for surgery or for additional treatment pre- or postoperatively. SIMILAR CONTENT BEING VIEWED BY OTHERS THE TUMOR BIOLOGICAL SIGNIFICANCE OF RNF43 AND LRP1B IN GASTRIC CANCER IS
COMPLEX AND CONTEXT-DEPENDENT Article Open access 23 February 2023 PROGNOSTIC SIGNIFICANCE OF HIGH NPC2 EXPRESSION IN GASTRIC CANCER Article Open access 24 November 2023 MOLECULAR AND
PATHOLOGICAL ANALYSES OF GASTRIC STUMP CANCER BY NEXT-GENERATION SEQUENCING AND IMMUNOHISTOCHEMISTRY Article Open access 18 February 2021 MAIN Patients with gastric carcinoma have a poor
prognosis, especially the patients with an advanced stage disease (Allum et al, 1989; Akoh et al, 1991; Wanebo et al, 1993). In TNM stages I and II, better survival rates are seen after a
curative resection: 5 year survival rates of 83–99% for stage I and 48–70% in stage II (Miwa, Japanese Research Society for Gastric Cancer, 1984; Hermanek, 1985). The TNM stage thus is an
important prognostic factor, but it can be assessed reliably only after surgery and is therefore of no use for patient selection before surgery. Furthermore, within stages I and II, a
significant number of patients will suffer from recurrent disease. Especially within this group there is a need for additional prognostic factors that can be determined preoperatively. Such
factors are potentially helpful in identifying patients that might benefit from additional therapeutic modalities, either pre- or post-operatively (Yu et al, 1998; Hermans et al, 1999). The
process of carcinogenesis and metastasis is complex. Since we were interested in factors, which can predict whether the tumour is beyond cure by surgery alone, we reasoned that especially
molecules involved in cell–cell and cell–extracellular matrix (ECM) interactions might be of high relevance. CD44, a hyaluronate receptor, is involved in cell migration through the ECM,
which can be viewed as highly relevant for tumour invasion and metastasis. Previous studies have indicated that CD44 isoform expression may be related to gastric tumour progression and poor
prognosis for the patient (Heider et al, 1993; Mayer et al, 1993; Mulder et al, 1994; Terpe et al, 1994; Günthert et al, 1995). Given the fact that progression of tumours is modulated by
changes in cell–cell interactions of the progressing tumour clones, we especially focused on the expression of Ep-CAM, an epithelial cell adhesion molecule, involved in regulation of
cadherin adhesions, and, possibly, cell proliferation and invasion (Litvinov et al, 1994a, 1994b; Litvinov, 1995). Furthermore, we analysed whether the expression of two molecules often
involved in gastric cancer, p53 (involved in cell cycle control and apoptosis, and often overexpressed when mutations are present) and _neu_ (or erbB2, a receptor tyrosine kinase that can be
overexpressed due to the gene amplification, Falck and Gullick, 1989; Houldsworth et al, 1990) might be associated with prognosis in gastric cancer, even though they are presumably
associated with early events in gastric carcinogenesis. Evaluation of prognostic factors has to be based on high quality clinical and pathological data. In the Netherlands, a prospectively
randomised, multicenter trial was conducted to compare the therapeutic efficacy of _extended_ lymph node dissection with _limited_ lymph node dissection in patients with gastric cancer
(Bonenkamp et al, 1995, 1999). Strict quality control measures were taken to obtain optimal lymph node retrieval and thus postoperative staging. The prospectively collected clinical and
pathology data from this trial form an optimal basis to evaluate the usefulness of prognostic value of immunohistochemically determined protein expression in tumour cells. MATERIAL AND
METHODS PATIENT SELECTION Between August 1989 and July 1993 a prospective randomised multicenter trial was conducted in the Netherlands (Dutch Gastric Cancer Trial, DGCT) to compare the
therapeutic efficacy of _extended_ lymph node dissection (N1 and N2 levels, so-called D2) with that of _limited_ lymph node dissection (N1 level, so-called D1) in patients with gastric
cancer, operated on with curative intent (R0). For these patients (criteria for curative resectability were published earlier, Bonenkamp et al, 1995, 1999), presence of nodal involvement was
