Induction of apoptosis in human prostate cancer cell line, pc3, by 3,3′-diindolylmethane through the mitochondrial pathway
Induction of apoptosis in human prostate cancer cell line, pc3, by 3,3′-diindolylmethane through the mitochondrial pathway"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prostate cancer is the most common malignancy and the second leading cause of male death in Western countries. Prostate cancer mortality results from metastases to the bones and
lymph nodes and progression from androgen-dependent to androgen-independent disease. Although androgen ablation was found to be effective in treating androgen-dependent prostate cancer, no
effective life-prolonging therapy is available for androgen-independent cancer. Epidemiological studies have shown a strong correlation between consumption of cruciferous vegetables and a
lower risk of prostate cancer. These vegetables contain glucosinolates, which during metabolism give rise to several breakdown products, mainly indole-3-carbinol (I3C), which may be
condensed to polymeric products, especially 3,3′-diindolylmethane (DIM). It was previously shown that these indole derivatives have significant inhibitory effects in several human cancer
cell lines, which are exerted through induction of apoptosis. We have previously reported that I3C and DIM induce apoptosis in prostate cancer cell lines through p53-, bax-, bcl-2- and
fasL-independent pathways. The objective of this study was examination of the apoptotic pathways that may be involved in the effect of DIM in the androgen-independent prostate cancer cell
line, PC3, _in vitro_. Our results suggest that DIM induces apoptosis in PC3 cells, through the mitochondrial pathway, which involves the translocation of cytochrome _c_ from the
mitochondria to the cytosol and the activation of initiator caspase, 9, and effector caspases, 3 and 6, leading to poly ADP-ribose polymerase (PARP) cleavage and induction of apoptosis. Our
findings may lead to the development of new therapeutic strategies for the treatment of androgen-independent prostate cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS QUINALIZARIN INDUCES
AUTOPHAGY, APOPTOSIS AND MITOTIC CATASTROPHE IN CERVICAL AND PROSTATE CANCER CELLS Article Open access 12 February 2025 SINULARIN INDUCES AUTOPHAGY-DEPENDENT CELL DEATH BY ACTIVATING ULK1
AND ENHANCING FOXO3-ATG4A AXIS IN PROSTATE CANCER CELLS Article Open access 07 May 2025 PHARMACOLOGICALLY TARGETABLE VULNERABILITY IN PROSTATE CANCER CARRYING RB1-SUCLA2 DELETION Article 21
July 2020 MAIN Prostate cancer is the most common diagnosed malignancy and the second leading cause of male death in Western countries (Tang and Porter, 1997; Crawford, 2003). Mortality from
prostate cancer results from metastases to the bones and lymph nodes and progression from androgen-dependent disease to androgen-independent prostatic growth (Bruckheimer and Kyprianou,
2000). The androgen-dependent phase of prostate cancer may be effectively treated with androgen ablation therapies, which cause involution of the prostate gland, as a result of inhibition of
cellular proliferation and stimulation of apoptosis (Tang and Porter, 1997). However, in most of the patients, progression to the lethal stage of androgen independence, for which there is
no effective life-prolonging therapy, eventually occurs (Tang and Porter, 1997; Abate-Shen and Shen, 2000). Hence, intense investigations emerge, trying to better understand
androgen-independent disease and seeking effective means against this type of cancer. Several epidemiological studies have shown a strong correlation between consumption of diets rich in
fruits and vegetables and a lower risk of various cancers (Dragsted et al, 1993; Tavani and La Vecchia, 1995). A number of studies have demonstrated a reduced risk of developing prostate
cancer in humans consuming cruciferous vegetables, such as broccoli, Brussels sprouts, cabbage and cauliflower (Jain et al, 1999; Cohen et al, 2000; Kolonel et al, 2000). These vegetables
contain glucosinolates which, during metabolism, give rise to several breakdown products, mainly indole-3-carbinol (I3C) (Bradfield and Bjeldanes, 1987; Verhoeven et al, 1997). In a low pH
environment, I3C is converted into polymeric products, among which 3,3′-diindolylmethane (DIM) is the main one (Bradfield and Bjeldanes, 1987; De Kruif et al, 1991). It was previously shown
that these indole derivatives have inhibitory effects on the viability and proliferation of several human cancer cell lines (Ge et al, 1996; Fares et al, 1998; Bonnesen et al, 2001; Chen et
al, 2001; Chinni et al, 2001; Kedmi et al, 2003). These studies demonstrated that the indolic compounds exert their effects in the cancer cells through the induction of an apoptotic cell
response. Apoptosis, a programmed cell death, is critical for normal development, function and homeostasis of multicellular organisms, and is regulated by the expression of multiple genes
such as p53, bcl-2 family members and cytochrome _c_ (Cosulich et al, 1999; Sheikh and Fornace, 2000). Signalling for apoptosis occurs through multiple independent pathways that are
initiated by diverse extracellular and intracellular factors (Strasser et al, 2000). Activation of a family of cysteine proteases, caspases, plays a critical role in the execution of all
apoptosis signalling pathways (Nunez et al, 1998; Strasser et al, 2000). These enzymes cleave vital cellular proteins, resulting in distinct biochemical and morphological features, including
cell shrinkage, chromatin condensation and DNA fragmentation (Bortner et al, 1995; Strasser et al, 2000). We and others (Chinni et al, 2001; Kedmi et al, 2003) have previously shown that
indole derivatives originating from cruciferous vegetables, I3C and DIM, have significant inhibitory effects in prostate cancer cell lines, which are carried out by induction of apoptosis.
