Fda authorizes emergency use of remdesivir for covid-19
Fda authorizes emergency use of remdesivir for covid-19"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
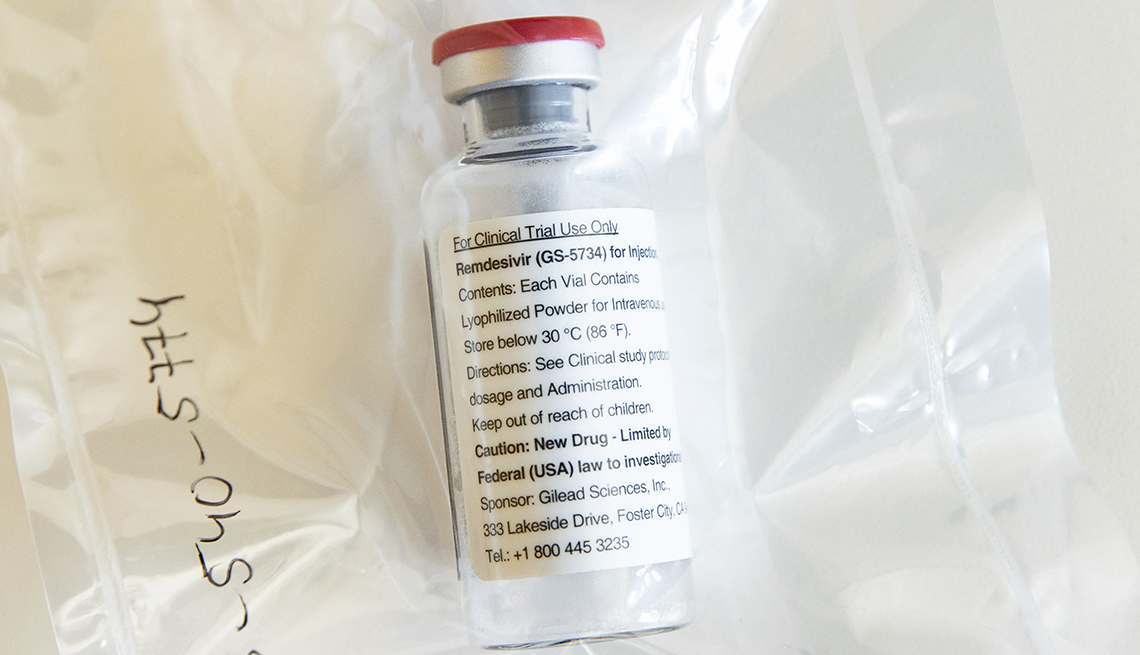
The Food and Drug Administration (FDA) on Friday issued an emergency use authorization for Gilead Sciences’ antiviral drug remdesivir as a treatment option for people who are hospitalized
with COVID-19, the illness caused by the new coronavirus. To date, the virus has killed more than 237,000 people worldwide. The news comes just days after the National Institutes of Health
announced preliminary results from an international clinical trial testing remdesivir as a potential treatment for COVID-19. Researchers found that patients with severe illness who received
the drug recovered faster than those who received a placebo. “Results also suggested a survival benefit,” the NIH said in a news release. The mortality rate for the group that received
remdesivir was lower than for the placebo group. The emergency use authorization — which is not the same as official FDA approval — “allows for remdesivir to be distributed in the U.S. and
administered intravenously by health care providers” to treat patients who have low blood oxygen levels or who need breathing support by way of a mechanical ventilator. "Based on
evaluation of the emergency use authorization criteria and the scientific evidence available, it was determined that it is reasonable to believe that remdesivir may be effective in treating
COVID-19, and that, given there are no adequate, approved, or available alternative treatments, the known and potential benefits to treat this serious or life-threatening virus currently
outweigh the known and potential risks of the drug's use,” the FDA said in a news release. During public health emergencies, the FDA has the power to bypass its normally lengthy
approval process to allow “unapproved medical products or unapproved uses of approved medical products” to diagnose, treat or prevent serious or life-threatening diseases or conditions “when
there are no adequate, approved, and available alternatives.” There is no approved treatment for COVID-19 and no vaccine for the coronavirus, which emerged in December and has since
sickened millions worldwide. In March, the FDA authorized the emergency use of two malaria drugs, chloroquine and hydroxychloroquine, to treat COVID-19. Less than a month later, on April 24,
the FDA warned doctors over use of the two antimalarials, citing serious and potentially life-threatening heart rhythm problems. In Friday's announcement, FDA Commissioner Stephen Hahn
said, “Today's action is an important step in our efforts to collaborate with innovators and researchers to provide sick patients timely access to new therapies where appropriate,
while at the same time supporting research to further evaluate whether they are safe and effective.”
Trending News
Crmage: crispr optimized mage recombineeringABSTRACT A bottleneck in metabolic engineering and systems biology approaches is the lack of efficient genome engineerin...
Medical records office | va providence health care | veterans affairsPer VHA Directives, we have 20 business days to process all requests. Requests are accepted in-person, through My Health...
Recent patents in hematopoietic cell gene therapyRIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Recent patents in hematopoietic cel...
Regional tuning of photoreceptor adaptation in the primate retinaABSTRACT Adaptation in cone photoreceptors allows our visual system to effectively operate over an enormous range of lig...
Video shows brutal hair-pulling brawl on flight over space in overhead binsWe all know that snagging space in a plane's overhead bins can be a stressful endeavor. But in one video circulatin...
Latests News
Fda authorizes emergency use of remdesivir for covid-19The Food and Drug Administration (FDA) on Friday issued an emergency use authorization for Gilead Sciences’ antiviral dr...
Holly willoughby: star's celebrity juice replacement disappoints fansCelebrity Juice continued last night on ITV, with comedian Chris Ramsey and singer Rita Ora stepping in for Holly Willou...
Homeless veteran care | veterans affairsIf you are a Veteran who is homeless or at risk of becoming homeless due to financial hardship, unemployment, addiction,...
Limousin society withdraws 15 jacot progeny - farmers weekly© Tim Scrivener A Lincolnshire Limousin herd has had 15 animals withdrawn from the breed herdbook owing to government co...
Page Not Found很抱歉,你所访问的页面已不存在了。 如有疑问,请电邮[email protected] 你仍然可选择浏览首页或以下栏目内容 : 新闻 生活 娱乐 财经 体育 视频 播客 新报业媒体有限公司版权所有(公司登记号:202120748H)...