assessed histologically and the pathologist recorded the actual number and location of the lymph nodes retrieved. Follow-up of the patients is at least 10 years. In the present study,
tumour tissue of 300 patients was used. Selection of these patients was based on the hospitals entering the largest number of patients, in order to have minimal variability in preparation
and preservation of patient material used in this study. IMMUNOHISTOCHEMISTRY In order to evaluate protein expression in adenocarcinoma of the stomach, formalin-fixed, paraffin-embedded
tissue blocks of the primary tumour were used. According to the allocated treatment, the specimens were obtained by D1 or D2 resection. If a curative resection in intent was not possible, a
palliative procedure was performed. From each resection specimen one tissue block was selected that contained the largest amount of tumour. Sections (4-_μ_m thick) were cut from
formalin-fixed, paraffin-embedded tissue blocks, mounted on precoated slides and kept at 37°C overnight. All paraffin sections were dewaxed in xylol for 20 min and endogenous peroxidase
activity was blocked by methanol/H2O2. The monoclonal antibodies of the following specificities were used: cell cycle regulator p53 (mAb NCL-p53-DO7, Novocastra Laboratories Ltd), Ep-CAM
(mAb 323/A3, Centocor, Malvern, PA, USA), E-Cadherin (mAb HECD-1, Thamer Diagnostica B.V.), CD44, splice variant v5 (VFF8), v6 (VFF7 and VFF18)9, and v9 (all from Bender Co., Vienna,
Austria) and _neu_ (Department of Pathology, Leiden). In negative controls, the primary antibody was replaced by phosphate-buffered saline (PBS). For Ep-CAM staining, the sections were
pretreated with a trypsin-solution (0.1% trypsin with 0.1% CaCl2), pH 7.4, at 37°C for 20 min. For p53, E-cadherin, CD44 variants and _neu_ staining, the sections were pretreated by
microwave in citrate buffer (pH 6.0) for 25 min. The pretreated sections were rinsed in PBS and blocked by normal goat serum to reduce nonspecific antibody binding. The primary antibody was
then applied and incubated overnight in its optimal dilutions in PBS/1% bovine serum albumin (BSA). The sections were washed with PBS prior to incubation with the secondary antibody. Then, a
double step detection system was used: biotinylated rabbit anti-mouse (RAM) IgG was followed by streptavidin-biotin-complexHRP (sABC); each incubation was for 45 min. The slides were
stained with 3,3′-diaminobenzidine/H2O2 (DAB) solution, and counterstained with Mayer's haematoxylin. SCORING OF THE SECTIONS The expression of the proteins was scored according to the
estimated percentage tumour cells in the total tissue section showing positive staining. For each marker a scoring system was developed after initial screening of the variation of expression
of each marker and taking systems used in the literature in account. This resulted in the following categories: p53 (0: 0–10%; 1: ⩾10–100%); VFF8, VFF7 and NEU (0: negative; 1: positive);
CD44v9 (0: 0–5%; 1: ⩾5–100%); VFF18 (0: 0–25%; 1: ⩾25–100%); E-Cadherin (0: <50%; 1: ⩾50%) and Ep-CAM (0: negative; 1: 1–99%; 2: 100%). The scoring was done by two independent observers
(IS and JHJMvK). Discrepancies were solved and consensus was reached by using a double-headed microscope. Incidentally, the marker scorings could not be performed because of missing
material. Clinical data were provided after obtaining immunohistochemical results. STATISTICAL ANALYSIS For statistical analysis the SPSS program was used. Kaplan–Meier survival curves are
compared using the log-rank test. Cox's regression was used to study the prognostic value for survival of tumour marker combinations. Differences were considered statistically
significant, when the _P_-value was less than 0.05. RESULTS The study group consisted of 181 male and 119 female patients. Mean age was 64.7 years (range 31–84). Of these patients, 154
received a D1 and 146 D2 lymphadenectomy; 254 patients (85%) had a resection with curative intent and the remaining 46 patients (15%) had noncurative procedures. As expected, the TNM stage
(not always available in noncurative procedures) was highly prognostic (see Table 1). Based on univariate analyses of the original scoring of the markers, all marker expressions were
dichotomised into more or less equal groups. Only Ep-CAM is divided into three groups: 1, negative; 2, any loss of expression (1–99% positive) and 3, no loss of expression (100% positive).