The purpose of our current study was to examine the apoptotic pathways that may be involved in the effect of DIM in the androgen-independent prostate cancer cell line, PC3, _in vitro_.
MATERIALS AND METHODS MATERIALS 3,3′-Diindolylmethane was purchased from Designed Nutritional Products (USA). Cell culture media and reagents were obtained from Biological Industries (Beit
Haemek, Israel). Anti-caspase 3 and anti-cytochrome _c_ monoclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-caspase 6 and 9 monoclonal antibodies
were purchased from Medical and Biological Laboratories (Japan). Anti-caspase 8 monoclonal antibody was purchased from Oncogene (Boston, MA, USA). Anti-actin monoclonal antibody was
purchased from ICN Biomedicals (Aurora, OH, USA). Secondary antibody peroxidase-conjugated goat anti-mouse IgG was purchased from Jackson Immune Research Laboratories (West Grove, PA, USA).
Colorimetric kits for the detection of caspase activity were purchased from Calbiochem (San Diego, CA, USA). All other chemicals were purchased from Sigma or other local sources. CELL
CULTURE Human prostate cancer cell line, PC3 (deficient in p53 gene, androgen-independent, poorly differentiated), was obtained from the American Type Culture Collection, Manassas, VA, USA.
Cells were grown in F-12 medium supplemented with 10% heat-inactivated foetal calf serum, and 100 U ml−1 penicillin/streptomycin (Beit Haemek, Israel). Cells were cultured at 37°C in an
atmosphere of 95% air and 5% of CO2. 3,3′-DIINDOLYLMETHANE STOCK SOLUTION 3,3′-Diindolylmethane stock solution was prepared by dissolving DIM powder in DMSO to yield a final concentration of
0.1 M. The final concentration of DMSO in the culture medium was 0.08% (v v−1). This concentration of DMSO was established as nontoxic to any cell line. TOTAL PROTEIN EXTRACTION PC3 cells
were treated with 10 ml of culture medium containing 75 _μ_ M DIM for 8, 16, 24, 48 and 72 h. The control cells were treated with 0.08% (v v−1) of DMSO solution. At the end of each
treatment, cells were collected and their total protein fraction was extracted as described previously (Kedmi et al, 2003). Protein concentrations of the cell lysates were quantified by the
method of Bradford (1976). CYTOSOL PROTEIN EXTRACTION PC3 cells were treated with 75 _μ_ M DIM for 8, 16, 24, 48 and 72 h. At the end of each treatment, cells were harvested and their
cytosol proteins were extracted, as described earlier (Bossy-Wetzel et al, 1998). Total cytosol proteins were precipitated with 10% TCA and harvested by centrifugation at 10 000 G and 4°C
for 15 min. The pellets were resuspended in 0.4 M NaOH to give a final protein concentration of 3 mg ml−1, as described previously (Grubb et al, 2001). WESTERN BLOT ANALYSIS In all, 60 _μ_g
of protein from each sample were separated by 15% SDS–polyacrylamide gels (SDS–PAGE), electrophoretically transferred onto a nitrocellulose membrane filter and incubated with specific
antibodies, as described earlier (Kedmi et al, 2003). Results were quantified by densitometer analysis (Zilber Lurma, France) using Bio1D software, and are expressed as percentages of the
respective controls. Actin level (as standard protein occurring naturally in these cells) was used as a reference. CASPASE COLORIMETRIC ASSAY PC3 cells were treated with 75 _μ_ M DIM for 8,
16, 24, 48 and 72 h. At the end of each treatment, cells were collected, proteins were extracted and the activity of caspases 3, 6, 8 and 9 was determined using colorimetric kits, according