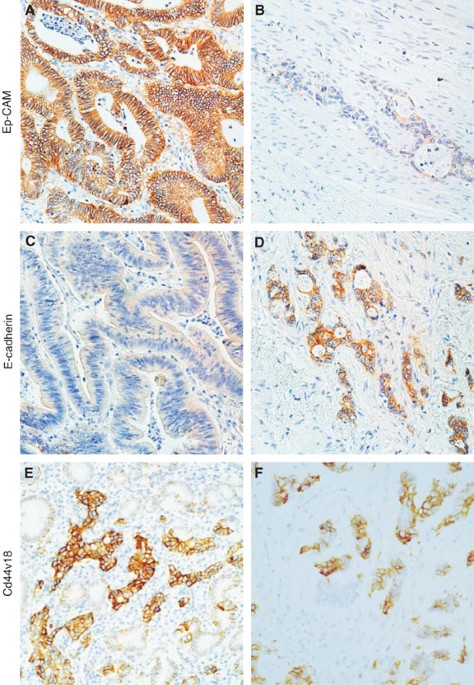
The results are reported in Table 1. Examples of the staining patterns for E-cadherin and CD44v6 are shown in Figure 1. From all the markers tested, only CD44v6 (VFF18 antibody) and Ep-CAM
had a statistically significant prognostic value for survival (see Table 1 and Figures 2 and 3). As a curative resection in intent is an important prognostic factor itself, we analysed this
group separately. Again, only the TNM stage, CD44v6 (VFF18) and Ep-CAM had a strong prognostic value (Table 2). The prognostic value of the markers was studied additional to the TNM stage
with a stepwise Cox's regression analysis. For this analysis the TNM stage was dichotomised into stages I+II _vs_ stages III+IV. Both CD44v6 (VFF18) and Ep-CAM were selected as having a
significant prognostic value additional to the TNM stage (Table 3). The prognostic value of the markers is especially evident in the stages I and II, see Figure 4A. In stages III and IV,
the survival rates are very poor, so Ep-CAM expression cannot reach a statistically significant difference between the groups, see Figure 4B. Also, CD44v6 (VFF18) expression has prognostic
relevance especially in early stages, see Figure 5. DISCUSSION Our study demonstrates that loss of Ep-CAM and CD44v6 (VFF18) expression in gastric cancer has additional prognostic value to
the TNM stage. If confirmed, these findings can be helpful in selecting patients for (neo-)adjuvant therapy for gastric cancer (Yu et al, 1998; Hermans et al, 1999). Although Ep-CAM is
present in many epithelial cell types, some, like squamous epithelia express this molecule only in embryogenesis or in neoplasia (reviewed by Litvinov, 1995). In gastric epithelium the
expression of Ep-CAM is low, and its increase is associated with very early stages of development of intestinal metaplasia (unpublished data). However, Ep-CAM loss associated with poor
prognosis, as demonstrated in our earlier (Songun et al, 1996) and the present study, differs from previous observations of increased Ep-CAM expression associated with poor prognosis in
breast cancer (Mirecka et al, 1995). Although not very strong, Ep-CAM mediated adhesions were able to suppress the scattering of cells embedded into matrigel (Litvinov, 1995). It was also
demonstrated that in carcinoma cells with low levels of E-cadherin, a role for Ep-CAM adhesions in interconnecting cells is increasing (Tandon et al, 1990; Basak et al, 1998). Basak et al
(1998) showed that in a model system Ep-CAM mediated adhesion can suppress invasion of tumour cells grafted in mice. Therefore, it seems quite possible that Ep-CAM negative cells are greatly
reduced in means of cell–cell adhesion, which promotes their metastasis. However, we did not find a prognostic impact for E-cadherin expression, in concordance with findings in colorectal
cancer (Van der Wurff et al, 1992, 1997). No studies have been carried out on gastric cancer previously, except for hereditary diffuse gastric cancer, which is extremely rare (Suriano et al,
2003). CD44 is a highly glycosylated cell surface molecule, which is involved in cell–cell and cell–matrix interactions (Haynes et al, 1989, 1991). It was proven that transfection with cDNA
encoding one isoform of CD44 converted nonmetastatic carcinoma and sarcoma rat cells into metastatic cells (Günthert et al, 1991). In human, high CD44 expression was shown to correlate with
tumour dissemination and poor prognosis in diffuse large cell lymphoma and in colorectal carcinoma (Koopman et al, 1993; Mulder et al, 1994). CD44 variants containing v6 were also
upregulated in activated lymphocytes (Koopman et al, 1993). However, it was also reported that CD44v6 is downregulated in tumours of squamocellular origin. Also, better differentiated
carcinomas displayed more intense reactivity than more undifferentiated ones (Salmi et al, 1993). Our data are in line with previous studies (Mirecka et al, 1995; Muller et al, 1997). Both