to the manufacturer's instructions (Calbiochem). STATISTICS Western blot analyses were repeated three times, and the quantitative evaluation of the protein levels using densitometeric
analysis is presented as means±standard error (s.e.). Caspase colorimetric activity determination was repeated three times, each performed in duplicate, and the data are presented as
means±s.e. Statistical analyses of the differences between controls and treated groups were performed using Student's _t_-test. RESULTS EFFECT OF DIM ON CASPASE PROTEIN LEVELS AND
ACTIVITIES In this study, we examined the effect of DIM on the levels and activities of initiator and effector caspases, in order to better characterise the pathway through which DIM exerts
its apoptotic effects in these cells. PC3 cells were treated with 75 _μ_ M DIM for 8, 16, 24, 48 and 72 h. At the end of each treatment, cells were harvested and their total protein fraction
was extracted. Determination of caspase 3, 6, 8 and 9 levels was conducted using Western blotting analysis with a quantitative analysis of three independent blots, using a densitometer, as
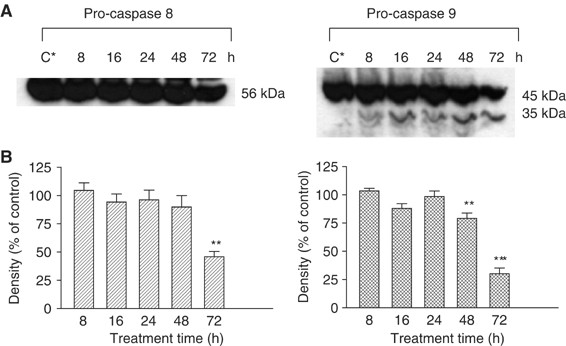
described in ‘Materials and methods’. The Western blot results shown in Figure 1 indicate the levels of the initiator caspases, pro-caspase 8 and 9. Treatment of PC3 cells with DIM for 72 h
causes a decrease in pro-caspase 8 (56 kDa) levels (Figure 1A), which was significant, according to the densitometer analysis (_P_<0.01), in comparison with the control (Figure 1B). There
was also a significant decrease in pro-caspase 9 (45 kDa) levels in these cells after exposure to DIM for 48 (_P_<0.01) and 72 h (_P_<0.001). Furthermore, the results indicate (Figure
1A) the appearance of one of the active subunits of caspase 9 (35 kDa), when cells were treated with DIM for 8 h. The levels of this subunit increase when the cells are exposed to DIM, in a
time-dependent manner. This protein is absent from the control group. Figure 2A indicates a decrease in the levels of both effector caspases, pro-caspase 3 (32 kDa) and 6 (34 kDa), after
exposure to DIM, in a time-dependent manner. Quantitative analysis of the results observed using a densitometer revealed (Figure 2B) that this decrease was significant when cells were
exposed to DIM for 48 or 72 h (_P_<0.001) in comparison with the controls. In order to strengthen the Western blot results, we further examined the activity of the above caspases using a
colorimetric detection assay kit with specific caspase substrates, as described in ‘Materials and methods’. Figure 3 demonstrates the activity of both effector and initiator caspases in PC3
cells treated with DIM. Our results indicate a significant increase in the activity of caspase 9 in these cells. The activity of this enzyme was twice that of the control when cells were
exposed to DIM for 16 h, and it increased with time and reached a level five-fold higher than that of the control after 24 h, nine-fold higher after 48 h (_P_<0.01) and 16-fold higher
after 72 h (_P_<0.01). The activity of caspase 8 also increased in these cells when exposed to DIM, starting from 24 h (twice that of the control), and reached a level eight times higher
than that of the control after 72 h. The results also show a significant increase in the activity of caspases 3 and 6, in a time-dependent manner. These caspase activities were found to be
more than 5 (_P_<0.01), 15 (_P_<0.001) and 30 (_P_<0.001) times higher when the PC3 cells were exposed to DIM for 16–24, 48 and 72 h, respectively, in comparison to the controls.