in the latter studies and in our study there was no association between CD44v6 expression (with VFF18) and presence of lymph node metastasis or tumour stage (data not shown). Our results
suggest that CD44v6 would not be responsible for invasive growth and metastasis formation. They rather suggest that CD44v6 containing tumours behave less aggressively, illustrated by the
fact that they are associated with significantly better survival. However, the VFF7 antibody, which is less sensitive in detecting CD44v6, was staining far fewer tumour cells and its
expression was less strong compared to the VFF18 antibody. This shows the importance of selection of the right antibodies in studies like the present one. The large amount of studies on p53
as prognostic marker gives very variable results. Our study, using just immunohistochemistry, the only generally applicable method, does not show clinical relevance. Our findings suggest the
following biological implications: loss of p53 protein or E-cadherin is an early event during oncogenesis and therefore not predictive of a metastatic behaviour (Van der Wurff et al, 1992),
whereas CD44 (v6) and Ep-CAM are late events, since Ep-CAM is positive in lymph node metastasis in patients with loss of Ep-CAM at the invasive front (unpublished observations). This
suggests that the loss we observed is not a genetic defect, but rather reflects the complex interactions of angiogenesis, adhesion, matrix degradation and inflammation, which take place
during the process of invasion and metastasis. We have demonstrated that Ep-CAM and CD44v6 provide prognostic information additional to the TNM stage in a large series of gastric cancer
patients with well documented, prospectively collected data from a randomised trial (Bonenkamp et al, 1995, 1999). Both CD44v6 and Ep-CAM expression may be helpful in identifying behaviour
of gastric adenocarcinoma. This information may be helpful in selecting patients suitable for surgery or for additional treatment pre- or postoperatively. However, additional studies are
required to establish the place of these markers in clinical management of patients with gastric cancer. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial
publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Akoh JA, Sedgwick DM, Macintyre IMC (1991) Improving results in the treatment of gastric
cancer: an 11-year audit. _Br J Surg_ 78: 349–351 Article CAS Google Scholar * Allum WH, Powell DJ, McConkey CC, Fielding JWL (1989) Gastric Cancer: a 25-year review. _Br J Surg_ 76:
535–540 Article CAS Google Scholar * Basak S, Speicher D, Eck S, Wunner W, Maul G, Simmons MS, Herlyn D (1998) Colorectal carcinoma invasion inhibition by CO17-1A/GA733 antigen and its
murine homologue. _J Natl Cancer Inst_ 90: 691–697 Article CAS Google Scholar * Bonenkamp JJ, Hermans J, Sasako M, van deVelde CJH (1999) Extended lymph node dissection for gastric
cancer. _N Engl J Med_ 340: 908–914 Article CAS Google Scholar * Bonenkamp JJ, Songun I, Hermans J, Welvaart K, van de Velde CJH, Sasako M, Plukker JTM, van Elk P, Obertop H, Gouma DJ,
Taat CW, van Lanschot J, Meyer S, de Graaf PW, von Meyenfeld MF, Tilanus H (1995) Randomized comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients.
_Lancet_ 345: 745–748 Article CAS Google Scholar * Falck VG, Gullick WJ (1989) c-erbB-2 oncogene product staining in gastric adenocarcinoma. An immunohistochemical study. _J Pathol_ 159:
107–111 Article CAS Google Scholar * Günthert BF, Liao H-X, Patton KL (1991) The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. _Cancer Cells_ 3: 347–350
Google Scholar * Günthert U, Hoffmann M, Rudy W, Reber S, Zoller M, Haussman I, Matzku S, Wenzel A, Ponta H, Herrlich P (1991) A new variant of glycoprotein CD44 confers metastatic
potential to rat carcinoma cells. _Cell_ 65: 13–24 Article Google Scholar * Günthert U, Stauder R, Mayer B, Terpe HJ, Finke L, Friedrichs K (1995) Are CD44 variants isoforms involved in
human tumour progression? _Cancer Surv_ 24: 19–24 PubMed Google Scholar * Haynes BF, Telen MJ, Hale LP, Denning SM (1989) CD44 – a molecule involved in leukocyte adherence and T-cell