RELEASE OF CYTOCHROME _C_ TO THE CYTOSOL Cytochrome _c_ is a mitochondrial protein, which is released to the cytosol in response to a variety of apoptotic signals and promotes caspase
cascade activation through caspase 9 (Nunez et al, 1998). Since our results indicated strong evidence for the activation of caspase 9 in PC3 cells after treatment with DIM, we examined
whether the apoptotic response in these cells involves the translocation of cytochrome _c_ from the mitochondria to the cytosol. For this purpose, the protein levels of cytochrome _c_ in the
cytosol were examined using Western blotting analysis and specific monoclonal antibody, as described in ‘Materials and methods’. The results indicated that treatment of the cells with 75
_μ_ M DIM induced cytochrome _c_ release to the cytosol after 8 h (Figure 4A). This release rose with time of exposure, until reaching a maximum after 48 h. In the control group, only a
minimal leakage of this protein was observed. Quantitative results, using a densitometeric analysis, displayed the significance of this protein release (Figure 4B). Treating cells with DIM
for 16 and 24 h caused a significant release of cytochrome _c_ to the cytosol (_P_<0.05) and this release was greatly increased with exposure times of 48 and 72 h (_P_<0.001). Actin
level was used as a reference of a standard protein occurring naturally in these cells ( Figure 6). The levels of this protein were equal in each of the samples. DISCUSSION In the present
study, we attempted to explore the mechanism of action involved in the beneficial effects of the indole derivative, DIM, on human androgen-independent prostate cancer cell line, PC3. We have
previously reported that this indole derivative has a suppressive effect on the growth of prostate as well as breast cancer cell lines. This effect was mediated through the induction of an
apoptotic process, as was observed through apoptotic morphological changes and the appearance of a typical DNA ladder within treated cells (Ge et al, 1996; Fares et al, 1998; Kedmi et al,
2003). Furthermore, we found that induction of apoptosis in PC3 cells by DIM occurred through a p53-, bax-, bcl-2- and fasL-independent pathway (Kedmi et al, 2003). In this study, we
examined the levels and activities of several caspases in the same cells in response to DIM exposure. Caspases are cysteine proteases which are formed constitutively in the cells and are
normally present as inactive proenzymes. Caspases are activated during apoptosis in a self-amplifying cascade (Saraste and Pulkki, 2000). Activation of upstream or initiator caspases, such
as caspases 8, 9 and 10, by proapoptotic signals leads to the proteolytic activation of downstream or effector caspases 3, 6 and 7. The effector caspases cleave a set of vital proteins and,
thus, initiate and execute the apoptotic degradation of the cell with the typical morphological and biochemical features. Two major pathways of caspase cascade activation have been
characterised. One is initiated by ligation of death receptors and the activation of caspase 8. In the other, mitochondrial pathway, cytochrome _c_ is released from mitochondria in response
to a variety of apoptotic stimuli. In the cytosol, cytochrome _c_ can bind to apaf-1 and, in the presence of dATP or ATP, it activates caspase 9 (Nunez et al, 1998; Saraste and Pulkki,
2000). In the current study, we provide evidence that the induction of apoptosis occurring in PC3 cells by DIM is exerted through the mitochondrial pathway. Schematic diagram is presented in
Figure 5. DIM triggers cytochrome _c_ translocation from the mitochondria to the cytosol (Figure 4), which promotes the activation of caspase 9, that activates in turn caspases 3 and 6
(Figures 1, 2 and 3) in a time-dependent manner. These effector caspases are responsible for the cleaving of vital cell proteins such as the PARP protein (Kaufmann et al, 1993). We have
previously shown the cleavage of this protein in these cells after exposure to DIM (Kedmi et al, 2003). In addition, there is a slight activation of caspase 8 in these cells as well, which
may amplify the apoptotic process. It has previously been reported that caspase 8 may cleave the proapoptotic bcl-2 family member, bid, which translocates to the mitochondria, where it