activation. _Immunol Today_ 10: 423–428 Article CAS Google Scholar * Heider K-H, Dämmrich J, Skroch-Angel P, Muller-Hermelinck HK, Vollmers HP, Herrlich P, Ponta H (1993) Differential
expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. _Cancer Res_ 53: 4197–4203 CAS PubMed Google Scholar * Hermanek P,
Marnyama K, Sobin LH (1985) Prognostic factors in stomach cancer. In: Hermanck P, Gospodarowicz MK, Henson DE, Hutter RVP, Sobin LH (eds) _Prognostic factor in cancer_. Berlin: Springer,
Verlag Google Scholar * Hermans J, Bonenkamp JJ, Boon MC, Bunt AMG, Lhyama S, Sasako M, van de Velde CJH (1999) Adjuvant therapy after curative resection for gastric cancer: meta-analysis
of randomized trials. _Classic Paper Curr Comments_ 3: 281–288 Google Scholar * Houldsworth J, Cordon-Cardo C, Ladanyi M, Kelsen DP, Chaganti RS (1990) Gene amplification in gastric and
esophageal adenocarcinoma. _Cancer Res_ 50: 6417–6422 CAS PubMed Google Scholar * Koopman G, Heider KH, Horst E, Adolf GR, van den Berg F, Ponta H, Herrlich P, Pals ST (1993) Activated
human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. _J Exp Med_ 177: 897–904 Article CAS Google Scholar *
Litvinov SV (1995) Ep-CAM: a homophilic cell–cell adhesion molecule with EGF-like domains. _Trends Glycosci Glycotechnol_ 7: 375–384 Article CAS Google Scholar * Litvinov SV, Bakker HAM,
Gourevitch MM, Velders MP, Warnaar SO (1994b) Evidence for a role of the epithelial glycorotein 40 (Ep-CAM) in epithelial cell–cell adhesion. _Cell Adhesion Commun_ 2: 417–428 Article CAS
Google Scholar * Litvinov SV, Velders MP, Bakker HAM, Fleuren GJ, Warnaar SO (1994a) Ep-CAM: a human epithelial antigen is a homophilic cell–cell adhesion molecule. _J Cell Biol_ 125:
437–446 Article CAS Google Scholar * Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP (1993) _De-novo_ expression of CD44 and survival in gastric cancer.
_Lancet_ 342: 1019–1022 Article CAS Google Scholar * Mirecka J, Marx D, Schauer A (1995) Immunohistochemical localization of CD44 variants 5 and 6 in human gastric mucosa and gastric
cancer. _Anticancer Res_ 15: 1459–1465 CAS PubMed Google Scholar * Miwa K, Japanese Research Society for Gastric Cancer (1984) Evaluation of the TNM classification of stomach cancer and
proposal for its rational stage grouping. _Jpn J Clin Oncol_ 14: 385–410 CAS PubMed Google Scholar * Mulder JWR, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJ,
Pals ST (1994) Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. _Lancet_ 344: 1470–1472 Article CAS Google Scholar * Muller W, Schneiders A, Heider KH,
Meier S, Hommel G, Gabbert HE (1997) Expression and prognostic value of the CD44 splicing variants v5 and v6 in gastric cancer. _J Pathol_ 183: 222–227 Article CAS Google Scholar * Salmi
M, Grön-Virta K, Sointu P, Grenman R, Kalimo H, Jalkanen S (1993) Regulated expression of exon v6 containing isoforms of CD44 in man: downregulation during malignant transformation of tumors
of squamocellular origin. _J Cell Biol_ 122: 431–442 Article CAS Google Scholar * Songun I, van de Velde CJ, Hermans J, Pals ST, Verspaget HW, Vis AN, Menon AG, Litvinov SV, van Krieken
JH (1996) Expression of oncoproteins and the amount of eosinophilic and lymphocytic infiltrates can be used as prognostic factors in gastric cancer. _Br J Cancer_ 74(11): 1783–1788 Article
CAS Google Scholar * Suriano G, Oliveira MJ, Huntsman D, Mateus AR, Ferreira P, Casares F, Oliveira C, Carneiro F, Machado JC, Mareel M, Seruca R (2003) E-cadherin germ line missence
mutations and cell phenotype: evidence for the independence of cell invasion on the motile capabilities of the cells. _Hum Mol Genet_ 12(22): 3007–3016 Article CAS Google Scholar * Tandon
AK, Clark GM, Chamness GC, McGuire WL (1990) Association of the 323/A3 surface glycoprotein with tumor characteristics and behavior in human breast cancer. _Cancer Res_ 50: 3317–3321 CAS
PubMed Google Scholar * Terpe H-J, Stark H, Prehm P, Günthert U (1994) CD44 variant isoforms are preferentially expressed in basal epithelia of non-malignant human fetal and adult tissues.