triggers cytochrome _c_ release and the activation of apaf-1/caspase 9 pathway (Lou et al, 1998). Several investigations have attempted to characterise the pathways involved in the apoptotic
responses in several cancer cells exposed to indole derivatives, which are found in cruciferous vegetables. These studies found that such compounds have pleiotropic anticarcinogenic
activities and may affect many biochemical pathways. Bonnesen et al (2001) demonstrated that indole compounds originating from crucifers prevent colon cancer cell lines growth through the
induction of several detoxification enzymes. Leong et al (2001) reported cytostatic effects of DIM in human endometrial cancer cells, which was mediated by the induction of a transforming
growth factor-_α_ expression and signal transduction pathway. Carter et al (2002) showed, using cDNA microarrays, that DIM altered the expression of more than 100 genes by at least two-fold
in human cervical cancer cells. Many of the stimulated genes encode to transcription factors and proteins involved in signalling, stress response and growth. Recently, Li et al (2003)
reported the gene expression profiles of I3C- and DIM-treated PC3 cells, as was determined by cDNA microarray analysis. These researchers found that these indole derivatives up- and
downregulate the expression of a large number of genes, which are significant in the regulation of critical events such as cell growth, cell cycle and apoptosis. Chinni and Sarkar (2002)
revealed that I3C-induced apoptosis in PC3 cells is partly mediated by the inhibition of Akt activation pathway and downregulation of BAD and bcl-xL. However, it has been reported that I3C
is unstable in tissue culture medium and under acidic conditions, and is partially converted to the very stable compound, DIM (Bradfield and Bjeldanes, 1987; Ge et al, 1999; Staub et al,
2002). Therefore, the _in vitro_ effect of I3C may be partially attributed to its dimeric product, DIM. Hence, Chinni and Sarkar's (2002) study on Akt inactivation may support our
findings regarding the apoptotic pathway of DIM in PC3 cell lines. Akt, a protein kinase, was shown to inhibit apoptosis and the processing of pro-caspases to their active forms, by delaying
mitochondrial changes. Akt promotes cell survival by inhibiting the release of cytochrome _c_ from the mitochondria (Kennedy et al, 1999) and phosphorylating and inactivating caspase 9
(Cardone et al, 1998). In conclusion, in this study we provide evidence that the indole derivative, DIM, induces apoptosis in human PC3 prostate cancer cells, through the mitochondrial
pathway. Our results may contribute to a better understanding of the molecular mechanisms by which DIM exerts its effects in prostate cancer cells and tumours. These findings may lead to the
development of new therapeutic strategies for the treatment of androgen-independent prostate malignancy, for which there is no effective life-prolonging therapy. CHANGE HISTORY * _ 16
NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Abate-Shen C, Shen MM (2000)
Molecular genetics of prostate cancer. _Genes Dev_ 14: 2410–2434 Article CAS Google Scholar * Bonnesen C, Eggleston IM, Hayes JD (2001) Dietary indoles and isothiocyanates that are
generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. _Cancer Res_ 61: 6120–6130 CAS Google Scholar *
Bortner CD, Oldenburg NBE, Cidlowski JA (1995) The role of DNA fragmentation in apoptosis. _Trends Cell Biol_ 5: 21–26 Article CAS Google Scholar * Bossy-Wetzel E, Newmeyer DD, Green DR
(1998) Mitochondrial cytochrome _c_ release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. _EMBO J_ 17:
37–49 Article CAS Google Scholar * Bradfield CA, Bjeldanes LF (1987) High-performance liquid chromatographic analysis of anticarcinogenic indoles in _Brassica oleracea_. _J Agric Food
Chem_ 35: 46–49 Article CAS Google Scholar * Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye
binding. _Anal Biochem_ 72: 248–254 Article CAS Google Scholar * Bruckheimer EM, Kyprianou N (2000) Apoptosis in prostate carcinogenesis. _Cell Tissue Res_ 301: 153–162 Article CAS
Google Scholar * Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. _Science_
282: 1318–1321 Article CAS Google Scholar * Carter TH, Liu K, Ralph W, Chen D, Qi M, Fan S, Yuan F, Rosen EM, Auborn KJ (2002) Diindolylmethane alters gene expression in human
keratinocytes _in vitro_. _J Nutr_ 132: 3314–3324 Article CAS Google Scholar * Chen DZ, Qi M, Auborn KJ, Carter TH (2001) Indole-3-carbinol and diindolylmethane induce apoptosis of human
cervical cancer cells and in murine HPV16-transgenic preneoplastic cervical epithelium. _J Nutr_ 131: 3294–3302 Article CAS Google Scholar * Chinni SR, Li Y, Upadhyay S, Koppolu PK,
Sarkar FH (2001) Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. _Oncogene_ 20: 2927–2936 Article CAS Google Scholar *
Chinni SR, Sarkar FH (2002) Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC3 cells. _Clin Cancer Res_ 8: 1228–1236 CAS PubMed Google Scholar * Cohen JH,
Kristal AR, Stanford JL (2000) Fruit and vegetable intakes and prostate cancer risk. _J Natl Cancer Inst_ 92: 61–68 Article CAS Google Scholar * Cosulich SC, Savoy PJ, Clarke PR (1999)
Bcl-2 regulates amplification of caspase activation by cytochrome _c_. _Curr Biol_ 9: 147–150 Article CAS Google Scholar * Crawford ED (2003) Epidemiology of prostate cancer. _Urology_
62: 3–12 Article Google Scholar * De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, Wortelboer HM (1991) Structure elucidation of acid reaction products of
indole-3-carbinol: detection _in vivo_ and enzyme induction _in vitro_. _Chem Biol Interact_ 80: 303–315 Article CAS Google Scholar * Dragsted LO, Strube M, Larsen JC (1993)
Cancer-protective factors in fruits and vegetables: biochemical and biological background. _Pharmacol Toxicol_ 72 (Suppl): 116–135 Article Google Scholar * Fares FA, Ge X, Yannai S,
Rennert G (1998) Dietary indole derivatives induce apoptosis in human breast cancer cells. _Adv Exp Med Biol_ 451: 153–157 Article CAS Google Scholar * Ge X, Fares FA, Yannai S (1999)
Induction of apoptosis in MCF-7 cells by indole-3-carbinol is independent of p53 and bax. _Anticancer Res_ 19: 3199–3204 CAS PubMed Google Scholar * Ge X, Yannai S, Rennert G, Gruener N,
Fares FA (1996) 3,3′-Diindolylmethane induces apoptosis in human cancer cells. _Biochem Biophys Res Commun_ 228: 153–158 Article CAS Google Scholar * Grubb DR, Ly JD, Vaillant F, Johnson
KL, Lawen A (2001) Mitochondrial cytochrome _c_ release is caspase-dependent and does not involve mitochondrial permeability transition in didemnin B-induced apoptosis. _Oncogene_ 20:
4085–4094 Article CAS Google Scholar * Jain MG, Hislop GT, Howe GR, Ghadirian P (1999) Plant foods, antioxidants, and prostate cancer risk: findings from case–control studies in Canada.
_Nutr Cancer_ 34: 173–184 Article CAS Google Scholar * Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase:
an early marker of chemotherapy-induced apoptosis. _Cancer Res_ 53: 3976–3985 CAS PubMed Google Scholar * Kedmi M, Yannai S, Haj A, Fares FA (2003) Indole-3-carbinol and
3,3′-diindolylmethane induce apoptosis in human prostate cancer cells. _Food Chem Toxicol_ 41: 745–752 Article Google Scholar * Kennedy SG, Kandel ES, Cross TK, Hay N (1999) Akt/protein
kinase B inhibits cell death by preventing the release of cytochrome _c_ from mitochondria. _Mol Cell Biol_ 19: 5800–5810 Article CAS Google Scholar * Kolonel LN, Hankin JH, Whittemore
AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS (2000) Vegetables, fruits, legumes and prostate cancer: a multiethnic case–control study. _Cancer
Epidemiol Biomarkers Prev_ 9: 795–804 CAS Google Scholar * Leong H, Firestone GL, Bjeldanes LF (2001) Cytostatic effects of 3,3′-diindolylmethane in human endometrial cancer cells result
from an estrogen receptor-mediated increase in transforming growth factor-_α_ expression. _Carcinogenesis_ 22: 1809–1817 Article CAS Google Scholar * Li Y, Li X, Sarkar FH (2003) Gene
expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. _J Nutr_ 133: 1011–1019 Article CAS Google Scholar * Lou X, Budihardjo
I, Zou H, Slaughter C, Wang X (1998) Bid, a bcl-2 interacting protein, mediates cytochrome _c_ release from mitochondria in response to activation of cell surface death receptors. _Cell_ 94:
481–490 Article Google Scholar * Nunez G, Benedict MA, Hu Y, Inohara N (1998) Caspases: the proteases of the apoptotic pathway. _Oncogene_ 17: 3237–3245 Article Google Scholar * Saraste