_Histochemistry_ 101: 79–89 Article CAS Google Scholar * Van der Wurff AA, ten Kate J, van der Linden EP, ten Kate J, Bosman FT (1992) L-CAM expression in normal, premalignant, and
malignant mucosa. _J Pathol_ 168(3): 287–291 Article CAS Google Scholar * Van der Wurff AA, Vermeulen SJ, van der Linden EP, Mareel MM, Bosman FT, Arends JW (1997) Patterns of alpha- and
beta-catenin and E-cadherin expression in colorectal adenomas and carcinomas. _J Pathol_ 182(3): 325–330 Article CAS Google Scholar * Wanebo HJ, Kennedy BJ, Chmiel J, Steele Jr G,
Winchester D, Osteen R (1993) Cancer of the stomach. A patient care study by the American College of Surgeons. _Ann Surg_ 218: 583–592 Article CAS Google Scholar * Yu W, Whang I, Suh I,
Averbach A, Chang D, Sugarbaker PH (1998) Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. _Ann Surg_ 3: 347–354
Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Surgery, Leiden University Medical Center, PO Box 9600, Leiden, 2300, RC, The
Netherlands I Songun & C J H van de Velde * Department of Pathology, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC, The Netherlands S V Litvinov * Department of
Pathology, Academic Medical Center, PO Box 22660, Amsterdam, 1100 DD, The Netherlands S T Pals * Department of Medical Statistics, Leiden University Medical Center, PO Box 9600, Leiden, 2300
RC, The Netherlands J Hermans * Department of Pathology, Radbond University, Nijmegen Medical Center, PO Box 9101, Nijimegen, 6500 HB, The Netherlands J H J M van Krieken Authors * I Songun
View author publications You can also search for this author inPubMed Google Scholar * S V Litvinov View author publications You can also search for this author inPubMed Google Scholar * C
J H van de Velde View author publications You can also search for this author inPubMed Google Scholar * S T Pals View author publications You can also search for this author inPubMed Google
Scholar * J Hermans View author publications You can also search for this author inPubMed Google Scholar * J H J M van Krieken View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to J H J M van Krieken. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the
Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Songun, I., Litvinov, S., van de Velde, C. _et al._ Loss of Ep-CAM (CO17-1A) expression predicts survival in patients with gastric cancer. _Br J Cancer_
92, 1767–1772 (2005). https://doi.org/10.1038/sj.bjc.6602519 Download citation * Received: 16 August 2004 * Revised: 07 February 2005 * Accepted: 21 February 2005 * Published: 03 May 2005 *
Issue Date: 09 May 2005 * DOI: https://doi.org/10.1038/sj.bjc.6602519 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Ep-CAM * CD44 *
immunohistochemistry * prognostic factor * survival * gastric cancer
Trending News
Editorial cartoon world b. P. Oil spillSIGN UP FOR THE WEEK'S FREE NEWSLETTERS From our morning news briefing to a weekly Good News Newsletter, get the be...
The hidden talents of 24 u. S. PresidentsBrooke Nelson Alexander Brooke is a two-time Emmy-nominated tech and consumer products reporter with nearly 10 years of ...
Extortionists suspected after bomb disguised as a gift kills 2 in guanajuatoTwo men were killed and five other people were injured Sunday when a bomb disguised as a birthday present exploded in Sa...
Britain will be best performing of largest economies in 2014, imf predictsBritain will be the best performing of the world's largest economies this year, the International Monetary Fund (IM...
Building a new scotland: our marine sector — scottish national partyScotland is a proud maritime nation and our marine sector is a national asset. Brexit has significantly impacted Scotlan...
Latests News
Loss of ep-cam (co17-1a) expression predicts survival in patients with gastric cancerABSTRACT Preoperative staging of gastric cancer is difficult and not optimal. The TNM stage is an important prognostic f...
Amy grant is finding her way back after 2022 bike accident | members onlyYOU’VE TALKED ABOUT HOW PHYSICAL HEALING REQUIRES YOU TO BE EMOTIONALLY GROUNDED. HOW DO YOU MANAGE THAT? I wait until t...
Can you collect unemployment and social security?Yes, you can. Collecting unemployment insurance does not prevent you from receiving Social Security retirement benefits ...
Pathanamthitta election result 2024 live updates: congress' anto antony has won this lok sabha seatPATHANAMTHITTA LOK SABHA ELECTION RESULT 2024 LIVE UPDATES: With the counting of votes for the 2024 Lok Sabha elections ...
John swinney’s first speech as scotland’s first minister — scottish national partyPresiding Officer When I stood down as Deputy First Minister in March last year, I believed that would be the last senio...