A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. _Cardiovasc Res_ 45: 528–537 Article CAS Google Scholar * Sheikh MS, Fornace AJ (2000) Role of p53 family members
in apoptosis. _J Cell Physiol_ 182: 171–181 Article CAS Google Scholar * Staub RE, Feng C, Onisko B, Bailey GS, Firestone GL, Bjeldanes LF (2002) Fate of indole-3-carbinol in cultured
human breast tumor cells. _Chem Res Toxicol_ 15: 101–109 Article CAS Google Scholar * Strasser A, O'Connor L, Dixit VM (2000) Apoptosis signaling. _Annu Rev Biochem_ 69: 217–245
Article CAS Google Scholar * Tang DG, Porter AT (1997) Target to apoptosis: a hopeful weapon for prostate cancer. _Prostate_ 32: 284–293 Article CAS Google Scholar * Tavani A, La
Vecchia C (1995) Fruit and vegetable consumption and cancer risk in a Mediterranean population. _Am J Clin Nutr_ 61: 1374S–1377S Article CAS Google Scholar * Verhoeven DTH, Verhagen H,
Goldbohm RA, van den Brandt PA, van Poppel GA (1997) Review of mechanisms underlying anticarcinogenicity by Brassica vegetables. _Chem Biol Interact_ 103: 79–129 Article CAS Google Scholar
Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Faculty of Food Engineering and Biotechnology, Technion-Israel Institute of Technology, Haifa, 32000, Israel M
Nachshon-Kedmi & S Yannai * Department of Biochemistry and Molecular Genetics, Carmel Medical Center, Haifa, Israel F A Fares * Faculty of Medicine, Technion-Israel Institute of
Technology, Haifa, Israel F A Fares Authors * M Nachshon-Kedmi View author publications You can also search for this author inPubMed Google Scholar * S Yannai View author publications You
can also search for this author inPubMed Google Scholar * F A Fares View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to F A Fares. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported
License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nachshon-Kedmi, M., Yannai, S.
& Fares, F. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. _Br J Cancer_ 91, 1358–1363 (2004).
https://doi.org/10.1038/sj.bjc.6602145 Download citation * Revised: 18 June 2004 * Accepted: 16 July 2004 * Published: 24 August 2004 * Issue Date: 04 October 2004 * DOI:
https://doi.org/10.1038/sj.bjc.6602145 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * 3,3′-diindolylmethane * apoptosis * caspases * PC3
cells * prostate cancer
Trending News
Clint Eastwood once branded ‘intimidating as hell’ by Tom Hanks: ‘Treats us like horses!''Where Eagles Dare', starring Clint Eastwood, airs on ITV 4 today. The World War 2 action film, written by Alistair MacL...
Aarp homefit video: hallways and stairwaysMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Mark bittman’s summer recipes for two | members onlyThere is a food-related reason people love the Riviera, which can be taken to mean Provence, France, and Liguria, Italy...
Reba mcentire's tasty mexican cornbread recipeMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Seale civil rights murder trial begins, 43 years onOpening statements are heard in the case of James Ford Seale, who has pleaded not guilty to federal kidnapping and consp...
Latests News
Induction of apoptosis in human prostate cancer cell line, pc3, by 3,3′-diindolylmethane through the mitochondrial pathwayABSTRACT Prostate cancer is the most common malignancy and the second leading cause of male death in Western countries. ...
The celebrities, family members with taylor swift at super bowl 2024EXPLORE MORE Taylor Swift didn’t show up to Super Bowl 2024 solo. The 14-time Grammy Award winner walked into Allegiant ...
Two people receive medical treatment after trench rescue near midway districtTwo people were taken to hospitals with unspecified injuries Tuesday after they were rescued from a construction-site tr...
Will the uk’s tactics to win a free-trade deal with india work?From India’s perspective, a deal would reduce its reliance on trade with China at a time of _very chilly relations_ betw...
Is early specialisation the worst feature of english education? | thearticleOnce upon a time, the traditional way to start a public debate in Britain was to write a letter to the Editor of The Tim...